Abstract
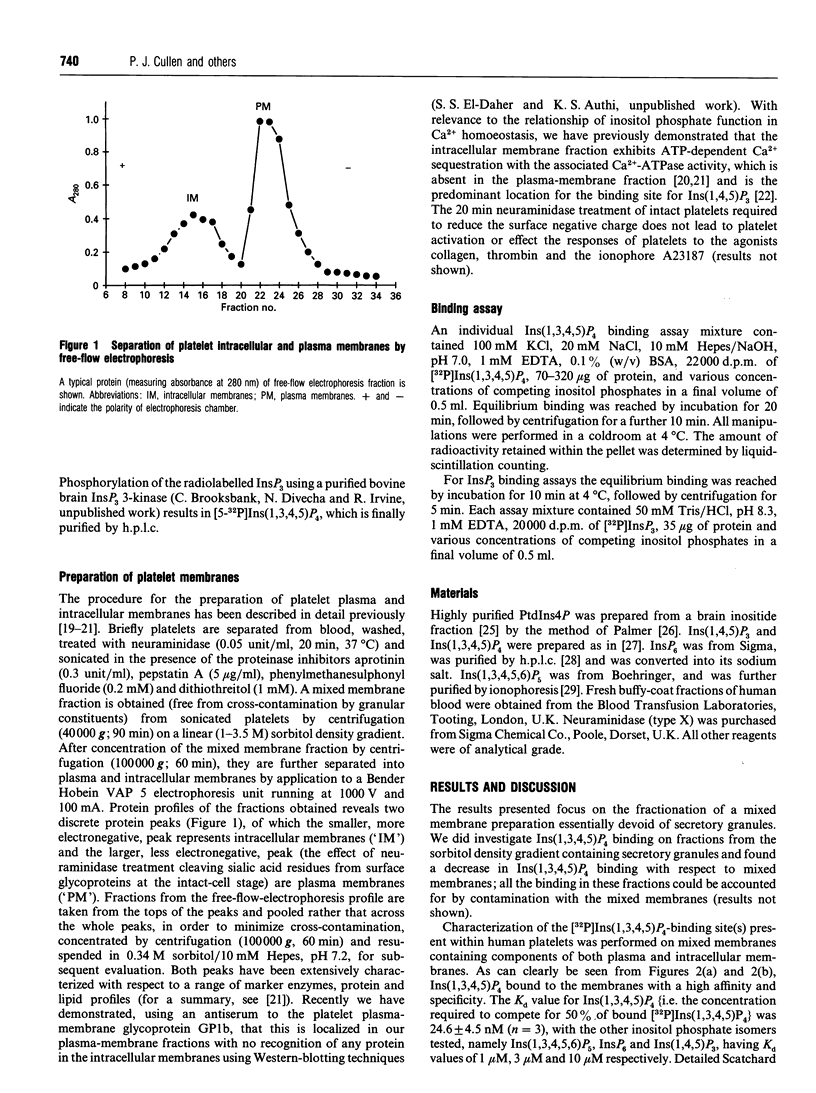
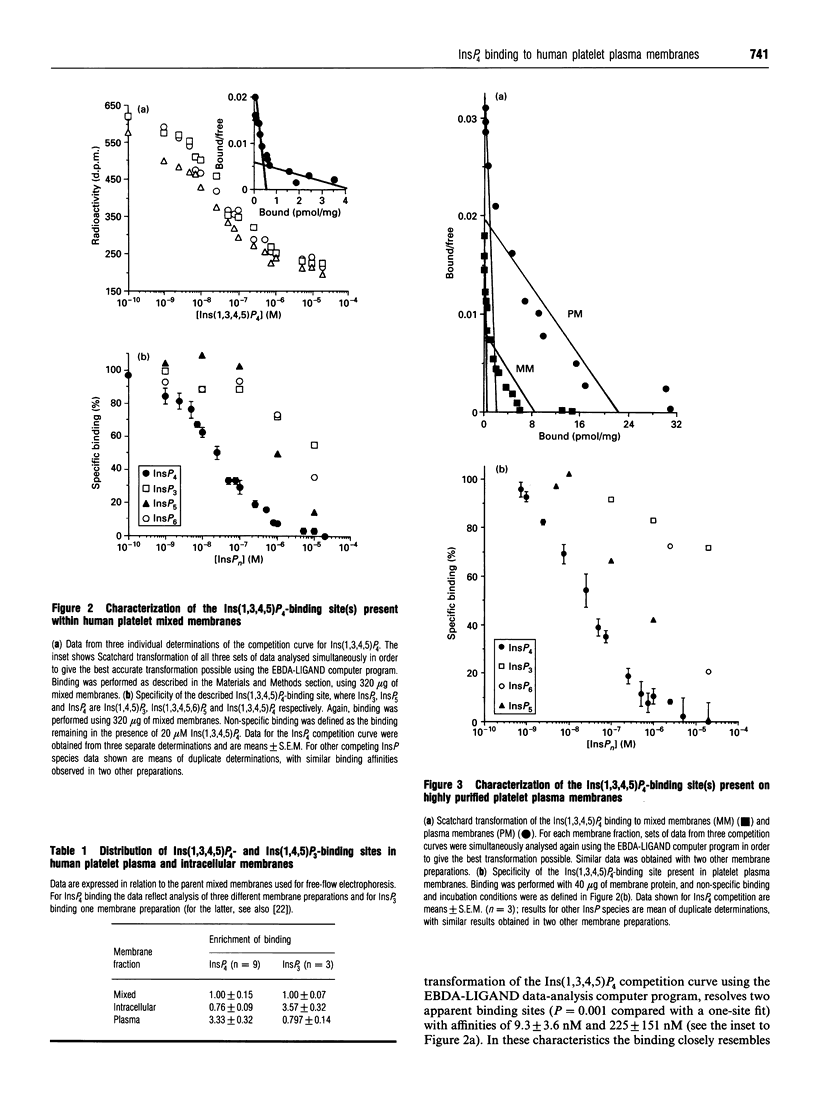
In the present study we describe the characterization and localization of Ins(1,3,4,5)P4-binding sites in human platelet membranes. Specific binding sites for Ins(1,3,4,5)P4 have been identified on mixed, plasma and intracellular membranes from neuraminidase-treated platelets using highly purified carrier-free [32P]Ins(1,3,4,5)P4. The displacement of Ins(1,3,4,5)P4 from these sites by Ins(1,4,5)P3 and InsP6 occurs at greater than two orders of magnitude higher concentrations and with Ins(1,3,4,5,6)P5 at about 40-fold higher concentrations than with Ins(1,3,4,5)P4. The membranes were further separated by free-flow electrophoresis into plasma and intracellular membranes. The Ins(1,3,4,5)P4-binding sites separated with plasma membranes, and showed similar affinities and specificities as mixed membranes, whereas Ins(1,4,5)P3-binding sites were predominantly in the intracellular membranes. These results suggest a predominantly plasma membrane location for putative Ins(1,3,4,5)P4 receptors in human platelets.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Authi K. S., Crawford N. Inositol 1,4,5-trisphosphate-induced release of sequestered Ca2+ from highly purified human platelet intracellular membranes. Biochem J. 1985 Aug 15;230(1):247–253. doi: 10.1042/bj2300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authi K. S. Localisation of the [32P]IP3 binding site on human platelet intracellular membranes isolated by high-voltage free-flow electrophoresis. FEBS Lett. 1992 Feb 24;298(2-3):173–176. doi: 10.1016/0014-5793(92)80049-m. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Nahorski S. R. Depolarization and agonist-stimulated changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass accumulation in rat cerebral cortex. J Neurochem. 1991 Sep;57(3):1042–1051. doi: 10.1111/j.1471-4159.1991.tb08255.x. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Crawford N., Authi K. S., Hack N. Isolation and characterization of platelet membranes prepared by free flow electrophoresis. Methods Enzymol. 1992;215:5–20. doi: 10.1016/0076-6879(92)15048-h. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate binding sites in neuronal and non-neuronal tissues. Properties, comparisons and potential physiological significance. Biochem J. 1992 Nov 15;288(Pt 1):149–154. doi: 10.1042/bj2880149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divecha N., Brooksbank C. E., Irvine R. F. Purification and characterization of phosphatidylinositol 4-phosphate 5-kinases. Biochem J. 1992 Dec 1;288(Pt 2):637–642. doi: 10.1042/bj2880637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donié F., Reiser G. A novel, specific binding protein assay for quantitation of intracellular inositol 1,3,4,5-tetrakisphosphate (InsP4) using a high-affinity InsP4 receptor from cerebellum. FEBS Lett. 1989 Aug 28;254(1-2):155–158. doi: 10.1016/0014-5793(89)81029-4. [DOI] [PubMed] [Google Scholar]
- Donié F., Reiser G. Purification of a high-affinity inositol 1,3,4,5-tetrakisphosphate receptor from brain. Biochem J. 1991 Apr 15;275(Pt 2):453–457. doi: 10.1042/bj2750453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Brown E., Williams G. Distinct binding sites for Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in bovine parathyroid glands. Biochem Biophys Res Commun. 1989 Feb 28;159(1):200–208. doi: 10.1016/0006-291x(89)92423-6. [DOI] [PubMed] [Google Scholar]
- Enyedi P., Williams G. H. Heterogenous inositol tetrakisphosphate binding sites in the adrenal cortex. J Biol Chem. 1988 Jun 15;263(17):7940–7942. [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Cullen P. J. Will the real IP4 receptor please stand up? Curr Biol. 1993 Aug 1;3(8):540–543. doi: 10.1016/0960-9822(93)90052-p. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. Inositol phosphates and Ca2+ entry: toward a proliferation or a simplification? FASEB J. 1992 Sep;6(12):3085–3091. doi: 10.1096/fasebj.6.12.1325932. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. Is inositol tetrakisphosphate the second messenger that controls Ca2+ entry into cells? Adv Second Messenger Phosphoprotein Res. 1992;26:161–185. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Berridge M. J. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J. 1986 Nov 15;240(1):301–304. doi: 10.1042/bj2400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. A., Steiner J. P., Snyder S. H. Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: selective enrichment in sialic acid and unique binding specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2849–2853. doi: 10.1073/pnas.89.7.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Menashi S., Weintroub H., Crawford N. Characterization of human platelet surface and intracellular membranes isolated by free flow electrophoresis. J Biol Chem. 1981 Apr 25;256(8):4095–4101. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Palmer F. B. Chromatography of acidic phospholipids on immobilized neomycin. J Lipid Res. 1981 Nov;22(8):1296–1300. [PubMed] [Google Scholar]
- Seiffert U. B., Agranoff B. W. Isolation and separation of inositol phosphates from hydrolysates of rat tissues. Biochim Biophys Acta. 1965 Jun 1;98(3):574–581. doi: 10.1016/0005-2760(65)90154-2. [DOI] [PubMed] [Google Scholar]
- Smith P. M. Ins(1,3,4,5)P4 promotes sustained activation of the Ca(2+(-dependent Cl- current in isolated mouse lacrimal cells. Biochem J. 1992 Apr 1;283(Pt 1):27–30. doi: 10.1042/bj2830027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theibert A. B., Estevez V. A., Ferris C. D., Danoff S. K., Barrow R. K., Prestwich G. D., Snyder S. H. Inositol 1,3,4,5-tetrakisphosphate and inositol hexakisphosphate receptor proteins: isolation and characterization from rat brain. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3165–3169. doi: 10.1073/pnas.88.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]


