Abstract
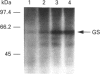
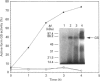
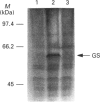
A monospecific anti-(glutamine synthetase) antibody raised against glutamine synthetase of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 immunoreacted with glutamine synthetase from the N2-fixing heterotrophic bacterium Azotobacter chroococcum. In Western-blotting experiments this antibody recognized a single protein of a molecular mass of 59 kDa corresponding to glutamine synthetase subunit. This protein was in vivo-labelled in response to addition of ammonium, both [3H]adenine and H(3)32PO4 preincubation of the cells being equally effective. Nevertheless, the amount of glutamine synthetase present in A. chroococcum was independent of the available nitrogen source. Modified, inactive glutamine synthetase was re-activated by treatment with snake-venom phosphodiesterase but not by alkaline phosphatase. L-Methionine-DL-sulphoximine, an inhibitor of glutamine synthetase, prevented the enzyme from being covalently modified. We conclude that, in A. chroococcum, glutamine synthetase is adenylylated in response to ammonium and that for the modification to take place ammonium must be metabolized.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatnagar L., Zeikus J. G., Aubert J. P. Purification and characterization of glutamine synthetase from the archaebacterium Methanobacterium ivanovi. J Bacteriol. 1986 Feb;165(2):638–643. doi: 10.1128/jb.165.2.638-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cejudo F. J., Paneque A. Short-term nitrate (nitrite) inhibition of nitrogen fixation in Azotobacter chroococcum. J Bacteriol. 1986 Jan;165(1):240–243. doi: 10.1128/jb.165.1.240-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejudo F. J., de la Torre A., Paneque A. Short-term ammonium inhibition of nitrogen fixation in Azotobacter. Biochem Biophys Res Commun. 1984 Sep 17;123(2):431–437. doi: 10.1016/0006-291x(84)90248-1. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Stadtman E. R. Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem. 1970 Oct 25;245(20):5206–5213. [PubMed] [Google Scholar]
- Fisher R., Tuli R., Haselkorn R. A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3393–3397. doi: 10.1073/pnas.78.6.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero M. G., Vega J. M., Leadbetter E., Losada M. Preparation and characterization of a soluble nitrate reductase from Azotobacter chroococcum. Arch Mikrobiol. 1973 Jun 25;91(4):287–304. doi: 10.1007/BF00425049. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Toukdarian A. Genetics of azotobacters: applications to nitrogen fixation and related aspects of metabolism. Annu Rev Microbiol. 1987;41:227–258. doi: 10.1146/annurev.mi.41.100187.001303. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Kleiner D. The glutamine synthetase from Azotobacter vinelandii: purification, characterization, regulation and localization. Eur J Biochem. 1978 Aug 15;89(1):51–60. doi: 10.1111/j.1432-1033.1978.tb20895.x. [DOI] [PubMed] [Google Scholar]
- Kombrink E., Schröder M., Hahlbrock K. Several "pathogenesis-related" proteins in potato are 1,3-beta-glucanases and chitinases. Proc Natl Acad Sci U S A. 1988 Feb;85(3):782–786. doi: 10.1073/pnas.85.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laane C., Krone W., Konings W., Haaker H., Veeger C. Short-term effect of ammonium chloride on nitrogen fixation by Azotobacter vinelandii and by bacteroids of Rhizobium leguminosarum. Eur J Biochem. 1980 Jan;103(1):39–46. doi: 10.1111/j.1432-1033.1980.tb04286.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maloy S., Stewart V. Autogenous regulation of gene expression. J Bacteriol. 1993 Jan;175(2):307–316. doi: 10.1128/jb.175.2.307-316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Stadtman E. R. Adenylylation state of glutamine synthetase and permeability properties of Pseudomonas fluorescens. Arch Biochem Biophys. 1986 May 1;246(2):622–632. doi: 10.1016/0003-9861(86)90318-8. [DOI] [PubMed] [Google Scholar]
- Mérida A., Candau P., Florencio F. J. Regulation of glutamine synthetase activity in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 by the nitrogen source: effect of ammonium. J Bacteriol. 1991 Jul;173(13):4095–4100. doi: 10.1128/jb.173.13.4095-4100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérida A., Leurentop L., Candau P., Florencio F. J. Purification and properties of glutamine synthetases from the cyanobacteria Synechocystis sp. strain PCC 6803 and Calothrix sp. strain PCC 7601. J Bacteriol. 1990 Aug;172(8):4732–4735. doi: 10.1128/jb.172.8.4732-4735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr J., Keefer L. M., Keim P., Nguyen T. D., Wellems T., Heinrikson R. L., Haselkorn R. Purification, physical characterization, and NH2-terminal sequence of glutamine synthetase from the cyanobacterium Anabaena 7120. J Biol Chem. 1981 Dec 25;256(24):13091–13098. [PubMed] [Google Scholar]
- Paneque A., Bárcena J. A., Cordero N., Revilla E., Llobell A. Benzyl viologen-mediated in vivo and in vitro inactivation of glutamine synthetase in Azotobacter chroococcum. Mol Cell Biochem. 1982 Nov 12;49(1):33–41. doi: 10.1007/BF00230993. [DOI] [PubMed] [Google Scholar]
- Siedel J., Shelton E. Purification and properties of Azotobacter vinelandii glutamine synthetase. Arch Biochem Biophys. 1979 Jan;192(1):214–224. doi: 10.1016/0003-9861(79)90086-9. [DOI] [PubMed] [Google Scholar]
- Son H. S., Rhee S. G. Cascade control of Escherichia coli glutamine synthetase. Purification and properties of PII protein and nucleotide sequence of its structural gene. J Biol Chem. 1987 Jun 25;262(18):8690–8695. [PubMed] [Google Scholar]
- Toukdarian A., Saunders G., Selman-Sosa G., Santero E., Woodley P., Kennedy C. Molecular analysis of the Azotobacter vinelandii glnA gene encoding glutamine synthetase. J Bacteriol. 1990 Nov;172(11):6529–6539. doi: 10.1128/jb.172.11.6529-6539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Ciardi J. E., Stadtman E. R. Comparative biochemical and immunological studies of bacterial glutamine synthetases. J Bacteriol. 1973 Sep;115(3):858–868. doi: 10.1128/jb.115.3.858-868.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]