Abstract
Background:
The approach to revision rhinoplasty is a challenge that plastic surgeons often face. The objective of this communication is to describe a surgical algorithm for patients undergoing revision rhinoplasty based on the stability of the nasal dorsum.
Methods:
The study included 18 patients, six men and 12 women, aged 19–54 years, who had previously undergone rhinoplasty and who visited our clinic to request a new procedure due to unsatisfactory results in those procedures. A surgical algorithm developed by the main author (N.A.) was followed for revision rhinoplasty, and then a validated rhinoplasty outcome evaluation questionnaire was applied 1 year after surgery to assess aesthetic outcomes.
Results:
The questionnaire was applied to all participants, showing a significant increase in patient satisfaction. Before surgery, a minimum value of six and a maximum of 21 (mean of 12) were found. After revision rhinoplasty following the proposed surgical algorithm, a minimum value of 21 and a maximum of 30 (mean of 29) were found, and this difference was statistically significant (P < 0.001).
Conclusions:
The surgical algorithm used for nasal dorsum reconstruction in patients undergoing revision rhinoplasty improved patient satisfaction and could be a feasible procedure to approach patients who have previously undergone rhinoplasty.
Takeaways
Question: Does the differential surgical algorithm provide good aesthetic satisfaction levels in patients with mestizo nose 1 year after surgery according to the rhinoplasty outcome evaluation questionnaire scale?
Findings: The satisfaction scores showed a significant increase with respect to the results of previous rhinoplasties (P < 0.001).
Meaning: The differential surgical algorithm improved patient satisfaction when compared with previous rhinoplasty.
INTRODUCTION
Improving the aesthetics of the nose is among the most sought-after procedures in plastic surgery, and rhinoplasty is among the most requested by patients who visit our practice. Satisfaction, as well as respiratory function, are key factors that often determine the decision to undergo new procedures.1
Revision rhinoplasty techniques have progressively evolved in recent years, expanding the range of techniques to improve the aesthetics and function of a nose previously operated on. Most cases present issues, including inverted-V, loss of nasal dorsal aesthetic lines, internal or external nasal valve collapse, wide or asymmetric nasal dorsum, saddle nose, asymmetric nostrils, alar retraction and collapse, nose tip drop, supratip, nasal synechiae, and septal perforations.2
Consistent and satisfactory long-term outcomes depend on the use of grafts with low resorption rates and sufficient strength to provide adequate support. Auricular cartilage, irradiated cartilage, and alloplastic materials have shown limited success, as opposed to costal cartilage, which has proven to be a reliable, abundant, and relatively accessible source to facilitate secondary rhinoplasty.3
The perception of dorsal aesthetic lines is important for patients because they are looking for beautiful and symmetric aesthetics from all angles. Some authors have recently advocated for a fusiform pattern with a flare around the keystone area over the more traditional straight/curvilinear shape for the ideal shape of the nasal dorsal aesthetic lines. The study by David Sterling et al describes the preferences for dorsal aesthetic lines, favoring the traditional concept of paired straight or curvilinear lines sweeping smoothly from the glabella to the nasal tip as more attractive. The fusiform nose was considered more natural.4 The objective of this communication is to describe a surgical algorithm for patients undergoing revision rhinoplasty, considering the anatomical characteristics of the nasal dorsum, to plan the surgical procedure. Patient satisfaction was assessed using a validated rhinoplasty outcome evaluation questionnaire (ROE); follow-up was for 1 year.5
MATERIALS AND METHODS
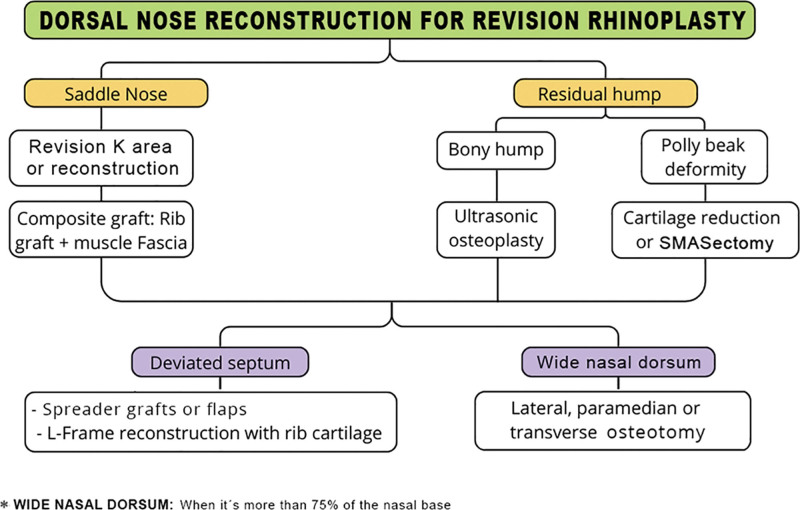
Revision rhinoplasty was performed on 18 patients (six men and 12 women) aged 19–54 years (mean of 31.94) in a private practice in Lima, Peru, during the months of February and April 2022, following a surgical algorithm developed by the author (N.A.). Different reconstruction techniques were performed according to the surgical algorithm shown in Figure 1. A Likert-type ROE questionnaire was then applied to all patients to assess their perception of the outcomes one year after surgery. [See appendix, Supplemental Digital Content 1, which shows the ROE questionnaire survey (English version) and the validated Spanish version conducted before surgery and 1 year later to measure satisfaction with the results. http://links.lww.com/PRSGO/D485.]
Fig. 1.
Nasal dorsum reconstruction algorithm in revision rhinoplasty.
All information was stored in a database in Microsoft Excel 16.77.1. The nonparametric Wilcoxon signed-rank test was used to assess patient satisfaction. Statistical analysis was carried out using SPSS version 25; a value of P less than 0.05 was considered a significant result.
All patients included in the study were given a detailed description of the surgical plan and, together with the surgeon, a decision was made to proceed with rhinoplasty according to the proposed surgical algorithm. Inclusion criteria were having had a previous rhinoplasty and dissatisfaction with the aesthetics of the nasal dorsum. Exclusion criteria were having a history of uncontrolled chronic diseases and a cardiac risk index (Goldman criteria) exceeding II.
The groups of patients according to the therapeutic scheme are described in Table 1. The informed consent was signed, including an authorization to use audiovisual material for the purposes of this study. These agreements complied with the principles of the Declaration of Helsinki, as well as with the authorizations of the local ethics committee of the institution where this study was carried out.
Table 1.
Descriptive Data of the Study Group
| Mean | SD | N | % | ||
|---|---|---|---|---|---|
| Age (y) | 31.94 | 8.71 | |||
| Sex | Female | 12 | 66.7% | ||
| Male | 6 | 33.3% | |||
| Anesthesia | General | 16 | 88.9% | ||
| Sedation with infiltration | 2 | 11.1% | |||
| Surgical time | Mins | 185.56 | 35.85 | ||
| Type of nasal dorsum | Saddle nose | 6 | 33.3% | ||
| Residual hump | 8 | 44.4% | |||
| Wide dorsum | 4 | 22.2% | |||
| Septal deviation | Yes | 13 | 72.2% | ||
| No | 5 | 27.8% | |||
| Technique | Open | 18 | |||
| Closed | 0 |
SURGICAL TECHNIQUE
Step 1: Preoperative Site Marking
With the patient in a standing position, the site for harvesting the cartilage, at the level of the intermammary sulcus in women and at the level of the fifth or sixth costal cartilage in men, was located.
Step 2: Cartilage Harvesting
Cartilage can be harvested on the same side or on the contralateral side if performed by two teams. The incision is done with a scalpel blade No. 15 and the incision size can range from 2.5 to 4 cm. Subcutaneous tissues were dissected using electrocautery up to the muscle fascia. The rectus abdominis muscle fascia was harvested in case a muscle fascia graft was required. Muscle fibers were dissected with Halstead forceps until reaching the perichondrium. Dissection continued until reaching the costochondral and chondrosternal junctions, and the perichondrium was harvested if necessary. A subperichondrial dissection of the inferior, superior, and lateral edges of the cartilage was then performed. The cartilage, measuring approximately 3–4 cm in length, was removed. Finally, the Valsalva maneuver was used to confirm that the chest wall remained intact, with no holes, to prevent a pneumothorax.
Step 3: Nasal Approach
An inverted-V transcolumellar incision was made, and the alar cartilages were dissected in the subperichondrial plane. Then, depending on the type of nasal dorsum, the following was done.
Residual Dorsal Hump
The nasal dorsum was dissected in the subperichondrical and subperiosteal plane, eliminating fibrosis, and separating the upper lateral cartilages or their remnants from the cartilaginous septum. If septal deviation of the L-frame was present, decompressing cuts were made in areas of tension and reinforcement were then done with costal cartilage grafts. For caudal septal deviation, the caudal septum was repositioned or replaced and fixed to the nasal spine with 5/0 Prolene. Osteotomy of the bony nasal dorsum was performed with a Piezotome, and the excess septal cartilage was removed with Caplan septum scissors. Next, paramedian, transverse, and lateral high-low-low osteotomies were performed with a Piezotome for a wide nasal dorsum. [See Video 1 (online), which displays the ultrasonic osteotomy.] The internal nasal valve was reconstructed using spreader flaps with the remaining upper lateral cartilages or, if insufficient material, with spreader grafts.
Video 1. displays the ultrasonic osteotomy.
Saddle Nose
The nasal dorsum was dissected in the subperichondrial and subperiosteal plane, with special caution in the keystone region. If required, the keystone region was reconstructed with transosseous sutures as described by Rezaeian.6 In cases of anterior septal deviation, the residual septum was reinforced with an L-frame and fixed to the nasal spine to provide greater stability. [See Video 2 (online), which displays the caudal septal stabilization.] Lateral, paramedian, and/or transverse osteotomies were performed in wide or asymmetric nasal dorsa with a Piezotome. In our experience, all cases require bilateral spreader grafts due to nasal valve insufficiency, inverted-V deformity, or loss of dorsal aesthetic lines. To recover nasal height, a carved rectangular costal cartilage graft measuring 8- to 10-mm wide, 2- to 3-cm long, and 1.5- to 3-mm thick was placed and then covered with the rectus abdominis fascia to restore the desired nasal height. [See Video 3 (online), which displays the Dorsal augmentation with costal graft.] Finally, a silicone splint was placed inside for 7 days for breathing reasons, as well as a thermoplastic splint.
Video 2. which displays the caudal septal stabilization.
Video 3. displays the Dorsal augmentation with costal graft.
Wide Nasal Dorsum
The nasal dorsum was dissected in the subperichondrial and subperiosteal plane. Next, a Piezotome was used for osteotomy and to eliminate irregularities in the nasal bones. Paramedian, transverse, and lateral high-low-low osteotomies were used for a wide nasal dorsum. If it was desirable to reduce the width of the cartilaginous vault, mild electrosurgical fulguration of the lateral flanges or scraping with a scalpel blade no. 11 was done. The internal nasal valve was reconstructed using spreader grafts in case of nasal valve insufficiency. For patients with thin skin, diced cartilage or muscle fascia can be used on the nasal dorsum to correct irregularities.
Postoperative Care
All procedures were ambulatory, having a continuous follow-up, part of our care protocol is developed as follows:
Bed rest at 45 degrees for the first 3 days, use of cold compresses on cheeks and forehead, a soft diet for the first 2 days, and then a normal diet.
Placement of thermoplastic splint on the nasal dorsum and Doyle splints to stabilize the septum, allowing adequate breathing in the postoperative period, which are removed on the seventh day.
Prophylactic antibiotics are used, the first choice being first-generation cephalosporin or ciprofloxacin in patients with allergies, for 5 days.
In our analgesic management, we use non-steroidal anti-inflammatory drugs for 3–5 days and/or opioids with severe pain conditionally.
We recommend no moderate-to-severe physical exertion and rest with headrest at 45 degrees.
We performed the first control, associated with nasal cleaning at 72 hours postoperatively, removal of splints on the seventh day, and follow-up at the first month, third month, sixth month and 12th month.
Compression of the nasal dorsum is indicated with the use of nasal packing for 1 month; the patient is instructed on adequate change and placement.
RESULTS
The study included 18 patients, 12 women (66.7%) and six men (33.3%). The minimum and maximum ages were 19 and 54 years, respectively (mean of 31.94 years). All patients had a cardiac risk index lower than II according to Goldman criteria (100%), and none of the participants reported adverse events. The minimum and maximum operative times were 130 and 240 minutes, respectively (mean of 185.56 minutes). General anesthesia was used in 16 patients (88.9%), and sedation with local anesthesia in two patients (11.1%; Table 1). Regarding donor cartilage, 14 costal cartilages were used (77.8%) and four septal cartilages (22.2%). Rectus abdominis fascia was used in 14 patients (77.8%), and spreader graft in 17 (94.4 %), with spreader flaps used in only one (5.6%) (Table 2; P < 0.001).
Table 2.
Cartilages Used in Nasal Dorsum Reconstruction
| No | Yes | |||
|---|---|---|---|---|
| n | % | n | % | |
| Costal cartilage | 4 | 22.2% | 14 | 77.8% |
| Rectus abdominis fascia | 4 | 22.2% | 14 | 77.8% |
| Spreader graft | 1 | 5.6% | 17 | 94.4% |
| Spreader flap | 17 | 94.4% | 1 | 5.6% P < 0.001 |
Differences exist between the operatory characteristics, with spreader graft standing out compared with others.
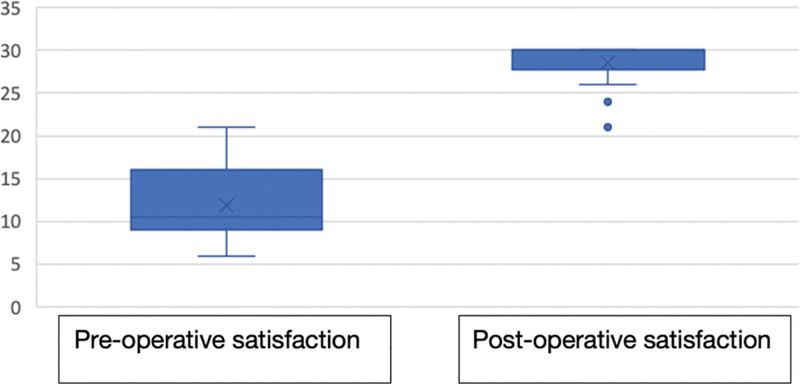
Regarding satisfaction before surgery, a minimum value of six and a maximum of 21 were found (mean of 11.89). After revision rhinoplasty following the proposed surgical algorithm, a minimum value of 21 and a maximum of 30 (mean of 28.50) were found, and this difference was statistically significant (P < 0.001; Table 3 and Fig. 2).
Table 3.
Satisfaction Scores before and after Surgery
| Satisfaction Score | n | Mean (SD) | Z | P |
|---|---|---|---|---|
| Preoperative | 11.89 (4.10) | |||
| 18 | -3.73 | <0.001 | ||
| Postoperative | 28.50 (2.55) |
Applying the nonparametric Wilcoxon test.
No normality in the data applying the Shapiro Wilk test.
Fig. 2.
Satisfaction with surgical algorithm.
Immediate Complications
There was one patient who had epistaxis on the seventh day who required anterior tamponade for 5 days and tranexamic acid orally; he evolved favorably.
Late Complications
We had retraction of the soft triangle in two patients who required revision with graft in this area. At the level of the dorsum, one patient presented supratip at 3 months, for which corticoid infiltration (triamcinolone two sessions and tape 1 month) evolved favorably.
DISCUSSION
The goals of a secondary rhinoplasty currently involve looking good in frontal, profile, and three-quarter views. Therefore, the study and knowledge of the anatomical and functional structures of the nasal dorsum (dorsal aesthetic lines, internal nasal valve) are important for performing a good nasal reconstruction.
The most frequent issues in the frontal view are loss of dorsal aesthetic lines, inverted-V deformity, and a wide or asymmetric dorsum. It is common to find bone irregularities in revision rhinoplasties, so it is necessary to correct these defects with precision to achieve the best possible outcomes.
This study proposes a surgical algorithm to reconstruct the nasal dorsum both aesthetically and functionally using various techniques, including spreader graft, spreader flap, diced cartilage with rectus abdominis fascia, and laminated costal cartilage wrapped in perichondrium and rectus abdominis fascia (Robotti and Leone).7 These tools help achieve more natural aesthetic dorsal lines and improve the function of the internal nasal valve in secondary or tertiary rhinoplasty. The upper third of the nasal dorsum in secondary rhinoplasty most often requires osteotomies and correction of irregularities by ultrasonic osteotomy.
The techniques used to reconstruct the nasal dorsum are based on the use of grafts carved from costal cartilage. Correction of low nasal dorsum requires total coverage of the costal graft with rectus abdominis fascia and perichondrium. Costal graft coverage is necessary to avoid deformity and tubular appearance. The alternative is using diced cartilage with deep temporalis fascia, but our practice does not achieve adequate dorsal aesthetic lines with this technique, so, in our opinion, this technique reconstructs the nasal dorsum more naturally.8 To minimize the deformity of the dorsal graft, the central part of the costal cartilage is used.9 Cases in which the nasal dorsum has a residual hump do not usually require dorsal augmentation grafts, but the internal nasal valve can be reconstructed with spreader grafts or spreader flaps. If the patient has thin skin, rectus abdominis fascia can be used to mask the spacer grafts (Table 4).
Table 4.
Anatomic Findings and Techniques in Revisional Rhinoplasty
| Findings | Technique Used |
|---|---|
| Low bony dorsum | Costal cartilage graft with muscle fascia |
| Irregular or wide bony dorsum | Osteoplasty and/or ultrasonic osteotomy |
| Cartilaginous dorsum with hump or supratip | Reduction of cartilaginous septum and/or spreader flaps |
| Low cartilaginous dorsum | Rib graft and/or reconstruction of keystone area with transosseous points in the nasal bone |
| S-shaped cartilaginous dorsum | Spreader graft or replacement with L-shaped rib graft |
| Absence of dorsal aesthetic lines | Paramedian, lateral and transverse osteotomy plus spreader graft |
| Deviated caudal septum | Reposition with transosseous stitches in the anterior nasal spine or replacement with costal cartilage |
| Septal spur or residual septal deviation | Secondary septoplasty |
| Turbinate hypertrophy | Dislocation and infiltration with corticosteroid |
| Internal synechiae | Release of synechiae and nasal splints for 7 d |
| Drooping nose tip | Bilateral septal extension graft (SEG) |
| Thick skin | SMAS and Pitanguy ligament removal and suture placement between supratip and SEG (Guyuron stitch) |
| Alar collapse | Alar rim graft and lateral crura tension steal with neodome positioning |
| Alar retraction | Auricular composite graft |
| External nasal valve dysfunction | Resting angle correction with a figure-8 suture between “W” septal point and lateral cruras. |
Several studies have analyzed the histological and biomechanical properties of costal cartilage, describing a lower cellular content, a greater amount of collagen, and lower elasticity with greater tensile strength.10 These properties vary according to the part of the cartilage used, either center or periphery, and its thickness. Therefore, using the center of the costal cartilage and thicknesses of up to 1.5 mm is recommended, thus providing properties similar to those of septal cartilage.10
The development of more versatile ultrasonic inserts allows the achievement of a precise paramedian and lateral osteotomy with limited detachment, as opposed to Gerbault et al, who proposed an extended detachment to avoid performing the osteotomy blindly.11 Regarding transverse osteotomy, the Robiony technique was used with a thin cutting ultrasonic insert that does not generate a visible scar, in addition to less inflammation, edema, and scarring.12
The mechanical force used with conventional osteotomes can cause irregularities in the fracture lines and damage to the surrounding mucosa.12 This avoids damaging the surrounding tissue with mechanical or thermal energy, while creating a precise fracture line.13,14 Given our experience with ultrasound, this technique is preferred because it causes less edema, swelling, and ecchymosis in the postoperative period.
When we want to reduce the width of the nasal dorsum, defined as a dorsum that exceeds 75% of the nasal base; the Piezotome helps us to generate less damage to the mucosa or skin and, therefore, less bleeding.14,15
A mean score of 12 was obtained with the survey before surgery. However, satisfactory outcomes were obtained with a mean score of 29, after performing the nasal dorsum reconstruction according to the surgical algorithm proposed in this study. This difference was statistically significant, so patient satisfaction improved (Figs. 3–5).
Fig. 3.
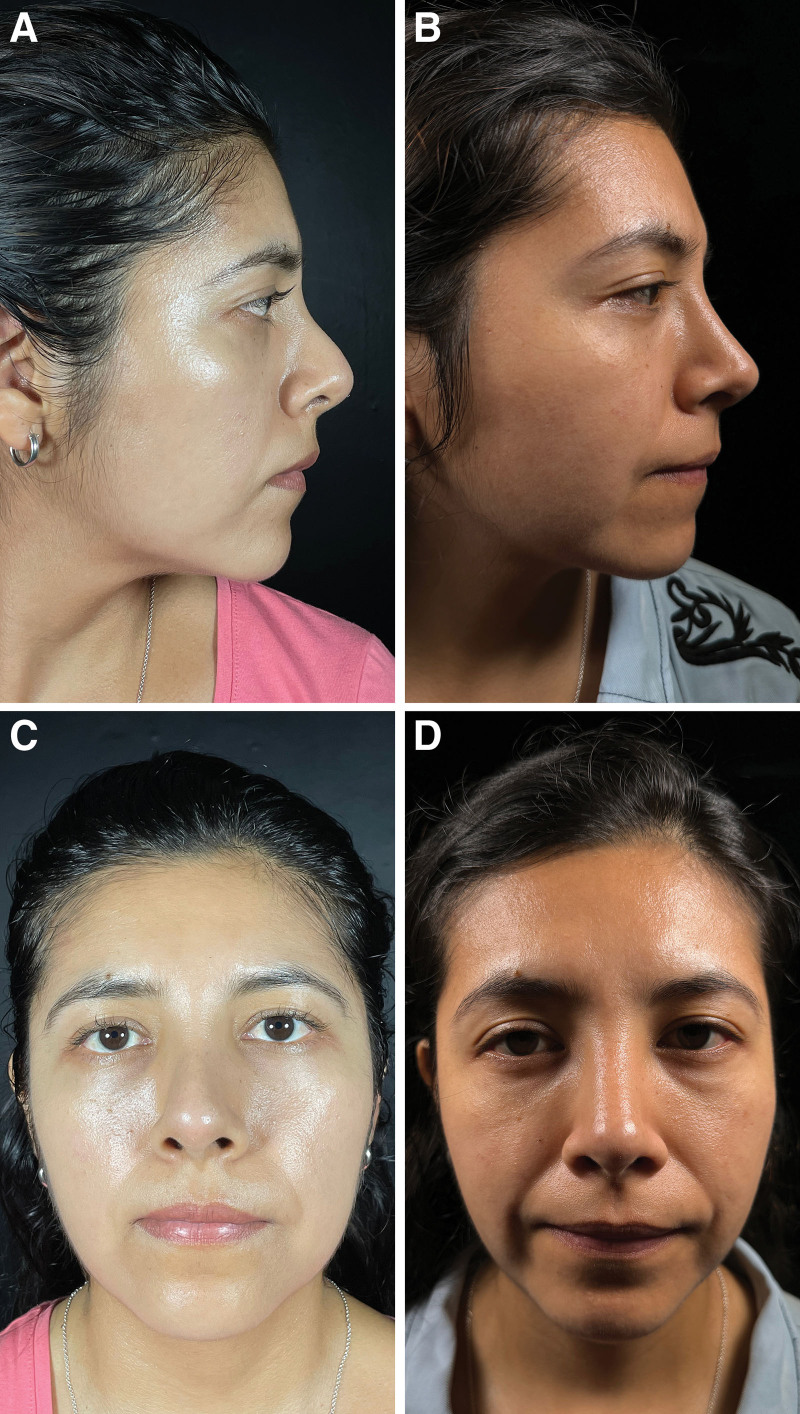
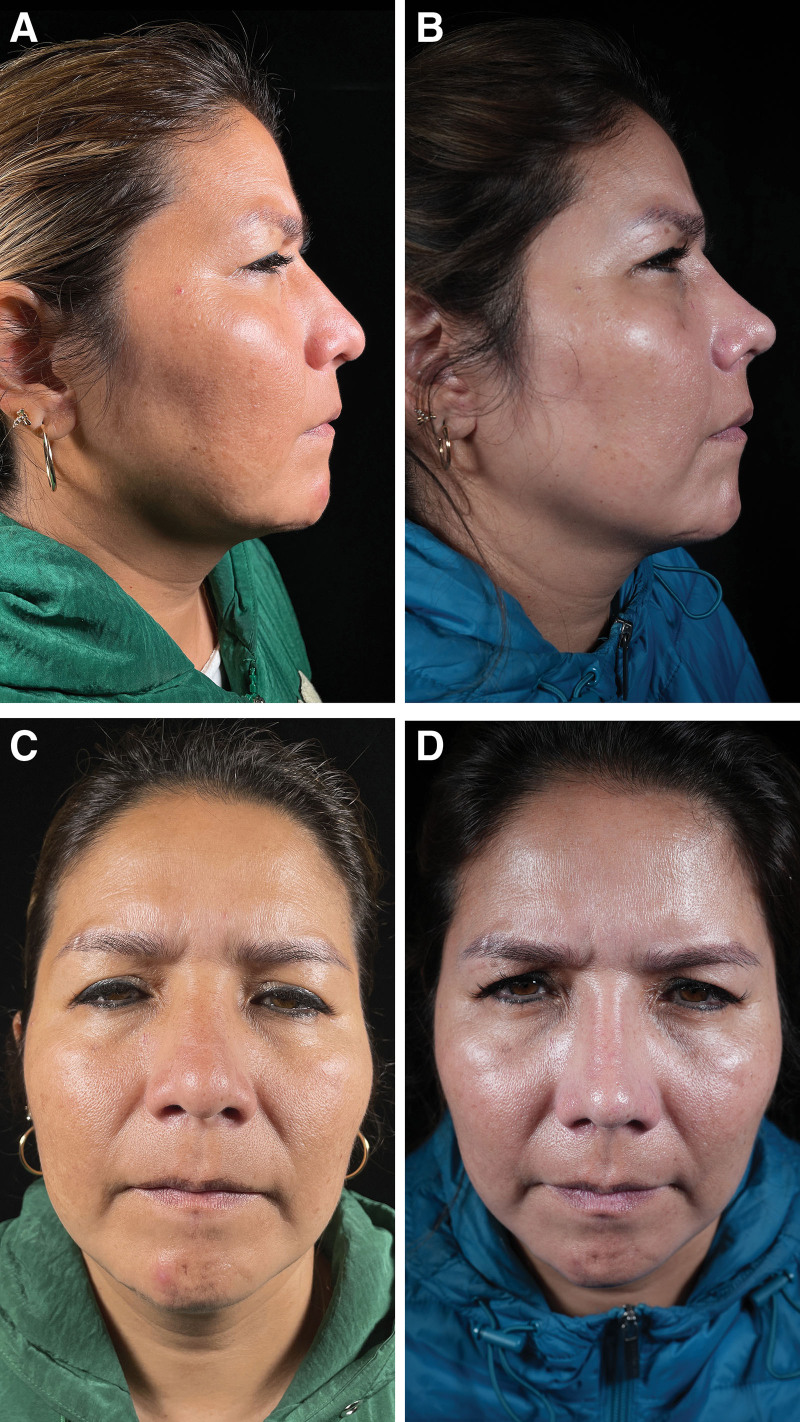
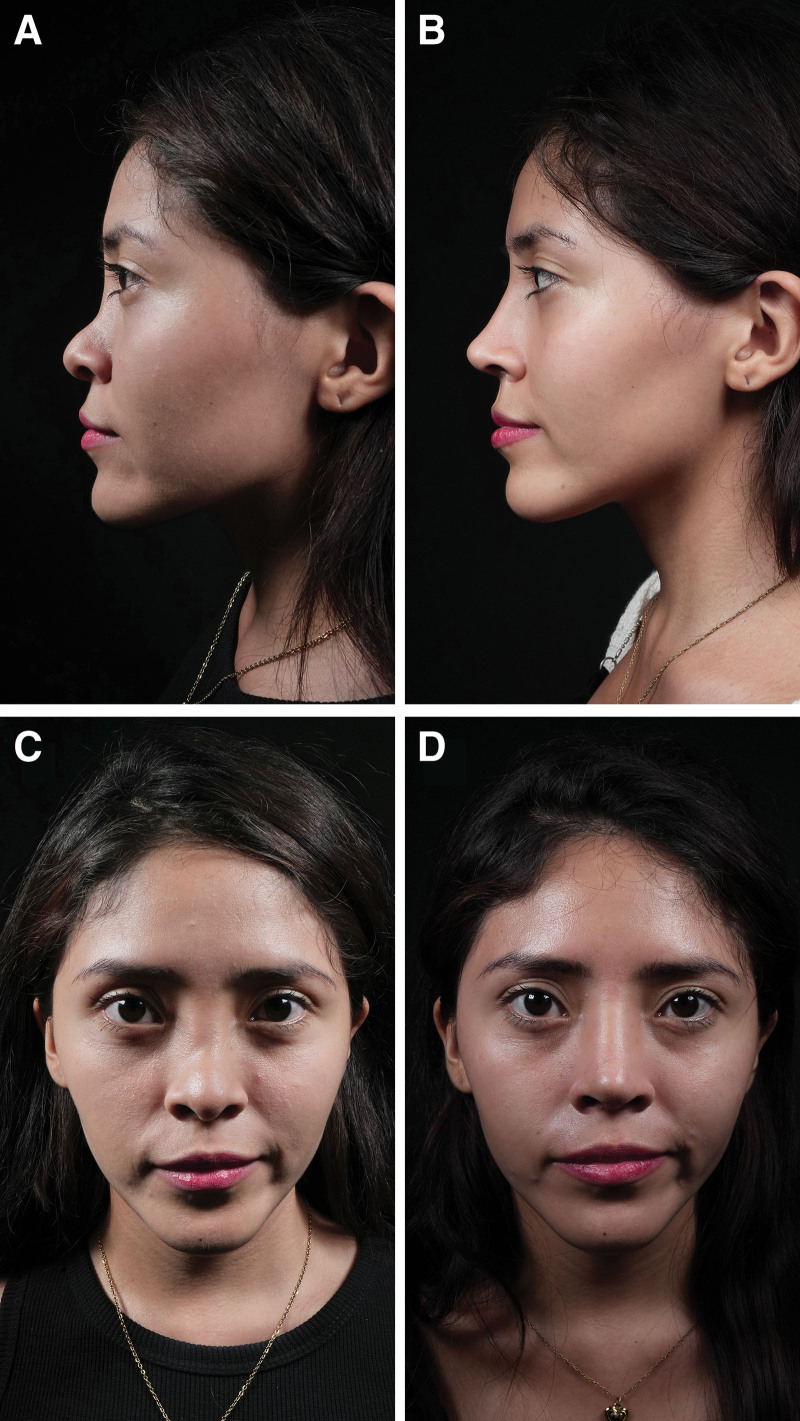
Outcomes one year after rhinoplasty. Residual dorsal hump. Dorsal septal humpectomy, bilateral spreader flaps, costal cartilage septal extension grafts, and lateral, paramedian, and transverse osteotomy. Before (A) and after (B) pictures in lateral view. Before (C) and after (D) pictures in frontal view.
Fig. 5.
Outcomes one year after rhinoplasty. Wide nasal dorsum residual. Ultrasonic osteotoplasty in the bony cap, paramedian, transverse, and lateral osteotomy, septal extension graft, and alar rim graft. Before (A) and after (B) pictures in lateral view. Before (C) and after (D) pictures in frontal view.
Fig. 4.
Outcomes one year after rhinoplasty. Saddle nose. Reconstruction of the keystone region with L-shaped costal cartilage fixed to nasal bones and anterior nasal spine, bilateral spreader grafts, costal graft of 30 × 8 mm wrapped in muscular fascia in nasal dorsum. Septal extension and alar rim graft. Paramedian, transverse, and lateral osteotomy. Before (A) and after (B) pictures in lateral view. Before (C) and after (D) pictures in frontal view.
The limitations of our study include the limited number of cases, but despite this, a statistically significant change in patient satisfaction 1 year after surgery was achieved.
CONCLUSIONS
The surgical algorithm proposed in this study for nasal dorsum reconstruction in patients who have had previous rhinoplasties is reproducible, with good satisfaction levels 1 year after surgery with statistically significant improvements (P < 0.001). [See Video 4 (online), which displays the final results.]
Video 4. displays the final results.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 6 September 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Sheen JH. Secondary rhinoplasty. Plast Reconstr Surg. 1975;56:137–145. [DOI] [PubMed] [Google Scholar]
- 2.Bracaglia R, Fortunato R, Gentileschi S. Secondary rhinoplasty. Aesthetic Plast Surg. 2005;29:230–239. [DOI] [PubMed] [Google Scholar]
- 3.Marin VP, Landecker A, Gunter JP. Harvesting rib cartilage grafts for secondary rhinoplasty. Plast Reconstr Surg. 2008;121:1442–1448. [DOI] [PubMed] [Google Scholar]
- 4.Sterling DA, Deot N, Davila RO. Perceptions of attractiveness in dorsal aesthetic lines of the nose: a crowd sourcing analysis. Facial Plast Surg Aesthet Med. 2022;24:424–429. [DOI] [PubMed] [Google Scholar]
- 5.Calderón G ME, Cuevas T P, Erazo C C, et al. Rinoplastía: resultados desde la perspectiva del paciente. Validación lingüística y psicométrica del rhinoplasty outcome evaluation instrument. Rev Chil Cir. 2013;65:30–34. [Google Scholar]
- 6.Rezaeian F, Gubisch W, Janku D, et al. New suturing techniques to reconstruct the keystone area in extracorporeal septoplasty. Plast Reconstr Surg. 2016;138:374–382. [DOI] [PubMed] [Google Scholar]
- 7.Robotti E, Leone F. The SPF-SPLF graft: building the ideal dorsum in revision rhinoplasty. Plast Reconstr Surg. 2020;145:1420–1424. [DOI] [PubMed] [Google Scholar]
- 8.Adamson PA, Warner J, Becker D, et al. Revision rhinoplasty: panel discussion, controversies, and techniques. Facial Plast Surg Clin North Am. 2014;22:57–96. [DOI] [PubMed] [Google Scholar]
- 9.Robotti E. Shaping the nasal dorsum. HNO. 2018;66:92–102. [DOI] [PubMed] [Google Scholar]
- 10.Alkan Z, Yigit O, Acioglu E, et al. Tensile characteristics of costal and septal cartilages used as graft materials. Arch Facial Plast Surg. 2011;13:322–326. [DOI] [PubMed] [Google Scholar]
- 11.Gerbault O, Daniel RK, Kosins AM. The role of piezoelectric instrumentation in rhinoplasty surgery. Aesthet Surg J. 2016;36:21–34. [DOI] [PubMed] [Google Scholar]
- 12.Robiony M, Lazzarotto A, Nocini R, et al. Piezosurgery: ten years’ experience of percutaneous osteotomies in rhinoplasty. J Oral Maxillofac Surg. 2019;77:1237–1244. [DOI] [PubMed] [Google Scholar]
- 13.Motamed S, Saberi A, Niazi F, et al. Complications of internal continuous and perforating external osteotomy in primary rhinoplasty. World J Plast Surg. 2017;6:164–169. [PMC free article] [PubMed] [Google Scholar]
- 14.Kisel J, Khatib M, Cavale N. A comparison between piezosurgery and conventional osteotomies in rhinoplasty on post-operative oedema and ecchymosis: a systematic review. Aesthetic Plast Surg. 2023;47:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orten SS, Hilger PA. Facial analysis of the rhinoplasty patient. In: Papel ID, ed. Facial Plastic and Reconstructive Surgery. New York, N.Y.: Thieme Medical Publishers; 2002:361–368. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.