Abstract
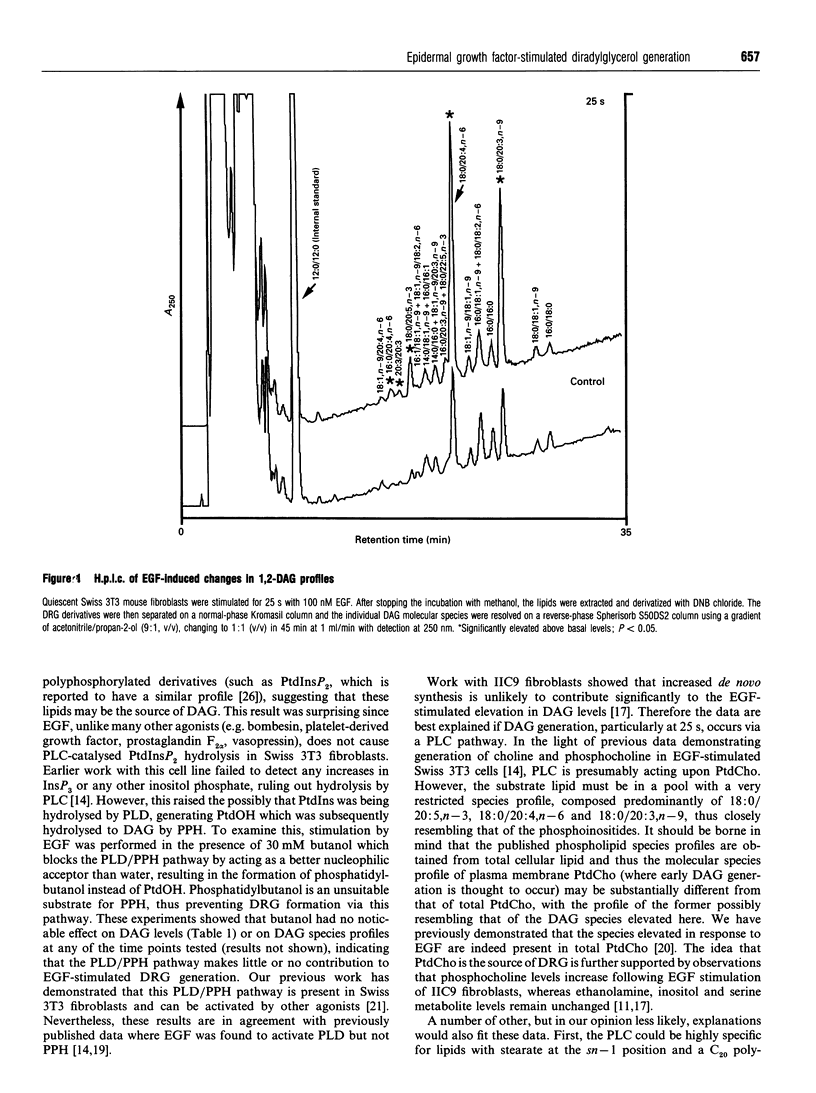
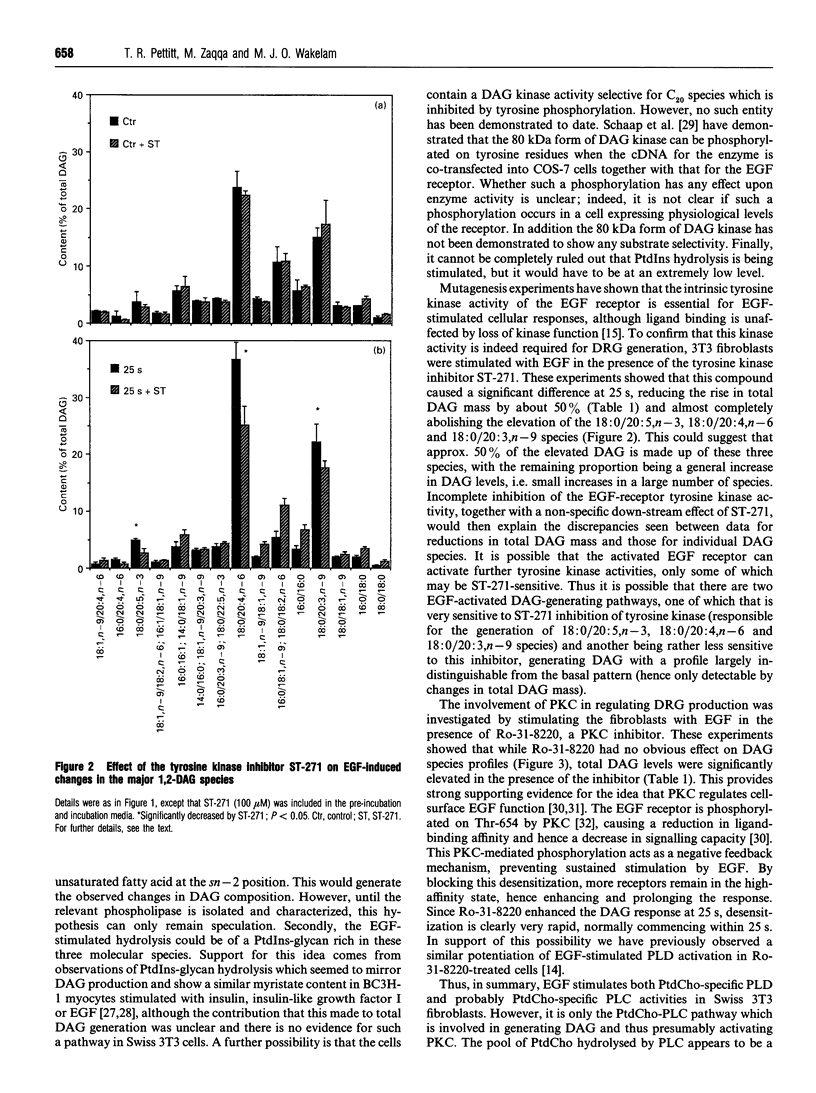
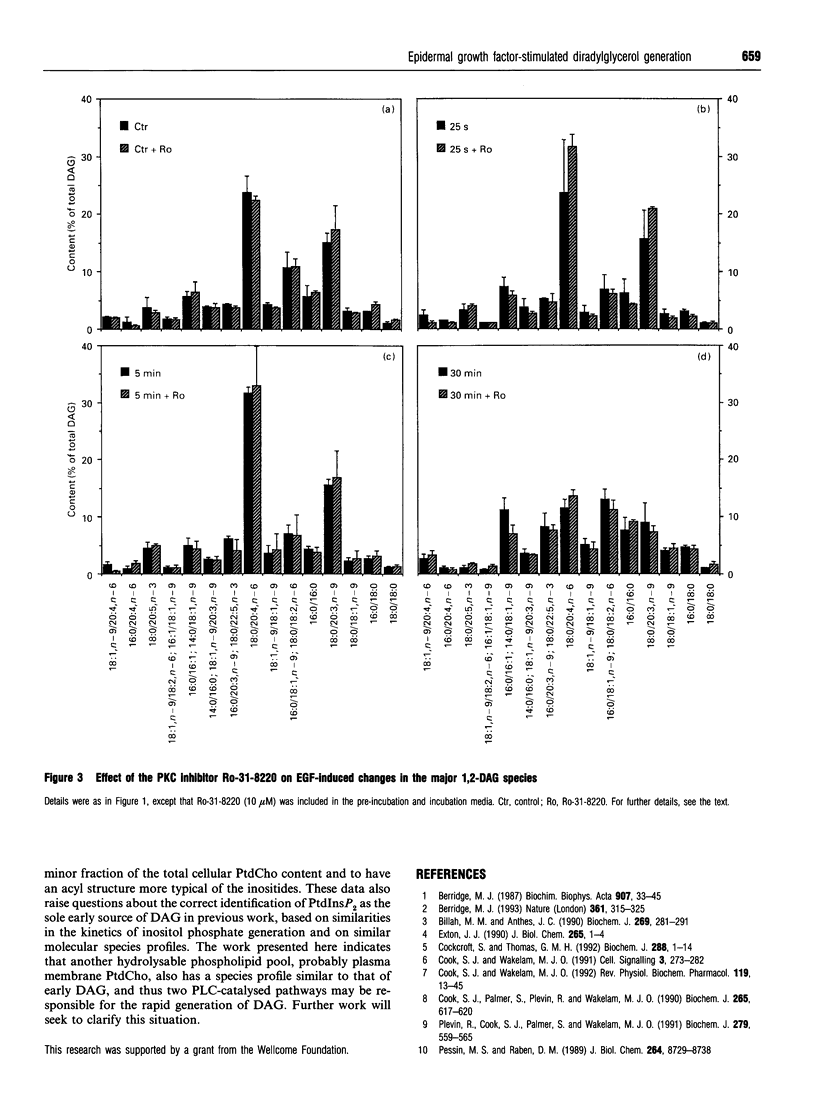
Stimulation of 3T3 fibroblasts with epidermal growth factor (EGF) results in an increase in 1,2-diacylglycerol (DAG) mass which is maximal at 25 s, declining at 1 min and returning to basal levels by 30 min. No changes in alkylacylglycerol or alkenylacylglycerol were detected. Three species account for most of this mass increase: 18:0/20:5,n-3, 18:0/20:4,n-6 and 18:0/20:3,n-9. These species are characteristic of the phosphoinositides; however, previous work failed to detect any EGF-stimulated rise in inositol phosphates in these cells [Cook and Wakelam (1992) Biochem. J. 285, 247-253]. This ruled out phosphoinositide hydrolysis by phospholipase C, but raised the possibility of phospholipase D/phosphatidate phosphohydrolase-catalysed hydrolysis of phosphatidylinositol. The inclusion of butanol in the incubation medium failed to block the diacylglycerol changes, indicating that the phospholipase D pathway is not involved and that DAG must be derived from another source, probably via phospholipase C-catalysed hydrolysis of a phosphatidylcholine pool that is particularly rich in these species. The tyrosine kinase inhibitor ST-271 almost abolished the elevation in 18:0/20:5,n-3, 18:0/20:4, n-6 and 18:0/20:3,n-9 at 25 s, but only reduced the rise in total DAG mass by about 50%. The protein kinase C (PKC) inhibitor Ro-31-8220 increased DAG levels at all time points but had no effect on the species profiles. This provides additional evidence for PKC-mediated regulation of cell-surface EGF receptors, since the inhibition of PKC would increase the availability and/or ligand binding affinity of receptors at the plasma membrane and hence increase and prolong the response to EGF.
Full text
PDF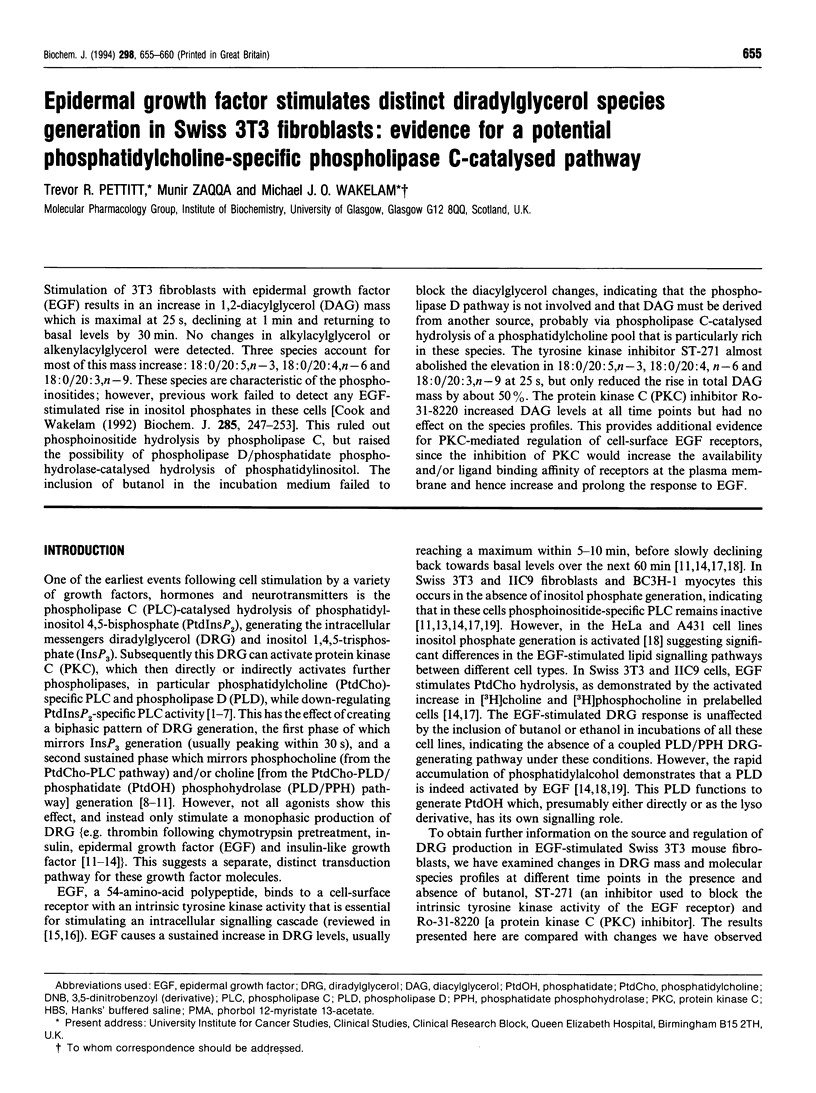


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beguinot L., Hanover J. A., Ito S., Richert N. D., Willingham M. C., Pastan I. Phorbol esters induce transient internalization without degradation of unoccupied epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1985 May;82(9):2774–2778. doi: 10.1073/pnas.82.9.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol lipids and cell proliferation. Biochim Biophys Acta. 1987 Apr 20;907(1):33–45. doi: 10.1016/0304-419x(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cockcroft S., Thomas G. M. Inositol-lipid-specific phospholipase C isoenzymes and their differential regulation by receptors. Biochem J. 1992 Nov 15;288(Pt 1):1–14. doi: 10.1042/bj2880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Briscoe C. P., Wakelam M. J. The regulation of phospholipase D activity and its role in sn-1,2-diradylglycerol formation in bombesin- and phorbol 12-myristate 13-acetate-stimulated Swiss 3T3 cells. Biochem J. 1991 Dec 1;280(Pt 2):431–438. doi: 10.1042/bj2800431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Palmer S., Plevin R., Wakelam M. J. Mass measurement of inositol 1,4,5-trisphosphate and sn-1,2-diacylglycerol in bombesin-stimulated Swiss 3T3 mouse fibroblasts. Biochem J. 1990 Jan 15;265(2):617–620. doi: 10.1042/bj2650617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Epidermal growth factor increases sn-1,2-diacylglycerol levels and activates phospholipase D-catalysed phosphatidylcholine breakdown in Swiss 3T3 cells in the absence of inositol-lipid hydrolysis. Biochem J. 1992 Jul 1;285(Pt 1):247–253. doi: 10.1042/bj2850247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Phospholipases C and D in mitogenic signal transduction. Rev Physiol Biochem Pharmacol. 1992;119:13–45. doi: 10.1007/3540551921_2. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Stimulated phosphatidylcholine hydrolysis as a signal transduction pathway in mitogenesis. Cell Signal. 1991;3(4):273–282. doi: 10.1016/0898-6568(91)90055-y. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farese R. V., Nair G. P., Sierra C. G., Standaert M. L., Pollet R. J., Cooper D. R. Insulin-like effects of epidermal growth factor and insulin-like growth factor-I on [3H]2-deoxyglucose uptake, diacylglycerol generation and protein kinase C activation in BC3H-1 myocytes. Biochem J. 1989 Aug 1;261(3):927–934. doi: 10.1042/bj2610927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Kaszkin M., Seidler L., Kast R., Kinzel V. Epidermal-growth-factor-induced production of phosphatidylalcohols by HeLa cells and A431 cells through activation of phospholipase D. Biochem J. 1992 Oct 1;287(Pt 1):51–57. doi: 10.1042/bj2870051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito M., Takamura H., Narita H., Urade R. A sensitive method for quantitative analysis of phospholipid molecular species by high-performance liquid chromatography. J Biochem. 1985 Aug;98(2):327–331. doi: 10.1093/oxfordjournals.jbchem.a135285. [DOI] [PubMed] [Google Scholar]
- Lee C., Fisher S. K., Agranoff B. W., Hajra A. K. Quantitative analysis of molecular species of diacylglycerol and phosphatidate formed upon muscarinic receptor activation of human SK-N-SH neuroblastoma cells. J Biol Chem. 1991 Dec 5;266(34):22837–22846. [PubMed] [Google Scholar]
- Nair G. P., Standaert M. L., Pollet R. J., Cooper D. R., Farese R. V. Effects of insulin and phorbol esters on diacylglycerol generation and synthesis and hydrolysis of phosphatidylcholine in BC3H-1 myocytes. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1345–1349. doi: 10.1016/0006-291x(88)90287-2. [DOI] [PubMed] [Google Scholar]
- Pessin M. S., Baldassare J. J., Raben D. M. Molecular species analysis of mitogen-stimulated 1,2-diglycerides in fibroblasts. Comparison of alpha-thrombin, epidermal growth factor, and platelet-derived growth factor. J Biol Chem. 1990 May 15;265(14):7959–7966. [PubMed] [Google Scholar]
- Pessin M. S., Raben D. M. Molecular species analysis of 1,2-diglycerides stimulated by alpha-thrombin in cultured fibroblasts. J Biol Chem. 1989 May 25;264(15):8729–8738. [PubMed] [Google Scholar]
- Pettitt T. R., Wakelam M. J. Bombesin stimulates distinct time-dependent changes in the sn-1,2-diradylglycerol molecular species profile from Swiss 3T3 fibroblasts as analysed by 3,5-dinitrobenzoyl derivatization and h.p.l.c. separation. Biochem J. 1993 Jan 15;289(Pt 2):487–495. doi: 10.1042/bj2890487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevin R., Cook S. J., Palmer S., Wakelam M. J. Multiple sources of sn-1,2-diacylglycerol in platelet-derived-growth-factor-stimulated Swiss 3T3 fibroblasts. Evidence for activation of phosphoinositidase C and phosphatidylcholine-specific phospholipase D. Biochem J. 1991 Oct 15;279(Pt 2):559–565. doi: 10.1042/bj2790559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Schaap D., van der Wal J., van Blitterswijk W. J., van der Bend R. L., Ploegh H. L. Diacylglycerol kinase is phosphorylated in vivo upon stimulation of the epidermal growth factor receptor and serine/threonine kinases, including protein kinase C-epsilon. Biochem J. 1993 Feb 1;289(Pt 3):875–881. doi: 10.1042/bj2890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. The epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry. 1988 May 3;27(9):3119–3123. doi: 10.1021/bi00409a002. [DOI] [PubMed] [Google Scholar]
- Takamura H., Kasai H., Arita H., Kito M. Phospholipid molecular species in human umbilical artery and vein endothelial cells. J Lipid Res. 1990 Apr;31(4):709–717. [PubMed] [Google Scholar]
- Uings I. J., Thompson N. T., Randall R. W., Spacey G. D., Bonser R. W., Hudson A. T., Garland L. G. Tyrosine phosphorylation is involved in receptor coupling to phospholipase D but not phospholipase C in the human neutrophil. Biochem J. 1992 Feb 1;281(Pt 3):597–600. doi: 10.1042/bj2810597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. M., Rangan L. A., Shin H. S., Raben D. M. Kinetic analysis of 1,2-diacylglycerol mass levels in cultured fibroblasts. Comparison of stimulation by alpha-thrombin and epidermal growth factor. J Biol Chem. 1988 Jul 5;263(19):9374–9380. [PubMed] [Google Scholar]
- Wright T. M., Shin H. S., Raben D. M. Sustained increase in 1,2-diacylglycerol precedes DNA synthesis in epidermal-growth-factor-stimulated fibroblasts. Evidence for stimulated phosphatidylcholine hydrolysis. Biochem J. 1990 Apr 15;267(2):501–507. doi: 10.1042/bj2670501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. M., Willenberger S., Raben D. M. Activation of phospholipase D by alpha-thrombin or epidermal growth factor contributes to the formation of phosphatidic acid, but not to observed increases in 1,2-diacylglycerol. Biochem J. 1992 Jul 15;285(Pt 2):395–400. doi: 10.1042/bj2850395. [DOI] [PMC free article] [PubMed] [Google Scholar]


