Abstract
The most severe consequences of dengue virus infection include shock, haemorrhage, and major organ failure; however, the frequency of these manifestations varies, and the relative contribution of pre-existing anti-dengue virus antibodies, virus characteristics, and host factors (including age and comorbidities) are not well understood. Reliable characterisation of the epidemiology of severe dengue first depends on the use of consistent definitions of disease severity. As vaccine trials have shown, severe dengue is a crucial interventional endpoint, yet the infrequency of its occurrence necessitates the inclusion of thousands of study participants to appropriately compare its frequency among participants who have and have not been vaccinated. Hospital admission is frequently used as a proxy for severe dengue; however, lack of specificity and variability in clinical practices limit the reliability of this approach. Although previous infection with a dengue virus is the best characterised risk factor for developing severe dengue, the influence of the timing between dengue virus infections and the sequence of dengue virus infections on disease severity is only beginning to be elucidated. To improve our understanding of the diverse factors that shape the clinical spectrum of disease resulting from dengue virus infection, prospective, community-based and clinic-based immunological, virological, genetic, and clinical studies across a range of ages and geographical regions are needed.
Introduction
The global incidence of dengue has doubled each decade for the past 30 years, with recent estimates of over 100 million infections and 50 million cases per year.1-3 Although clinically severe disease is an uncommon outcome of dengue virus (DENV) infection, more than 50% of the estimated US $8·9 billion global financial burden of dengue results from patients who are admitted to hospital or die.1,2 The epidemiological characteristics of dengue are variable and complex, and many facets are incompletely understood.4 Studies that have used crosssectional surveillance data frequently report the proportion of patients with clinically severe disease, generally considering patients who have been admitted to hospital to have severe disease; however, because many patients with dengue either do not seek care or are not admitted for care,5 there is little clarity about the actual frequency of severe disease among all ill individuals infected with any of the four dengue viruses (DENV-1–4). Such data have been reported from few prospective, mostly paediatric, cohort studies,6 as well as from multi-site evaluations of paediatric dengue vaccine candidates;7,8 however, the factors affecting the occurrence of clinically severe dengue (a term which, as used in this Personal View, includes both severe dengue9 and dengue haemorrhagic fever and dengue shock syndrome10), and particularly the interplay between these factors, are not well understood. An improved understanding of the risk factors associated with developing clinically severe dengue is needed to optimise the design and evaluation of effective and safe clinical interventions and vaccine interventions, to reduce the morbidity and mortality of dengue.11,12
Status of past infection with a DENV (ie, serostatus) is perhaps the most well known risk factor for developing clinically severe dengue, considering that individuals with secondary DENV infection are typically over-represented among patients with clinically severe dengue;12 however, clinically severe dengue can also occur after primary DENV infection in children and adults.13 Studies with prospective data and mathematical models further show the importance of previous DENV infection as a risk factor for clinically severe dengue.14,15
Multiple mechanisms probably contribute to increased disease severity during secondary DENV infection. Nonneutralising antibody binding to the virus, followed by uptake in Fc receptor-bearing monocytes, might result in higher and longer magnitude of viraemia (ie, antibody-dependent enhancement). An accompanying exacerbated immune response might also occur, in which activated natural killer cells and memory T cells trigger inflammatory mediators that contribute to intravascular leakage.16 The viral protein nonstructural protein 1 is secreted from infected cells and is independently associated with vascular leakage by damaging the endothelial glycocalyx and disrupting endothelial cell junctions. This process might be exacerbated during secondary infection due to heightened viraemia.17
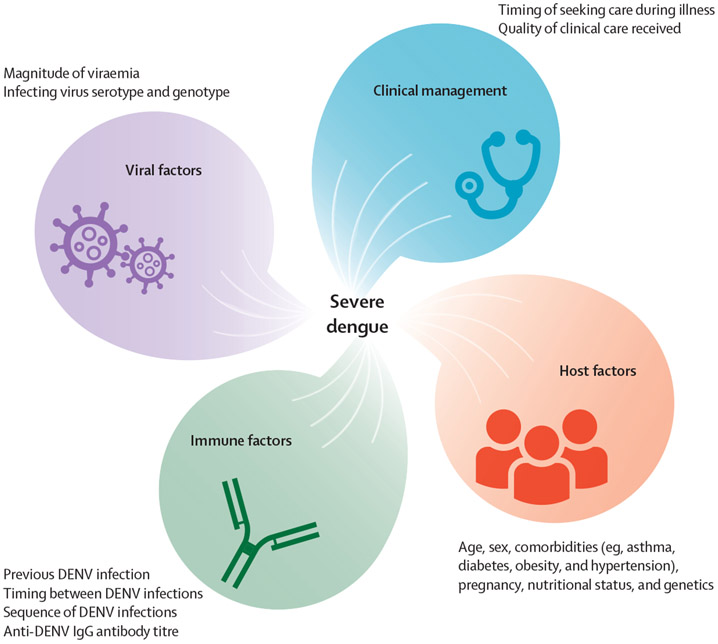
Multiple studies have also shown important roles for various viral and host factors in disease severity (figure). Accumulating evidence for all four DENVs suggests that genotype-specific viral factors can result in phenotypic changes in viraemia, disease severity, and epidemic potential.18-22 Additionally, host genetics has long been thought to have a role in disease severity, which has been evidenced by case-control studies.23,24 Vascular leakage and shock tend to occur more frequently in children than in adults.25 Several other risk factors for developing clinically severe dengue have been identified, including sex, underlying comorbidities (eg, asthma, obesity, diabetes, and cardiac disorders), pregnancy, virus serotype, and sequence of and interval between DENV infections (panel 1). Because multiple epidemiological factors are associated with disease severity, much effort has focused on the discovery of simple, generalisable biomarkers that reliably identify patients who will progress to clinically severe dengue, which has been elusive.43
Figure: Factors for which epidemiological evidence has shown an association with the clinical severity of disease resulting from DENV infection.
DENV=dengue virus.
Panel 1: Factors associated with clinical severity of disease resulting from dengue virus (DENV) infection.
Identification of pathophysiological risk factors that affect the development of clinically severe dengue is complicated by the unclear relative contribution of previous DENV infection, spatial and temporal heterogeneities in historic and current levels of population-level DENV transmission, and both host and viral characteristics. Although longitudinal cohort studies have proven to be instrumental in identifying and describing factors associated with disease severity,6 these studies are resource-intensive and, by design, focus on individuals at high risk for infection (ie, children in highly endemic areas). This focus limits the generalisability of findings to other age groups, epidemiological contexts, and populations with variable frequencies of genetic predisposition to disease and prevalence of comorbid conditions, all of which might also influence disease severity. For example, the epidemiology of clinically severe dengue in Africa and in Afro Latino individuals has not been sufficiently investigated.44 Resolving the factors that contribute to both the pathophysiology and the observed epidemiology of clinically severe dengue has gained attention after the results of the first licensed vaccine against dengue.
CYD-TDV (Dengvaxia), developed by Sanofi Pasteur, is a three-dose, live-attenuated, tetravalent vaccine.45 Phase 3 clinical trials were completed among more than 30 000 paediatric participants from Asia and Latin America.46,47 Under advice from the Strategic Advisory Group of Experts in April, 2016,48,49 because of a safety signal in children aged 2–5 years in year 3 of the Asian trial, WHO initially recommended that the vaccine only be used in populations with DENV seroprevalence of 70% or greater by age 9.49 In November, 2017, Sanofi Pasteur released new analyses of 60 months of follow-up data indicating that, despite substantial benefit among seropositive individuals, vaccination of seronegative individuals increased the risk of developing more severe disease (defined using definitions of either severe dengue or dengue haemorrhagic fever and dengue shock syndrome) on subsequent natural infection.47,50 Consequently, WHO revised its recommendation for Dengvaxia such that only individuals who have been tested and shown to have evidence of previous DENV infection should be vaccinated.51,52 In 2019, the US Food and Drug Administration approved the use of Dengvaxia in children aged 9–16 years who have evidence of previous DENV infection and live in areas of the USA where dengue is endemic.53 The requirement for prevaccination screening and the current absence of a fully evaluated and available screening test with high specificity complicates the implementation of Dengvaxia in national vaccine programmes.
The outcomes of the trials of Dengvaxia and subsequent follow-up studies show the complexity and importance of elucidating the factors that contribute to dengue severity.54 Such findings will be of continued importance during evaluation of Dengvaxia and additional dengue vaccine candidates, including Takeda’s vaccine, TAK-003, for which the initial results are promising but not without concern regarding unequal protection by serotype and serostatus of potential vaccinees.8 Other vaccine candidates have raised the concern of intraserotype antigenic variability potentially affecting vaccine effectiveness and either protection from or progression to clinically severe dengue.55,56 The role of genotype variation on vaccine efficacy has also been reported for Dengvaxia.57,58 In this Personal View, we describe current limitations that affect our understanding of the epidemiology of clinically severe dengue and make recommendations regarding how such challenges might be resolved.
Case definitions to identify and study clinically severe dengue
Case definitions are the metric by which clinical and epidemiological studies assess and compare outcomes; however, the use of consistent and comparable definitions has been an impediment to dengue research since the 19th century.59 Of paramount importance is accurately diagnosing dengue by reliably identifying and differentiating acute, recent, and historic DENV infection through the detection of several factors: viral nucleic acid by RT-PCR; nonstructural protein 1 by ELISA; anti-DENV IgM or IgG antibody by ELISA, in some cases followed by confirmation with a neutralising antibody test; and viral antigen or antibodies by rapid diagnostic test.60 Doing so is not trivial, given that much variation exists in assays used to define DENV infection and serostatus. Furthermore, many studies also seek to identify which individuals develop symptomatic DENV infection (ie, dengue), the definition of which also varies between studies. Some studies consider dengue to be any illness that meets a specified clinical case definition regardless of whether the individual sought medical care or had laboratory diagnostic evidence of dengue, whereas other studies consider symptomatic dengue to be a clinically apparent disease, and other studies refer to subclinical infection as any infection for which the infected individual did not seek clinical care regardless of the presence or absence of disease. Similarly, various studies use different denominators for the calculation of dengue case fatality rates, including all DENV infections, all symptomatic DENV infections, all clinically apparent cases, or all people admitted to the hospital.1,2,61-64 Hence, a need remains for the common use of terminology and case definitions to enable comparison across studies, to obtain a more holistic understanding of the pathophysiology and global burden of dengue.
These ambiguities are exacerbated when considering clinically severe manifestations of DENV infection. Nearly all epidemiological studies define disease severity according to either the WHO 1997 case definition for dengue haemorrhagic fever and dengue shock syndrome10 or the 2009 revised case classification that reframed all patients with clinically severe disease into a single category of severe dengue9 (panel 2). Notably, the WHO 1997 case definition of dengue haemorrhagic fever and dengue shock syndrome focuses on thrombocytopenia, haemorrhagic manifestations, plasma leakage, and shock as the metrics of severe disease in patients with dengue, and although the 2009 classification of severe dengue similarly includes bleeding and plasma leakage, this 2009 classification also includes other life-threatening manifestations of DENV infection (eg, meningoencephalitis and myocarditis) not captured by the definition of dengue haemorrhagic fever and dengue shock syndrome that might occur from pathophysiological processes distinct from those resulting in plasma leakage. Although systematic review of dengue case classification studies suggested that the 2009 classification is more sensitive,66 evaluations in multiple jurisdictions have shown that both definitions have clinical merit as well as additional use for research studies.66-68 In particular, while vaccine trials and other clinical studies need to monitor the impact of vaccine and other interventions on reducing the risk and costs of all disease and especially severe dengue, immunopathogenesis studies might be better served by use of the dengue haemorrhagic fever and dengue shock syndrome definition. Consequently, clinical research studies, and specifically vaccine trials, should ideally evaluate results using outcomes of both severe dengue and dengue haemorrhagic fever and dengue shock syndrome. In all cases, assessing factors associated with disease severity to identify generalisable epidemiological trends will be greatly assisted by use of consistent measures of clinical outcomes.
Panel 2: Dengue clinical* case definitions as established by WHO in 1997 and 2009.
WHO 1997
Dengue fever:
- Fever, along with at least two of the following:
- Headache
- Retro-orbital pain
- Myalgia
- Arthralgia
- Rash
- Haemorrhagic manifestations
- Leukopenia
Dengue haemorrhagic fever:
Fever or history of fever lasting 2–7 days
- Haemorrhagic tendencies, including at least one of the following:
- Positive tourniquet test
- Petechiae, ecchymoses, or purpura
- Bleeding from the mucosa, gastrointestinal tract, injection sites, or other locations
- Haematemesis or melena
Thrombocytopenia (≤100 000 cells per μL)
- Evidence of plasma leakage due to increased vascular permeability, manifested by at least one of the following:
- An increase in haematocrit equal to or greater than 20% above average for age, sex, and population
- A decrease in haematocrit following volume replacement treatment equal to or greater than 20% of baseline
- Signs of plasma leakage, such as pleural effusions, ascites, and hypoproteinaemia
Dengue shock syndrome:
- All four criteria for dengue haemorrhagic fever, plus evidence of circulatory failure manifested by:
- Rapid and weak pulse and narrow pulse (<20 mmHg); OR
- Hypotension for age, cold, clammy skin, and restlessness
WHO 2009
Dengue:
- Fever, and two of the following:
- Nausea, vomiting
- Rash
- Aches and pains
- Tourniquet test positive
- Leukopenia
- Any warning sign
Dengue with warning signs:
- Meet criteria for dengue, plus any of the following:
- Abdominal pain or tenderness
- Persistent vomiting
- Clinical fluid accumulation
- Mucosal bleed
- Lethargy, restlessness
- Liver enlargement (>2 cm)
- Increase in haematocrit concurrent with rapid decrease in platelet count
Severe dengue:†
- Meet criteria for dengue, plus any of the following:
- Severe plasma leakage leading to:
- Shock
- Fluid accumulation with respiratory distress
- Severe bleeding, as evaluated by clinician
- Severe organ involvement:
- Liver: aspartate transaminase or alanine aminotransferase more than or equal to 1000 units
- CNS: impaired consciousness
- Heart or other organ
* Completion of full case definitions also require completion of relevant epidemiological and laboratory criteria not specified here. †Refined by Tomashek and colleagues.65
Incidence of clinically severe dengue
The large cohorts from the Dengvaxia vaccine trials in five Asian and five Latin American countries provide the best available estimates of the frequency of dengue, dengue haemorrhagic fever and dengue shock syndrome, and severe dengue across regions.5,7 Among children aged 2–16 years, approximately 10% of febrile episodes were attributed to virologically-confirmed dengue (VCD), with 4·6 and 2·9 episodes of VCD per 100 person-years occurring in Asian and Latin American cohorts, respectively. The incidence of dengue haemorrhagic fever was less than 0·3 episodes per 100 person-years in each cohort; 61 (19·1%) of 319 VCD episodes in the Asian cohort and 43 (11·1%) 389 of VCD episodes in the Latin American cohort required hospital admission. Among comparable age groups (9–12 years and 13–16 years), the burden of dengue was higher in Asia than Latin America.
Other manifestations of severe dengue include myocarditis, liver failure, and neurological complications, including meningoencephalitis and Guillain-Barré syndrome. Such manifestations appear to be uncommon compared with shock and hemorrhage,65,69,70,71 although reliable estimation of their prevalence requires enrolment of a large number of patients with dengue. Hence, only the Dengvaxia vaccine trial has yielded potentially generalisable estimates of the prevalence of uncommon but severe manifestations of dengue among non-vaccinated children aged 2–16 years (ie, among 1094 cases of VCD, 13 [1·2%] included visceral manifestations).50
Although clinical case definitions of non-shock severe manifestations of disease have historically been variable, which has further complicated estimation of their prevalence, suggested case definitions have been developed by a panel of clinical experts in 2018.65 With the use of these definitions developed by the panel, the frequency of the various alternative manifestations of severe dengue should be assessed in both adult and paediatric populations, considering that the underlying prevalence of comorbidities that contribute to development of severe and fatal dengue (eg, chronic liver, kidney, or heart disease) differs in children and adults. Notably, accurate evaluations of such definitions are expected to be complicated in patients coinfected with DENV and other pathogens. For example, coinfection with DENV and chikungunya virus, Leptospira spp bacteria, or parasites of the genus Plasmodium might modulate clinical presentation.72-76 Similarly, patients who are admitted to hospital are at increased risk for poor outcome due to nosocomial infections.77 Although it would be ideal to systematically test patients with severe dengue for a wide array of other pathogens representing potential nosocomial infections or coinfections, geographical and temporal heterogeneity in the possibilities make this approach infeasible. An alternative approach would be banking of blood at different times during illness for targeted retrospective investigations.
Patient-specific risk factors for clinically severe dengue
Changes in clinical suspicion of dengue in adults and changing demographics in some countries have led to a renewed recognition of the burden of clinically severe dengue in adults.4,78 Such observations have shown that the clinical features of dengue, and possibly its pathophysiology, might differ between children and adults, including the likelihood of progressing to symptomatic infection and developing the most common manifestations of severe dengue (eg, plasma leakage and shock are more common in children, whereas adults more frequently experience haemorrhage).25,68,79-81 A variety of intrinsic and modifiable risk factors might predispose adults for severe dengue. For example, adults might be more likely to develop bleeding due to underlying peptic ulcer disease or anticoagulant medications,82 and might also be more likely to have comorbidities, such as renal failure or heart failure, that complicate fluid management.64
Similarly, risk of developing uncommon but severe manifestations of DENV infection, such as myocarditis and meningoencephalitis, might increase with age or underlying comorbidities. However, few data are available that have identified risk factors for developing such manifestations or estimated their relative frequency compared with shock and haemorrhage. Identification of these risk fators is complicated by the dearth of reports that reliably quantify and differentiate the proportion of patients with severe dengue that meet case definitions for severe organ involvement, as well as infrequent occurrence of such cases in cohort studies with well defined data on comorbidities, demographics, and infection history.
Consequently, studies that enrol both children and adults are needed to elucidate the mechanisms of progression to clinically severe dengue, including age-specific effects of serostatus.26
Interplay between disease severity and transmission intensity
Recent models have shown the global variability in DENV transmission intensity (ie, force of infection) and its strong effect on the observed epidemiology of both dengue and severe dengue.3,83 The duration of protective immunity might be extended through boosting of antibody titres after re-exposure either to a DENV serotype with which the individual was previously infected or to a new DENV serotype.29,84 Moreover, an individual’s titre of cross-reactive neutralising antibodies affects their likelihood of progression to symptomatic infection,15,29,85,86 possibly as a function of the interval between infections.30,87,88 Accordingly, cross-protection from progression to dengue following heterotypic infection occurs for a short period of time (6 months to 2 years);30,88 however, when infections occur more than 2 years apart, and specifically when mid-range antibody titres are present, the risk of developing severe dengue increases.14,15,54,88 One model suggested that a sustained high force of infection might result in an overall lower incidence of symptomatic infection and severe dengue, whereas a mid-level force of infection could result in a higher proportion of both symptomatic infection and severe dengue.89 If true, the potential effect of a dengue vaccine on these trends is unclear. If a high level of protective immunity against all four DENVs is not achieved, a dengue vaccine or other interventions that effectively reduce the overall force of infection could, in theory, increase the proportion of patients with dengue who develop severe dengue.90,91
Although post-secondary DENV infections are less likely to result in symptomatic infection,92,93 the effect of previous infection with other flaviviruses on the severity of dengue is only beginning to be understood.94 In Thailand, pre-existing antibodies against Japanese encephalitis virus were associated with an increased risk of developing symptomatic DENV infection,95 whereas early studies from Sabin showed that Japanese encephalitis virus antibodies protected against symptomatic DENV infection.87 The original Japanese encephalitis virus vaccine efficacy study observed a nonsignificant decrease in dengue haemorrhagic fever among vaccinees, and disease severity among individuals with dengue was reduced.96 Interestingly, dengue haemorrhagic fever-like illness was reported in a patient with West Nile virus infection and historic DENV infection.97 Conversely, although data from Colombia and Puerto Rico showed no apparent effect of pre-existing anti-DENV antibodies on the magnitude of viraemia during Zika virus infection in vivo,98,99 recent reports from Brazil and Nicaragua showed that DENV crossreactive immunity protects against symptomatic Zika virus infection.28,100,101 By contrast, recent findings have shown that previous Zika virus infection increases the risk of subsequent symptomatic infection with DENV-2 and worsens disease severity.28 Overall, potential immune interactions and asymmetries between DENV and other flaviviruses are of interest for both better epidemiological understanding and vaccine development, and require further investigation.94
Accuracy of hospital admission as a proxy for disease severity
Clinically severe dengue is a crucial clinical endpoint, but this endpoint is not readily targeted in either clinical or community-based cohort studies because it requires very large numbers of study participants. Instead, multiple vaccine trials have used hospital admission as the most readily available clinical endpoint to evaluate potential increases in disease severity. Although reasonable with respect to study design and cost effectiveness, in practice many factors affect rates of hospital admission among patients with dengue, including age and sex of the patient,102,103 comorbidities,9 status of hydration,104 occurrence of an epidemic,105,106 presence of dengue warning signs,9 clinical acuity,102,103 and socioeconomic status.107 When trials are done in multiple jurisdictions in which both patient characteristics and hospital admission practices differ, hospital admission as an outcome is not a precise or reliable indicator of disease severity. Consequently, differences in disease severity based on the observed frequency of hospital admission should be interpreted with caution, particularly when comparing between regions. The use of dengue warning signs is also limited as a measure of disease severity, given that some warning signs are variably defined (eg, abdominal pain or lethargy), and the presence of warning signs does not clearly represent a true increase in disease severity.
Because appropriate clinical management can result in substantial decreases in both morbidity and mortality among patients with dengue,41,108,109 patient outcomes can also be worsened by attitudes regarding seeking care for dengue-like illness, access to care, and biases in hospital admission of patients with dengue by age, sex, and other characteristics.4,107 Patients’ risk of developing clinically severe dengue is also affected by a variety of factors beyond their control, including the experience of medical personnel managing the patient and the availability of clinical and diagnostic resources, including intensive care facilities. In areas with poor health-care infrastructure or other societal disruptions that limit the patients’ ability to receive appropriate medical care, dengue patient outcomes suffer and case fatality rates increase.110 These variables, as well as infrastructure for case reporting,13,44 hamper the comparison of the burden and epidemiology of clinically severe dengue between regions and over time and affect estimates of the global burden of dengue, disability-adjusted life years lost to dengue, and ultimately the effectiveness of vaccines and other interventions.
Conclusions
Although large cohorts to evaluate dengue vaccine efficacy have provided valuable insight into the epidemiology of dengue in endemic areas, major gaps in study methods and knowledge persist and preclude a thorough understanding of the epidemiology of clinically severe dengue. These gaps include several factors: inconsistent use of case definitions; unknown generalisability and relative contribution of demographic, virological, immunological, genetic, and clinical characteristics on the risk of developing clinically severe dengue; unclear comparability of hospital admission rates between and within regions; and absence of generalisable data on the frequency of severe dengue and death due to dengue, which are the major drivers of the human and economic burden of dengue (table). Moving forward, use of uniform measures of disease severity, including case definitions and clinical endpoints, will provide the most reasonable measure by which to make comparisons.111 Multi-partner consortiums should be formed to better elucidate the generalisable aspects of clinically severe dengue and identify key determinants of disease severity by combining and comparing data from paediatric and adult prospective cohort studies in multiple jurisdictions and by integrating these data with findings from facility-based enhanced surveillance.112 Mathematical models combined with data from both seroprevalence and cohort studies will aid in estimating the parameters governing clinically severe dengue by explicitly incorporating the similarities and differences between cohorts and by including the uncertainty from different types of data.66,113 As vaccines and other interventions likely to affect the intensity of DENV transmission are introduced, a thorough understanding of the factors affecting the occurrence of clinically severe dengue will be of increasing importance to assess and implement interventions for, and define progress in, reducing the disease burden resulting from DENV infection.
Table:
Knowledge gaps in the epidemiology of clinically severe dengue
| Relevance | Challenges | Next steps | |
|---|---|---|---|
| How best to implement use of uniform measures of disease severity? | Comparability of study findings and burden of dengue in different jurisdictions. | Disagreement in the field regarding if separate or unified definitions should exist for clinical management, case classification, and research studies. | Definitions for research studies should differentiate between complications of DENV infection and exacerbation of underlying illnesses to appropriately measure patient outcomes. |
| What is the frequency of the different manifestations of clinically severe dengue (eg, shock vs haemorrhage vs encephalitis vs myocarditis)? | Although shock and haemorrhage are the most common manifestations, the relative frequency of the other manifestations has not been defined. Evaluation of vaccine safety will necessitate an appropriate capture of clinically severe dengue in all populations. |
Large sample sizes are needed to reliably capture and describe the frequency of uncommon events, such as clinically severe dengue. Less common manifestations might be more common in individuals with comorbidities. |
Multi-year, multi-centre study from sites managing large numbers of patients with clinically severe dengue. Case-control study to compare patients with uncommon manifestations of clinically severe dengue to matched patients with non-severe dengue. |
| What demographic and epidemiological variables affect progression to clinically severe dengue? | Many factors have been identified, but few have been consistently identified across studies. Manifestations of clinically severe dengue might differ by age, sex, serostatus, genetic backgrounds, and infecting DENV serotype and genotype; comorbidities (eg, asthma, diabetes, and obesity) increase risk of severe dengue, but it is unclear how. Co-infection might increase disease severity, particularly in patients who are admitted to hospital. Unclear if or how factors interact. Mechanism of pathogenesis are not fully understood, complicating vaccine design and evaluation. |
Severe dengue is an uncommon occurrence. Diagnostic testing for co-infection might not be feasible in or done on patients with dengue. Enrolling a sufficient number of individuals to evaluate outcomes while controlling for interactions is prohibitive. |
Combine data from studies in various settings after ensuring equivalent collection and definition of relevant variables. Develop outbreak protocols to systematically address these issues. |
| How does disease severity affect care-seeking, hospital admission, and detection by surveillance? | Case surveillance is often biased by severity of disease. Surveillance in many jurisdictions is the sole indicator used to estimate burden of disease, disability-adjusted life years lost to dengue, and potential vaccine impact. | Modifying surveillance is both infeasible and will probably affect analysis of long-term trends. Collection of community-based data is resource-intensive. |
Conduct enhanced surveillance to better define the frequency of clinically apparent dengue, which might be used as a jurisdictional multiplier. Conduct rapid community-based surveys during epidemics to estimate frequency with which individuals with dengue seek care, are admitted to hospital, and are identified by surveillance. |
| Can hospital admission be used as a proxy for clinically severe dengue? | Hospital admission is a more common outcome and easier to document than clinically severe dengue. Hospital admission is an important measure for vaccine evaluation. |
Hospital admission practices can differ widely between and within jurisdictions. | Evaluate data from individual settings to determine if trends in clinically severe dengue are reflected by those of patients with dengue who have been hospital admission. |
DENV=dengue virus.
Key messages.
A holistic understanding of the myriad factors that affect progression to clinically severe dengue is needed to optimise the design and evaluation of safe and effective vaccines, to reduce the morbidity and mortality of dengue.
Multiple, oftentimes disparate, case definitions have been used to define patients with clinically severe dengue, which complicates the comparison of findings from diverse studies. To overcome this impediment in the field, clinical research studies, and specifically vaccine trials, should evaluate results using outcomes of both severe dengue and dengue haemorrhagic fever and dengue shock syndrome.
Multiple disease manifestations constitute clinically severe dengue (eg, shock, haemorrhage, encephalitis, and myocarditis) that might arise from diverse pathophysiological pathways resulting from dengue virus (DENV) infection, which in turn are affected by factors specific to the individual, virus, and population. Combined, these pathways and the diverse factors that contribute to them obscure both the incidence and causes of clinically severe dengue.
Although previous infection with a heterologous DENV is the best characterised risk factor for developing severe dengue, currently, there is only a nascent understanding of the complex interplay between disease severity and transmission intensity, including both the timing between infections and sequence of infections.
Hospital admission is a frequently used but unreliable indicator of patients with clinically severe disease. Prospective cohort studies of children and adults in geographically diverse settings are needed to better elucidate the diverse factors that contribute to clinically severe dengue, which in turn will improve both the design and the evaluation of dengue vaccines.
Acknowledgments
EH and LCK were supported in part by the National Institutes of Health grant (P01 AI106695). KBA was supported in part by the National Institutes of Health grant (P01 AI034533). The views expressed in this Personal View are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention or the US Public Health Service.
Footnotes
Declaration of interests
KBA reports serving as a consultant for Emergent Biosolutions and Eli Lilly, outside of the submitted work. EH reports grants from the National Institute of Allergy and Infectious Diseases (National Institutes of Health), during the conduct of this Personal View. LCK reports grants from the National Institutes of Health, during the conduct of this Personal View. ACM reports grants from the National Institutes of Health, during the conduct of this Personal View. All other authors declare no competing interests.
Contributor Information
Tyler M Sharp, Dengue Branch, Centers for Disease Control and Prevention, San Juan, PR, USA; United States Public Health Service, Silver Springs, MD, USA.
Kathryn B Anderson, Institute for Global Health and Translational Sciences and Department of Medicine, and Department of Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, NY, USA; Department of Virology, Armed Forces Research Institute for Medical Sciences, Bangkok, Thailand.
Leah C Katzelnick, Division of Infectious Diseases and Vaccinology, School of Public Health, University of California, Berkeley, Berkeley, CA, USA; Department of Biology, University of Florida, Gainesville, FL, USA.
Hannah Clapham, Saw Swee Hock School of Public Health, National University of Singapore, Singapore; Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK.
Michael A Johansson, Dengue Branch, Centers for Disease Control and Prevention, San Juan, PR, USA.
Amy C Morrison, Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, University of California, Davis, Davis, CA, USA.
Eva Harris, Division of Infectious Diseases and Vaccinology, School of Public Health, University of California, Berkeley, Berkeley, CA, USA.
Gabriela Paz-Bailey, Dengue Branch, Centers for Disease Control and Prevention, San Juan, PR, USA.
Stephen H Waterman, Dengue Branch, Centers for Disease Control and Prevention, San Juan, PR, USA; United States Public Health Service, Silver Springs, MD, USA.
References
- 1.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis 2016; 16: 935–41. [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016; 16: 712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattarino L, Rodriguez-Barraquer I, Imai N, Cummings DAT, Ferguson NM. Mapping global variation in dengue transmission intensity. Sci Transl Med 2020; 12: eaax4144. [DOI] [PubMed] [Google Scholar]
- 4.Sharp TM, Tomashek KM, Read JS, Margolis HS, Waterman SH. A new look at an old disease: recent insights into the global epidemiology of dengue. Curr Epidemiol Rep 2017; 4: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nealon J, Taurel AF, Capeding MR, et al. Symptomatic dengue disease in five southeast Asian countries: epidemiological evidence from a dengue vaccine trial. PLoS Negl Trop Dis 2016; 10: e0004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzelnick LC, Harris E. The use of longitudinal cohorts for studies of dengue viral pathogenesis and protection. Curr Opin Virol 2018; 29: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.L’Azou M, Moureau A, Sarti E, et al. Symptomatic dengue in children in 10 Asian and Latin American Countries. N Engl J Med 2016; 374: 1155–66. [DOI] [PubMed] [Google Scholar]
- 8.Biswal S, Reynales H, Saez-Llorens X, et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med 2019; 381: 2009–19. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 10.WHO. Dengue haemorrhagic fever: diagnosis, treatment, and control. Geneva: World Health Organization, 1997. [Google Scholar]
- 11.Achee NL, Grieco JP, Vatandoost H, et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis 2019; 13: e0006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilder-Smith A, Ooi EE, Horstick O, Wills B. Dengue. Lancet 2019; 393: 350–63. [DOI] [PubMed] [Google Scholar]
- 13.Huy NT, Van Giang T, Thuy DH, et al. Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Negl Trop Dis 2013; 7: e2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzelnick LC, Gresh L, Halloran ME, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017; 358: 929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salje H, Cummings DAT, Rodriguez-Barraquer I, et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 2018; 557: 719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srikiatkhachorn A, Mathew A, Rothman AL. Immune-mediated cytokine storm and its role in severe dengue. Semin Immunopathol 2017; 39: 563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasner DR, Puerta-Guardo H, Beatty PR, Harris E. The good, the bad, and the shocking: the multiple roles of dengue virus nonstructural protein 1 in protection and pathogenesis. Annu Rev Virol 2018; 5: 227–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manokaran G, Finol E, Wang C, et al. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 2015; 350: 217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.OhAinle M, Balmaseda A, Macalalad AR, et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med 2011; 3: 114ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinheiro TM, Mota MTO, Watanabe ASA, et al. Viral immunogenicity determines epidemiological fitness in a cohort of DENV-1 infection in Brazil. PLoS Negl Trop Dis 2018; 12: e0006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messer WB, Gubler DJ, Harris E, Sivananthan K, de Silva AM. Emergence and global spread of a dengue serotype 3, subtype III virus. Emerg Infect Dis 2003; 9: 800–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett SN, Holmes EC, Chirivella M, et al. Selection-driven evolution of emergent dengue virus. Mol Biol Evol 2003; 20: 1650–58. [DOI] [PubMed] [Google Scholar]
- 23.Khor CC, Chau TN, Pang J, et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet 2011; 43: 1139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boillat-Blanco N, Klaassen B, Mbarack Z, et al. Dengue fever in Dar es Salaam, Tanzania: clinical features and outcome in populations of black and non-black racial category. BMC Infect Dis 2018; 18: 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trung DT, Thao TT, Dung NM, et al. Clinical features of dengue in a large Vietnamese cohort: intrinsically lower platelet counts and greater risk for bleeding in adults than children. PLoS Negl Trop Dis 2012; 6: e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clapham HE, Cummings DAT, Johansson MA. Immune status alters the probability of apparent illness due to dengue virus infection: evidence from a pooled analysis across multiple cohort and cluster studies. PLoS Negl Trop Dis 2017; 11: e0005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soo KM, Khalid B, Ching SM, Chee HY. Meta-analysis of dengue severity during infection by different dengue virus serotypes in primary and secondary infections. PLoS One 2016; 11: e0154760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katzelnick LC, Narvaez C, Arguello S, et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020; 369: 1123–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci USA 2016; 113: 728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montoya M, Gresh L, Mercado JC, et al. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis 2013; 7: e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000; 181: 2–9. [DOI] [PubMed] [Google Scholar]
- 32.Libraty DH, Endy TP, Houng HS, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 2002; 185: 1213–21. [DOI] [PubMed] [Google Scholar]
- 33.Pinto RC, Castro DB, Albuquerque BC, et al. Mortality predictors in patients with severe dengue in the State of Amazonas, Brazil. PLoS One 2016; 11: e0161884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zulkipli MS, Dahlui M, Jamil N, et al. The association between obesity and dengue severity among pediatric patients: a systematic review and meta-analysis. PLoS Negl Trop Dis 2018; 12: e0006263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang J, Hsu JP, Yeo TW, Leo YS, Lye DC. Diabetes, cardiac disorders and asthma as risk factors for severe organ involvement among adult dengue patients: a matched case-control study. Sci Rep 2017; 7: 39872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen TH, Nguyen TL, Lei HY, et al. Association between sex, nutritional status, severity of dengue hemorrhagic fever, and immune status in infants with dengue hemorrhagic fever. Am J Trop Med Hyg 2005; 72: 370–74. [PubMed] [Google Scholar]
- 37.Anders KL, Nguyet NM, Chau NV, et al. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg 2011; 84: 127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouliot SH, Xiong X, Harville E, et al. Maternal dengue and pregnancy outcomes: a systematic review. Obstet Gynecol Surv 2010; 65: 107–18. [DOI] [PubMed] [Google Scholar]
- 39.Vouga M, Chiu YC, Pomar L, et al. Dengue, Zika and chikungunya during pregnancy: pre- and post-travel advice and clinical management. J Travel Med 2019; 26: taz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trang NTH, Long NP, Hue TTM, et al. Association between nutritional status and dengue infection: a systematic review and meta-analysis. BMC Infect Dis 2016; 16: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam PK, Tam DT, Diet TV, et al. Clinical characteristics of dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis 2013; 57: 1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Temprasertrudee S, Thanachartwet V, Desakorn V, Keatkla J, Chantratita W, Kiertiburanakul S. A multicenter study of clinical presentations and predictive factors for severe manifestation of dengue in adults. Jpn J Infect Dis 2018; 71: 239–43. [DOI] [PubMed] [Google Scholar]
- 43.Lee YH, Leong WY, Wilder-Smith A. Markers of dengue severity: a systematic review of cytokines and chemokines. J Gen Virol 2016; 97: 3103–19. [DOI] [PubMed] [Google Scholar]
- 44.Jaenisch T, Junghanss T, Wills B, et al. Dengue expansion in Africa—not recognized or not happening? Emerg Infect Dis 2014; 20: e140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SAGE working group on dengue vaccines and WHO secretariat. Background paper on dengue vaccines. 2018. http://www.who.int/immunization/sage/meetings/2018/april/2_DengueBackgrPaper_SAGE_Apr2018.pdf (accessed June 24, 2021). [Google Scholar]
- 46.Capeding MR, Tran NH, Hadinegoro SR, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384: 1358–65. [DOI] [PubMed] [Google Scholar]
- 47.Villar L, Dayan GH, Arredondo-García JL, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372: 113–23. [DOI] [PubMed] [Google Scholar]
- 48.WHO. Meeting of the Strategic Advisory Group of Experts on immunization, April 2016—conclusions and recommendations. Wkly Epidemiol Rec 2016; 91: 266–84. [PubMed] [Google Scholar]
- 49.WHO. Dengue vaccine: WHO position paper—July 2016. Wkly Epidemiol Rec 2016; 91: 349–64. [PubMed] [Google Scholar]
- 50.Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 2018; 379: 327–40. [DOI] [PubMed] [Google Scholar]
- 51.Dengue vaccine: WHO position paper, September 2018—recommendations. Vaccine 2019; 37: 4848–49. [DOI] [PubMed] [Google Scholar]
- 52.Wilder-Smith A, Hombach J, Ferguson N, et al. Deliberations of the Strategic Advisory Group of Experts on Immunization on the use of CYD-TDV dengue vaccine. Lancet Infect Dis 2019; 19: e31–38. [DOI] [PubMed] [Google Scholar]
- 53.US Food and Drug Administration. First FDA-approved vaccine for the prevention of dengue disease in endemic regions. 2019. https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-dengue-disease-endemic-regions (accessed June 24, 2021).
- 54.Anderson KB, Endy TP, Thomas SJ. The dynamic role of dengue cross-reactive immunity: changing the approach to defining vaccine safety and efficacy. Lancet Infect Dis 2018; 18: e333–38. [DOI] [PubMed] [Google Scholar]
- 55.Martinez DR, Yount B, Nivarthi U, et al. Antigenic variation of the dengue virus 2 genotypes impacts the neutralization activity of human antibodies in vaccinees. Cell Rep 2020; 33: 108226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katzelnick LC, Fonville JM, Gromowski GD, et al. Dengue viruses cluster antigenically but not as discrete serotypes. Science 2015; 349: 1338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Juraska M, Magaret CA, Shao J, et al. Viral genetic diversity and protective efficacy of a tetravalent dengue vaccine in two phase 3 trials. Proc Natl Acad Sci USA 2018; 115: E8378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabaa MA, Girerd-Chambaz Y, Duong Thi Hue K, et al. Genetic epidemiology of dengue viruses in phase III trials of the CYD tetravalent dengue vaccine and implications for efficacy. eLife 2017; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuno G. Emergence of the severe syndrome and mortality associated with dengue and dengue-like illness: historical records (1890 to 1950) and their compatibility with current hypotheses on the shift of disease manifestation. Clin Microbiol Rev 2009; 22: 186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharp TM, Fischer M, Muñoz-Jordán JL, et al. Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep 2019; 68: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gutierrez-Barbosa H, Medina-Moreno S, Zapata JC, Chua JV. Dengue infections in Colombia: epidemiological trends of a hyperendemic country. Trop Med Infect Dis 2020; 5: e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Low GK, Ogston SA, Yong MH, Gan SC, Chee HY. Global dengue death before and after the new World Health Organization 2009 case classification: a systematic review and meta-regression analysis. Acta Trop 2018; 182: 237–45. [DOI] [PubMed] [Google Scholar]
- 63.Sharp TM, Ryff KR, Santiago GA, Margolis HS, Waterman SH. Lessons learned from dengue surveillance and research, Puerto Rico, 1899–2013. Emerg Infect Dis 2019; 25: 1522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalayanarooj S, Rothman AL, Srikiatkhachorn A. Case management of dengue: lessons learned. J Infect Dis 2017; 215 (suppl 2): S79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomashek KM, Wills B, See Lum LC, et al. Development of standard clinical endpoints for use in dengue interventional trials. PLoS Negl Trop Dis 2018; 12: e0006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horstick O, Jaenisch T, Martinez E, et al. Comparing the usefulness of the 1997 and 2009 WHO dengue case classification: a systematic literature review. Am J Trop Med Hyg 2014; 91: 621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barniol J, Gaczkowski R, Barbato EV, et al. Usefulness and applicability of the revised dengue case classification by disease: multi-centre study in 18 countries. BMC Infect Dis 2011; 11: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenberger KD, Alexander N, Martinez E, et al. Severe dengue categories as research endpoints—results from a prospective observational study in hospitalised dengue patients. PLoS Negl Trop Dis 2020; 14: e0008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yacoub S, Griffiths A, Chau TT, et al. Cardiac function in Vietnamese patients with different dengue severity grades. Crit Care Med 2012; 40: 477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carod-Artal FJ, Wichmann O, Farrar J, Gascón J. Neurological complications of dengue virus infection. Lancet Neurol 2013; 12: 906–19. [DOI] [PubMed] [Google Scholar]
- 71.Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet 2000; 355: 1053–59. [DOI] [PubMed] [Google Scholar]
- 72.Pérez Rodríguez NM, Galloway R, Blau DM, et al. Case series of fatal Leptospira spp./dengue virus co-infections—Puerto Rico, 2010—2012. Am J Trop Med Hyg 2014; 91: 760–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salam N, Mustafa S, Hafiz A, Chaudhary AA, Deeba F, Parveen S. Global prevalence and distribution of coinfection of malaria, dengue and chikungunya: a systematic review. BMC Public Health 2018; 18: 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bagno FF, Figueiredo MM, Villarreal J, Pereira GC, Godoi LC, da Fonseca FG. Undetected Chikungunya virus co-infections in a Brazilian region presenting hyper-endemic circulation of dengue and Zika. J Clin Virol 2019; 113: 27–30. [DOI] [PubMed] [Google Scholar]
- 75.Silva MMO, Tauro LB, Kikuti M, et al. Concomitant transmission of dengue, chikungunya, and Zika viruses in Brazil: clinical and epidemiological findings from surveillance for acute febrile illness. Clin Infect Dis 2019; 69: 1353–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waggoner JJ, Gresh L, Vargas MJ, et al. Viremia and clinical presentation in Nicaraguan patients infected with Zika virus, chikungunya virus, and dengue virus. Clin Infect Dis 2016; 63: 1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rowe EK, Leo YS, Wong JG, et al. Challenges in dengue fever in the elderly: atypical presentation and risk of severe dengue and hospital-acquired infection [corrected]. PLoS Negl Trop Dis 2014; 8: e2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cummings DA, Iamsirithaworn S, Lessler JT, et al. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med 2009; 6: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Egger JR, Coleman PG. Age and clinical dengue illness. Emerg Infect Dis 2007; 13: 924–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thai KT, Nishiura H, Hoang PL, et al. Age-specificity of clinical dengue during primary and secondary infections. PLoS Negl Trop Dis 2011; 5: e1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammond SN, Balmaseda A, Pérez L, et al. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg 2005; 73: 1063–70. [PubMed] [Google Scholar]
- 82.Huang WC, Lee IK, Chen YC, Tsai CY, Liu JW. Characteristics and predictors for gastrointestinal hemorrhage among adult patients with dengue virus infection: emphasizing the impact of existing comorbid disease(s). PLoS One 2018; 13: e0192919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nealon J, Bouckenooghe A, Cortes M, et al. Dengue endemicity, force of infection and variation in transmission intensity in 13 endemic countries J Infect Dis 2020; 25: jiaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clapham HE, Rodriguez-Barraquer I, Azman AS, et al. Dengue virus (DENV) neutralizing antibody kinetics in children after symptomatic primary and postprimary DENV Infection. J Infect Dis 2016; 213: 1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Endy TP, Anderson KB, Nisalak A, et al. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis 2011; 5: e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, de Silva AD, de Silva AM. Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis 2015; 211: 590–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg 1952; 1: 30–50. [DOI] [PubMed] [Google Scholar]
- 88.Anderson KB, Gibbons RV, Cummings DA, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J Infect Dis 2014; 209: 360–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagao Y, Koelle K. Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proc Natl Acad Sci USA 2008; 105: 2238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferguson NM, Rodríguez-Barraquer I, Dorigatti I, Mier-Y-Teran-Romero L, Laydon DJ, Cummings DA. Benefits and risks of the Sanofi–Pasteur dengue vaccine: modeling optimal deployment. Science 2016; 353: 1033–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferguson NM, Kien DTH, Clapham H, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 2015; 7: 279ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olkowski S, Forshey BM, Morrison AC, et al. Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis 2013; 208: 1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gibbons RV, Kalanarooj S, Jarman RG, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 2007; 77: 910–13. [PubMed] [Google Scholar]
- 94.Katzelnick LC, Bos S, Harris E. Protective and enhancing interactions among dengue viruses 1–4 and Zika virus. Curr Opin Virol 2020; 43: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderson KB, Gibbons RV, Thomas SJ, et al. Preexisting Japanese encephalitis virus neutralizing antibodies and increased symptomatic dengue illness in a school-based cohort in Thailand. PLoS Negl Trop Dis 2011; 5: e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hammon WM, Tigertt WD, Sather GE, Berge TO, Meiklejohn G. Epidemiologic studies of concurrent virgin epidemics of Japanese B encephalitis and of mumps on Guam, 1947–1948, with subsequent observations including dengue, through 1957. Am J Trop Med Hyg 1958; 7: 441–67. [DOI] [PubMed] [Google Scholar]
- 97.Paddock CD, Nicholson WL, Bhatnagar J, et al. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis 2006; 42: 1527–35. [DOI] [PubMed] [Google Scholar]
- 98.Terzian ACB, Schanoski AS, Mota MTO, et al. Viral load and cytokine response profile does not support antibody-dependent enhancement in dengue-primed Zika virus-infected patients. Clin Infect Dis 2017; 65: 1260–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santiago GA, Sharp TM, Rosenberg E, et al. Prior dengue virus infection is associated with increased viral load in patients infected with dengue but not Zika virus. Open Forum Infect Dis 2019; 6: ofz320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gordon A, Gresh L, Ojeda S, et al. Prior dengue virus infection and risk of Zika: a pediatric cohort in Nicaragua. PLoS Med 2019; 16: e1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodriguez-Barraquer I, Costa F, Nascimento EJM, et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019; 363: 607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.da Silva NS, Undurraga EA, da Silva Ferreira ER, Estofolete CF, Nogueira ML. Clinical, laboratory, and demographic determinants of hospitalization due to dengue in 7613 patients: a retrospective study based on hierarchical models. Acta Trop 2018; 177: 25–31. [DOI] [PubMed] [Google Scholar]
- 103.Coelho GE, Leal PL, Cerroni MP, Simplicio AC, Siqueira JB Jr. Sensitivity of the dengue surveillance system in Brazil for detecting hospitalized cases. PLoS Negl Trop Dis 2016; 10: e0004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris E, Pérez L, Phares CR, et al. Fluid intake and decreased risk for hospitalization for dengue fever, Nicaragua. Emerg Infect Dis 2003; 9: 1003–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dechant EJ, Rigau-Pérez JG. Hospitalizations for suspected dengue in Puerto Rico, 1991–1995: estimation by capture-recapture methods. Am J Trop Med Hyg 1999; 61: 574–78. [DOI] [PubMed] [Google Scholar]
- 106.Tomashek KM, Biggerstaff BJ, Ramos MM, Pérez-Guerra CL, Garcia Rivera EJ, Sun W. Physician survey to determine how dengue is diagnosed, treated and reported in Puerto Rico. PLoS Negl Trop Dis 2014; 8: e3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carabali M, Hernandez LM, Arauz MJ, Villar LA, Ridde V. Why are people with dengue dying? A scoping review of determinants for dengue mortality. BMC Infect Dis 2015; 15: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rocha C, Silva S, Gordon A, et al. Improvement in hospital indicators after changes in dengue case management in Nicaragua. Am J Trop Med Hyg 2009; 81: 287–92. [PubMed] [Google Scholar]
- 109.Mayurasakorn S, Suttipun N. The impact of a program for strengthening dengue hemorrhagic fever case management on the clinical outcome of dengue hemorrhagic fever patients. Southeast Asian J Trop Med Public Health 2010; 41: 858–63. [PubMed] [Google Scholar]
- 110.Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jaenisch T, Tam DT, Kieu NT, et al. Clinical evaluation of dengue and identification of risk factors for severe disease: protocol for a multicentre study in 8 countries. BMC Infect Dis 2016; 16: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beatty ME, Stone A, Fitzsimons DW, et al. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl Trop Dis 2010; 4: e890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodriguez-Barraquer I, Salje H, Cummings DA. Opportunities for improved surveillance and control of dengue from age-specific case data. eLife 2019; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]