Chronic kidney disease (CKD) is a relatively common condition in patients with type 2 diabetes (T2D), and its prevalence augments with age [1]. It is well established that older adults have a higher risk of frailty with functional impairment and adverse events [2]. Moreover, frail older adults have many comorbidities that further contribute to both cognitive and functional impairment [2]. Empagliflozin is an inhibitor of sodium glucose cotransporter 2 (SGLT2), which has shown nephroprotective and cardioprotective actions and other salutary effects [3–7].
The actual effects of empagliflozin on cognitive impairment in elderly patients remain to be fully determined [8]; specifically, there are no studies exploring this aspect in frail older adults with T2D and CKD. In order to fill this knowledge gap, we enrolled consecutive frail older adults with confirmed diagnoses of T2D and CKD presenting at the Local Health Company of the Italian Ministry of Health of Avellino (ASL AV) from May 2021 to July 2022. Every patient (or legally authorized representative) signed a written informed consent. All subjects fulfilled the following criteria: Diagnosis of diabetes, frailty, and CKD with glomerular filtration rate (GFR) > 30 and < 60; age > 65; no previous myocardial infarction or revascularization procedures, EF > 50%; Montreal Cognitive Assessment (MoCA) Score < 26.
Patients were divided into two groups according to their antidiabetic treatment: empagliflozin 10 mg in addition to standard therapy vs. no empagliflozin. We performed this investigation following the ethical standards of the 1964 Declaration of Helsinki and its later amendments. We obtained formal IRB approval from Campania-Nord and we registered the trial in clinicaltrials.gov (Identifier-NCT04962841).
We evaluated global cognitive function at baseline and after 6-months using the MoCA test: scores range from zero-to-30; a score > 25 is considered normal [9]. We diagnosed physical frailty when at least three of the following Fried criteria [9] were present: exhaustion (poor endurance and energy); weakness (handgrip strength in the lowest 20% quintile, adjusted for sex and BMI); slowness (walking speed under the lowest quintile adjusted for sex and height); weight loss (unintentional loss ≥4.5 kg in the past 12-months); low physical activity (lowest quintile of kilocalories of physical activity during the previous 7-days). A 5-meter gait speed test (GST) was performed in all patients, as described [9]. We assessed differences for continuous variables using the Wilcoxon signed-rank test, a non-parametric alternative to paired t-test. We used chi-square to evaluate associations between dichotomous and categorical variables. Multivariable regression analysis was applied to adjust for potential confounders. We considered significant a P < 0.05 for two-sided comparisons.
We screened 166 frail elders with diabetes and CKD. Since 47 subjects did not fully meet the inclusion/exclusion criteria mentioned above and/or were unwilling to provide information, 119 patients entered the study. We divided our cohort in 2 groups based on the antidiabetic treatment: empagliflozin plus standard therapy (59 patients) and no-empagliflozin (60 patients).
Importantly, there were no significant differences between groups at baseline. We tested the MoCA scores in the 2 groups at baseline and at 6-month follow-up: 19.7 ± 3.68 vs. 21.4 ± 3.66 (p: 0.009) in the empagliflozin-group; 19.4 ± 3.76 vs. 19.5 ± 3.67 (p: 0.119) in the no-empagliflozin-group (Fig. 1A). We then performed a multivariable regression analysis using the improvement of MoCA score as dependent variable, adding to the model potential confounders, confirming the significant effect of empagliflozin treatment on the amelioration of cognitive impairment (Fig. 1B).
Fig. 1.
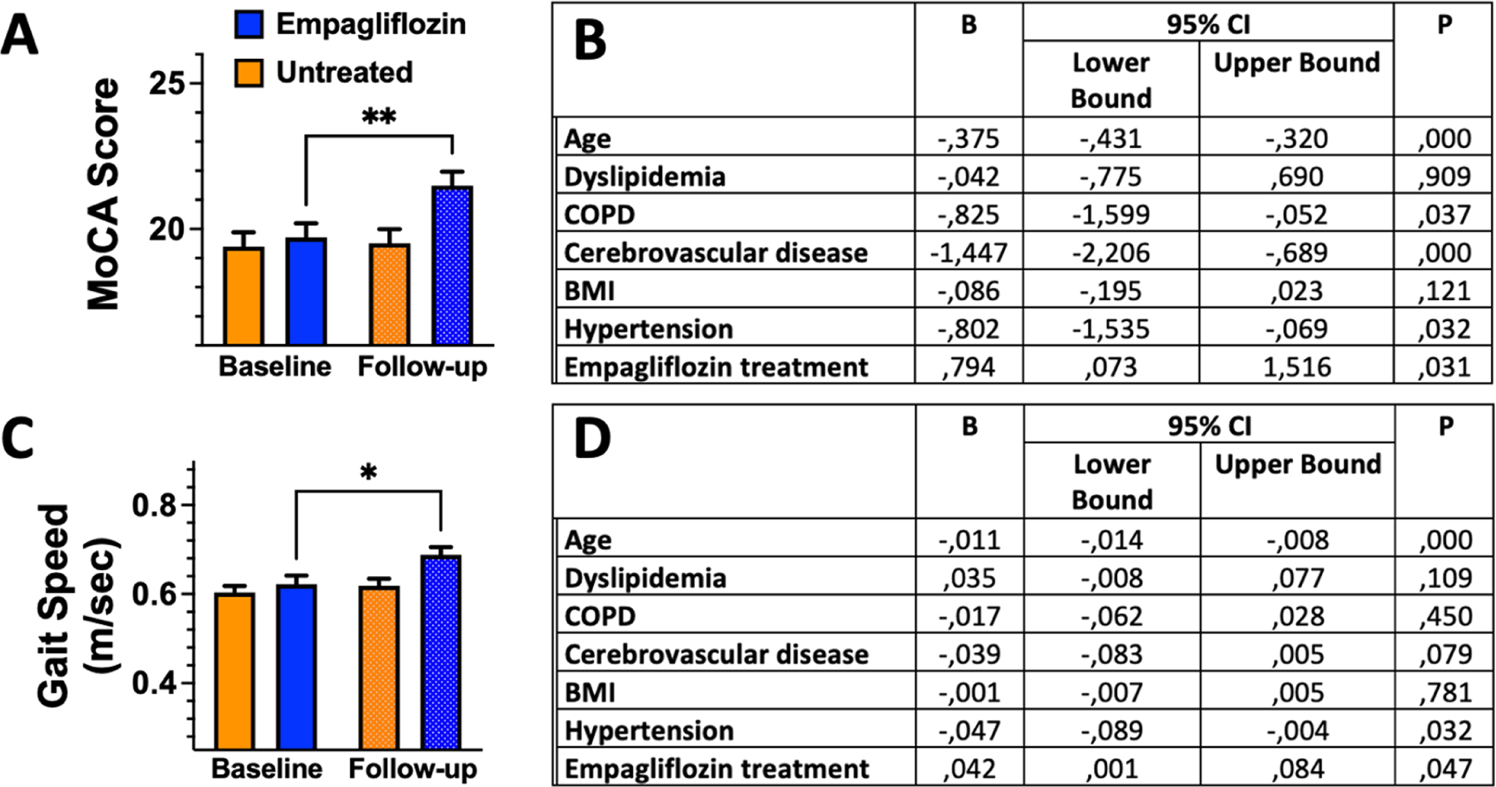
Effects of empagliflozin on cognitive and physical impairment. A) Montreal Cognitive Assessment (MoCA) Score at baseline and follow-up in patients treated or not with empagliflozin; B) multivariable regression analysis using the improvement in MoCA score as dependent variable; C) Gait Speed at baseline and follow-up in patients treated or not with empagliflozin; D) multivariable regression analysis using the improvement in GST score as dependent variable. *: 0.05, **: p<0.01; paired samples Wilcoxon test.
We also analyzed GST scores in the 2 groups at baseline and at 6-month follow-up: 0.603±0.11 vs. 0.688±0.13 (p<0.001) in the empagliflozin-group; 0.618±0.12 vs. 0.627±0.15 (p: 0.687) in the no-empagliflozin-group (Fig. 1C). We confirmed these results via a multivariable regression analysis using the improvement in GST as dependent variable (Fig. 1D).
The management of diabetic complications is a rapidly rising health problem. Kidney disease is a typical complication in T2D patients, which increases the risk of adverse events, including cognitive and physical impairment, particularly in frailty. In this scenario, we evaluated the effects of empagliflozin treatment. We observed a favorable action on both cognitive and physical function. Salutary vascular properties have been proposed for SGLT2-inhibitors, and we have previously observed beneficial effects on physical and cognitive function in hypertensive patients, by improving endothelial dysfunction and reducing mitochondrial oxidative stress [10]. To the best of our knowledge, we are the first group exploring the effects of empagliflozin on cognitive and physical impairment in frail older adults with diabetes and CKD, further supporting our view that SGLT2-inhibitors may be considered anti-frailty drugs. Nevertheless, additional studies with larger follow-up and larger sample size are warranted to confirm our data.
Funding
The Santulli’s Lab is currently supported in part by the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Center for Advancing Translational Sciences (NCATS: UL1-TR002556-06, UM1-TR004400) to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). This publication was also produced with the co-funding European Union – Next Generation EU, in the context of The National Recovery and Resilience Plan, Investment Partenariato Esteso PE8 “Conseguenze e sfide dell’invecchiamento”, Project Age-It (Ageing Well in an Ageing Society).
Footnotes
CRediT authorship contribution statement
Pansini Antonella: Investigation. Marro Anna: Data curation, Investigation. Frullone Salvatore: Investigation. Taurino Alessandro: Investigation, Methodology. Mone Pasquale: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. Guerra Germano: Data curation, Validation. Santulli Gaetano: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. Lombardi Angela: Methodology, Resources, Validation, Visualization. Illario Maddalena: Validation, Visualization. Iaccarino Guido: Validation, Visualization. Sorriento Daniela: Validation, Visualization. Verri Veronica: Investigation, Software.
Declaration of Competing Interest
None.
Contributor Information
Pasquale Mone, Department of Medicine, Einstein-Mount Sinai Diabetes Research Center (ES-DRC), Fleischer Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, Albert Einstein College of Medicine, New York City, New York, USA, ASL Avellino, Italy; University of Molise, Campobasso, Italy.
Germano Guerra, University of Molise, Campobasso, Italy; International Translational Research and Medical Education (ITME) Consortium, Academic Research Unit, Naples, Italy.
Angela Lombardi, Department of Medicine, Einstein-Mount Sinai Diabetes Research Center (ES-DRC), Fleischer Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, Albert Einstein College of Medicine, New York City, New York, USA.
Maddalena Illario, “Federico II” University, Naples, Italy.
Antonella Pansini, ASL Avellino, Italy.
Anna Marro, ASL Avellino, Italy.
Salvatore Frullone, ASL Avellino, Italy.
Alessandro Taurino, University of Bari, Italy.
Daniela Sorriento, Department of Advanced Biomedical Sciences, “Federico II” University, Naples, Italy.
Veronica Verri, University of Bari, Italy.
Guido Iaccarino, “Federico II”University, Naples, Italy.
Gaetano Santulli, Department of Advanced Biomedical Sciences, “Federico II” University, Naples, Italy; International Translational Research and Medical Education (ITME) Consortium, Academic Research Unit, Naples, Italy; Department of Medicine, Einstein-Mount Sinai Diabetes Research Center (ES-DRC), Fleischer Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, Albert Einstein College of Medicine, New York City, New York, USA; Department of Molecular Pharmacology, Wilf Family Cardiovascular Research Institute, Einstein Institute for Neuroimmunology and Inflammation (INI), Albert Einstein College of Medicine, New York City, New York 10461, USA.
References
- [1].Kristofi R, Bodegard J, Norhammar A, Thuresson M, Nathanson D, Nystrom T, Birkeland KI, Eriksson JW, Cardiovascular and renal disease burden in type 1 compared with type 2 diabetes: a two-country nationwide observational study, Diabetes Care 44 (5) (2021) 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP, Frailty: implications for clinical practice and public health, Lancet 394 (10206) (2019) 1365–1375. [DOI] [PubMed] [Google Scholar]
- [3].Nagasu H, Yano Y, Kanegae H, Heerspink HJL, Nangaku M, Hirakawa Y, Sugawara Y, Nakagawa N, Tani Y, Wada J, Sugiyama H, Tsuruya K, Nakano T, Maruyama S, Wada T, Yamagata K, Narita I, Tamura K, Yanagita M, Terada Y, Shigematsu T, Sofue T, Ito T, Okada H, Nakashima N, Kataoka H, Ohe K, Okada M, Itano S, Nishiyama A, Kanda E, Ueki K, Kashihara N, Kidney Outcomes Associated With SGLT2 inhibitors versus other glucose-lowering drugs in real-world clinical practice: the Japan chronic kidney disease database, Diabetes Care 44 (11) (2021) 2542–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Group E-KC, Effects of empagliflozin on progression of chronic kidney disease: a prespecified secondary analysis from the empa-kidney trial, Lancet Diabetes Endocrinol. 12 (1) (2024) 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anan G, Hirose T, Kikuchi D, Takahashi C, Endo A, Ito H, Sato S, Nakayama S, Hashimoto H, Ishiyama K, Kimura T, Takahashi K, Sato M, Mori T, Inhibition of sodium-glucose cotransporter 2 suppresses renal stone formation, Pharm. Res. 186 (2022) 106524. [DOI] [PubMed] [Google Scholar]
- [6].Qiu M, Ding LL, Zhang M, Lin JH, Gu JS, Zhou X, Tang YX, Wei XB, Liu SY, SGLT2 inhibitors for prevention of cardiorenal events in people with type 2 diabetes without cardiorenal disease: a meta-analysis of large randomized trials and cohort studies, Pharm. Res. 161 (2020) 105175. [DOI] [PubMed] [Google Scholar]
- [7].Sanchez-Garcia A, Simental-Mendia M, Millan-Alanis JM, Simental-Mendia LE, Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: a systematic review and meta-analysis of 48 randomized controlled trials, Pharm. Res 160 (2020) 105068. [DOI] [PubMed] [Google Scholar]
- [8].Mone P, Varzideh F, Jankauskas SS, Pansini A, Lombardi A, Frullone S, Santulli G, SGLT2 inhibition via empagliflozin improves endothelial function and reduces mitochondrial oxidative stress: insights from frail hypertensive and diabetic patients, Hypertension 79 (8) (2022) 1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mone P, Gambardella J, Pansini A, Martinelli G, Minicucci F, Mauro C, Santulli G, Cognitive dysfunction correlates with physical impairment in frail patients with acute myocardial infarction, Aging Clin. Exp. Res. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Santulli G, Varzideh F, Forzano I, Wilson S, Salemme L, de Donato A, Lombardi A, Rainone A, Nunziata L, Jankauskas SS, Tesorio T, Guerra G, Kansakar U, Mone P, Functional and clinical importance of SGLT2-inhibitors in frailty: from the kidney to the heart, Hypertension 80 (9) (2023) 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]


