Abstract
Background:
Surgical rhinoplasty is a highly complex cosmetic procedure with significant revision rates. Unfortunately, surgical revision rhinoplasty is associated with many challenges. Nonsurgical correction of surgical rhinoplasty complications with injectable hyaluronic acid fillers is an alternative with less cost and downtime. In this article, we present the first author’s experience with 2088 cases of nonsurgical revision rhinoplasty, including technical considerations, patient-reported outcomes, and adverse events.
Methods:
A retrospective chart review was completed on patients 18 years and older who received nonsurgical rhinoplasty treatment between March 2018 and August 2022. Patient demographic data, and data on indications for treatment, volume of filler used, patient-reported satisfaction, and adverse events (including erythema, infection, vascular occlusion, and necrosis) were collected up to 1 year after the initial injection.
Results:
A total of 2088 patient cases are included in this study. The most common indications for treatment included bridge collapse or asymmetry (49.0%), an under-projected tip (44.0%), and surface irregularity/scarring (35.4%). The mean volume of filler used at initial treatment was 0.49 mL (SD 0.19). Median patient satisfaction immediately after treatment was 9 (visual analog scale ranging from 1 to 10). The most common adverse event reported at the 2-week follow-up was erythema (36.4%). Three patients presented with skin necrosis (0.47%). All three of these were transient and self-resolving.
Conclusions:
Nonsurgical correction of rhinoplasty complications with hyaluronic acid fillers can be a safe, minimally invasive option with high patient satisfaction and immediate and predictable results. This should be considered first line before surgical revision.
Takeaways
Question: Is injection with hyaluronic acid filler, in patients with aesthetic complications after a rhinoplasty, a viable and attractive alternative to surgical revision rhinoplasty?
Findings: In a retrospective look at the first author’s cohort of 2088 patients, a relatively modest mean volume of injected hyaluronic acid filler resulted in a median patient satisfaction of 9 of 10 and no serious adverse outcomes.
Meaning: Correction of aesthetic complications after rhinoplasty can be achieved with careful injection of small amounts of hyaluronic acid filler. This is a cost-effective alternative to surgical revision rhinoplasty that affords immediate results, minimal downtime and high patient satisfaction.
INTRODUCTION
The popularity of surgical rhinoplasty is indisputable: in the United States it was ranked as the single most common cosmetic surgery in 2019 and 2020, and it is consistently ranked amongst the top five most common cosmetic surgical procedures worldwide.1–3 Despite its popularity, surgical rhinoplasty is considered a challenging facial cosmetic procedure, one where both aesthetic and functional outcomes determine patient satisfaction. The multiple interdependent anatomical structures of the nose and the three-dimensional forces that must be accounted for contribute to the complexity of surgical rhinoplasty.4,5 Even in experienced hands, the long-term outcomes can be unpredictable, as some complications occur postoperatively due to scarring and contracture.6,7
Months and years after surgical rhinoplasty, patients may experience suboptimal outcomes.6–10 The nasal dorsum may exhibit a residual hump due to under-resection; a low profile and/or “scooped” appearance due to overresection; collapse; asymmetry; the well-described “inverted V” deformity; open roof deformity; or a high radix in the case of augmentation procedures. In the supratip, common issues include the pollybeak deformity/supratip fullness, nasal asymmetry, and contour irregularities. At the nasal tip, aesthetic complications include asymmetry, a pinched tip, overrotated tip or under-projection, alar retraction, or alar rim deformity. Postsurgical scarring includes columellar contracture and overall tightness of the soft tissue envelope.
Surgical revision rhinoplasty incidence is estimated at 5%–20% in the published literature.11 This procedure is more complex than primary rhinoplasty due to many factors including postsurgical scarring and structural compromise; the possible need for grafting; and the psychological state of patients considering revision rhinoplasty, which is often characterized as being traumatized, hesitant and exhibiting diminished hope.5,12,13 The literature suggests that patient satisfaction rates with revision rhinoplasty are lower when compared with primary rhinoplasty.5
Given the pitfalls of surgical revision rhinoplasty as well as the high cost and invasive nature of the procedure, it follows that nonsurgical rhinoplasty (NSR) with soft tissue injectable fillers offers a viable and attractive alternative. NSR is versatile because it can address most of the common aesthetic issues that present after surgical rhinoplasty. This procedure also affords instantaneous results at a relatively low cost. The issue of unpredictable scarring in the nose after surgical revision rhinoplasty and the associated aesthetic and functional complications are obviated with NSR. However, care must be taken to respect the postsurgical anatomic changes in the nose.
In a nonoperated nose, four distinct layers exist between the skin and the underlying osseocartilaginous frame: the subcutaneous fat, the nasal SMAS, the deep areolar/fatty layer, and the perichondrium/periosteum.10,13–17 Variations in the course of the dorsal nasal arteries are known to exist, including bilateral paramedian dorsal nasal arteries (34%), a subcutaneous plexus randomly distributed on the upper two-thirds of the nose (38%), and a single dorsal nasal artery. Despite these variations, the depth of the major vessels is fairly consistently in the subcutaneous plane.15,17 A history of surgical rhinoplasty can severely impact the behavior and flexibility of the soft tissue, as well as the location of blood vessels.10,11,13
Surgery obliterates the natural soft tissues layers of the nose, resulting in unpredictable anatomy. After surgery, there is extensive scarring in the soft tissue envelope without discernable layers. The nasal tissues are tighter, resulting in a reduced ability for filler to accommodate in the deep injection plane. The nasal tip is often the most severely impacted. The tight scarring also means there is less flexibility in the soft tissues surrounding the vessels and an increased risk for compression with small volumes of filler injection. The risk of vascular compromise due to both external compression and intraluminal occlusion is increased. Overall, the postsurgical nose presents multiple challenges for revision via injectable fillers. The objectives of this article are (a) to discuss the first author’s clinical experience and approach to performing NSR in the postsurgical population and (b) to present a retrospective review of patients who received this procedure, including indications for treatment and outcomes.
METHODS
Study Population
Patients 18 years or older were included if they were seen between March 2018 and August 2022 and received injectable hyaluronic acid soft tissue filler for treating aesthetic complications after surgical rhinoplasty. Patients were excluded if they had contraindications such as pregnancy, breastfeeding, active autoimmune disease, active infection at the injection site, or allergy to components of the injected product. Patients were also excluded if they exhibited absence of pliability/excessive tightness of the soft tissue envelope, severe asymmetry, or severe deformities of the alar complexes presenting as a primary concern. All patients provided verbal and written consent for the procedure as per the treating clinician’s standard protocol.
Materials
Cross-linked hyaluronic acid gels [Teosyal Puresense Ultradeep 25 mg/mL with lidocaine (Teoxane Laboratories, Geneva, Switzerland); Belotero Intense 25.5 mg/mL with lidocaine (Merz Pharma, Frankfurt, Germany)] were used due to demonstrated high elasticity (G’), cohesivity, longevity, and reversibility. Skin was prepared with antiseptic chlorhexidine gluconate 2% w/v/ isopropyl alcohol 70% v/v before injection. Topical numbing was not used in any case. Sterile 0.3 mL Becton-Dickinson (Franklin Lakes, N.J.) syringes with 30G needles were used to decant product from the original filler syringes using a “no-touch” aseptic technique. These syringes allow very small aliquots of 0.01–0.02 mL of filler to be injected easily and precisely.
Although decanting hyaluronic acid into 0.3-mL Becton-Dickinson syringes with 30G needles is a technique developed and extensively used by the first author under strict aseptic conditions, it is important to note that this practice is not generally recommended by manufacturers of the hyaluronic acid material or the syringe. This article is descriptive of the first author’s current practice, and we caution readers and novice injectors against emulating this decanting technique.
Although each injection point involves boluses of 0.01–0.02 mL of filler, multiple microboluses are administered through a single skin entry site without fully retracting the needle. Instead, the needle tip moves within a few millimeters’ radius, staying on the deep periosteal or perichondrial plane. This process is repeated a few times at each skin insertion site to reduce pain and soft tissue trauma associated with excessive skin injections. Multiple syringes are decanted and ready for treatment.
Data Collection
Demographic and baseline patient data were collected from patient charts, including age, sex, ethnicity, presenting concerns, number of previous surgical rhinoplasties, time since last surgical rhinoplasty, and whether a graft was used. Treatment data were collected, including volume of filler used, treatment time, and patient-rated satisfaction on a visual analogue scale (0–10). In addition, data on immediate complications were collected, including the presence of excessive bleeding (defined as an amount greater than expected from the procedure and requiring a pause in treatment), bruising, patient-rated pain score on a visual analogue scale (0–10), and the presence of vascular emergency. For a subset of patients, the Nose Pliability Assessment Scale (NPAS) was used to categorize the soft tissue envelope of the nose before treatment planning. This scale has been described in previous work published by the authors and involves grading on a scale of 1, denoting an inflexible soft tissue envelope with tight skin and immobility of the soft tissues, to 5, characterized by a soft tissue envelope that is loose with an ability to lift the skin (“skin pinch”) 10 mm or more.18 At the 2-weeks follow-up, data were collected on complications, patient-reported satisfaction, and the need for top-up treatment and volume. Lastly, at the 12-month follow-up point, data were collected on complications and patient satisfaction.
Technique
The technique developed by the first author (A.H.) has been previously described in a published retrospective review of 5000 patients.19 In the postsurgical population, there are important caveats and considerations. However, amid all the complicating factors cited above, one thing remains constant: the perichondrium and periosteum remain the deepest layer and the target for injection. A micro-bolus technique is used with deliberately slow, low-pressure injection of volumes of 0.01–0.02 mL per point. This allows for improved precision, a better aesthetic result, and reduced risk. Although each injection point involves boluses of 0.01–0.02 mL of filler, multiple injections are administered through a single skin injection site without fully retracting the needle. Instead, the needle tip moves a millimeter or two on either side of the initial injection, staying on the deep periosteal or perichondreal plane, before a new bolus of 0.01–0.02mL is delivered. This is repeated a few times at each skin insertion site to avoid the soft tissue trauma and bruising associated with excessive superficial soft tissue injections.
The injection locations remain mainly in the midline but may deviate from this to target areas with irregular contours and deviations. The goal is to hide irregular shadows, create an uninterrupted light reflex, restore bridge contour, symmetrize the tip, and reduce the visible stigmata of surgery.
Statistical Methods
Demographic, baseline, and treatment outcome data were summarized using descriptive statistical methods. For continuous data, medians (range, IQR) and means (SD) are used as appropriate. For categorical data, frequency counts and proportions are reported.
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. All patients provided verbal and written informed consent for treatment as per the treating clinician’s standard protocol. Verbal and written informed consent via photograph-release agreements was obtained for all patient images displayed in this publication. Images were taken before and immediately after the procedure on a Sony alpha-5000 ILCE camera (Sony Group Corporation, Tokyo, Japan) and securely stored.
RESULTS
A total of 2088 patients are included in this retrospective review. The mean age of patients was 31.5 years (SD 8.9), and the majority were of female sex (95.4%). The three most common indications for treatment included bridge collapse or asymmetry (49.0%), under-projected tip (44.0%), and surface irregularity/scarring (35.4%). The NPAS was used to grade the pliability of the soft tissue envelope in 681 patients (32.6% of total cohort). The majority of patients (78.9%) displayed no or minimal pliability of the soft tissue envelope (NPAS 1 or 2). In 31.6% of cases, patients had undergone more than one surgical rhinoplasty, and the mean time since last surgery was 29.0 months with a range of 11–360 months. A graft was used in just over half of the cases in this cohort. See Table 1 for further details on patient demographics, presenting indications, and surgical history.
Table 1.
Patient Demographics and Surgical History
| Demographics | |
| Age (y) | |
| Mean (SD) | 31.5 (8.9) |
| Median (range) | 30 (19–93) |
| Sex | |
| Female (n, %) | 1992 (95.4%) |
| Ethnicity (n, %) | |
| White | 1344 (64.4%) |
| Asian | 160 (7.7%) |
| Arab | 510 (24.4%) |
| Black | 25 (1.2%) |
| Mixed race | 49 (2.3%) |
| Other | 0 (0%) |
| Presenting indications (n, %) | |
| Under-resection/residual dorsal hump | 236 (11.3%) |
| Bridge overresection | 712 (34.1%) |
| Bridge collapse or asymmetry | 1024 (49.0%) |
| Under-projected tip | 918 (44.0%) |
| Tip asymmetry | 504 (24.1%) |
| Surface irregularity/scarring | 740 (35.4%) |
| Nostril asymmetry | 301 (14.4%) |
| Nose Pliability Assessment Scale (n = 681) | |
| Grade 1 | 99 (14.5%) |
| Grade 2 | 439 (64.4%) |
| Grade 3 | 121 (17.8%) |
| Grade 4 | 22 (3.2%) |
| Grade 5 | 0 (0%) |
| Surgical history | |
| No. previous surgical rhinoplasties (n,%) | |
| One | 1428 (68.4%) |
| Two | 644 (30.8%) |
| Three | 16 (0.8%) |
| Months since last surgical rhinoplasty | |
| Mean (SD) | 29.0 (22.4) |
| Median (range) | 26 (11–360) |
| Graft used (n,%) | 1063 (50.9%) |
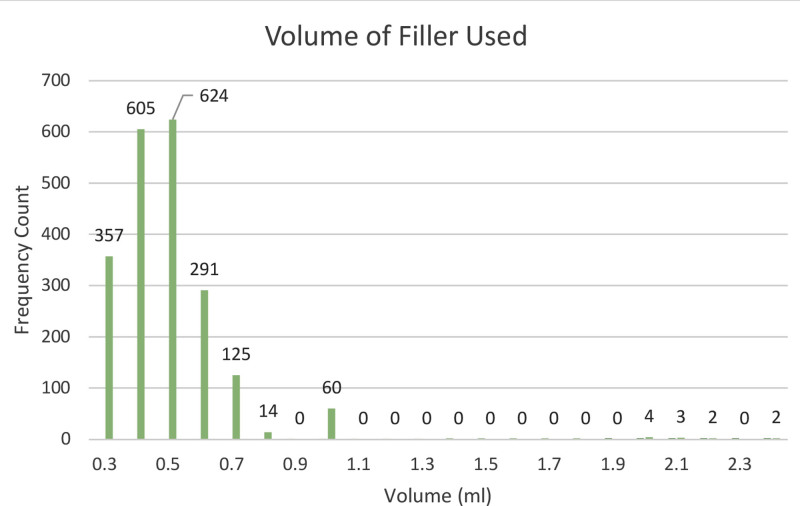
The mean volume of filler used at initial treatment was 0.49 mL (SD 0.19) with a range of 0.3–2.4 mL. See Figure 1 for a graphical representation of volumes used during the procedure. The mean treatment time was 11.8 minutes (SD 0.10), the median pain score (on a scale of 0–10) was 2, and median patient satisfaction was 9 of 10. A vascular emergency requiring treatment with hyaluronidase occurred in 1.1% of cases. See Table 2 for details on immediate treatment outcomes. Representative before and after images are shown in Figures 2–4.
Fig. 1.
A bar graph depicting filler volumes used at initial treatment.
Table 2.
Immediate Treatment Outcomes
| Treatment time (min) | |
| Mean (SD) | 11.8 (0.1) |
| Median (range) | 11 (5–24) |
| Volume of filler used (mL) | |
| Mean (SD) | 0.49 (0.19) |
| Median (range) | 0.50 (0.3–2.4) |
| Patient satisfaction (0–10) | |
| Mean (SD) | 8.9 (0.02) |
| Median (range) | 9 (7–10) |
| Pain scores (0–10) | |
| Mean (SD) | 8.9 (1.9) |
| Median (range) | 9 (range 7–10) |
| Adverse events (n,%) | |
| Bleeding | 218 (10.4%) |
| Bruising | 1121 (53.7%) |
| Vascular emergency | 24 (1.1%) |
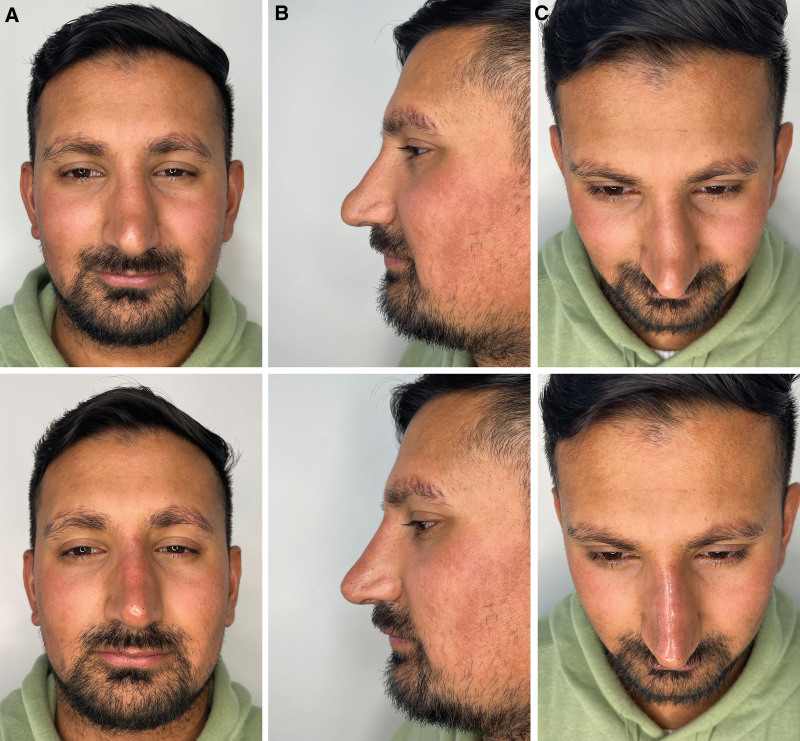
Fig. 2.
Illustrative before and after results for correction of aesthetic complications after surgical rhinoplasty with hyaluronic acid filler injections. Before and immediately after treatment in a male patient from (A) front view, (B) side profile view, and (C) bird’s eye view.
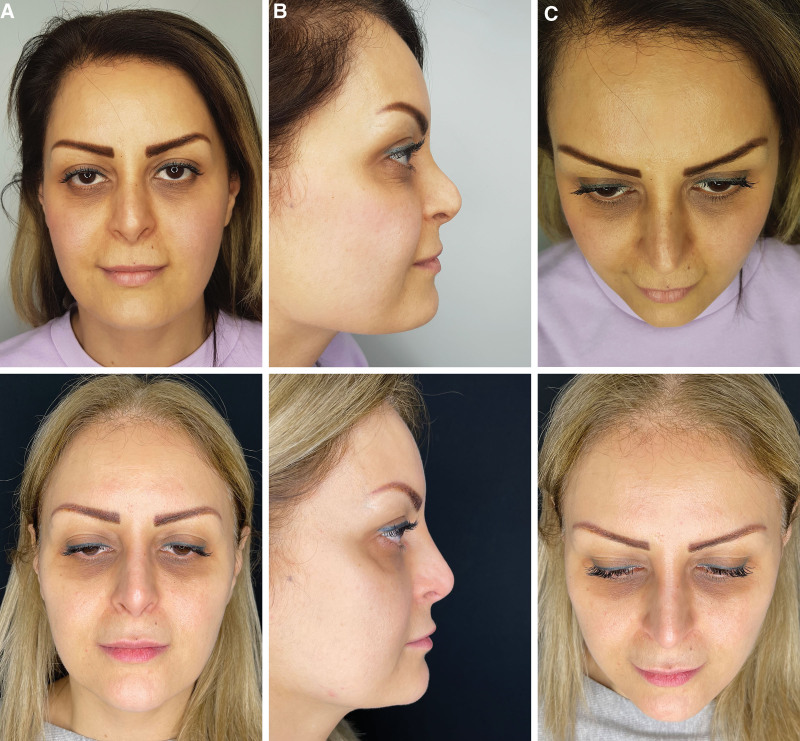
Fig. 4.
Illustrative before and after results for correction of aesthetic complications after surgical rhinoplasty with hyaluronic acid filler injections. Before and at 3-year follow-up without further treatment in a female patient from (A) front view, (B) side profile view, and (C) bird’s eye view.
Fig. 3.
Illustrative before and after results for correction of aesthetic complications after surgical rhinoplasty with hyaluronic acid filler injections. Before and at 2-year follow-up without further treatment in a female patient from (A) front view, (B) side profile view, and (C) bird’s eye view.
A total of 643 patients presented for follow-up 2 weeks after initial treatment (30.8% of the total cohort). The most common adverse event reported at this time point was erythema (36.4%). Three patients experienced skin necrosis (0.47%), which presented as mild skin sloughing which healed completely without further intervention required at the 2-week review. Mean patient-reported satisfaction was 7.4 (SD 1.2). Twenty-four patients (3.7%) received a touch-up treatment, with a range of 0.1–0.2 mL of filler being injected at the 2-week follow-up. See Table 3 for further details of outcomes observed at the 2-week follow-up appointment. A total of 372 patients presented for a 6-week follow-up, and 290 (78.0%) received top-up treatment with a mean volume of 0.23 mL (SD 0.11, range 0.1–0.4). This brings the total mean volume for this subset of patients to approximately 0.72 mL over two treatment sessions, underscoring the variability in treatment needs. There were no observed cases of skin necrosis, and mean patient satisfaction was 8.1 (SD 1.3) at this time point. A total of 790 patients followed up 1 year after initial treatment (37.8%), and 474 (60%) received retreatment. In the other 40%, resorption was not significant enough to warrant retreatment. We cannot provide an overall comment on the average timeline of resorption given that less than half of the original group presented for a 1-year follow-up. However, it is possible that the high level of maintained satisfaction contributed to the lack of need for further treatment. In this subgroup of patients who did return for review and retreatment at 1 year, no persistent adverse effects were observed. Mean patient-reported satisfaction 1 year after the procedure was 7.9 (SD 1.2).
Table 3.
Outcomes at 2-Week Follow-up (n = 643)
| Adverse events (n,%) | |
| Erythema | 234 (36.4%) |
| Swelling | 159 (24.7%) |
| Infection | 0 (0%) |
| Skin necrosis | 3 (0.47%) |
| Headache | 3 (0.47%) |
| Patient satisfaction (0–10) | |
| Mean (SD) | 7.4 (1.2) |
| Additional treatment? (n, %) | 24 (3.7%) |
| Treatment volume, mL (range) | (0.1–0.2 mL) |
At each follow-up time point, patients were asked if they were dissatisfied with their results (“yes/no”). At the 2-week, 6-week, 6-month, and 1-year time points, the number of patients presenting for follow-up were 643, 372, 145, and 790, respectively. At all of these time points, zero patients reported dissatisfaction with their results.
DISCUSSION
A total of 2088 patients were included in this study on nonsurgical rhinoplasty with injectable hyaluronic acid fillers, making it the largest to date. The mean volume of filler used was 0.49 mL, and the mean treatment time was 11.8 minutes. The median pain score was 2 of 10, and median patient satisfaction was 9 of 10.
There are few published studies on NSR for correction of complications after surgical rhinoplasty. A review published in 2020 included 15 cohort studies and found patient satisfaction to range from 80% to 100%. The authors found that “injectable retouching” of the nose can reduce the need for surgical revision.20 In a retrospective review, Heden studied a cohort of patients who underwent injectable rhinoplasty with hyaluronic acid, 27% of whom had a history of surgical rhinoplasty. Sixty-five percent of patients indicated they were “very satisfied” with treatment; 35% indicated they were “satisfied”; and in most patients, the effects of treatment lasted for more than 1 year.21
The most common presenting indications for NSR treatment include bridge collapse/asymmetry, under-projection of the tip, surface irregularity/scarring, bridge overresection, and tip asymmetry. This is similar to presenting cosmetic indications for surgical revision rhinoplasty,10,11,22,23 indicating that this is a very comparable patient population. Furthermore, it suggests the suitability of the nonsurgical approach for most people considering revision rhinoplasty.
Abbas compared satisfaction rates of 54 primary rhinoplasty patients with 54 patients who received revision rhinoplasty using the Rhinoplasty Outcome Evaluation score and found that the revision group had statistically significantly lower satisfaction scores.5 A single-site US study published in 2021 similarly compared patients receiving primary versus revision rhinoplasty and found rates of dissatisfaction to be higher in the revision group: 29.6% of the revision rhinoplasty cohort stated they were “very” or “somewhat” dissatisfied with their result, whereas only 44.4% stated they were “very satisfied.”22 In comparison, none of the patients in this study who presented for follow-up assessment reported being dissatisfied with their NSR result at any time point ranging from 2 weeks to the final follow-up time of 1 year. Although we cannot directly compare the satisfaction scores in this study with those of previously published papers on surgical revision rhinoplasty due to differences in evaluation methods, the mean satisfaction score in this study of 8.9 of 10 immediately after treatment and 7.9 of 10 1 year later suggest high satisfaction scores with durability over time.
In the first study looking at the incidence of skin compromise in patients receiving surgical rhinoplasty, 7% of patients were found to have vascular compromise intraoperatively or in the early postoperative phase, and the majority (92.6%) of these were in patients undergoing a revision surgery.24 These patients were treated with a multi-step protocol including surgical release of tension, hyperbaric oxygen therapy, leech therapy, nitroglycerin ointment, and pentoxifylline. At the final follow-up time (average of 392 days), 13 patients (3.4%) had persistent skin discoloration requiring further treatment. One patient in this study underwent revision surgery directly related to skin necrosis, and a second was considering revision at the time of study completion. Thus, skin necrosis can and does occur in surgical rhinoplasty, but this is rarely discussed in the literature and there is no standard nomenclature for this adverse event. In comparison, our study reports a much lower rate of vascular compromise of 1.1%, and skin necrosis of 0.47% with NSR, all of which completely resolved without intervention.
Revision rhinoplasty is a very complex procedure, and one where extranasal grafts are often required and the changes in the soft tissue envelope and structural compromise of the nose contributes significantly to the challenge of the surgery. Patients who have had a revision rhinoplasty are more likely to have further surgical rhinoplasties compared with primary rhinoplasty patients, seemingly leading to a cycle of invasive procedures with increasing complexity.25 The psychological burden on the patient is not to be overlooked as a complicating factor when deciding to undergo surgical revision. Patients considering revision rhinoplasty typically have negative perspectives about surgery compared with those considering primary rhinoplasty: they are disappointed with their previous surgery and hesitant and leery to undergo another surgical procedure.13,26,27 The cost also contributes to the burden of surgical revision rhinoplasty. In comparison, NSR obviates a lot of these issues: the procedure typically is a fraction of the cost of surgical revision, is done without anesthesia, and is much less technically challenging and invasive. In addition, the result is immediate and more predictable when compared with surgical rhinoplasty, where scarring and contracture over time can change the initial result.
The patient cohort in this study was complex: 50.9% of the patients in this study had a graft, and 31.6% had undergone two or more surgical rhinoplasty procedures. The majority of patients displayed no or minimal pliability of the soft tissue envelope (NPAS 1 or 2). In this context, surgical options become more challenging and the likelihood of satisfaction with further surgical revision is diminished. However, for many patients, it can be untenable to live with the stigmata of unsatisfactory surgical rhinoplasty results. NSR is well-placed to offer an attractive solution.
Although filler injections incur a cost, the financial burden is significantly lower than that of revision rhinoplasty, even when considering recurrent treatments. Most patients do not return for retreatment for a year or longer after the initial treatment, and the cost of filler treatments is a small fraction of the cost of potential surgical interventions. Additionally, clinical experience suggests that results tend to last longer after the second treatment, with a duration lasting a few to several years.
One limitation of this study is the focus on immediate posttreatment outcomes, which, although providing valuable insights, does not fully capture the long-term clinical outcomes of nonsurgical rhinoplasty with hyaluronic acid fillers. Although follow-up data were collected, the response rate at various time points was limited. Future studies should aim for more robust follow-up to better assess long-term outcomes and complications. Future prospective studies could also compare the rates of surgical revision rhinoplasty in patients who did and did not receive injectable revision treatment to answer an important question: can NSR reduce the need for revision rhinoplasty?
CONCLUSIONS
In this large retrospective review, we demonstrated high patient satisfaction scores, safety, and versatility of NSR in a patient group with suboptimal surgical outcomes. NSR should be considered first line before surgical revision because of the demonstrated effectiveness, safety, satisfaction, and longevity of this low-cost and expeditious procedure. Furthermore, NSR is well-suited for managing the majority of commonly observed aesthetic complications of surgical rhinoplasty.
DISCLOSURES
Ayad Harb was previously a paid consultant for Teoxane SA. Amane Abdul-Razzak has no financial interests to declare in relation to the content of this article.
PATIENT CONSENT
The patients provided written consent for the use of their images.
ACKNOWLEDGMENTS
This study involves the use of cross-linked hyaluronic acid gel [Teosyal Puresense Ultradeep 25 mg/mL with lidocaine (Teoxane Laboratories, Geneva, Switzerland)], and Belotero Intense 25.5 mg/mL with lidocaine (Merz Pharma, Frankfurt, Germany)] as well as sterile 0.3 mL Becton-Dickinson (Franklin Lakes, N.J.) syringes with 30G needles.
Footnotes
Published online 6 September 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Heilbronn C, Cragun D, Wong BJF. Complications in rhinoplasty: a literature review and comparison with a survey of consent forms. Facial Plast Surg Aesthet Med. 2020;22:50–56. [DOI] [PubMed] [Google Scholar]
- 2.ASPS American Society of Plastic Surgeons 2020 plastic surgery statistics report. Available at https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf. Accessed January 4, 2023. [Google Scholar]
- 3.ISAPS international survey on aesthetic/cosmetic procedures performed in 2020. Available at https://www.isaps.org/media/dzjfg50s/isaps-global-survey_2020.pdf. Accessed January 4, 2023. [Google Scholar]
- 4.Wu B, Chen S, Sun K, et al. Complications associated with rhinoplasty: an umbrella review of meta-analyses. Aesthetic Plast Surg. 2022;46:805–817. [DOI] [PubMed] [Google Scholar]
- 5.Abbas OL. Revision rhinoplasty: measurement of patient-reported outcomes and analysis of predictive factors. Springerplus. 2016;5:1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surowitz JB, Most SP. Complications of rhinoplasty. Facial Plast Surg Clin North Am. 2013;21:639–651. [DOI] [PubMed] [Google Scholar]
- 7.Eytan DF, Wang TD. Complications in rhinoplasty. Clin Plast Surg. 2022;49:179–189. [DOI] [PubMed] [Google Scholar]
- 8.Gryskiewicz JM, Hatef DA, Bullocks JM, et al. Problems in rhinoplasty. Clin Plast Surg. 2010;37:389–399. [DOI] [PubMed] [Google Scholar]
- 9.Bermudez P, Quereshy FA. Nasal tip deformities after primary rhinoplasty. Oral Maxillofac Surg Clin North Am. 2021;33:111–117. [DOI] [PubMed] [Google Scholar]
- 10.Nassab R, Matti B. Presenting concerns and surgical management of secondary rhinoplasty. Aesthet Surg J. 2015;35:137–144. [DOI] [PubMed] [Google Scholar]
- 11.East C, Kwame I, Hannan SA. Revision rhinoplasty: what can we learn from error patterns? An analysis of revision surgery. Facial Plast Surg. 2015;32:409–415. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberger ES, Toriumi DM. Controversies in revision rhinoplasty. Facial Plast Surg Clin North Am. 2016;24:337–345. [DOI] [PubMed] [Google Scholar]
- 13.Adamson PA, Warner J, Becker D, et al. Revision rhinoplasty: panel discussion, controversies, and techniques. Facial Plast Surg Clin North Am. 2014;22:57–96. [DOI] [PubMed] [Google Scholar]
- 14.Moon HJ. Use of fillers in rhinoplasty. Clin Plast Surg. 2016;43:307–317. [DOI] [PubMed] [Google Scholar]
- 15.Alfertshofer MG, Frank K, Ehrl D, et al. The layered anatomy of the nose: an ultrasound-based investigation. Aesthet Surg J. 2022;42:349–357. [DOI] [PubMed] [Google Scholar]
- 16.Jung GS, Chu SG, Lee JW, et al. A safer non-surgical filler augmentation rhinoplasty based on the anatomy of the nose. Aesthetic Plast Surg. 2019;43:447–452. [DOI] [PubMed] [Google Scholar]
- 17.Tansatit T, Apinuntrum P, Phetudom T. Facing the worst risk: confronting the dorsal nasal artery, implication for non-surgical procedures of nasal augmentation. Aesthetic Plast Surg. 2017;41:191–198. [DOI] [PubMed] [Google Scholar]
- 18.Harb A, Abdul-Razzak A, Twijiri S. ‘It’s all in the pinch’: a description of a novel method for assessing nasal soft tissue pliability for nonsurgical rhinoplasty with hyaluronic acid filler. J Cosmet Dermatol. 2023;00:1–3. [DOI] [PubMed] [Google Scholar]
- 19.Harb A, Brewster C. The nonsurgical rhinoplasty: a retrospective review of 5000 treatments. Plast Reconst Surg. 2020;145:661–667. [DOI] [PubMed] [Google Scholar]
- 20.Bouaoud J, Belloc JB. Use of injectables in rhinoplasty retouching: towards an evolution of surgical strategy? Literature review. J Stomatol Oral Maxillofac Surg. 2020;121:550–555. [DOI] [PubMed] [Google Scholar]
- 21.Hedén P. Nasal reshaping with hyaluronic acid: an alternative or complement to surgery. Plast Reconstr Surg Glob Open. 2016;4:e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loghmani S, Loghmani A, Maraki F. Secondary rhinoplasty: aesthetic and functional concerns. Plast Surg (Oakv). 2019;27:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan N, Alexander AJ, Sepehr A, et al. Patient complaints with primary versus revision rhinoplasty: analysis and practice implications. Aesthet Surg J. 2011;31:775–780. [DOI] [PubMed] [Google Scholar]
- 24.Irvine LE, Azizzadeh B, Kerulos JL, et al. Outcomes of a treatment protocol for compromised nasal skin in primary and revision open rhinoplasty. Facial Plast Surg Aesthet Med. 2021;23:118–125. [DOI] [PubMed] [Google Scholar]
- 25.Hacker S, Pollock J, Gubisch W, et al. Differences between primary and revision rhinoplasty: indications, techniques, grafts and outcomes. Plast Reconstr Surg. 2021;148:532–541. [DOI] [PubMed] [Google Scholar]
- 26.Ambro BT, Wright RJ. Psychological considerations in revision rhinoplasty. Facial Plast Surg. 2008;24:288–292. [DOI] [PubMed] [Google Scholar]
- 27.Günel C, Omurlu IK. The effect of rhinoplasty on psychosocial distress level and quality of life. Eur Arch Otorhinolaryngol. 2015;272:1931–1935. [DOI] [PubMed] [Google Scholar]