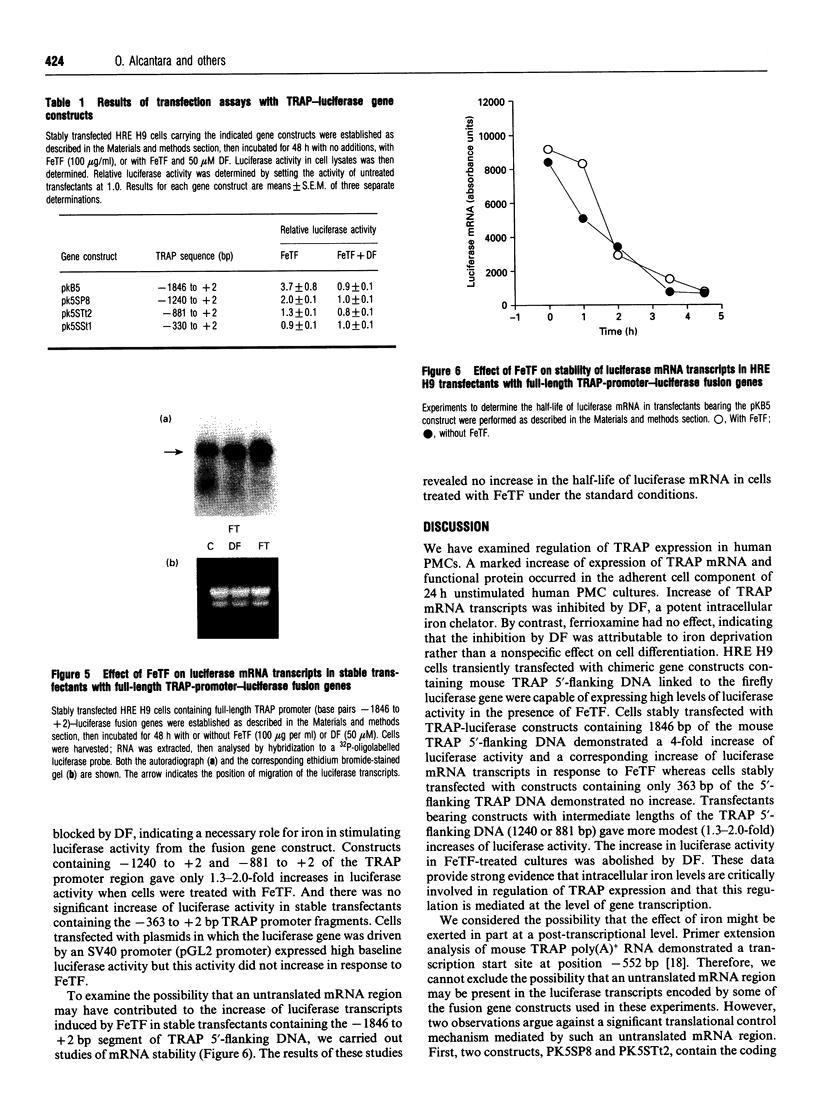
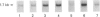Abstract
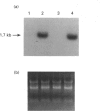
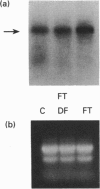
Tartrate-resistant acid phosphatase (TRAP) was first identified in cells from patients with hairy cell leukaemia. Subsequently, it has been found in other leukaemias, B-lymphoblastoid cell lines, osteoclasts and subsets of normal lymphocytes, macrophages, and granulocytes. Recent data indicate that TRAP and porcine uteroferrin, a placental iron-transport protein, represent a single gene product. However, the intracellular role of TRAP is unknown. We used a full-length human placental TRAP cDNA probe to examine TRAP expression in human peripheral mononuclear cells (PMCs). TRAP mRNA increased 50-75-fold after 24 h in unstimulated PMC cultures. Cell-fractionation experiments indicated that monocytes were the main cell population accounting for increased TRAP mRNA transcripts, and this was confirmed by histochemical staining for TRAP enzyme activity. Because expression of other iron-binding and -transport proteins is controlled by iron availability, we examined the role of iron in regulating TRAP expression. Increase of TRAP mRNA transcripts in PMCs was inhibited by 50 microM desferrioxamine, a potent iron chelator. The 5' flanking region of the TRAP gene was cloned from a mouse genomic library. In preliminary transient transfection experiments, it was determined that the 5'-flanking region of the TRAP gene contained iron-responsive elements. Therefore, a series of stably transfected HRE H9 cell lines was developed bearing genetic constructs containing various segments of the murine TRAP 5' promoter region driving a luciferase reporter gene. Treatment of transfectants with 100 micrograms/ml iron-saturated human transferrin (FeTF) was performed to assess iron responsiveness of the constructs. Constructs containing a full-length TRAP promoter (comprising base pairs -1846 to +2) responded to FeTF with a 4-5-fold increase of luciferase activity whereas constructs containing only base pairs -363 to +2 of the TRAP promoter did not respond. Constructs containing 1240 or 881 bp of the TRAP promoter gave only a 1.5- to 2-fold increase of luciferase activity with FeTF. In all cases, increase of luciferase activity was blocked by desferrioxamine. Cells transfected with another luciferase construct driven by a simian virus 40 promoter did not show any increase of luciferase activity with FeTF. These data indicate that expression of TRAP is regulated by iron and that this regulation is exerted at the level of gene transcription. The transfection experiments also suggest that the region of the TRAP 5'-flanking sequence between base pairs -1846 and -1240 contains an iron regulatory element.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Smith A. Heme-hemopexin-mediated induction of metallothionein gene expression. J Biol Chem. 1992 Aug 15;267(23):16379–16384. [PubMed] [Google Scholar]
- Alcantara O., Denham C. A., Phillips J. L., Boldt D. H. Transcriptional regulation of transferrin receptor expression by cultured lymphoblastoid T cells treated with phorbol diesters. J Immunol. 1989 Mar 1;142(5):1719–1726. [PubMed] [Google Scholar]
- Alcantara O., Javors M., Boldt D. H. Induction of protein kinase C mRNA in cultured lymphoblastoid T cells by iron-transferrin but not by soluble iron. Blood. 1991 Mar 15;77(6):1290–1297. [PubMed] [Google Scholar]
- Boldt S., Skinner A. M., Kornfeld S. Studies of two subpopulations of human lymphocytes differing in responsiveness to concanavalin A. J Clin Invest. 1972 Dec;51(12):3225–3234. doi: 10.1172/JCI107149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia E. M., Profita V., Fiorucci G., Romeo G., Affabris E., Testa U., Hentze M. W., Battistini A. Modulation of ferritin H-chain expression in Friend erythroleukemia cells: transcriptional and translational regulation by hemin. Mol Cell Biol. 1992 Jul;12(7):3015–3022. doi: 10.1128/mcb.12.7.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Drexler H. G., Gaedicke G., Minowada J. Occurrence of particular isoenzymes in fresh and cultured leukemia-lymphoma cells. I. Tartrate-resistant acid phosphatase isoenzyme. Cancer. 1986 May 1;57(9):1776–1782. doi: 10.1002/1097-0142(19860501)57:9<1776::aid-cncr2820570911>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Fliss A. E., Michel F. J., Chen C. L., Hofig A., Bazer F. W., Chou J. Y., Simmen R. C. Regulation of the uteroferrin gene promoter in endometrial cells: interactions among estrogen, progesterone, and prolactin. Endocrinology. 1991 Aug;129(2):697–704. doi: 10.1210/endo-129-2-697. [DOI] [PubMed] [Google Scholar]
- Grimes R., Reddy S. V., Leach R. J., Scarcez T., Roodman G. D., Sakaguchi A. Y., Lalley P. A., Windle J. J. Assignment of the mouse tartrate-resistant acid phosphatase gene (Acp5) to chromosome 9. Genomics. 1993 Feb;15(2):421–422. doi: 10.1006/geno.1993.1079. [DOI] [PubMed] [Google Scholar]
- Ketcham C. M., Baumbach G. A., Bazer F. W., Roberts R. M. The type 5, acid phosphatase from spleen of humans with hairy cell leukemia. Purification, properties, immunological characterization, and comparison with porcine uteroferrin. J Biol Chem. 1985 May 10;260(9):5768–5776. [PubMed] [Google Scholar]
- Ketcham C. M., Roberts R. M., Simmen R. C., Nick H. S. Molecular cloning of the type 5, iron-containing, tartrate-resistant acid phosphatase from human placenta. J Biol Chem. 1989 Jan 5;264(1):557–563. [PubMed] [Google Scholar]
- Kühn L. C. mRNA-protein interactions regulate critical pathways in cellular iron metabolism. Br J Haematol. 1991 Sep;79(1):1–5. doi: 10.1111/j.1365-2141.1991.tb07998.x. [DOI] [PubMed] [Google Scholar]
- Lam K. W., Li O., Li C. Y., Yam L. T. Biochemical properties of human prostatic acid phosphatase. Clin Chem. 1973 May;19(5):483–487. [PubMed] [Google Scholar]
- Ling P., Roberts R. M. Uteroferrin and intracellular tartrate-resistant acid phosphatases are the products of the same gene. J Biol Chem. 1993 Apr 5;268(10):6896–6902. [PubMed] [Google Scholar]
- Lord D. K., Cross N. C., Bevilacqua M. A., Rider S. H., Gorman P. A., Groves A. V., Moss D. W., Sheer D., Cox T. M. Type 5 acid phosphatase. Sequence, expression and chromosomal localization of a differentiation-associated protein of the human macrophage. Eur J Biochem. 1990 Apr 30;189(2):287–293. doi: 10.1111/j.1432-1033.1990.tb15488.x. [DOI] [PubMed] [Google Scholar]
- Rangarajan P. N., Padmanaban G. Regulation of cytochrome P-450b/e gene expression by a heme- and phenobarbitone-modulated transcription factor. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3963–3967. doi: 10.1073/pnas.86.11.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S. V., Scarcez T., Windle J. J., Leach R. J., Hundley J. E., Chirgwin J. M., Chou J. Y., Roodman G. D. Cloning and characterization of the 5'-flanking region of the mouse tartrate-resistant acid phosphatase gene. J Bone Miner Res. 1993 Oct;8(10):1263–1270. doi: 10.1002/jbmr.5650081015. [DOI] [PubMed] [Google Scholar]
- Roberts R. M., Raub T. J., Bazer F. W. Role of uteroferrin in transplacental iron transport in the pig. Fed Proc. 1986 Sep;45(10):2513–2518. [PubMed] [Google Scholar]
- Robinson D. B., Glew R. H. A tartrate-resistant acid phosphatase from Gaucher spleen. Purification and properties. J Biol Chem. 1980 Jun 25;255(12):5864–5870. [PubMed] [Google Scholar]
- Simmen R. C., Srinivas V., Roberts R. M. cDNA sequence, gene organization, and progesterone induction of mRNA for uteroferrin, a porcine uterine iron transport protein. DNA. 1989 Oct;8(8):543–554. doi: 10.1089/dna.1989.8.543. [DOI] [PubMed] [Google Scholar]
- Srivastava G., Borthwick I. A., Maguire D. J., Elferink C. J., Bawden M. J., Mercer J. F., May B. K. Regulation of 5-aminolevulinate synthase mRNA in different rat tissues. J Biol Chem. 1988 Apr 15;263(11):5202–5209. [PubMed] [Google Scholar]
- Theil E. C. The ferritin family of iron storage proteins. Adv Enzymol Relat Areas Mol Biol. 1990;63:421–449. doi: 10.1002/9780470123096.ch7. [DOI] [PubMed] [Google Scholar]
- Troy K., Cuttner J., Reilly M., Grabowski G., Desnick R. Tartrate-resistant acid phosphatase staining of monocytes in Gaucher disease. Am J Hematol. 1985 Jul;19(3):237–244. doi: 10.1002/ajh.2830190305. [DOI] [PubMed] [Google Scholar]
- White K., Munro H. N. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988 Jun 25;263(18):8938–8942. [PubMed] [Google Scholar]
- Wu Y. J., Noguchi C. T. Activation of globin gene expression by cDNAs from induced K562 cells. Evidence for involvement of ferritin in globin gene expression. J Biol Chem. 1991 Sep 15;266(26):17566–17572. [PubMed] [Google Scholar]
- Yam L. T. Clinical significance of the human acid phosphatases: a review. Am J Med. 1974 May;56(5):604–616. doi: 10.1016/0002-9343(74)90630-5. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Lam K. W. Tartrate-resistant acid phosphatase isoenzyme in the reticulum cells of leukemic reticuloendotheliosis. N Engl J Med. 1971 Feb 18;284(7):357–360. doi: 10.1056/NEJM197102182840704. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Moonga B., Moss D. W., MacIntyre I. Inhibition of osteoclastic acid phosphatase abolishes bone resorption. Biochem Biophys Res Commun. 1989 Feb 28;159(1):68–71. doi: 10.1016/0006-291x(89)92405-4. [DOI] [PubMed] [Google Scholar]