Abstract
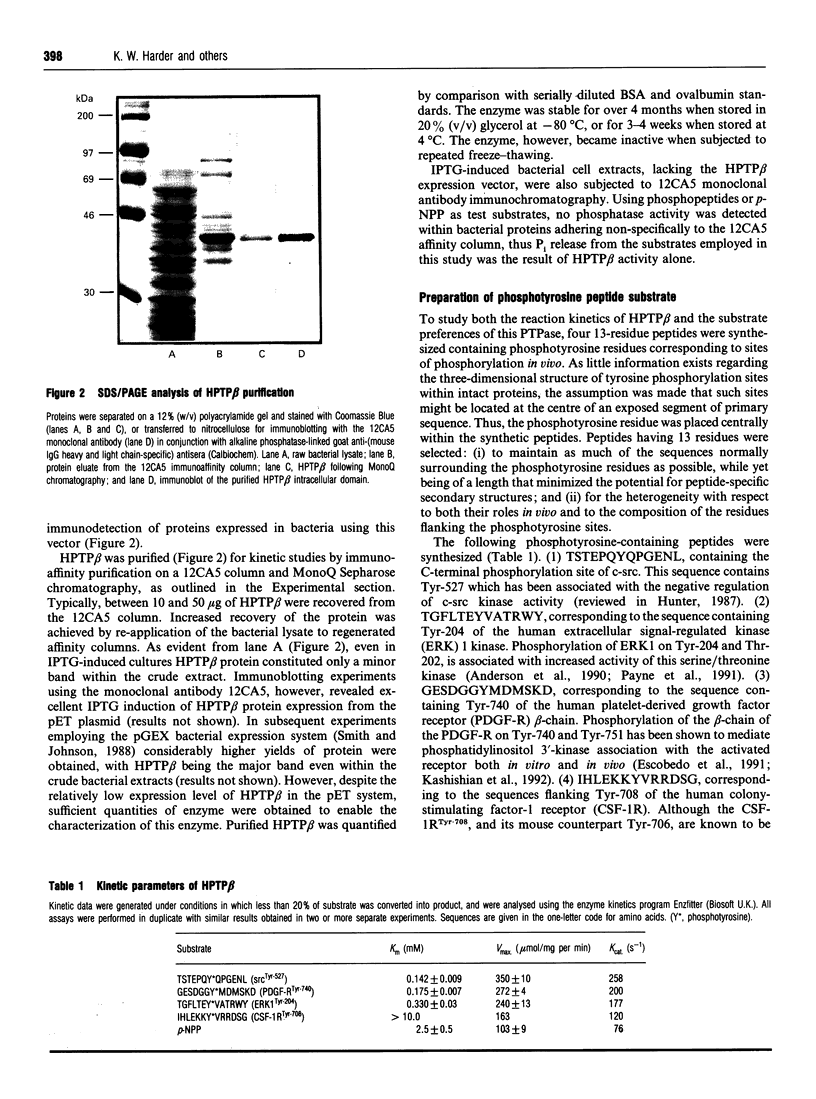
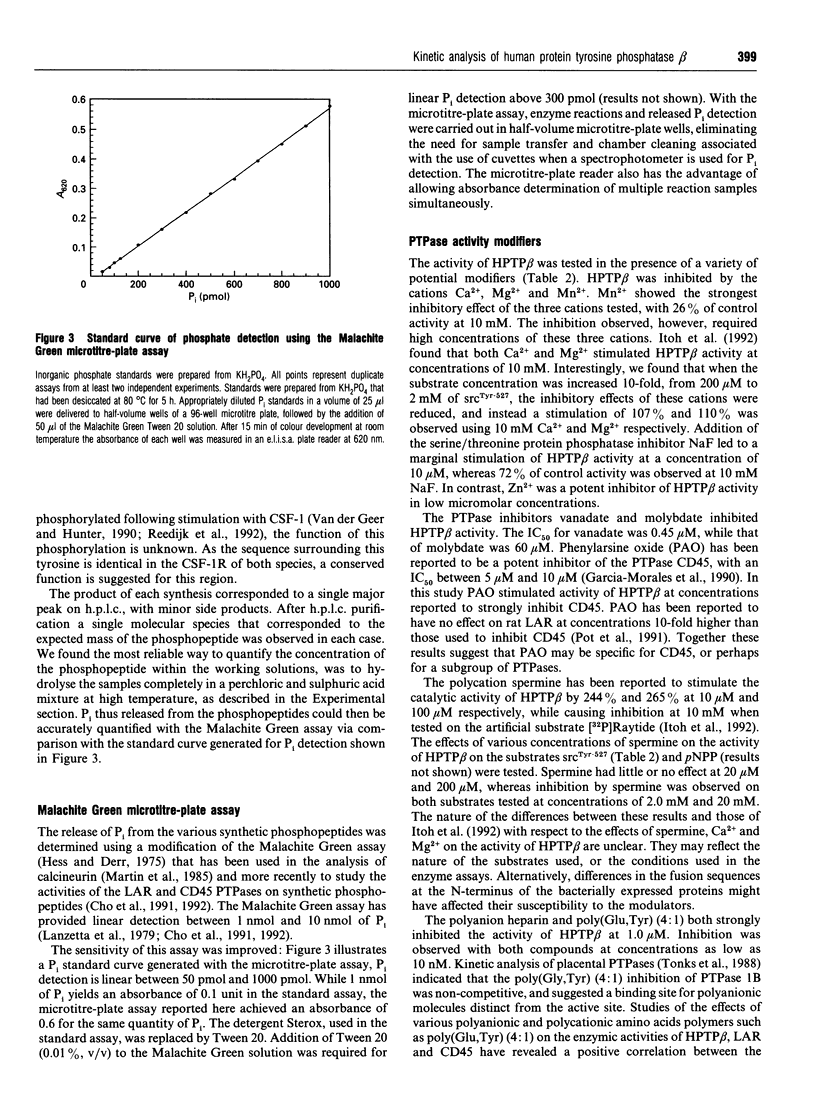
The intracellular domain of human protein tyrosine phosphatase beta (HPTP beta) (44 kDa) was expressed in bacteria, purified using epitope 'tagging' immunoaffinity chromatography, and characterized with respect to kinetic profile, substrate specificity and potential modulators of enzyme activity. A chromogenic assay based on the Malachite Green method was employed for the detection of inorganic phosphate (Pi) released from phosphopeptides by HPTP beta. This assay, modified so as to improve its sensitivity, was adapted to a 96-well microtitre plate format, and provided linear detection between 50 and 1000 pmol of Pi. The cytoplasmic domain of HPTP beta was strongly inhibited by vanadate, molybdate, heparin, poly(Glu, Tyr) (4:1) and zinc ions. In order to explore the substrate preferences of this PTPase, we generated 13-residue synthetic phosphotyrosine-containing peptides that corresponded to sites of physiological tyrosine phosphorylation. HPTP beta demonstrated kcat. values between 76 and 258 s-1 using four different phosphopeptides. The substrate preference of HPTP beta was in the order srcTyr-527 > PDGF-RTyr-740 > ERK1Tyr-204 >> CSF-1RTyr-708 with Km values ranging from 140 microM to greater than 10 mM. The variations in affinity were probably due to differences among the four phosphopeptides compared, particularly with respect to the character of the charged amino acids flanking the phosphotyrosine residue.
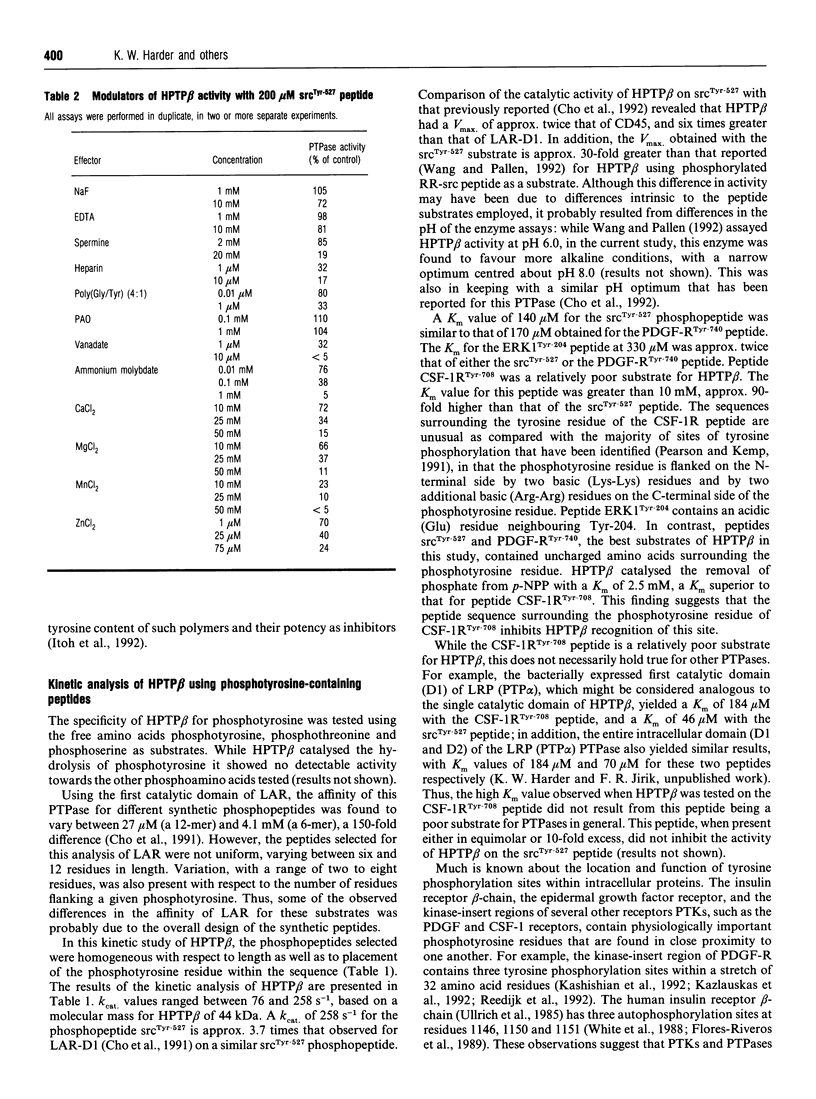
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Ramer S. E., Itoh M., Winkler D. G., Kitas E., Bannwarth W., Burn P., Saito H., Walsh C. T. Purification and characterization of a soluble catalytic fragment of the human transmembrane leukocyte antigen related (LAR) protein tyrosine phosphatase from an Escherichia coli expression system. Biochemistry. 1991 Jun 25;30(25):6210–6216. doi: 10.1021/bi00239a019. [DOI] [PubMed] [Google Scholar]
- Cho H., Ramer S. E., Itoh M., Kitas E., Bannwarth W., Burn P., Saito H., Walsh C. T. Catalytic domains of the LAR and CD45 protein tyrosine phosphatases from Escherichia coli expression systems: purification and characterization for specificity and mechanism. Biochemistry. 1992 Jan 14;31(1):133–138. doi: 10.1021/bi00116a019. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Moser B., Walz A., Baggiolini M., Scott G. J., Aebersold R. Chemical synthesis, purification, and characterization of two inflammatory proteins, neutrophil activating peptide 1 (interleukin-8) and neutrophil activating peptide. Biochemistry. 1991 Mar 26;30(12):3128–3135. doi: 10.1021/bi00226a021. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. CAMs and Igs: cell adhesion and the evolutionary origins of immunity. Immunol Rev. 1987 Dec;100:11–45. doi: 10.1111/j.1600-065x.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Kaplan D. R., Kavanaugh W. M., Turck C. W., Williams L. T. A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol Cell Biol. 1991 Feb;11(2):1125–1132. doi: 10.1128/mcb.11.2.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Flores-Riveros J. R., Sibley E., Kastelic T., Lane M. D. Substrate phosphorylation catalyzed by the insulin receptor tyrosine kinase. Kinetic correlation to autophosphorylation of specific sites in the beta subunit. J Biol Chem. 1989 Dec 25;264(36):21557–21572. [PubMed] [Google Scholar]
- Garcia-Morales P., Minami Y., Luong E., Klausner R. D., Samelson L. E. Tyrosine phosphorylation in T cells is regulated by phosphatase activity: studies with phenylarsine oxide. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9255–9259. doi: 10.1073/pnas.87.23.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Broyles S. S., Dixon J. E. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature. 1991 Mar 28;350(6316):359–362. doi: 10.1038/350359a0. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Deschenes R. J., Qiu H., Dixon J. E. Cloning and expression of a yeast protein tyrosine phosphatase. J Biol Chem. 1991 Jul 15;266(20):12964–12970. [PubMed] [Google Scholar]
- Harder K. W., Anderson L. L., Duncan A. M., Jirik F. R. The gene for receptor-like protein tyrosine phosphatase (PTPRB) is assigned to chromosome 12q15-->q21. Cytogenet Cell Genet. 1992;61(4):269–270. doi: 10.1159/000133419. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Chuang P. T., Rubin G. M. Cloning and characterization of a receptor-class phosphotyrosine phosphatase gene expressed on central nervous system axons in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11266–11270. doi: 10.1073/pnas.88.24.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Parniak M., Kaufman S. Determination of the phosphate content of purified proteins. Anal Biochem. 1982 Mar 1;120(2):360–364. doi: 10.1016/0003-2697(82)90358-x. [DOI] [PubMed] [Google Scholar]
- Hess H. H., Derr J. E. Assay of inorganic and organic phosphorus in the 0.1-5 nanomole range. Anal Biochem. 1975 Feb;63(2):607–613. doi: 10.1016/0003-2697(75)90388-7. [DOI] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Itoh M., Streuli M., Krueger N. X., Saito H. Purification and characterization of the catalytic domains of the human receptor-linked protein tyrosine phosphatases HPTP beta, leukocyte common antigen (LCA), and leukocyte common antigen-related molecule (LAR). J Biol Chem. 1992 Jun 15;267(17):12356–12363. [PubMed] [Google Scholar]
- Kashishian A., Kazlauskas A., Cooper J. A. Phosphorylation sites in the PDGF receptor with different specificities for binding GAP and PI3 kinase in vivo. EMBO J. 1992 Apr;11(4):1373–1382. doi: 10.1002/j.1460-2075.1992.tb05182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., Kashishian A., Cooper J. A., Valius M. GTPase-activating protein and phosphatidylinositol 3-kinase bind to distinct regions of the platelet-derived growth factor receptor beta subunit. Mol Cell Biol. 1992 Jun;12(6):2534–2544. doi: 10.1128/mcb.12.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Martin B., Pallen C. J., Wang J. H., Graves D. J. Use of fluorinated tyrosine phosphates to probe the substrate specificity of the low molecular weight phosphatase activity of calcineurin. J Biol Chem. 1985 Dec 5;260(28):14932–14937. [PubMed] [Google Scholar]
- Matthews R. J., Flores E., Thomas M. L. Protein tyrosine phosphatase domains from the protochordate Styela plicata. Immunogenetics. 1991;33(1):33–41. doi: 10.1007/BF00211693. [DOI] [PubMed] [Google Scholar]
- Ota I. M., Varshavsky A. A gene encoding a putative tyrosine phosphatase suppresses lethality of an N-end rule-dependent mutant. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2355–2359. doi: 10.1073/pnas.89.6.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottilie S., Chernoff J., Hannig G., Hoffman C. S., Erikson R. L. A fission-yeast gene encoding a protein with features of protein-tyrosine-phosphatases. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3455–3459. doi: 10.1073/pnas.88.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pot D. A., Dixon J. E. A thousand and two protein tyrosine phosphatases. Biochim Biophys Acta. 1992 Jul 22;1136(1):35–43. doi: 10.1016/0167-4889(92)90082-m. [DOI] [PubMed] [Google Scholar]
- Pot D. A., Woodford T. A., Remboutsika E., Haun R. S., Dixon J. E. Cloning, bacterial expression, purification, and characterization of the cytoplasmic domain of rat LAR, a receptor-like protein tyrosine phosphatase. J Biol Chem. 1991 Oct 15;266(29):19688–19696. [PubMed] [Google Scholar]
- Ramachandran C., Aebersold R., Tonks N. K., Pot D. A. Sequential dephosphorylation of a multiply phosphorylated insulin receptor peptide by protein tyrosine phosphatases. Biochemistry. 1992 May 5;31(17):4232–4238. doi: 10.1021/bi00132a012. [DOI] [PubMed] [Google Scholar]
- Reedijk M., Liu X., van der Geer P., Letwin K., Waterfield M. D., Hunter T., Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3'-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992 Apr;11(4):1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Thai T., Tang M., Saito H. Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. 1990 Aug;9(8):2399–2407. doi: 10.1002/j.1460-2075.1990.tb07415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tian S. S., Tsoulfas P., Zinn K. Three receptor-linked protein-tyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophila embryo. Cell. 1991 Nov 15;67(4):675–685. doi: 10.1016/0092-8674(91)90063-5. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wang Y., Pallen C. J. Expression and characterization of wild type, truncated, and mutant forms of the intracellular region of the receptor-like protein tyrosine phosphatase HPTP beta. J Biol Chem. 1992 Aug 15;267(23):16696–16702. [PubMed] [Google Scholar]
- Wang Y., Pallen C. J. The receptor-like protein tyrosine phosphatase HPTP alpha has two active catalytic domains with distinct substrate specificities. EMBO J. 1991 Nov;10(11):3231–3237. doi: 10.1002/j.1460-2075.1991.tb04886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- Yang X. H., Seow K. T., Bahri S. M., Oon S. H., Chia W. Two Drosophila receptor-like tyrosine phosphatase genes are expressed in a subset of developing axons and pioneer neurons in the embryonic CNS. Cell. 1991 Nov 15;67(4):661–673. doi: 10.1016/0092-8674(91)90062-4. [DOI] [PubMed] [Google Scholar]
- van der Geer P., Hunter T. Identification of tyrosine 706 in the kinase insert as the major colony-stimulating factor 1 (CSF-1)-stimulated autophosphorylation site in the CSF-1 receptor in a murine macrophage cell line. Mol Cell Biol. 1990 Jun;10(6):2991–3002. doi: 10.1128/mcb.10.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]