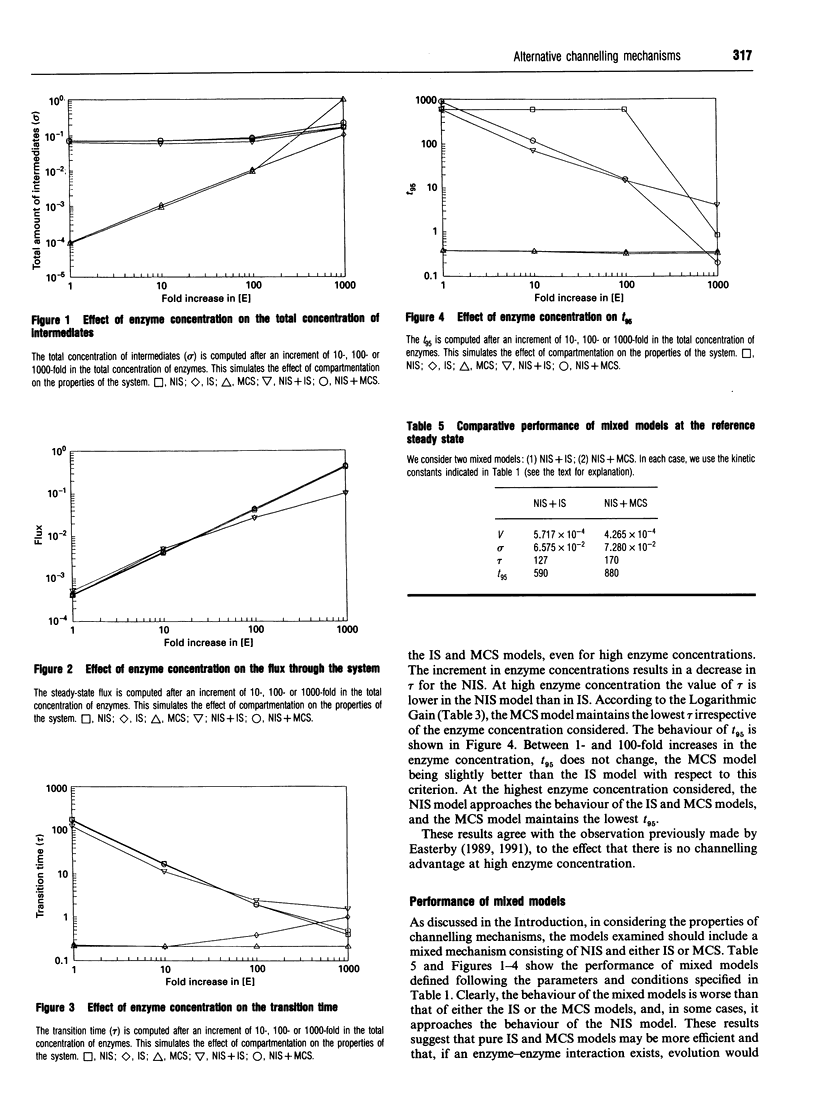
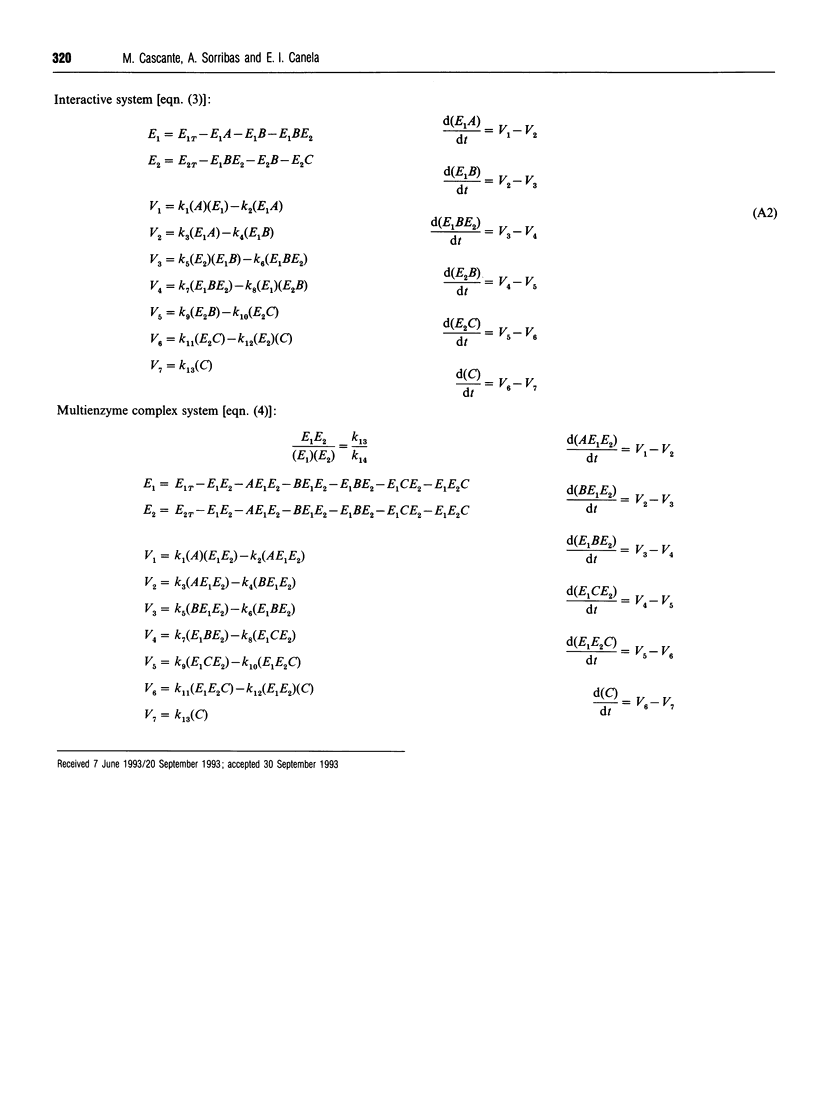
Abstract
Metabolite channelling may result from different kinetic mechanisms in which enzyme-enzyme interactions occur, so that intermediates are not released into the bulk solution and cannot be used by enzymes outside the channel. From an evolutionary point of view, the emergence of such mechanisms may provide new functional possibilities for the system, which would result in a selective advantage. Hence, it would be useful to evaluate the objective advantages provided by the various options by considering different criteria for functional effectiveness. Following this strategy, the goal of this paper is to compare a model for a free-diffusion two-enzyme system with two different models with inclusion of enzyme-enzyme interactions. In addition, models with simultaneous free and interacting branches are also analysed, and their advantages or disadvantages are presented. Basic guidelines are suggested that help in predicting the occurrence of specific mechanisms in different circumstances, and provide theoretical evidence in support of the hypothesis that no single solution simultaneously optimizes all the possible desired properties of the system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung C. W., Cohen N. S., Raijman L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J Biol Chem. 1989 Mar 5;264(7):4038–4044. [PubMed] [Google Scholar]
- Clegg J. S., Jackson S. A. Evidence for intermediate channelling in the glycolytic pathway of permeabilized L-929 cells. Biochem Biophys Res Commun. 1989 May 15;160(3):1409–1414. doi: 10.1016/s0006-291x(89)80161-5. [DOI] [PubMed] [Google Scholar]
- Clegg J. S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am J Physiol. 1984 Feb;246(2 Pt 2):R133–R151. doi: 10.1152/ajpregu.1984.246.2.R133. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Cárdenas M. L. Channelling can affect concentrations of metabolic intermediates at constant net flux: artefact or reality? Eur J Biochem. 1993 Apr 1;213(1):87–92. doi: 10.1111/j.1432-1033.1993.tb17737.x. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. Failure of channelling to maintain low concentrations of metabolic intermediates. Eur J Biochem. 1991 Jan 1;195(1):103–108. doi: 10.1111/j.1432-1033.1991.tb15681.x. [DOI] [PubMed] [Google Scholar]
- Easterby J. S. Coupled enzyme assays: a general expression for the transient. Biochim Biophys Acta. 1973 Feb 15;293(2):552–558. doi: 10.1016/0005-2744(73)90362-8. [DOI] [PubMed] [Google Scholar]
- Easterby J. S. Homeostasis, flexibility and conflict in the kinetic advantage of channelling. J Theor Biol. 1991 Sep 7;152(1):47–48. doi: 10.1016/s0022-5193(05)80507-7. [DOI] [PubMed] [Google Scholar]
- Easterby J. S. The analysis of metabolite channelling in multienzyme complexes and multifunctional proteins. Biochem J. 1989 Dec 1;264(2):605–607. doi: 10.1042/bj2640605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R., Canela E. I. A program for the numerical integration of enzyme kinetic equations using small computers. Int J Biomed Comput. 1984 Nov-Dec;15(6):419–432. doi: 10.1016/0020-7101(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Franco R., Canela E. I. Computer simulation of purine metabolism. Eur J Biochem. 1984 Oct 15;144(2):305–315. doi: 10.1111/j.1432-1033.1984.tb08465.x. [DOI] [PubMed] [Google Scholar]
- Giersch C. Pillow strategy. J Theor Biol. 1991 Sep 7;152(1):71–71. doi: 10.1016/s0022-5193(05)80513-2. [DOI] [PubMed] [Google Scholar]
- Heinrich R., Schuster S. Is metabolic channelling the complicated solution to the easy problem of reducing transient times? J Theor Biol. 1991 Sep 7;152(1):57–61. doi: 10.1016/s0022-5193(05)80510-7. [DOI] [PubMed] [Google Scholar]
- Hervé G. Semantics of channeling and microcompartmentation. J Theor Biol. 1991 Sep 7;152(1):131–132. doi: 10.1016/s0022-5193(05)80526-0. [DOI] [PubMed] [Google Scholar]
- Irvine D. H., Savageau M. A. Network regulation of the immune response: alternative control points for suppressor modulation of effector lymphocytes. J Immunol. 1985 Apr;134(4):2100–2116. [PubMed] [Google Scholar]
- Irvine D. H., Savageau M. A. Network regulation of the immune response: modulation of suppressor lymphocytes by alternative signals including contrasuppression. J Immunol. 1985 Apr;134(4):2117–2130. [PubMed] [Google Scholar]
- Keleti T., Batke J., Ovádi J., Jancsik V., Bartha F. Macromolecular interactions in enzyme regulation. Adv Enzyme Regul. 1976;15:233–265. doi: 10.1016/0065-2571(77)90019-x. [DOI] [PubMed] [Google Scholar]
- Keleti T., Ovádi J., Batke J. Kinetic and physico-chemical analysis of enzyme complexes and their possible role in the control of metabolism. Prog Biophys Mol Biol. 1989;53(2):105–152. doi: 10.1016/0079-6107(89)90016-3. [DOI] [PubMed] [Google Scholar]
- Keleti T., Ovádi J. Control of metabolism by dynamic macromolecular interactions. Curr Top Cell Regul. 1988;29:1–33. doi: 10.1016/b978-0-12-152829-4.50003-3. [DOI] [PubMed] [Google Scholar]
- Kell D. B. On the physiological significance of metabolite channelling: if, how, and where, but not why. J Theor Biol. 1991 Sep 7;152(1):49–51. doi: 10.1016/s0022-5193(05)80508-9. [DOI] [PubMed] [Google Scholar]
- Meléndez-Hevia E., Montero F. Channelling and evolution of metabolism. J Theor Biol. 1991 Sep 7;152(1):77–79. doi: 10.1016/s0022-5193(05)80515-6. [DOI] [PubMed] [Google Scholar]
- Mendes P., Kell D. B., Westerhoff H. V. Channelling can decrease pool size. Eur J Biochem. 1992 Feb 15;204(1):257–266. doi: 10.1111/j.1432-1033.1992.tb16632.x. [DOI] [PubMed] [Google Scholar]
- Nichol L. W., Kuchel P. W., Jeffrey P. D. The detection and consequences of association of two enzymes involved in catalysing consecutive reactions. Biophys Chem. 1974 Dec;2(4):354–358. doi: 10.1016/0301-4622(74)80062-1. [DOI] [PubMed] [Google Scholar]
- Ovádi J. Physiological significance of metabolic channelling. J Theor Biol. 1991 Sep 7;152(1):1–22. [PubMed] [Google Scholar]
- Savageau M. A. Metabolite channeling: implications for regulation of metabolism and for quantitative description of reactions in vivo. J Theor Biol. 1991 Sep 7;152(1):85–92. doi: 10.1016/s0022-5193(05)80517-x. [DOI] [PubMed] [Google Scholar]
- Savageau M. A. Optimal design of feedback control by inhibition. J Mol Evol. 1974 Nov 29;4(2):139–156. doi: 10.1007/BF01732019. [DOI] [PubMed] [Google Scholar]
- Savageau M. A., Sorribas A. Constraints among molecular and systemic properties: implications for physiological genetics. J Theor Biol. 1989 Nov 8;141(1):93–115. doi: 10.1016/s0022-5193(89)80011-6. [DOI] [PubMed] [Google Scholar]
- Shulman R. G. Is channeling a disproveable hypothesis? J Theor Biol. 1991 Sep 7;152(1):133–134. doi: 10.1016/s0022-5193(05)80527-2. [DOI] [PubMed] [Google Scholar]
- Sorribas A., Savageau M. A. A comparison of variant theories of intact biochemical systems. I. Enzyme-enzyme interactions and biochemical systems theory. Math Biosci. 1989 Jun;94(2):161–193. doi: 10.1016/0025-5564(89)90064-3. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Welch G. R. On the role of organized multienzyme systems in cellular metabolism: a general synthesis. Prog Biophys Mol Biol. 1977;32(2):103–191. [PubMed] [Google Scholar]
- Westerhoff H. V., Melandri B. A., Venturoli G., Azzone G. F., Kell D. B. A minimal hypothesis for membrane-linked free-energy transduction. The role of independent, small coupling units. Biochim Biophys Acta. 1984 Dec 17;768(3-4):257–292. doi: 10.1016/0304-4173(84)90019-3. [DOI] [PubMed] [Google Scholar]


