Abstract
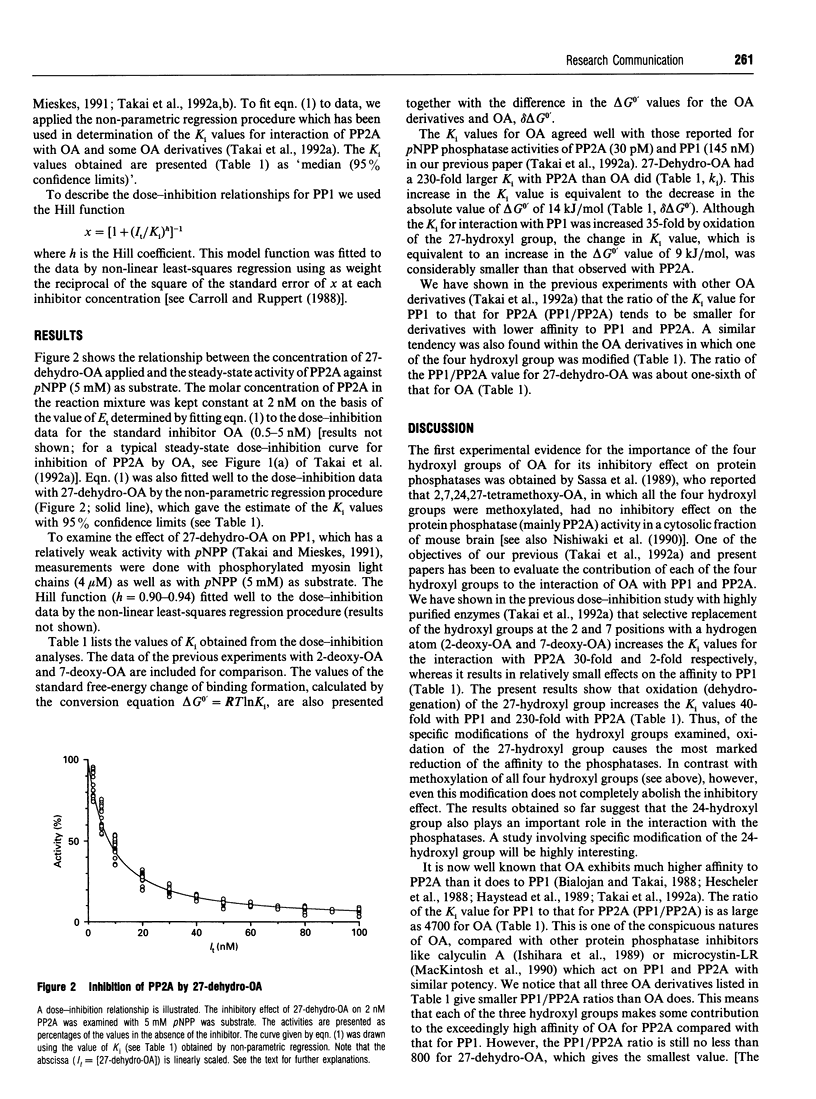
Okadaic acid (OA), a potent inhibitor of type-1 and type-2A protein phosphatases (PP1 and PP2A), has four hydroxyl groups at 2, 7, 24 and 27 positions (see Figure 1). By chemical treatment of OA we synthesized a derivative, in which the 27-hydroxyl group was specifically oxidized (27-dehydro-OA). The inhibitory effect of this OA derivative was examined on the activities of PP1 and PP2A, which were inhibited by intact OA with dissociation constants (Ki) of 150 nM and 32 pM respectively. We found that the affinity of OA was decreased 40-fold (Ki = 6 microM) with PP1 and 230-fold (Ki = 7.3 nM) with PP2A after oxidation of the 27-hydroxyl group. According to the model of the three-dimensional conformation of OA on the basis of X-ray analyses, the 27-hydroxyl group appears to be present in a position relatively free from intramolecular bonding formation, in comparison with the other three hydroxyl groups. The marked increases in the Ki values for PP1 and PP2A, which indicate the reduction of the absolute values of the free energy of binding by 9 kJ/mol and 14 kJ/mol respectively, may imply that the 27-hydroxyl group serves as a binding site with the phosphatase molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Lambert S. J. Myosin transitions in the bovine and human heart. A developmental and anatomical study of heavy and light chain subunits in the atrium and ventricle. Circ Res. 1986 Jun;58(6):846–858. doi: 10.1161/01.res.58.6.846. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Carruthers C. A., Walsh M. P. Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem J. 1984 Mar 15;218(3):863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki S., Fujiki H., Suganuma M., Furuya-Suguri H., Matsushima R., Iida Y., Ojika M., Yamada K., Uemura D., Yasumoto T. Structure-activity relationship within a series of okadaic acid derivatives. Carcinogenesis. 1990 Oct;11(10):1837–1841. doi: 10.1093/carcin/11.10.1837. [DOI] [PubMed] [Google Scholar]
- Sassa T., Richter W. W., Uda N., Suganuma M., Suguri H., Yoshizawa S., Hirota M., Fujiki H. Apparent "activation" of protein kinases by okadaic acid class tumor promoters. Biochem Biophys Res Commun. 1989 Mar 31;159(3):939–944. doi: 10.1016/0006-291x(89)92199-2. [DOI] [PubMed] [Google Scholar]
- Takai A., Mieskes G. Inhibitory effect of okadaic acid on the p-nitrophenyl phosphate phosphatase activity of protein phosphatases. Biochem J. 1991 Apr 1;275(Pt 1):233–239. doi: 10.1042/bj2750233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai A., Murata M., Torigoe K., Isobe M., Mieskes G., Yasumoto T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem J. 1992 Jun 1;284(Pt 2):539–544. doi: 10.1042/bj2840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai A., Ohno Y., Yasumoto T., Mieskes G. Estimation of the rate constants associated with the inhibitory effect of okadaic acid on type 2A protein phosphatase by time-course analysis. Biochem J. 1992 Oct 1;287(Pt 1):101–106. doi: 10.1042/bj2870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung H. Y., Resink T. J., Hemmings B. A., Shenolikar S., Cohen P. The catalytic subunits of protein phosphatase-1 and protein phosphatase 2A are distinct gene products. Eur J Biochem. 1984 Feb 1;138(3):635–641. doi: 10.1111/j.1432-1033.1984.tb07962.x. [DOI] [PubMed] [Google Scholar]


