Abstract
Background
The impact of migration on HIV risk among non-migrating household members is poorly understood. We measured HIV incidence among non-migrants living in households with and without migrants in Uganda.
Methods
We used four survey rounds of data collected from July 2011 to May 2018 from non-migrant participants aged 15–49 years in the Rakai Community Cohort Study. Non-migrants were individuals with no-migration between surveys or at the prior survey. Household migration was defined as ≥1 household member migrating into or out of the house from another community between surveys (∼18 months). Incident HIV was defined as testing HIV seropositive following a negative result. Incidence rate ratios (IRRs) were estimated using Poisson regression with generalized estimating equations. Analyses were stratified by gender, migration into or out of the household and the relationship between non-migrants and migrants (e.g. spouse, child).
Results
About 11 318 non-migrants (5674 women) were followed for 37 320 person-years. Twenty-eight percent (6059/21 370) of non-migrant person-visits had recent migration into or out of the household, and 240 HIV incident cases were identified. Overall, non-migrants in migrant households were not at greater risk of acquiring HIV than non-migrants in households without any migration. However, men were significantly more likely to acquire HIV if their spouse had recently migrated in [adjusted IRR: 2.12; 95% confidence interval (CI): 1.05–4.27] or out (adjusted IRR: 4.01; 95% CI, 2.16–7.44) compared with men with no spousal migration.
Conclusions
HIV incidence is higher among non-migrant men with migrant spouses. Targeted HIV testing and prevention interventions like pre-exposure prophylaxis could be considered for men with migrant spouses.
Keywords: Migration, household, incidence, HIV, Uganda
Key Messages.
Despite high levels of migration and an established association between migration and HIV risk among migrants, there is very limited data on the broader societal impacts of migration on HIV acquisition risk among non-migrant populations.
Using prospective data from over 11 000 non-migrating individuals in an open, population-based HIV surveillance cohort in southern Uganda, we report that spousal migration into or out of the household was associated with 2- to 4-fold greater HIV incidence among non-migrating men.
Our results suggest targeted HIV testing and prevention interventions, such as self-testing interventions and pre-exposure prophylaxis, be considered for men with migrant spouses.
Introduction
Migration is increasingly common in Africa and linked to higher risk of HIV.1,2 Previous studies in sub-Saharan Africa have shown that migrants have a higher risk of HIV acquisition, are more likely to report riskier sexual behaviours and are less likely to be virally suppressed if living with HIV.2–4 However, less is known about how migration impacts HIV risk for non-migrant household members. Migration may be a broadly disruptive event for the household, changing non-migrants’ relationships, access to resources and support, thereby increasing their risk of HIV acquisition.5 As generalized African HIV epidemics decline,6,7 identifying population sub-groups at highest HIV risk will be critical for effective targeting of limited HIV prevention resources.
Migration may impact HIV risk through changes to non-migrant residents’ sexual networks and their own individual behaviours. Non-migrants may become more stressed as a result of partner, child or parent migration. Some individuals may cope with the greater stress, separation of partners or reduced parental supervision by increasing sexual risk behaviours resulting in higher risk of HIV acquisition.8–11 Previous studies have shown that non-migrants with migrant spouses are more likely to report riskier sexual behaviours, and acquire HIV or other sexually transmitted infections.10,12,13 However, most previous studies focus on non-migrant wives whose male spouses migrate for work for long periods of time.14
Here, we evaluated whether HIV incidence is higher among non-migrants living in households that migrants enter or leave using data from the Rakai Community Cohort Study in south-central Uganda. In addition, we also examined the effect on non-migrants with migrant spouses, and parents with migrant children. Based on existing literature, we hypothesized that non-migrants living in migrant households, especially those with migrant spouses, would be more likely to acquire HIV.
Methods
Study overview
We used data collected from July 2011 to May 2018 from non-migrant participants 15–49 years in the Rakai Community Cohort Study (RCCS), an open, population-based cohort in southern Uganda. The RCCS collects data via a census and survey (see Supplementary Text for additional details). Briefly, the census records household members’ relationship to head of household and any migration into or out of the household since the last census. The survey, conducted ∼2 weeks after the census, collects demographic and self-reported sexual behaviours from residents who have been living in the community for at least six months or at least one month with the intention to stay. At time of survey, HIV testing and counselling are also offered. During each RCCS survey round, communities are censused and surveyed sequentially over a period of ∼18 months. Once all communities have been censused surveyed, the next survey round begins.7,15
Our analysis included 38 continuously surveyed communities with varying HIV seroprevalence, including 34 semi-urban and agrarian inland communities (range: 9–26% HIV seroprevalence) and four Lake Victoria fishing communities with extremely high HIV seroprevalence (range: 38–43%), over four survey rounds.15 Community boundaries were established previously through household mapping and in consultation with community leaders for inland communities as part of a randomized controlled trial in 1994 and for fishing communities in 2011.15
The present study was restricted to RCCS non-migrant individuals who initially tested HIV seronegative. Non-migrants were those who had reported not moving at two sequential survey visits, allowing for one skipped visit. Individuals who reported moving within communities or returning to communities were classified as mobile and excluded.
Exposure
The primary exposure was a household migration event, defined as one member moving into (in-migrants) or out of the household (out-migrants) to/from another community between surveys (∼18 months), staying at their destination for at least one month or less than one month with the intention to stay for at least six months. Intention to stay was ascertained by asking the migrants themselves, the head of household, or a proxy respondent. Migration events were assessed from census and survey data irrespective of age.
Households were initially categorized into three groups: (i) no-migration households where no recent migration event was documented; (ii) any household in-migration where member(s) migrated into the household and (iii) any household out-migration where member(s) migrated out. Households with experiencing in- and out-migration in the same visit-interval contributed to both in- and out-analyses.
We further determined the relationship between non-migrants and migrants using their current or previous census household roster. This household roster was used to classify relationships between out-migrants and non-migrant residents. Spousal relationships were defined as long-term consensual unions or marital relationships. We examined relationships where the spouse or child migrated as previous studies suggest that spousal migration may impact sexual behaviour and networks for the non-migrant spouse;10,12,13 and a child migrating may impact support available for the parent.16 Four types of relationships between non-migrant and migrants were evaluated: (i) non-migrants with an in-migrating spouse; (ii) non-migrants with an out-migrating spouse; (iii) non-migrant parents with an in-migrating child and (iv) non-migrant parents with an out-migrating child. Those who experienced both an in- and out-migration of their spouse or child in the same visit-interval contributed to both in- and out-analyses.
Outcome
Our outcome was incident HIV infection, defined as a first positive HIV test preceded by an HIV negative test at the prior visit. Those who missed two consecutive survey rounds were excluded from the analysis. HIV status was determined using a validated testing algorithm which included three rapid tests and enzyme immunoassays or polymerase chain reaction tests to confirm HIV status.15
Statistical methods
We first compared the demographics of men and women included in our analytic sample at baseline to those who were lost-to-follow-up. We then contrasted the demographics for each visit-interval included in our analysis, comparing any household in-migration to no-migration visit-intervals and any household out-migration to no-migration visit-intervals.
Next, we assessed the association between HIV incidence and migration events occurring during the same visit-interval. In these primary analyses, we assumed migration always preceded the HIV incident event. Incidence rate ratios (IRR) with 95% confidence intervals (95% CIs) were estimated using generalized estimating equations Poisson regression models with person-time as the offset. Robust standard errors were calculated assuming an exchangeable correlation structure.17 An exchangeable correlation structure generated the best model fit as measured by QIC (Quasilikelihood under the Independence model Criterion).18 Incident HIV was assumed to occur with equal probability through the visit-interval so, on average, HIV acquisition was assumed to occur at the midpoint of the visit-interval. However, we assessed if results were sensitive to this assumption by assessing if results changed when assuming HIV acquisition occurred at the end of the of the interval. Furthermore, we evaluated HIV incidence in the visit-interval following spousal migration, because in primary analyses we were not able to determine if migration preceded or followed acquisition of HIV. For the following visit-interval to be included in this sensitivity analysis, non-migrants had to remain non-migrants after spousal migration and not experience any in- or out-spousal migration.
We stratified analyses by: (i) gender as correlates for HIV seropositivity and viremia differ between men and women19,20; (ii) migration into or out of the household; (iii) the relationship between non-migrants and migrants (i.e. any household migration, spouse, child) as spouse migrations were theorized to have a more direct impact on HIV incidence than other types of migration5,13 and (iv) by community type (inland vs fishing) as local HIV epidemic dynamics differ.15 To account for differences in demographics across exposed and unexposed groups, we adjusted for confounders like five-year age group, education, marital status (for non-spousal analyses), survey round and residency in an inland or fishing community (for analyses not stratified by community type).
In sub-group analyses, we also assessed how the relationship between spousal migration and HIV acquisition differed by marital status as relationship dissolution is associated with spouses migrating out and relationship formation is associated with spouses in-migrating. We first restricted spousal analyses to those currently married. Then we assessed HIV incidence among those who were previously married and had a spouse migrate out compared with those who were previously married but did not have any spousal migration.
Lastly, we evaluated whether sexual behaviour and related factors (i.e. condom use with casual partners, number of sexual partners in the past year or presence of genital ulcers) changed following in- or out-spousal migration. We compared the sexual behaviours at the start of the visit-interval prior to spousal migration to those at the end of the visit-interval following spousal migration. We classified sexual behaviours into lower and higher risk categories (Supplementary Text).
Analyses were conducted using Stata 17 and R version 4.1.1.
Results
Overview
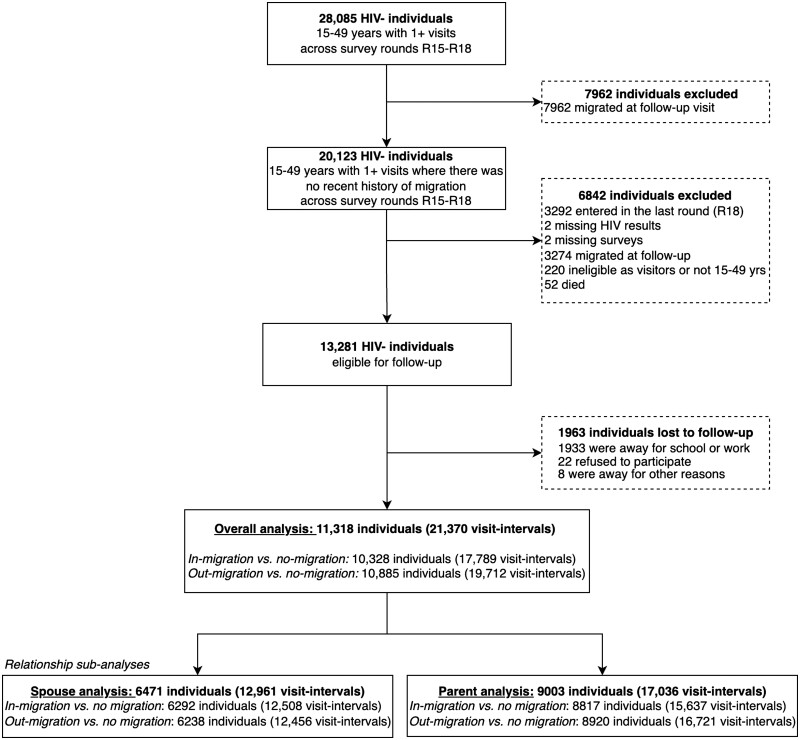
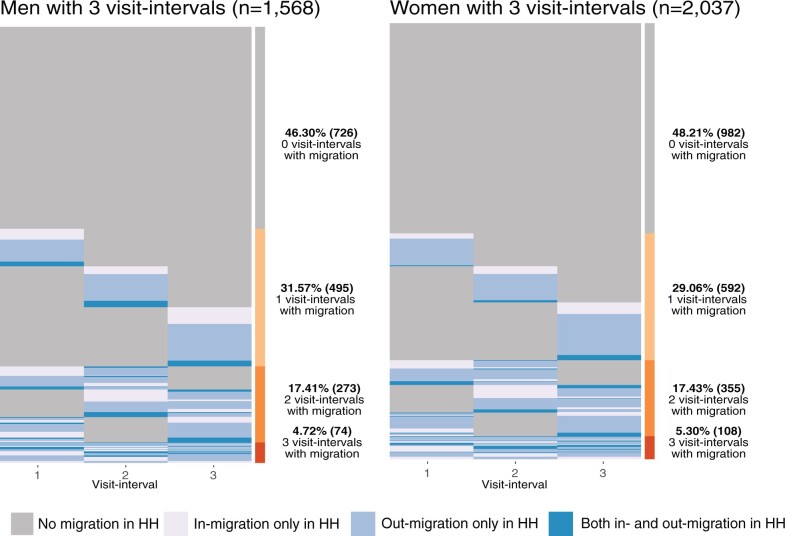
Of the 28 085 individuals in the cohort, 20 123 (71.7%) individuals had no recent history of migration observed across the four survey waves and were eligible for our analysis (Figure 1). A further 13 281 were eligible to be surveyed at more than one study visit. Of those, 1963 were lost to follow-up and they were more likely to be men, younger and never married (Supplementary Table S1). In the remaining analytic cohort, 11 318 non-migrant participants (5674 women) were followed for 37 320 person-years contributing 21 370 visit-intervals. Migration into or out of the household occurred within 28% (6059/21 370) of visit-intervals. Out of those who contributed three visit-intervals, 52.6% had at least one visit where there was migration into or out of the household (Figure 2).
Figure 1.
Flowchart of analysis
Figure 2.
Longitudinal migration in the household patterns for non-migrant men and women contributing three visit-intervals to the analysis. Each line represents an individual’s trajectory across the three visit-intervals. HH = household
At baseline, over half of participants were currently married and most individuals lived in inland communities (Table 1). Visit-intervals from fishing communities were more likely to report migration into the household (Supplementary Table S2). In total 13% (2820/21 370) of visit-intervals had either a spouse or child migrate. A limited number of visit-intervals (820/21 370; 3.84%) experienced both in- and out-migration in the same period contributing to both in-migration and out-migration households.
Table 1.
Baseline characteristics of the 11 318 included non-migrant men and women
| Men | Women | |
|---|---|---|
| [n/N (%)] | [n/N (%)] | |
| Individuals | 5644 | 5674 |
| Education | ||
| None to primary | 3779/5644 (67%) | 3445/5674 (61%) |
| Secondary school and beyond | 1865/5644 (33%) | 2229/5674 (39%) |
| Mean age in years (SD) | 29 (9) | 30 (9) |
| Age group (years) | ||
| 15–29 | 2952/5644 (52%) | 2809/5674 (50%) |
| 30–39 | 1678/5644 (30%) | 1943/5674 (34%) |
| 40–49 | 1014/5644 (18%) | 922/5674 (16%) |
| Marital status | ||
| Currently married | 3030/5644 (54%) | 3567/5674 (63%) |
| Previously married | 550/5644 (9.7%) | 777/5674 (14%) |
| Never married | 2064/5644 (37%) | 1330/5674 (23%) |
| Community type | ||
| Inland | 4482/5644 (79%) | 4894/5674 (86%) |
| Fishing | 1162/5644 (21%) | 780/5674 (14%) |
SD = standard deviation.
Migration in the household and HIV incidence
There were 240 HIV incident cases identified in non-migrating household members, including 68 (28%) that occurred during the same visit-interval as a household migration event. Overall, there was no association between migration of any household member with risk of HIV acquisition among either men or women (Table 2). Child migration was not associated with increased risk of HIV incidence among parents of either gender, although there were very few incident HIV infections among men with migrating children. However, in analyses of spousal relationships, we observed substantially higher HIV incidence among those with migrating spouses, especially men. Among men who had a spouse who in-migrated or out-migrated into the household, HIV incidence was 1.3 and 2.1 per 100 person-years, respectively, compared with 0.4 per 100 person-years among men whose wives had not migrated. After adjusting for demographics, men with an in-migrating spouse were 2.1-fold (95% CI, 1.0–4.3) more likely to acquire HIV and men with out-migrating spouses were 4.0-fold more likely (95% CI, 2.2–7.4) to acquire HIV compared with men whose spouses did not migrate (Table 2). We found that 14% of all non-migrant men had migrating spouses (n = 773/5644). Out of all HIV incident cases for non-migrant men, 23% (26/111) were for men with migrating spouses. HIV incidence was also elevated among women whose spouses migrated into or out of the household compared with women without migrating spouses; however, differences were not statistically significant. To assess whether primary results were sensitive to the assumed timing of HIV acquisition, we ran our primary regressions again but assumed that HIV was acquired at the end of a visit-interval, and this did not alter our findings (Supplementary Table S3).
Table 2.
Incident rates and ratios for migration in the household
| Exposed |
Referencea,b,c |
|||||||
|---|---|---|---|---|---|---|---|---|
| Incident cases/pyrs | IR per 100 pyrs (95% CI) | Incident cases/pyrs | IR per 100 pyrs (95% CI) | Crude IRR (95% CI) | P | Adjusted IRRd(95% CI) | P | |
| Men | ||||||||
| Any in-migrationa | 14/2343 | 0.60 (0.35–1.01) | 77/12 655 | 0.61 (0.49–0.76) | 0.98 (0.56–1.74) | 0.95 | 0.85 (0.48–1.5) | 0.58 |
| Any out-migrationa | 25/3890 | 0.64 (0.43–0.95) | 77/12 655 | 0.61 (0.49–0.76) | 1.06 (0.67–1.66) | 0.81 | 1.20 (0.77–1.88) | 0.43 |
| Parent with in-migrating childb | 0/181 | .. | 43/11 586 | 0.37 (0.28–0.50) | .. | .. | .. | .. |
| Parent with out-migrating childb | 2/796 | 0.25 (0.06–1.01) | 43/11 586 | 0.37 (0.28–0.50) | 0.68 (0.16–2.80) | 0.59 | 0.79 (0.19–3.26) | 0.74 |
| In-migrating spousec | 13/998 | 1.31 (0.76–2.26) | 34/8534 | 0.40 (0.28–0.56) | 3.27 (1.73–6.19) | <0.01 | 2.12 (1.05–4.27) | 0.04 |
| Out-migrating spousec | 18/871 | 2.07 (1.30–3.28) | 34/8534 | 0.40 (0.28–0.56) | 5.19 (2.93–9.19) | <0.01 | 4.01 (2.16–7.44) | <0.01 |
| Women | ||||||||
| Any in-migrationa | 12/2027 | 0.59 (0.34–1.04) | 95/13 895 | 0.68 (0.56–0.84) | 0.87 (0.48–1.58) | 0.64 | 0.88 (0.48–1.62) | 0.67 |
| Any out-migrationa | 24/3992 | 0.60 (0.40–0.90) | 95/13 895 | 0.68 (0.56–0.84) | 0.88 (0.56–1.38) | 0.58 | 0.97 (0.61–1.55) | 0.90 |
| Parent with in-migrating childb | 2/405 | 0.49 (0.12–1.98) | 106/15 191 | 0.70 (0.58–0.84) | 0.71 (0.17–2.87) | 0.63 | 0.62 (0.15–2.52) | 0.50 |
| Parent with out-migrating childb | 6/1754 | 0.34 (0.15–0.76) | 106/15 191 | 0.70 (0.58–0.84) | 0.49 (0.22–1.12) | 0.09 | 0.80 (0.32–2.00) | 0.62 |
| In-migrating spousec | 3/228 | 1.32 (0.42–4.10) | 56/12 016 | 0.47 (0.36–0.61) | 2.82 (0.88–9.03) | 0.08 | 2.38 (0.72–7.89) | 0.16 |
| Out-migrating spousec | 3/257 | 1.17 (0.37–3.63) | 56/12 016 | 0.47 (0.36–0.61) | 2.50 (0.78–8.00) | 0.12 | 2.33 (0.72–7.48) | 0.16 |
IR = incidence rate, IRR = incidence rate ratio, pyrs = person-years, CI = confidence interval regression did not converge. Bold values indicate P < 0.05.
Compared with no-migration households.
Compared with parents with non-migrating children.
Compared with spouses with non-migrating spouses.
Adjusted for age category-education-study round-fishing or inland community and marital status for non-spouse regressions.
Impact of migration in inland and fishing communities
Next, we assessed if analyses by type of community had an effect on the relationship between household migration and HIV incidence. Within inland communities only, men with in- and out-migrant spouses were more likely to acquire HIV compared with men without migrant spouses (Supplementary Table S4). For women in fishing communities, those who experienced any in-migration into the household were 2.28-fold more likely to acquire HIV than women in no-migration households in adjusted analyses (95% CI, 1.04–5.01, Supplementary Table S5).
Marital status and HIV incidence
Examination of spousal migration events showed that 80% (1086/1352) were associated with the breakdown of a relationship or start of a new relationship. To assess whether change in marital status was potentially driving the association between spousal migration and HIV incidence, we stratified analyses by the current marital status of the non-migrant (Supplementary Table S6). Among currently married men, HIV incidence was higher for men whose spouse had in-migrated [adjusted incident rate ratio (adjIRR), 1.75; 95% CI, 0.76–4.01] but not out-migrated compared with currently married men who did not experience a spousal migration. Men who reported being previously married at the time of survey and who reported spouse out-migration during the visit-interval were three times as likely to acquire HIV than previously married men who did not experience spousal migration after adjusting for demographics [3.20/100 person-years (pyrs) vs 1.23/100 pyrs; adjusted incident rate ratio (adjIRR), 2.95; 95% CI, 1.44–6.04]. In addition, we assessed if primary results were sensitive to the inclusion of those with both in- and out- migrating spouses (Supplementary Table S6). Whereas most results did not appreciably change, the IRR for men with in-migrating spouses was no longer statistically significant when excluding those whose spouses migrated in and out [adjIRR, 1.7 (95% CI, 0.8–4.0)].
Effect of spousal migration on HIV incidence in the visit-interval following spousal migration
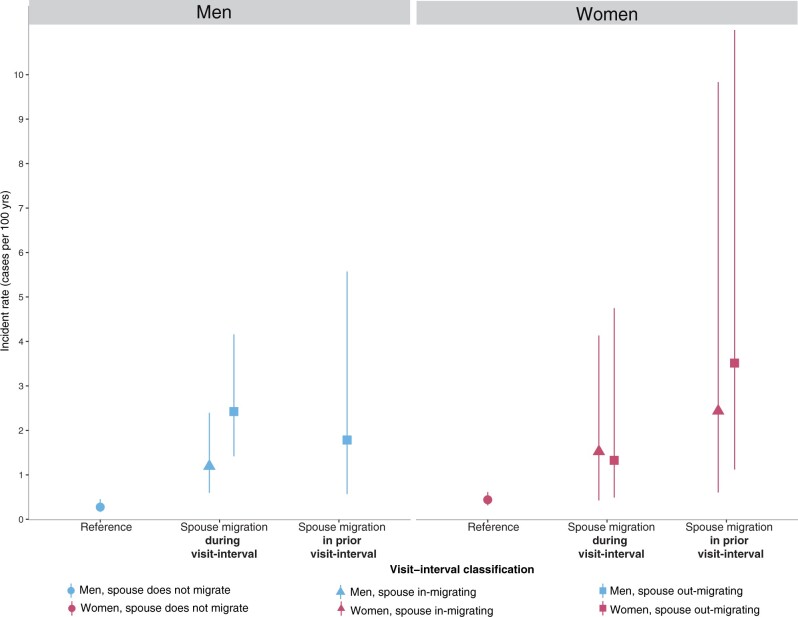
Next, we evaluated whether spousal migration in the visit-interval preceding HIV incidence was associated with higher risk of HIV acquisition, as the timing of migration and HIV acquisition events occurring during the same visit-interval was unknown, and HIV incidence may cause spousal migration (Figure 3, Supplementary Table S7). For men, the HIV incidence rate in the visit-interval following spousal out-migration was 1.78 per 100 person-years (95% CI, 0.57–5.57). Whereas this was lower than the estimated incident rate when spousal out-migration and HIV incidence occurred during same visit-interval [2.40 per 100 person-years (95% CI, 1.39–4.13)] it was still substantially higher than that among men whose spouses did not migrate [0.27 per 100 person-years (95% CI, 0.17–0.46)]. We were unable to assess HIV incidence among men whose spouse migrated into the household in the prior visit-interval as there were no subsequent incident cases. For women, HIV incidence rates in the visit-interval following spousal migration into or out of the household were higher than incidence rates where no spousal migration occurred [following spousal in-migration: 2.44/100 pyrs (95% CI, 0.60–9.83); out-migration: 3.51/100 pyrs (95% CI, 1.12–11.00), Supplementary Table S7].
Figure 3.
HIV incidence rates and 95% confidence intervals during a spousal migration and following a spousal migration. Note: the incidence rate for men with in-migrating spouses in the prior visit-interval were not able to be estimated as there were zero incident cases. Reference refers to those with spouses that did not migrate
Impact of spousal migration on sexual behaviour
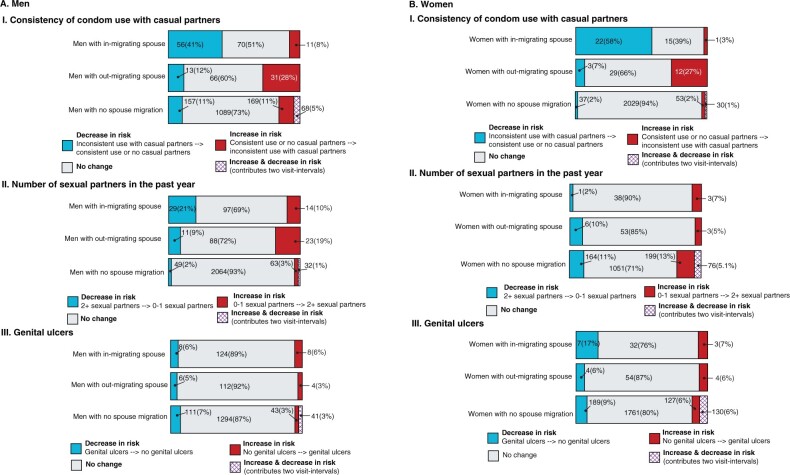
Lastly, as spousal migration was associated with higher HIV incidence, we compared sexual behaviours prior to spousal migration (ti-k) to after spousal migration at the end of the visit-interval (ti) (Figure 4). Spousal out-migration was linked to an increase in inconsistent condom use with casual partners for 28% of men and 27% of women. For men but not women with migrant spouses, an increased number of sexual partners in the past year was observed (Figure 4).
Figure 4.
Change [n(%)] in sexual behavior following spousal migration among non-migrant spouses
Discussion
In this population-based study, we found that spousal migration, but not other types of household migration, substantially increased HIV risk among non-migrating household members, especially among men. HIV incidence rates remained elevated among men experiencing spousal migration irrespective of marital status and in the visit-interval following spousal migration, suggesting spousal migration may lead to HIV acquisition. Furthermore, changes in relationship status tended to accompany increases in risk associated with spousal migration. Taken together, our results suggest that spousal migration may be associated with an increased risk of HIV acquisition.
There has been little research on the effect of migration on HIV incidence for non-migrants living with them. Only one 2006 study from Sub-Saharan Africa examined HIV incidence, using data from couples in Tanzania but this used data from collected between 1994 and 2000 prior to scale-up of HIV treatment and services. Consistent with our findings, they found that non-migrant men with long-term mobile partners were 4.22-fold more likely to acquire HIV compared with men where both partners were residents.10
Prior studies suggest that relationship dissolution, which is associated with spousal migration, is associated with higher rates of incident HIV.21–23 Studies in Uganda also have shown that previously married men and women are more likely to acquire HIV,21 and that those who are living with HIV are more likely to experience marital dissolution.24 We also found that that those whose wives had migrated recently were significantly more likely to acquire HIV. This suggests that previously married men are at greatest risk of acquiring HIV in the lead up to and immediately following spousal migration. Women and men whose spouses migrated into the household were also more likely to acquire HIV. This could be due to an increased likelihood for non-migrants, especially men, to partner with migrants with untreated HIV.25 Even though incidence rates were not statistically significantly higher for women with migrant spouses, there was still over a 2-fold increase in risk after adjusting for demographics. Instead, the lack of statistical significance likely reflects the small number of HIV incident cases rather than the absence of an association. Indeed, some studies from Africa demonstrate higher incident HIV and riskier sexual behaviours among migrant men alongside widening sexual networks for women who remain behind, suggesting a potential reason why women with migrant spouses are at higher risk of acquiring HIV but further research is needed.2,12,26 Our results further corroborate with other studies from Sub-Saharan Africa, demonstrating that changes in sexual behaviour following spousal migration are common for men and women, with non-migrants reporting riskier sexual behaviours such as reduced condom use and an increased number of sexual partners.10,11,13
Taken together, our results suggest that non-migrants with migrant spouses may benefit from more focused HIV prevention strategies that mitigate the temporary increase in risk of acquiring HIV during periods surrounding spousal migration. In particular, the use of pre-exposure prophylaxis may benefit non-migrants, especially men, whose spouses have migrated in or out recently. Risk-screening questionnaires are seen as an essential part of making sure that pre-exposure prophylaxis is able to be accessed by those who need it most.27 Our findings suggest that such screening tools could include questions on individuals whose spouses have recently migrated. However, further validation and analysis are required to see if screening based on spousal migration is effective.
Our results further suggest that efforts to prevent and treat HIV among migrants are also likely to be beneficial for non-migrants with whom they partner. Migrants are more likely to experience disruptions to HIV treatment due to the challenges associated with migration including reduced time and money and additional structural barriers that make it difficult to transfer care between facilities.28,29 Several studies suggest that providing testing and treatment outside of health facilities within communities, for extended hours, or that reduce the frequency of refills for antiretroviral therapy by dispensing larger amounts may be especially useful for migrants.28,29
There were several limitations to this study. First, the relative timing of migration into or out of the household and incident HIV was unclear in primary analyses as both were measured during the same survey. However, in secondary analyses, HIV incidence was still elevated in the visit-interval following spousal migration. Second, sexual behaviours were self-reported and may have been underreported. We also restricted analyses of sexual behaviours and related factors to only those with a migrating spouse, which resulted in a limited sample size. Third, our analyses only go up to 2018. Since 2018, combination HIV interventions have been further scaled up, and so it is unclear if we would observe similar relationships with more recent data. Furthermore, as 15% (1933/13 281) of our cohort were lost-to-follow-up, with most being away for school of work, we cannot rule out the possibility that selection biases may have affected our findings. In addition, we were unable to control for individual wealth, the HIV status of new sexual partners for non-migrants, especially those with in-migrant spouses, and the number of previous marriages, all of which may impact HIV incidence. We excluded other types of migration, e.g. circular, smaller-scale mobility and return migration, from our analysis. Finally, our analysis is context-specific and as the types, reasons for and broader context for migration varies by setting, our findings may not be generalizable to other settings.
In conclusion, we find that men and women whose spouses migrate into or out of the household are more likely to acquire HIV than those with non-migrant spouses. Our results suggest that HIV prevention interventions should be directed towards individuals with a spouse who recently moved into or out of the household.
Ethics approval
The RCCS protocol was reviewed and approved by the Uganda National Council for Science and Technology, Uganda Virus Research Institute Research Ethics Committee, Johns Hopkins School of Medicine Institutional Review Board, and Western Institutional Review Board.
Supplementary Material
Acknowledgements
We thank the cohort participants and many staff and investigators who made this study possible. This work was presented as an e-poster at AIDS 2022, was part of RY’s doctoral dissertation and a preprint version is available on medRxiv. We would also like to thank the reviewers for this manuscript, your feedback was invaluable.
Contributor Information
Ruth Young, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Joseph Ssekasanvu, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Joseph Kagaayi, Rakai Health Sciences Program, Kalisizo, Uganda.
Robert Ssekubugu, Rakai Health Sciences Program, Kalisizo, Uganda.
Godfrey Kigozi, Rakai Health Sciences Program, Kalisizo, Uganda.
Steven J Reynolds, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA; Division of Intramural Research, National Institute for Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MA, USA.
Maria J Wawer, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Rakai Health Sciences Program, Kalisizo, Uganda; Department of Population, Family, and Reproductive Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Bareng Aletta Sanny Nonyane, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Betty Nantume, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Thomas C Quinn, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA; Division of Intramural Research, National Institute for Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MA, USA.
Aaron A R Tobian, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA; Department of Pathology, Johns Hopkins School of Medicine, Baltimore, MD, USA.
John Santelli, Population and Family Health and Pediatrics, Columbia Mailman School of Public Health, New York, NY, USA.
Larry W Chang, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Caitlin E Kennedy, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Ligia Paina, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Philip A Anglewicz, Department of Population, Family, and Reproductive Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
David Serwadda, Department of Disease Control and Environmental Health, Makerere University School of Public Health, Kampala, Uganda.
Fred Nalugoda, Rakai Health Sciences Program, Kalisizo, Uganda.
Mary Kate Grabowski, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Pathology, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Data availability
A de-identified version of the data can be provided to interested parties subject to completion of the Rakai Health Sciences Program data request form and signing of a Data Transfer Agreement. Inquiries should be directed to datarequests@rhsp.org.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
Ruth Young and Mary Kate Grabowski conceived and designed the study. Ruth Young, Mary Kate Grabowski, Joseph Ssekasanvu, Joseph Kagaayi, Robert Ssekubugu, Godfrey Kigozi, Steven J Reynolds, Maria J Wawer, Bareng Aletta Sanny Nonyane, Betty Nantume, Thomas C Quinn, Aaron AR Tobian, John Santelli, Larry W Chang, Caitlin E Kennedy, Ligia Paina, Philip A Anglewicz, David Serwadda and Fred Nalugoda contributed to the acquisition, analysis and interpretation of the data for the study. Ruth Young wrote the initial draft manuscript. Ruth Young, Joseph Ssekasanvu, Joseph Kagaayi, Robert Ssekubugu, Godfrey Kigozi, Steven J Reynolds, Maria J Wawer, Bareng Aletta Sanny Nonyane, Betty Nantume, Thomas C Quinn, Aaron AR Tobian, John Santelli, Larry W Chang, Caitlin E Kennedy, Ligia Paina, Philip A Anglewicz, David Serwadda, Fred Nalugoda and Mary Kate Grabowski contributed to the draft of the manuscript, provided substantial inputs, critical comments and suggested additional analyses. Ruth Young and Mary Kate Grabowski finalized the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (grant numbers R01AI114438, R01AI110324, R01AI123002, R01AI128779, R01AI143333, R01AI155080, R01AI087409, U01AI075115, U01AI100031, K25AI114461, K01AI125086), the National Institute of Mental Health (grant numbers R01MH107275, R01MH105313, R01MH099733, F31MH095649), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant numbers R01HD091003, R01HD050180, R01HD070769), the National Institute on Alcohol Abuse and Alcoholism (K01AA024068), the Division of Intramural Research of the National Institute for Allergy and Infectious Diseases, the Bill and Melinda Gates Foundation (grant number OPP1175094) and the President's Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention (grant number NU2GGH000817). RY was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (grant number F31HD102287). We thank the personnel at the Office of Cyberinfrastructure and Computational Biology at the National Institute of Allergy and Infectious Diseases for data management support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
None declared.
References
- 1. Abubakar I, Aldridge RW, Devakumar D. et al. ; UCL–Lancet Commission on Migration and Health. The Lancet Commissions The UCL-Lancet Commission on Migration and Health: the health of a world on the move. Lancet 2018;392:2606–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dzomba A, Tomita A, Govender K, Tanser F.. Effects of migration on risky sexual behavior and HIV acquisition in South Africa: a systematic review and meta-analysis, 2000–2017. AIDS Behav 2018;23:1396–430. [DOI] [PubMed] [Google Scholar]
- 3. Olawore O, Tobian AAR, Kagaayi J. et al. Migration and risk of HIV acquisition in Rakai, Uganda: a population-based cohort study. Lancet HIV 2018;5:e181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Billioux VG, Chang LW, Reynolds SJ. et al. Human immunodeficiency virus care cascade among sub-populations in Rakai, Uganda: an observational study. JIAS 2017;20:21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young R. Migration and Health: the effect of migration on HIV outcomes for non-migrants and a qualitative exploration of barriers to health services for migrants in Rakai, Uganda. PhD Thesis. Johns Hopkins University, Baltimore, Maryland 2022.
- 6. Vandormael A, Akullian A, Siedner M, T de O, Bärnighausen T, Tanser F.. Declines in HIV incidence among men and women in a South African population-based cohort. Nat Commun 2019;10:5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grabowski MK, Serwadda DM, Gray RH. et al. ; Rakai Health Sciences Program. HIV prevention efforts and incidence of HIV in Uganda. N Engl J Med 2017;377:2154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hulland EN, Brown JL, Swartzendruber AL, Sales JM, Rose ES, Diclemente RJ.. The association between stress, coping, and sexual risk behaviors over 24 months among African-American female adolescents. Psychol Health Med 2015;20:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turpin R, Brotman RM, Miller RS, Klebanoff MA, He X, Slopen N.. Perceived stress and incident sexually transmitted infections in a prospective cohort. Ann Epidemiol 2019;32:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kishamawe C, Vissers DCJ, Urassa M. et al. Mobility and HIV in Tanzanian couples: both mobile persons and their partners show increased risk. AIDS 2006;20:601–8. [DOI] [PubMed] [Google Scholar]
- 11. Palk L, Blower S.. Mapping divided households and residency changes: the effect of couple separation on sexual behavior and risk of HIV infection. Sci Rep 2015;5:17598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lurie MN, Williams BG, Khangelani Z. et al. Who infects whom? HIV-1 concordance and discordance among migrant and non-migrant couples in South Africa. AIDS 2003;17:2245–52. [DOI] [PubMed] [Google Scholar]
- 13. Vissers DCJ, Voeten HACM, Urassa M. et al. Separation of spouses due to travel and living apart raises HIV risk in Tanzanian couples. Sex Transm Dis 2008;35:714–20. [DOI] [PubMed] [Google Scholar]
- 14. Agadjanian V, Hayford SR.. Men’s migration, women’s autonomy, and union dissolution in rural Mozambique. J Fam Issues 2018;39:1236–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang LW, Quinn TC, Reynolds SJ. et al. Heterogeneity of the HIV epidemic in agrarian, trading, and fishing communities in Rakai, Uganda: an observational epidemiological study. Lancet HIV 2016;3:e388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thapa DK, Visentin D, Kornhaber R, Cleary M.. Migration of adult children and mental health of older parents ‘left behind’: an integrative review. PLoS One 2018;13:e0205665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang K, Zeger S.. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 18. Cui J. QIC program and model selection in GEE analyses. Stata J 2007;7:209–20. [Google Scholar]
- 19. Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep 2018;15:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta GR, Parkhurst JO, Ogden JA, Aggleton P, Mahal A.. Structural approaches to HIV prevention. Lancet 2008;372:764–75. [DOI] [PubMed] [Google Scholar]
- 21. Nalugoda F, Guwatudde D, Bwaninka JB. et al. Marriage and the risk of incident HIV infection in Rakai, Uganda. J Acquir Immune Defic Syndr 2014;65:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schumann H, Rubagumya K, Rubaihayo J, Harms G, Wanyenze RK, Theuring S.. The incidence of HIV and associated risk factors among pregnant women in Kabarole District, Uganda. PLoS One 2020;15:e0234174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shisana O, Risher K, Celentano DD. et al. Does marital status matter in an HIV hyperendemic country? Findings from the 2012 South African National HIV prevalence, incidence and behaviour survey. AIDS Care 2016;28:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Porter L, Hao L, Bishai D. et al. ; Rakai Project Team. HIV status and union dissolution in Sub-Saharan Africa: the case of Rakai, Uganda. Demography 2004;41:465–82. [DOI] [PubMed] [Google Scholar]
- 25. Brophy JE, Lessler J, Ssekubugu R. et al. Prevalence of untreated HIV and associated risk behaviors among the sexual partners of recent migrants and long-term residents in Rakai, Uganda. J Acquir Immune Defic Syndr 2021;88:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dladla AN, Hiner CA, Qwana E, Lurie M.. Speaking to rural women: the sexual partnerships of rural South African women whose partners are migrants. Soc Trans 2001;32:79–82. [Google Scholar]
- 27. Verguet S, Stalcup M, Walsh JA.. Where to deploy pre-exposure prophylaxis (PrEP) in sub-Saharan Africa? Sex Transm Infect 2013;89:628–34. [DOI] [PubMed] [Google Scholar]
- 28. Faturiyele I, Karletsos D, Ntene-Sealiete K. et al. ; EQUIP Innovation for Health Team. Access to HIV care and treatment for migrants between Lesotho and South Africa: a mixed methods study. BMC Public Health 2018;18:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernardo EL, Nhampossa T, Clouse K. et al. Patterns of mobility and its impact on retention in care among people living with HIV in the Manhiça District, Mozambique. PLoS One 2021;16:e0250844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A de-identified version of the data can be provided to interested parties subject to completion of the Rakai Health Sciences Program data request form and signing of a Data Transfer Agreement. Inquiries should be directed to datarequests@rhsp.org.