Abstract
Background:
Tiny arteriovenous (AV) shunts of 10–150 µm (0.01–0.15 mm) are documented in the hands and feet. Larger shunts up to 0.5 mm (500 µm) have been discovered by the authors in the inner canthus and the human eye. This study seeks their possible existence in the upper limb.
Methods:
Radiographic lead oxide cadaver injection and dissection studies of 14 archival and six new upper limbs were examined.
Results:
AV shunts of 0.1–0.5 mm were discovered between the brachial, ulnar, and radial arteries and their venae comitantes and between their arterial perforators and the subcutaneous veins.
Conclusion:
This pilot study provides insight into the possible function of these large AV shunts associated with blood flow variation in temperature, blood pressure, tissue transfer, flap prefabrication, and flap necrosis.
Takeaways
Question: Large arteriovenous shunts up to 0.5 mm have been discovered in the inner canthus and eye. This study seeks their existence in the upper extremity.
Findings: Radiographic cadaver injections and dissections revealed shunts 0.1–0.5 mm between (1) the brachial, radial, and ulnar arteries and their venae comitantes and (2) their arterial perforators and the subcutaneous veins.
Meaning: This study provides insight into the possible function of these arteriovenous shunts, much larger than those documented in glabrous skin, associated with sudden blood flow variations in temperature, blood pressure, tissue transfer, flap prefabrication, and flap necrosis.
INTRODUCTION
Arteriovenous (AV) anastomoses or shunts are direct connections between arteries and veins. In the integument, they are well documented in the glabrous skin of the hands and feet where they are involved in temperature control in the thermoneutral range between 26°C and 36°C. They are short, tiny, well-innervated, low-resistance connections, situated in the precapillary zone with diameters between 10 and 150 µm1,2 (0.01–0.15 mm). Although suggested,1 they have not been definitively documented elsewhere in the integument.
However, recent human and nonhuman investigations, combined with a review of our archival studies, have revealed that larger AV shunts of 0.1–0.5 mm (100–500 µm) were situated more proximal in the vascular “tree.” These include (1) the finding of connections between the radial artery and a vena comitans during a free flap dissection (Fig. 1), (2) the discovery of AV shunts in the skin of the inner canthus between the facial artery and vein3 (Fig. 2), and (3) the large number of shunts found in the giraffe skin between its patch arteries and their circumferential veins4 (Fig. 3).
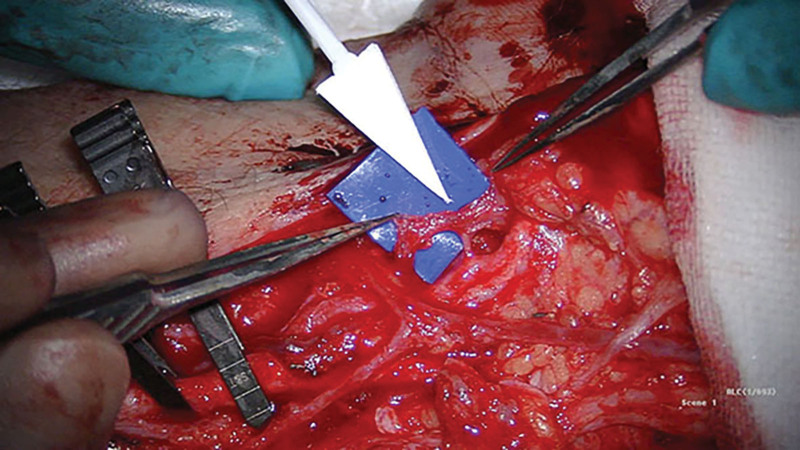
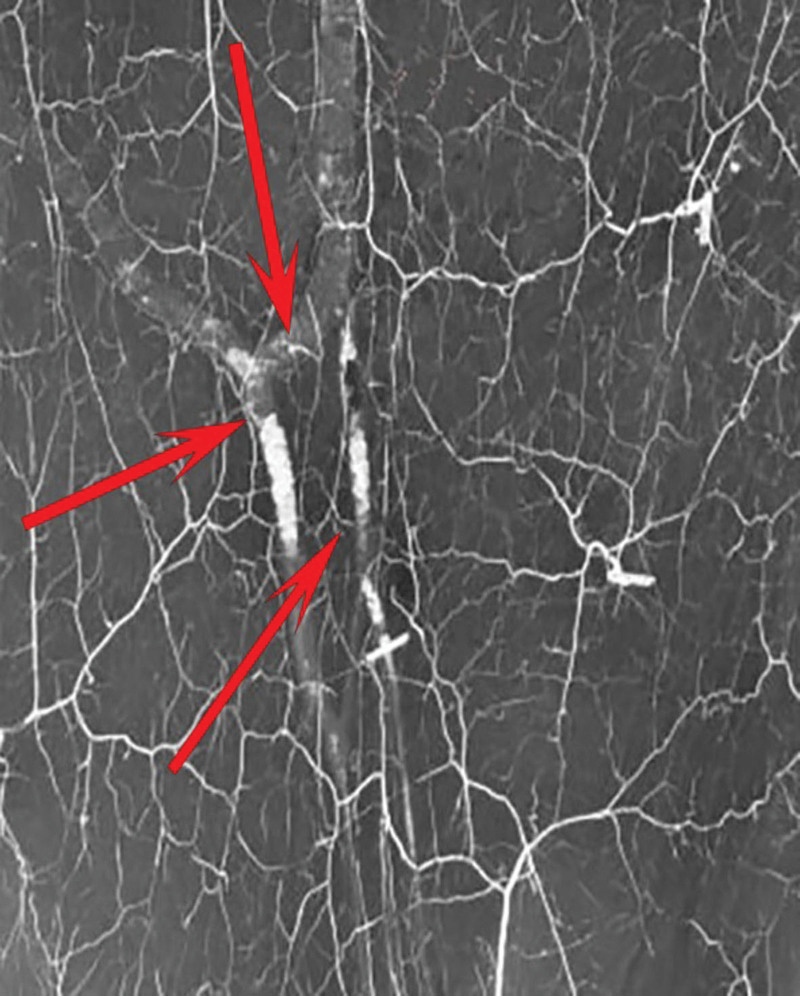
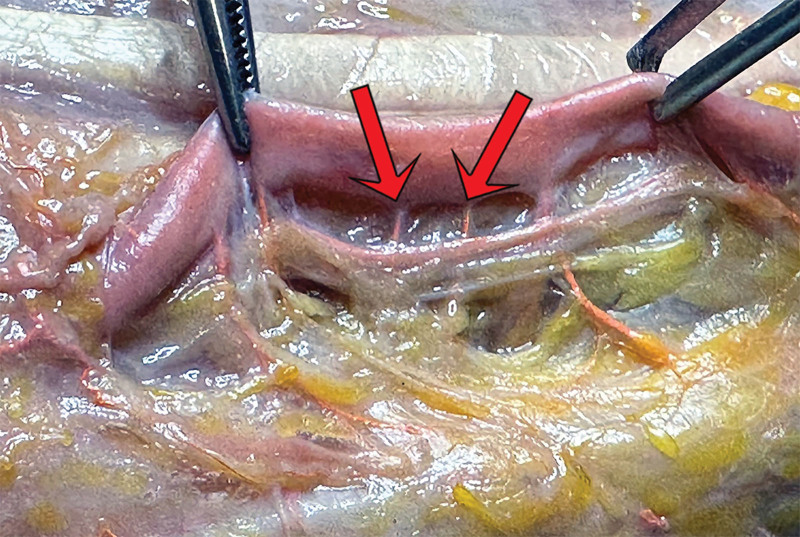
Fig. 1.
AV shunt between radial artery and vein found at operation while dissecting the flap pedicle.
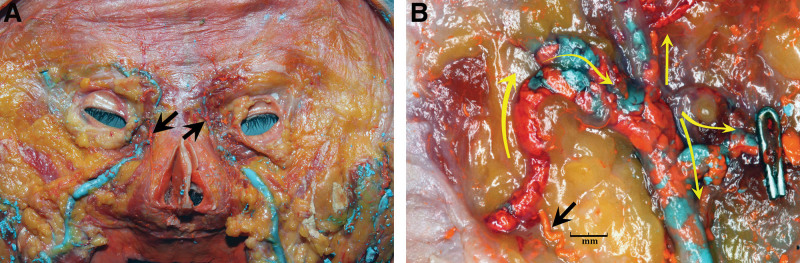
Fig. 2.
A, Deep surface of the face of a combined arterial and venous fresh human body donor injection study revealing the bilateral sites of AV shunts (arrows) with the right side of the figure enlarged (B), demonstrating large globules of orange lead-oxide that have filled through a 0.5-mm AV shunt (black arrow) into the blue barium-filled vein and its metal clipped branch to the orbit (yellow arrows). Reproduced from Plast Reconstr Surg. 2020;146:745–755.3 Copyright © 2020 by the American Society of Plastic Surgeons. Used with permission of Wolters Kluwer Health.
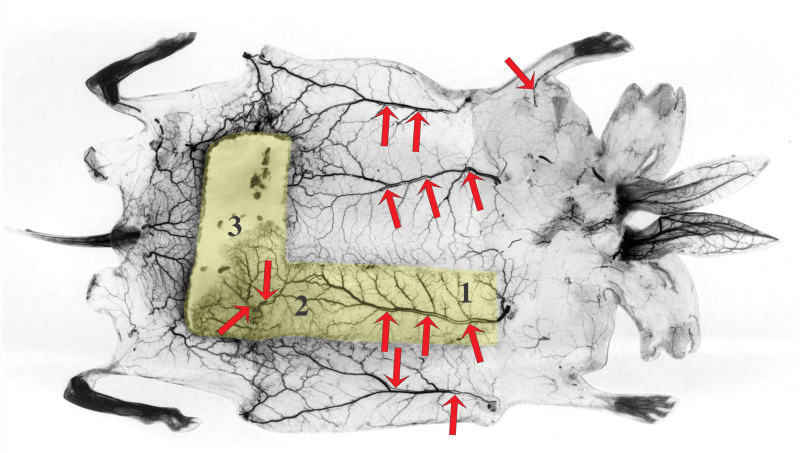
Fig. 3.
Venous filling by lead oxide overflow from the arterial injection via AV shunts in the skin of the giraffe neck. A, Vein segments backfilled until arrested by valves between patches (large arrows) and their arteriae comitantes (small arrows). B, Arteriae comitantes connected to patch arteries (large arrows) with several AV shunts identified (short arrows). Reproduced from Plast Reconstr Surg. 2023;152:669–680.4 Copyright © 2023 by the American Society of Plastic Surgeons. Used with permission of Wolters Kluwer Health.
These shunts have been suggested where there has been overflow of the lead oxide arterial injection, with particle size too large to pass through the capillary bed into large veins rather than their tributaries: for example, into the axial vein draining the rabbit flank flap, especially after a delay procedure5 (Fig. 4); the saphenous veins in the leg while studying their neurovascular relationships6 (Fig. 5); and the forearm veins while documenting the effect of increased perfusion pressure on anastomotic vessels.7
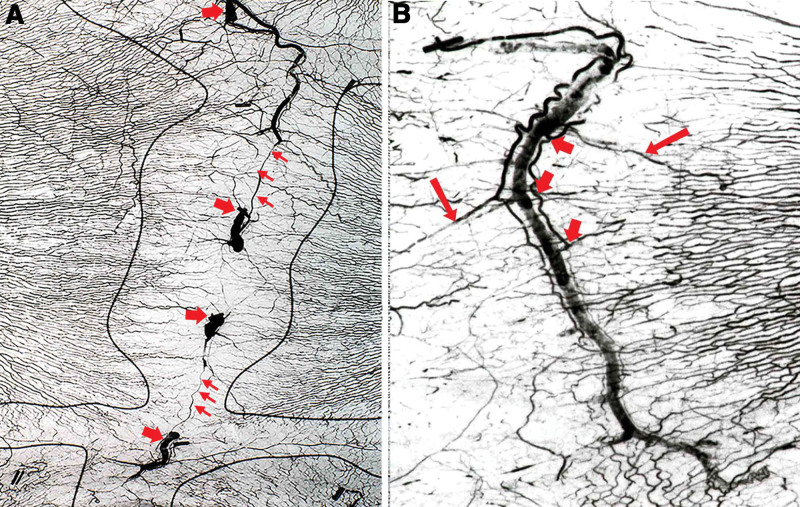
Fig. 4.
A three-territory rabbit flap with well-defined necrosis line between territories 2 and 3 and overflow of the lead oxide arterial injection into the pedicle vein of all vessels within, and divided around, the flap circumference as well as a cutaneous vein in the forelimb (arrows).
Fig. 5.
Early lead oxide filling of short saphenous veins with globules of lead oxide via AV shunts (arrows). Note arterial perforator branches arranged parallel to the veins, similar to the giraffe (compared with Fig. 3).
Situated strategically in the arterial pathway well before the capillary bed, it seems likely that these “macro” shunts, many times larger than those in the glabrous skin, have evolved to “intercept” and respond to sudden physiological or pathological changes in the circulation. Already it has been shown experimentally8 and clinically3,9,10 that they are involved in the diversion of fat or hyaluronic acid accidently injected into an artery to be diverted into large veins and diluted.
Are they involved in the homeostasis of a tissue transfer, where, for example, it was noted that the venous return in the radial vein of a radial nerve free flap was rapid and almost instantaneous, especially in this example where the capillary bed was small?11 Do they define the almost linear necrosis line of a flap or that of a digit exposed to frostbite? Is the flow only unidirectional from artery to vein? Clearly there are two main questions to be answered: where are these AV shunts located; and what is their function?
This article is a preliminary report of a new anatomical discovery. It focuses on our previous study of the upper limb7 because the location of the AV shunts was not identified in the skin, nor were the deep tissues studied.
METHODOLOGY
Fourteen archival and six new upper limbs were examined. Fresh cadavers were obtained through our institutions body donor program. Each limb was perfused with our lead oxide mixture12 using a peristaltic pump via a rigid ureteric catheter inserted into the brachial artery. To control for variables which may affect the flow of the contrast solution, it was heated before injection, used immediately, and maintained in solution using a magnetic stirrer. Leakage was minimized by a proximal tourniquet.
Archival
With a pressure transducer (Hospira Transpac IV; ICU Medical, Inc., San Clemente, Calif.) placed in the radial artery at the wrist and calibrated using a mercury sphygmomanometer, the forearm specimens were perfused with an identical volume of contrast solution at low or high rates achieving pressures ranging from 50 to 211 mm Hg. In each, the integument was removed superficial to the deep fascia and radiographed. These specimens were not dissected, nor were the vessels in the deep tissues studied.
Current
Six upper limbs were perfused and then disarticulated at the shoulder so that the arm and the forearm could be studied. Four were injected at low rate, and the rate was increased in the other two. Previous injection pressure studies indicated that low-rate injections achieved perfusion pressures of between 51 and 103 mm Hg, whereas high-rate injections achieved pressures of 155–211 mm Hg.7 Each limb was x-rayed and incised along the ulnar border of the arm and forearm. The skin was removed superficial to the deep fascia, noting the sites of lead oxide–filled perforators as they emerged from the deep arteries. The integument was x-rayed again. It was then dissected under loupe magnification, tracing the major veins to locate their AV connections with the arterial perforators. Vein segments containing the lead oxide, noted on x-ray and during dissection, facilitated identification of these AV shunts.
Next, the deep tissues were x-rayed and dissected. The brachial, radial, and ulnar arteries, together with their comitante veins, were traced from the axilla to the wrist.
RESULTS
Combining the dissection and all radiographic studies, both current and archival, AV shunts were identified.
Superficial
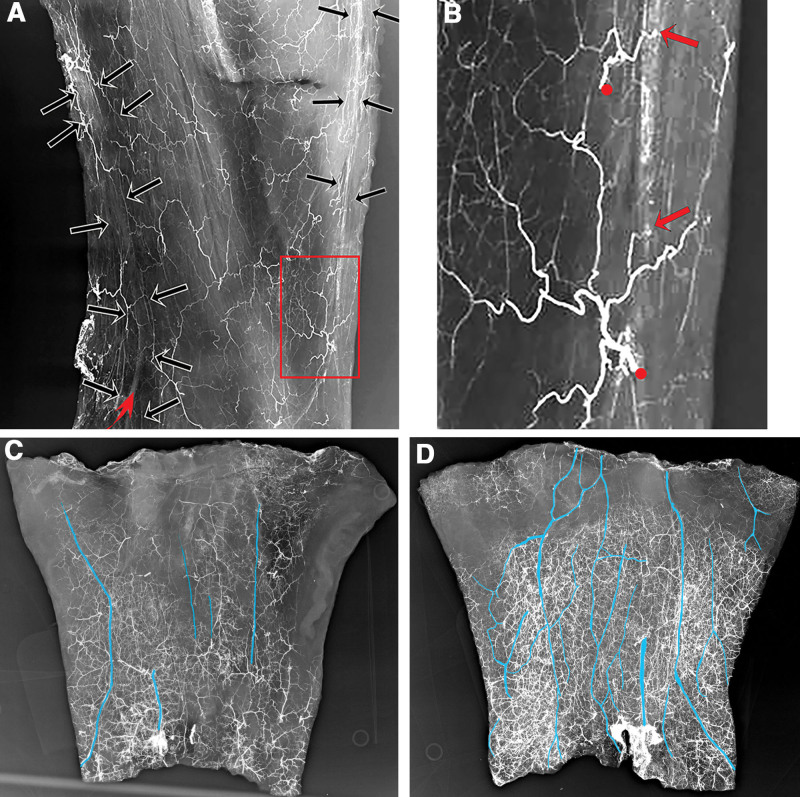
Major shunts up to 0.5 mm in diameter were located between perforators of the under lying arteries and the major subcutaneous veins, especially the cephalic, basilic, and their interconnecting venous network. They were more common in the forearm than the arm, where the cutaneous veins are more superficial. Initially, the major veins, not their tributaries, were filled with lead oxide commencing distally in the forearm. It was easier to identify the AV shunts in this early filling phase. Then, as the pressure of the injectant increased, more veins filled, along with their tributaries, making it harder to distinguish them from arteries on x-ray (Fig. 6).
Fig. 6.
Arterial injection studies of the forearm integument at (A) early low pressure, (C) medium pressure, and (D) high pressure. Note (A and B) early filling of cephalic and basilic veins highlighted in red with accompanying arterial branches arranged in parallel (black arrows), and (B) arterial perforators (red dots) and their AV shunts (arrows) with (C and D) lead oxide-filled veins (colored blue to distinguish them from arteries) that increase in number and extent as the perfusion pressure increases.
Dissection revealed that these AV shunts arose from branches of arterial perforators, especially from the radial and ulnar arteries. These branches often linked together to form a parallel network on either side of the vein—not unlike that seen in the giraffe4 (Figs. 3–7). So evident was this arrangement that even without lead oxide in the vein this “arterial railway” often identified the vein’s location (Fig. 6A). Notably, branches of the medial and lateral cutaneous nerves of the forearm often accompanied the veins.
Fig. 7.
Dissection (A) and diagram (B) showing 2 shunts from radial artery perforators (arrows) into the cephalic vein. Note the connection between branches of the arterial perforators that parallel the vein and lead oxide in the vein’s lumen.
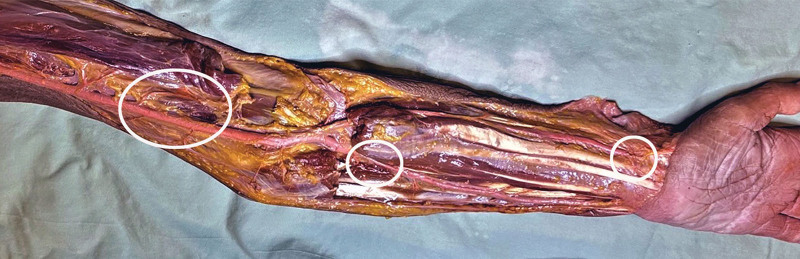
Deep
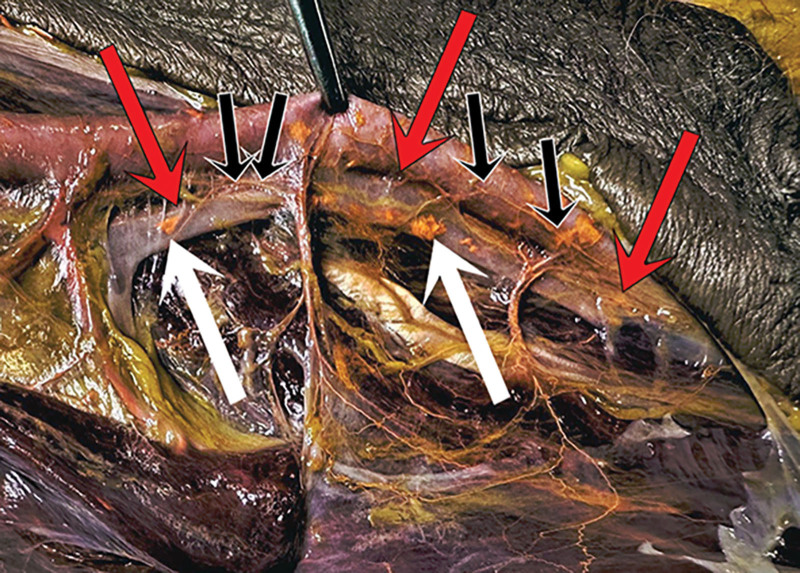
AV shunts were located between the brachial, radial, and ulnar arteries and their venae comitantes (Fig. 8). They were of two types:
Fig. 8.
One of the upper limb dissections showing the sites (outlined) of radial, ulnar, and brachial AV shunts.
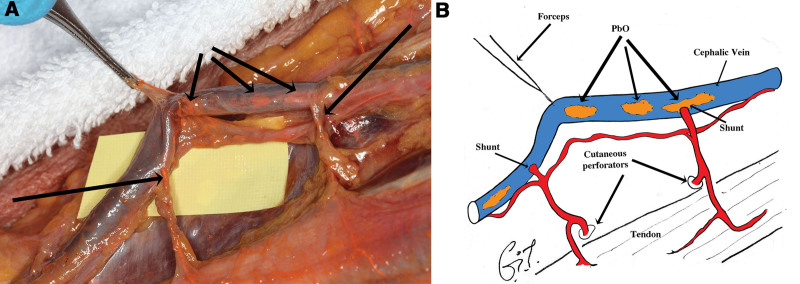
Large diameter of 0.2–0.5 mm short connections between the radial and ulnar arteries and their venae comitantes. They were more common in the distal forearm (Fig. 9).
Small short 0.1-mm-diameter AV shunts. They arose as branches from a longitudinal network along the brachial artery, which was formed by its vasa vasorum. These shunts released “blobs” of lead oxide into the lumen of the brachial vein (Fig. 10).
Fig. 9.
Dissection showing two shunts (arrows) between the radial artery and one of its veins.
Fig. 10.
Dissection showing the vasa vasorum on the surface of the brachial artery (black arrows) and their shunts (red arrows) releasing globules of lead oxide into the accompanying vein (white arrows).
DISCUSSION
These findings confirm our previous radiological studies, which have suggested the presence of sizeable AV shunts of 0.1–0.5 mm in the upper limb7 of sizes similar to those documented in the heart and abdominal viscera.2 Their size and site have been identified by dissection as well as x-ray (Fig. 11) and confirm with other studies where we have revealed their location elsewhere in the human face3 (Fig. 2) and leg6 (Fig. 5) as well as in the skin of the giraffe4 (Fig. 3). These shunts highlight the third anatomical pathway for arterial flow in a flap. The other two are those that have either reduced caliber “choke” anastomoses linking perforators along the flap or true anastomoses without reduction in caliber. Their discovery, location, potential function, and clinical significance need to be considered.
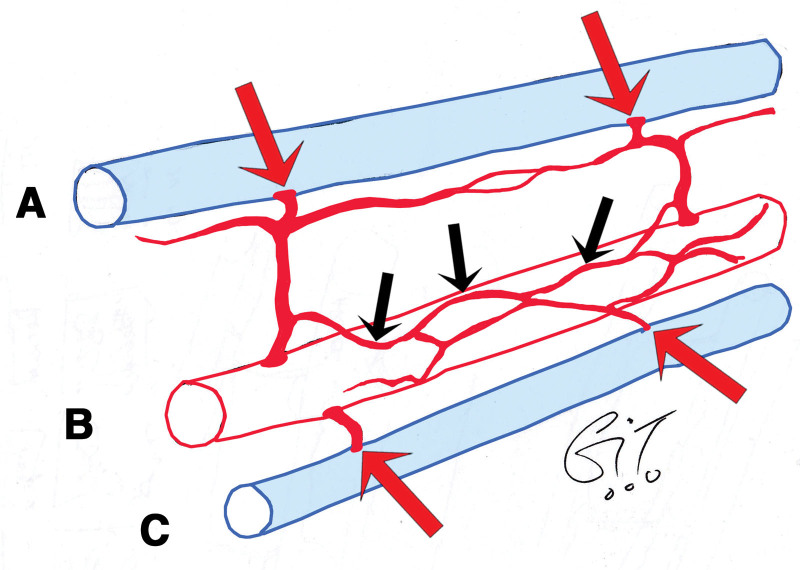
Fig. 11.
Summary diagram showing AV shunts (red arrows) between (A) subcutaneous veins and arterial perforators and between (B) axial arteries and their (C) accompanying veins, either directly or via their vas vasorum (black arrows).
It seems that the main purpose of these AV shunts is to provide a short circuit to maintain the body’s homeostasis, by responding to sudden challenges that may impact the physical or chemical equilibrium of the vascular system. This may be one of the reasons why the arteries and veins have developed anatomically in juxtaposition. We suggest the following functions of AV shunts.
Temperature Regulation
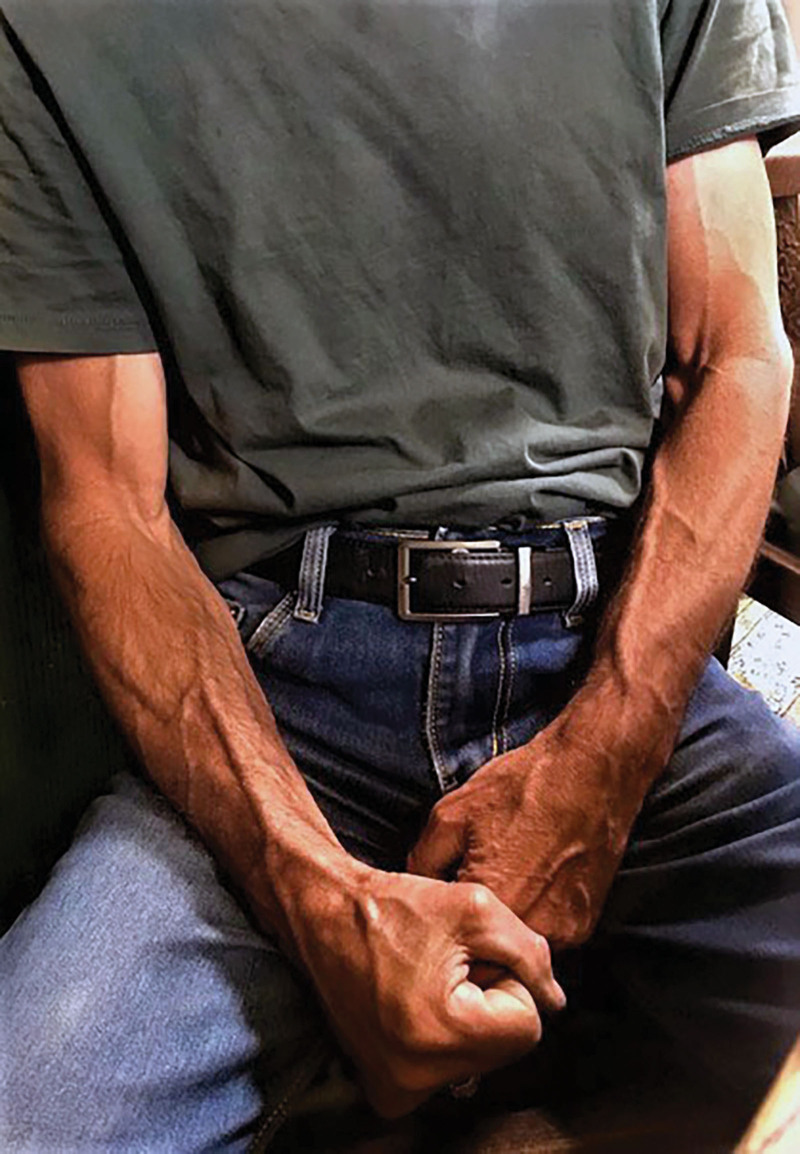
Already documented in the fingertips as glomus bodies,1,2 these tiny innervated shunts control temperature between 26°C and 36°C. However, temperatures can sometimes reach as high as 40°C with severe exercise.1 In such cases, these large AV shunts could short circuit the capillary bed and divert overheated blood into the large, engorged subcutaneous veins, typical of the athlete or hard manual worker (Fig. 12).
Fig. 12.
Distended veins in the upper limb following heavy work.
Blood Pressure Flow Control
It has been suggested that AV shunts may be involved in any sudden increase of blood pressure, functioning as a “pressure release” valve in response to a variety of neural, humoral, and mechanical controls.1,2 We observed that the number of veins filling with lead oxide increased with pressure (Fig. 6). Not only does this support the suggestion that AV shunts could be involved with pressure changes but that they unknowingly may be involved in a radial or ulnar free flap transfer from the forearm. In each case, a large artery provides few small branches to the skin, well below the potential field of supply of that artery. Raised usually under tourniquet, it has always been our practice, after its release, for a “coffee break” to allow the flap to “drink.” Unbeknownst to us, AV shunts may have been involved in the flap pedicle to adjust to the sudden high flow to the skin to maintain homeostasis and prevent arterial thrombosis. Not only could this occur at the donor site but may be involved in reanastomosis to recipient vessels where flow adjustments may be required because of vessel mismatch. This would suggest that wide separation of the pedicle artery and vein should be avoided.
Flap Necrosis
When necrosis occurs in a skin flap or frostbite, the remarkable feature in each is a clear line defining viable from deceased tissue. [See figure, Supplemental Digital Content 1, which displays the distinct necrosis line separating viable from necrotic tissue shown (left) in island flaps in the pig, isolated on a single perforator (arrows) and (right) frostbite of the fingers. http://links.lww.com/PRSGO/D477.] In all of our animal and human studies, this necrosis line was seen to occur anatomically in the anastomotic zone linking adjacent perforator angiosome territories, provided they were reduced caliber (choke) anastomoses.5
We have shown in the pig and rabbit that these choke anastomoses are functional.5 They are capable of spasm and control (permit or prevent) flow between angiosomes. It is conceivable that AV shunts could not only be involved with maintaining homeostasis in the flap but could provide the pathway to “short circuit” the flow to its tip and define the necrotic line of the flap. This is suggested in Figure 4, where overflow of lead oxide into the accompanying vein in the three territory flap is noted, not only at its base but close to the necrotic line between territories 2 and 3 that were connected by choke vessels.
Hence, a revisit to the delay phenomenon to study these shunts may be timely because previously our focus had been concentrated only on changes in the anastomotic vessels.13 It may have some application to the following procedures.
Venous Flow-through Flaps
Venous flow-through flaps are commonly used for reconstruction of defects in the hand,14,15 providing readily accessible, thin, pliable tissue for resurfacing of digits. However, their vascular basis is not well understood, deterring some surgeons from their use. Our identification of AV anastomoses between superficial veins and perforating arteries may provide a rationale for their vascularity, as high pressure in-flow from an artery into the venous network may be shunted into the arterial circulation. A second venous return anastomosis is recommended,15 supported by its use earlier in a successful face replant where a suitable artery could not be identified in the amputated part.16 We would recommend using tissue from close to the distal forearm for these flaps, preferably incorporating one of the large superficial veins (cephalic or basilic) as these seem to be the richest sites of AV shunts.
AV Flaps
A pedicled or free AV “railway” has been dissected, with a cuff of surrounding connective tissue, sometimes including a small sleeve of muscle to prefabricate or neovascularize a flap.17,18 With the distal end of this AV flap ligated, it has been transposed either locally into the proposed donor area or detached as a free flap with reanastomoses at a distant site. In this situation, the presence of AV shunt bypass may be critical to maintain homeostasis of the AV flap and prevent arterial thrombosis, especially with a large feeding artery and the runoff of a relatively small capillary bed. In each case, the AV pedicle is placed in a subcutaneous pocket of the desired tissue. Sometimes sandwiched over a tissue expander, this has the advantage of a larger, thinner flap, easier closure of the donor site and a delay effect on the vessels in the overlying skin.19 After a period of at least 8 weeks, the prefabricated flap with its implanted AV pedicle is ready for transfer, either locally or as a free flap to a distant site. Vascular reanastomosis occurs spontaneously either between capillary loops that sprout from the vasa vasora of the vascular pedicle and the subdermal plexus of the overlying skin or by inosculation with the axial vessels of the recipient bed or a combination of both (Fig. 13). Donor sites for this procedure include the radial AV free flap.20
Fig. 13.
Angiogram of prefabricated rat abdominal wall flap showing (A) transposed femoral AV pedicle, (B) vessel proliferation connecting with (C) native epigastric artery perfusing the entire skin. Note the pedicle vein has filled as well as the artery compatible with AV shunting (small arrow).
The current study has several limitations. It is small and descriptive in nature, and its findings need to be confirmed in a large cadaveric study to determine the distribution of AV shunts in the upper limb, as well as elsewhere in the body. Nevertheless, it has identified the location of these large AV shunts. In vivo studies are required to reveal their function.
CONCLUSIONS
This preliminary study has revealed significant AV shunts up to 0.5 mm in diameter in the integument and deep tissues of the upper limb, much larger than those documented in the fingertips. Their potential involvement in sudden changes in temperature, blood pressure, free tissue transfer, and flap necrosis is discussed. Some of their functions are proven, whereas others may be speculative. However, “without speculation we may never discover fact.” (G. Ian Taylor, 2024).
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
The authors wish to acknowledge the Department of Anatomy and Physiology, The University of Melbourne, and the Department of Plastic and Reconstructive Surgery, The Royal Melbourne Hospital, for facilities and support to conduct the work. The authors also acknowledge the body donors and their families. Without their generosity, this work would not be possible.
Supplementary Material
Footnotes
Published online 6 September 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Walloe L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature (Austin, Tex.). 2016;3:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman JL. Normal arteriovenous anastomoses. Medicine (Baltim). 1963;42:247–267. [DOI] [PubMed] [Google Scholar]
- 3.Taylor GI, AO, Shoukath S, Gascoigne A, et al. The functional anatomy of the ophthalmic angiosome and its implications in blindness as a complication of cosmetic facial filler procedures. Plast Reconstr Surg. 2020;146:745–755. [DOI] [PubMed] [Google Scholar]
- 4.Taylor GI, Dodwell P, Gascoigne A, et al. Thermoregulation not just camouflage. The unique vasculature of giraffe patches: a cadaver injection study with clinical implications. Plast Reconstr Surg. 2023;152:669–680. [DOI] [PubMed] [Google Scholar]
- 5.Taylor GI, Corlett RJ, Ashton MW. The functional angiosome: clinical implications of the anatomical concept. Plast Reconstr Surg. 2017;140:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Gascoigne A, Taylor GI, Corlett RJ, et al. The relationship of superficial cutaneous nerves and interperforator connections in the leg: a cadaveric anatomical study. Plast Reconstr Surg. 2017;139:994e–1002e. [DOI] [PubMed] [Google Scholar]
- 7.Gascoigne AC, Taylor GI, Corlett RJ, et al. Increasing perfusion pressure does not alter the calibre of cutaneous perforating arteries and their anastomotic branches but reveals arteriovenous shunting in cadaveric studies. Plast Reconstr Surg Global Open. 2020;8:e2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang Y, Yang M, Liu C. An islanded rabbit auricular skin flap model of hyaluronic acid injections-induced embolism. Aesth Plast Surg. 2016;40:421. [DOI] [PubMed] [Google Scholar]
- 9.Schelke LW, Velthuis P, Kadouch J, et al. Early ultrasound for diagnosis and treatment of vascular adverse events with hyaluronic acid fillers. J Am Acad Dermatol. 2023;88:79–85. [DOI] [PubMed] [Google Scholar]
- 10.Schelke LW. Personal Demonstration. 2019, Victoria, Australia: University of Melbourne. [Google Scholar]
- 11.Taylor GI, Ham FJ. Vascularised nerve graft. Plast Reconstr Surg. 1976;57:413–426. [PubMed] [Google Scholar]
- 12.Rees MJW, Taylor GI. A simplified lead oxide cadaver injection technique. Plast Reconstr Surg. 1986;77:141–145. [DOI] [PubMed] [Google Scholar]
- 13.Dhar SC, Taylor GI. The delay phenomenon: the story unfolds. Plast Reconst Surg. 1999;104:2079–2091. [DOI] [PubMed] [Google Scholar]
- 14.Goldschlager R, Rozen WM, Ting JWC. The nomenclature of venous flow-through flaps: updated classification and review of the literature. Microsurg. 2012;32:497–501. [DOI] [PubMed] [Google Scholar]
- 15.Rozen WR, Ting WJC, Gilmour RF, et al. The arterialized saphenous venous flow-through flap with dual venous drainage. Microsurg. 2012;32:281–288. [DOI] [PubMed] [Google Scholar]
- 16.Morris SF, MacGill KA, Taylor GI. Scalp replantation by arterialised venous network flow through. BJPS. 1992;45:187–192. [DOI] [PubMed] [Google Scholar]
- 17.Falco NA, Pribaz JJ, Eriksson E. Vascularization of skin following implantation of an arteriovenous pedicle: implications in flap prefabrication. Microsurgery. 1992;13:249–254. [DOI] [PubMed] [Google Scholar]
- 18.Pribaz JJ, Fine NA. Prefabricated and prelaminated flaps in head and neck reconstruction. Clin Plast Surg. 2001;28:261–272. [PubMed] [Google Scholar]
- 19.Taylor GI, Corlett RJ, Caddy CM, et al. An anatomic review of the delay phenomenon: 11. Clinical applications. Plast Reconstr Surg. 1992;89:408–416, discussion 417–418. [PubMed] [Google Scholar]
- 20.Guo L, Pribaz JJ. Clincial flap prefabrication. Plast Reconstr Surg. 2009;124:e340–e350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.