Abstract
Introduction
Some studies suggest that the monovalent mRNA-1273 vaccine is more effective than BNT162b2 in producing higher levels of antibodies. However, limited data are available, and the methods used are not directly comparable.
Material and methods
Blood samples were obtained before the booster (third dose) and after 14, 90, and 180 days in two similar cohorts who received the original BNT162b2 or mRNA-1273 vaccine designed to target wild type SARS-CoV-2. The aim of our study is to compare their effectiveness by assessing the levels of binding and neutralizing antibodies specifically against each of the BA.1 variant, BA.5 variant, and the XBB.1.5 subvariant.
Results
Once the peak was reached after two weeks, a drastic decline in binding and neutralizing antibodies was observed up to 6 months after the homologous booster administration. The humoral response was however more sustained with the mRNA-1273 booster, with half-lives of 167, 55, and 48 days for binding, BA.1, and BA.5 neutralizing antibodies compared to 144, 30, and 29 days for the BNT162b2 booster, respectively. Compared to the BA.1 variant, the neutralizing capacity was significantly decreased at 6 months with the BA.5 variant (fold-decrease: 1.67 to 3.20) and the XBB.1.5. subvariant (fold-decrease: 2.86 to 5.48).
Conclusion
Although the decrease in the humoral response was observed with both mRNA vaccines over time, a more sustained response was observed with the mRNA-1273 vaccine. Moreover, the emergence of Omicron-based variants causes a reduced neutralizing capacity, notably with the XBB.1.5. subvariant. The administration of subsequent boosters would therefore be needed to restore a sufficiently high neutralizing response.
Keywords: SARS-CoV-2, Vaccine booster, Humoral response, BNT162b2, mRNA-1273, Omicron
Highlights
-
•
Comparative analysis of immunogenicity profiles between mRNA-1273 and BNT162b2 vaccines.
-
•
mRNA-1273 shows prolonged humoral response compared to BNT162b2.
-
•
Subsequent boosters are crucial for restoring effective neutralization, particularly against XBB.1.5.
1. Introduction
The messenger RNA (mRNA) vaccines developed by Moderna (mRNA-1273) and Pfizer-BioNTech (BNT162b2) were developed in record time to respond to a global health emergency in an unprecedented way. Unparalleled in terms of accelerating technological innovation and mobilizing all nations to fight the COVID-19 pandemic which was not limited to health but affected all aspects of our society [1]. Of note, the establishment of the technology transfer center for mRNA vaccines by the World Health Organization (WHO) in 2021 was a crucial step in global health efforts, which enabled manufacturers in low- and middle-income countries to produce their own vaccines [2]. The deployment of vaccination clearly represented one of the major challenges in controlling the pandemic and its consequences. More than 2 years after the arrival of the first vaccines, on March 10, 2023, the number of the vaccine doses administered worldwide had reached 13,338,833,198 [3]. Among the different vaccines available, doses of BNT162b2 were the most distributed within the European Union/European Economic Area (EU/EEA) with 683,103,370 doses (47.1 %) over a total number of 1,449,042,265 and those of mRNA-1273 represented 13.5 % (n = 195,676,132). Interestingly, the vast majority of the population received a monovalent formula. The doses of the bivalent types distributed only reached 4.9 % (n = 71,078,400) for the BNT162b2 and 2.1 % (n = 30,004,771) for the mRNA-1273 [4].
The vaccine efficacy of BNT162b2 and mRNA-1273 was nearly equivalent in phase III trials, respectively 95 % [5] and 94.5 % [6], however this effectiveness and equivalence was questioned [7].The emergence of new variants has shown a progressive loss of efficacy of these vaccines and the need to adapt their formulation with the circulating variants [8,9]. According to the ECDC, for the last fortnight of October, Among the 18 countries reporting at least 10 results from SARS-CoV-2 sequencing or genotyping for weeks 40–41 (2 October to 15 October 2023), the estimated distribution (median and interquartile range (IQR) of country proportions) of variants of concern (VOC) or of interest (VOI) was 65.5 % (56.2–70.1 %) for XBB.1.5+F456L, 25.8 % (21.4–31.8 %) for XBB.1.5, 2.4 % (0.9–4.8 %) for BA.2 (most of which is due to BA.2.86) and 2.0 % (0.8–3.1 %) for BA.2.75) [10].
Several independent studies suggest that the monovalent mRNA-1273 vaccine is slightly more effective than BNT162b2 in producing higher levels of antibodies and providing longer protection against infection and hospitalization. However, limited data are available, and the methods used are not directly comparable [[11], [12], [13], [14], [15]].
Moreover, the correlation between levels of binding antibodies generated after vaccination and viral neutralization is questioned [16]. The difficulty lies in the inevitable appearance of new mutations, which make the virus evolution uncertain and require adaptation of biological tests to assess the immunogenicity of vaccines in real time. The routine serological tests currently available are based on a single or combined detection of antibodies directed against the spike protein and the nucleocapsid and are not specific for a variant of the virus. At the dawn of the new vaccination campaign, it is important to ensure continuous monitoring of the effectiveness of available vaccines, but also to compare the durability of their responses to offer the best vaccination strategy to the population. From an immunogenicity point of view, the question arises to know which vaccine is the best.
The study aimed at providing data on seroneutralisation capacity following vaccination with either the BNT162b2 or the mRNA-1273. This study compares the humoral response against the BA.1, BA.5 and XBB.1.5 variants between 2 cohorts of comparable healthcare workers who received 3 doses exclusively of the same COVID-19 vaccine: either mRNA-1273 or BNT162b2.
2. Material and methods
2.1. Study design and participants
A total of 126 participants were included in this investigation. The subjects were initially enrolled in two separate clinical studies. Fifty-eight participants (46 % of the cohort) came from the CRO‐VAX-HCP study, a prospective and multicenter study that was created to evaluate the humoral response in healthcare workers aged 18 to 65 who received at least 3 doses of the BNT162b2 mRNA COVID‐19 vaccine (Comirnaty, Pfizer‐BioNTech) [17]. The study has been approved by a central ethical committee (CHU UCL Namur, Yvoir, Belgium; approval number: 2020‐006149‐21). Volunteers were given the first vaccine dose between 18 January and 17 February 2021. The second one was administered after 21 days. Afterwards, they received a homologous booster (third dose) between November 8, 2021 and 14 January 2022 [18]. Lithium heparin and serum separator tubes were used to collect the blood (BD Vacutainer, Becton Dickinson, New Jersey, USA).
The 68 other participants (54 % of the cohort) were initially enrolled in another prospective and interventional study that was designed to assess the humoral response in a population of healthcare workers from 18 years of age having received two doses of the mRNA-1273 COVID‐19 vaccine (Spikevax, Moderna). The study has been approved by the Ethical Committee of the HIS-IZZ (ethical agreement number: CEHIS/2021-007). Volunteers were given the first vaccine dose between 18 January and 6 February 2021. The second one was administered after 28 days. Afterwards, they received a homologous booster between 6 October 2021 and 28 December 2021 [18]. Serum collection tubes were used to collect the blood (BD Vacutainer SST II advance, Becton Dickinson, New Jersey, USA).
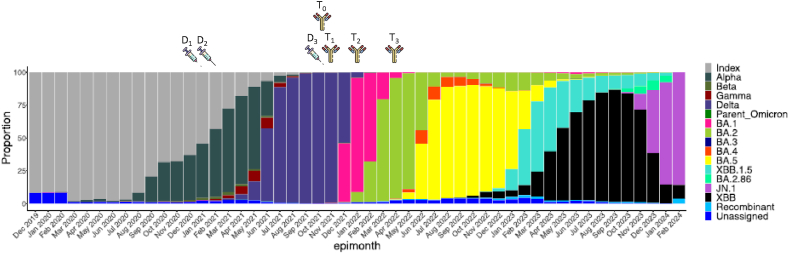
According to the design of the two initial trials, all participants were collected (1) within 2 days before the homologous booster administration (T0), (2) after 14 days (T1), (3) after 90 days (T2), and (4) after 180 days or 6 months (T3). Samplings performed earlier or later compared to the expected blood collection times were allowed with a maximal allowed variation of maximum 10 % (e.g. 180 days = 18 days). Participants who missed one sampling were not excluded from the study (Fig. 1). Participants with positive antibodies against the SARS‐CoV‐2 nucleocapsid (NCP) antigen before the injection of the homologous booster were considered seropositive and therefore had history of SARS‐CoV‐2 infection while those with negative antibodies against NCP classified as seronegative (i.e., COVID-19 naïve).
Fig. 1.
Comparison of vaccine administration and immune response timelines with the global SARS-CoV-2 variants distribution.
2.2. Analytical procedures
A pseudovirus‐neutralization test was used to assess the neutralization potency of vaccines‐elicited antibodies against the Omicron BA.1, BA.5, and XBB.1.5 (sub)variants. Pseudoviruses were obtained from eEnzyme (Maryland, USA). SARS‐CoV‐2 Pseudoviral Particles are replication‐deficient Maloneymurine leukemia virus (MLV or MuLV) pseudotyped with the SARS‐CoV‐2 spike protein that carry the BA.1, BA.5, XBB.1.5 Omicron genotypes. They contain the open reading frame for firefly luciferase used as a reporter. In short, HEK293T hACE2 cells were seeded in a white 384‐well cell culture plate at the density of 8500 cells/well. The sera used from participants were heat‐inactivated in a water bath (54 °C, 30 min) and then serially diluted in a specific culture medium (Dulbecco's modified Eagle medium supplemented with 10 % of fetal bovine serum). Afterwards, samples were mixed with pseudovirus (1:4 ratio) and incubated (37 °C, 2 h). This mix was added to the cells and then incubated (37 °C, 48 h). The reading was performed after the addition of a reagent to measure the luciferase activity which was proportional to the number of cells infected by the pseudovirus. Raw data were obtained in relative luminescence units (RLU) and were normalized against the positive control (i.e., cells incubated with pseudovirus in the absence of any serum). The antibody titer referred to the serum dilution at which 50 % of the infectivity is inhibited (IC50) as determined by a nonlinear sigmoid regression model obtained by serial dilution of the sample in the same testing condition. A sample with a titer of less than 1:20 is considered negative [19,20].
The neutralizing antibodies against the XBB.1.5 Omicron subvariant was only measured at 6 months in a subset of 20 participants randomly selected among those who did not develop a breakthrough (BK) infection during the study. BK cases were defined as participants with a positive Reverse Transcriptase – quantitative Polymerase Chain Reaction (RT–qPCR) along with the generation of anti-NCP antibodies in individuals without a history of SARS-CoV-2 infection in the past or by an increase of anti-NCP antibodies in previously infected individuals. Among them, 6 were men and 14 were women.
At each time point, two different analytical methods were used for the measurement of binding antibodies. Firstly, total antibodies against the RBD of the S1 subunit were measured using the Elecsys Anti‐SARS‐CoV‐2 S assay (Roche Diagnostics, Basel, Switzerland, positivity cut‐off = 0.8 BAU/mL). The measuring range of 0.4–250 BAU/mL was automatically extended to 25,000 BAU/mL using a 1:100 dilution in case of a result >250 BAU/mL. Secondly, total antibodies against the NCP were measured using the Elecsys Anti‐SARS‐CoV‐2 assay ((Roche Diagnostics, positivity cut-off index = 0.165) [21].
2.3. Statistical analyses
Demographic data were presented using median and IQR while the humoral response was presented using mean and 95 % confidence intervals (95 % CI). A Fisher's exact test was performed to assess any significant difference between two proportions between the vaccine groups (i.e., for binary data including number of BK infection, number of previous infections, and sex). Antibody levels were compared using a mixed-effect analysis, with the Geisser-Greenhouse correction for adjusting for lack of sphericity. An unpaired t-test was used to assess the potential difference between antibody titers at each time point between mRNA vaccines. The difference between participants with a history of prior infection or not was evaluated with a Mann-Withney test.
The kinetic models for neutralizing and binding antibodies were performed using the following equation on non-log transformed data: (a × b)/[(a − b × basal response) × Exp(−Days since vaccination×c)] + [b × Exp(Days since vaccination×d)], where “a” stands for the maximal antibody response, “b” for the baseline response, “c” for the antibody production rate, and “d” for the antibody elimination rate. Half‐life (T1/2) were calculated based on the elimination rate of the model. The time to maximal concentration (Tmax) as well as the mean time required to cross the positivity threshold were also determined based on this model. Blood samples corresponding to a BK infection were excluded from the kinetics. Levels of binding and neutralizing antibodies were compared using a Pearson's correlation. Statistical analyses were performed using GraphPad Prism 9.5.1 (GraphPad Software, Massachusetts, USA) and JMP Pro 16.0.0 (JMP, version 16.0.0. SAS Institute Inc., North Carolina, USA). p < 0.05 was considered significant.
3. Results
3.1. Demographic data
A total of 126 participants were included in the study and followed up to 6 months after the homologous booster. Among the participants, 58 received the BNT162b2 booster and 68 the mRNA-1273 booster. The sex repartition in each group was comparable (69.0 % of females and 31.0 % of males in the BNT162b2 group and 72.1 % of females and 27.9 % of males in the mRNA-1273 group) (Fisher p = 0.76). Ages between the two vaccine groups were significantly different for females with a lower median age in the BNT162b2 group (45.5 versus 51.3 years, p = 0.04) but not for males (37.5 versus 48.5 years, p = 0.30). A total of 26 subjects (44.8 %) had a previous history of SARS-CoV-2 infection before the BNT162b2 booster administration. For the mRNA-1273 group, 35 subjects (51.5 %) contracted the virus before the homologous booster administration. Proportions were non-significantly different (Fisher p = 0.48). The median time between first and third vaccine dose was 287 days (IQR = 273–294 days) for the BNT162b2 vaccine and 288 days (IQR = 285–295 days) for the mRNA-1273 vaccine. In the BNT162b2 group, 23 participants (39.7 %) developed a BK infection after the homologous booster as evidenced by the new development or the rising of antibodies against the NCP. In the mRNA-1273 vaccine group, 23 participants (33.8 %) developed a BK infection. Proportions were non-significantly different (Fisher p = 0.58) (Table 1).
Table 1.
Demographic characteristics of participants.
| Homologous booster (third dose) | BNT162b2 | mRNA-1273 |
|---|---|---|
| Participants – no. (%) | 58 (46.0 %) | 68 (54.0 %) |
| Age (years) | ||
| Median | 44.5 | 51.2 |
| Inter-quartile range | 35.8–52.5 | 44.1–57.4 |
| Min–max | 23.0–63.0 | 26.5–68.9 |
| Sex – no. (%) | ||
| Female | 40 (69.0 %) | 49 (72.1 %) |
| Male | 18 (31.0 %) | 19 (27.9 %) |
| Previous infection history – no. (%) | 26 (44.8 %) | 35 (51.5 %) |
| Time of booster administration (date) | ||
| Median | 25/11/21 | 03/12/21 |
| Min–max | 08/11/21–14/01/22 | 06/10/21–28/12/21 |
| Time between first and third vaccine dose (days) | ||
| Median | 287 | 288 |
| Inter-quartile range | 273–294 | 285–295 |
| Min-max | 259–332 | 232–313 |
| Total number of blood samples obtained – no. (%) | 202 (48.1 %) | 218 (51.9 %) |
3.2. Neutralizing antibody response against BA.1 and BA.5
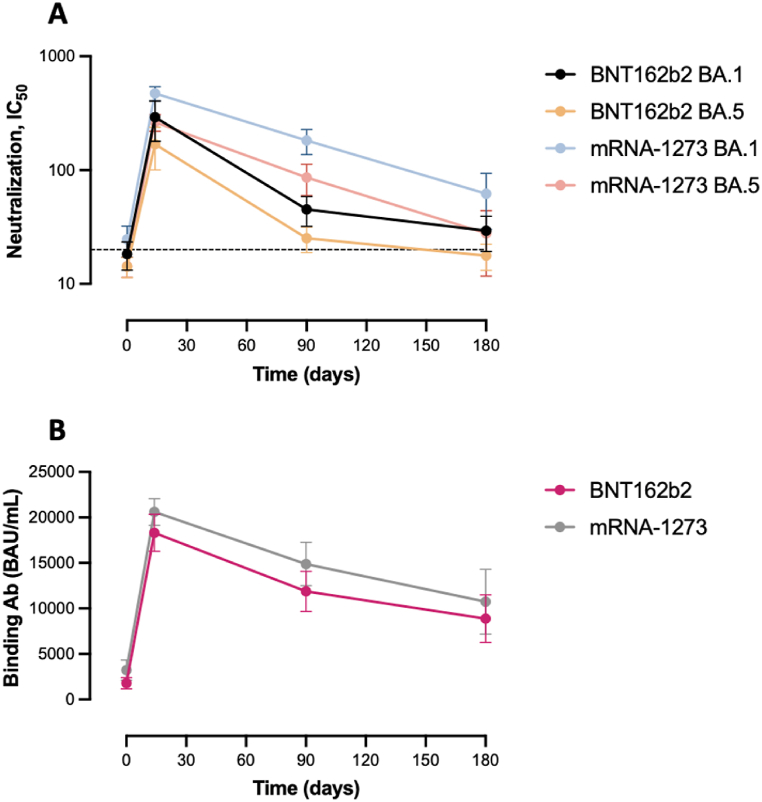
The maximal measured neutralizing capacity against the BA.1 and BA.5 variants was reached at day 14 after the BNT162b2 booster with a mean of 291 (95 % CI = 179–404) and of 169 (95 % CI = 100–237), representing a significant 15.9 and 11.9‐fold increase from baseline (i.e., 18.3; 95 % CI = 13.2–23.4, p < 0.0001 and 14.2; 95 % CI = 11.3–17.0, p = 0.0001), respectively. A substantial decrease was then observed up to 180 days with an observed mean of 29.3 for BA.1 (95 % CI = 19.3–39.3, p = 0.0049) and of 17.8 for BA.5 (95 % CI = 13.1–22.4, p = 0.0085), which represents a 9.9 and 9.5‐fold decrease. The neutralizing capacity at 180 days was non-significantly different compared to baseline (p = 0.1304 and 0.5503) (Fig. 2A, Table 2).
Fig. 2.
Kinetics of the humoral response (neutralizing (A) and binding (B) antibodies) after the homologous booster (BNT162b2 versus mRNA-1273). The dotted line represents the positivity cut‐off for neutralizing antibodies (dilution titer of 1:20). Mean and 95 % confidence intervals (95 % CI) are used to present the data.
Table 2.
Levels of binding and neutralizing antibodies (either against BA.1 and BA.5) just before the booster administration and after 14, 90, and 180 days. Levels of binding antibodies are expressed in BAU/mL and neutralizing antibodies in a dilution titer−1. NS, *, **, and *** refer to a p-value >0.05 (non-significant), 0.05 to 0.01, 0.01 to 0.001, and 0.001 to <0.0001.
|
Days |
Binding Ab (BAU/mL) |
BA.1 NAbs (dilution−1) |
BA.5 NAbs (dilution−1) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| mRNA-1273 | BNT162b2 | P-value | mRNA-1273 | BNT162b2 | P-value | mRNA-1273 | BNT162b2 | P-value | |
| 0 | 3234 (2118–4349) | 1792 (1170–2414) | 0.0244, * | 24.6 (17.1–32.2) | 18.3 (13.2–23.4) | 0.1650, NS | 14.3 (11.4–17.2) | 14.2 (11.3–17.0) | 0.9516, NS |
| 14 | 20,601 (19,147–22,054) | 18,318 (16,292–20,343) | 0.0621, NS | 473.0 (406.0–540.0) | 291.0 (179.0–404.0) | 0.0039, ** | 263.0 (219.0–307.0) | 169.0 (100.0–237.0) | 0.0166, * |
| 90 | 14,890 (12,506–17,275) | 11,880 (9677–14,082) | 0.0648, NS | 182.0 (137.0–228.0) | 45.2 (32.0–39.3) | <0.0001, *** | 86.2 (59.8–113.0) | 25.2 (18.9–31.5) | <0.0001, *** |
| 180 | 10,747 (7190–14,303) | 8887 (6268–11,505) | 0.3799, NS | 62.0 (30.1–93.9) | 29.3 (19.3–39.3) | 0.0354, * | 27.8 (11.7–43.9) | 17.8 (13.1–22.4) | 0.1696, NS |
A significant increase in the neutralizing capacity after the mRNA-1273 booster was also observed after 14 days with a 19.2 and 18.4‐fold increase from baseline (i.e., 24.6; 95 % CI = 17.1–32.2, p < 0.0001 and 14.3; 95 % CI = 11.4–17.2, p < 0.0001), for BA.1 and BA.5, respectively. After 6 months, a significant decline was observed with an observed mean of 62.0 for BA.1 (95 % CI = 30.1–93.9, p < 0.0001) and of 27.8 for BA.5 (95 % CI = 11.7–43.9, p < 0.0001), which represents a 7.6 and 9.5‐fold decrease. The neutralizing capacity at 180 days was still significantly higher compared to baseline (p = 0.0164 and 0.0078) (Fig. 2A, Table 2).
Based on the kinetic model, the estimated t1/2 for neutralizing antibodies against BA.1 and BA.5 after the BNT162b2 booster were 30 (95 % CI = 20–60) and 29 days (95 % CI = 19–63). A mean time of 129 (95 % CI = 76–174) and 104 days (95 % CI = 63–145) would be required to exceed the dilution titer threshold of 1:20. For mRNA-1273 booster, the t1/2 for neutralizing antibodies against BA.1 and BA.5 were 55 (95 % CI = 45–72) and 48 days (95 % CI = 37–66), longer compared to BNT162b2. A mean time of 268 (95 % CI = 206–317) and 192 days (95 % CI = 144–235) would be needed to exceed the dilution titer threshold of 1:20 (Table 3, Supplementary Fig. 2). Accordingly, the proportion of detectable neutralizing antibodies at each timepoint was systematically higher for the mRNA-1273 booster compared to the BNT162b2 booster (Supplementary Fig. 3).
Table 3.
Pharmacokinetic data derived from the models used to evaluate the kinetics of antibodies after the administration of either BNT162b2 or mRNA-1273 mRNA vaccines.
| Half-life (days) |
Cmax (IC50 or BAU/mL) |
Tmax (days) |
Time to negative (days) |
|
|---|---|---|---|---|
| BNT162b2 | Mean (95 % CI) | Mean (95 % CI) | Mean (95 % CI) | Mean (95 % CI) |
| Binding Ab | 144 (112–203) | 18,047 (16,470–19,625) | 13 (8–18) | 174 (142–218) |
| BA.1 NAbs | 30 (20–60) | 305 (240–369) | 10 (5–16) | 129 (76–174) |
|
BA.5 NAbs |
29 (19–63) |
175 (136–214) |
11 (4–17) |
104 (63–145) |
|
mRNA-1273 |
Mean (95 % CI) |
Mean (95 % CI) |
Mean (95 % CI) |
Mean (95 % CI) |
| Binding Ab | 167 (128–242) | 20,517 (19,001–22,031) | 15 (9–20) | 231 (184–298) |
| BA.1 NAbs | 55 (45–72) | 481 (437–526) | 11 (7–15) | 268 (206–317) |
| BA.5 NAbs | 48 (37–66) | 269 (249–299) | 11 (7–15) | 192 (144–235) |
3.3. Neutralizing antibody response against XBB.1.5
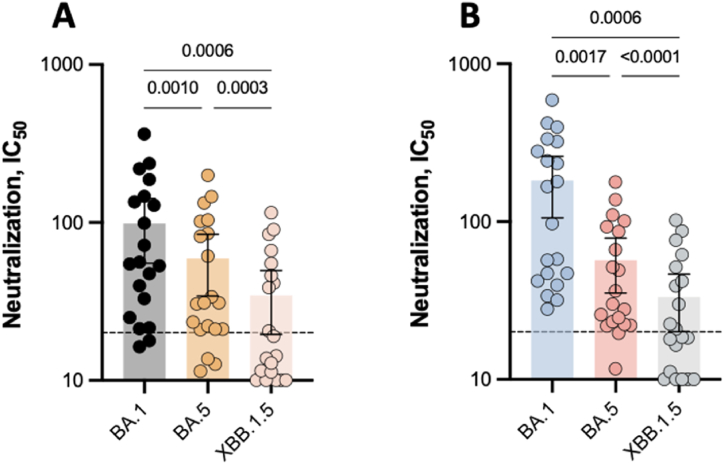
Six months after the BNT162b2 booster, the mean neutralizing capacity against BA.1, BA.5, and XBB.1.5 in a subset of 20 participants were 98.6 (95 % CI: 55.2–142), 59.2 (95 % CI: 34.2–84.2), and 34.5 (95 % CI: 19.5–49.6). The neutralization of BA.5 and XBB.1.5 were 1.67 and 2.86-fold lower compared to the BA.1 variant (p = 0.001 and 0.0006, respectively)). The proportion of detectable neutralizing antibodies was 90 %, 85 % and 45 %, respectively (Fig. 3A). The mean neutralizing capacity 6 months after the mRNA-1273 booster were 182 (95 % CI: 106–259), 57.0 (95 % CI: 35.3–78. 6), and 33.3 (95 % CI: 20.1–46.5) against BA.1, BA.5, and XBB.1.5. The neutralizing capacity was also reduced against BA.5 (3.20-fold decrease; p = 0.0017) and XBB.1.5 (5.48-fold decrease; p = 0.0006) as compared to the BA.1 variant. The proportion of detectable neutralizing antibodies was 100 %, 90 % and 50 %, respectively (Fig. 3B).
Fig. 3.
Comparison of the neutralizing capacity against the BA.1, BA.5, and XBB.1.5 Omicron variants in a population of 20 healthy-volunteers 6 months after having received the homologous booster. The dotted line represents the positivity cut‐off for neutralizing antibodies (dilution titer of 1:20). A. BNT162b2. B. mRNA-1273. Mean and 95 % confidence intervals 95 % CI are used to present the data.
3.4. Binding antibody response
The peak of the binding antibody response was identified after 14 days, both after the BNT162b2 and the mRNA-1273 booster (18,318 (95 % CI: 16,292–20,343) and 20,601 (95 % CI: 19,147–22,054)), representing a significant 10.2 and 6.4-fold increase from baseline (i.e., 1792 (95 % CI = 1170–2414; p < 0.0001) and 3234 (95 % CI = 2118–4349; p < 0.0001), respectively). As for neutralizing antibodies, a significant decline was observed up to 6 months after the homologous booster (2.1 and 1.9-fold decrease for the BNT162b2 and mRNA-1273). The level of binding antibodies after 6 months was superior to the one observed at baseline, for both homologous boosters (p < 0.0001 and p = 0.0002). Except at baseline, the binding antibody response was non-significantly different up to 6 months according to the type of homologous booster administered (Fig. 2B, Table 2).
According to the kinetic model, the estimated t1/2 for binding antibodies after the BNT162b2 booster was 144 days (95 % CI = 112–203). Based on the model, a mean time of 174 (95 % CI = 142–218) would be required to exceed the dilution titer threshold of 1:20. Considering the mRNA-1273 booster, the t1/2 was estimated at 167 days (95 % CI = 128–242) and a mean time of 231 days (95 % CI = 184–298) would be needed to exceed the dilution titer threshold of 1:20 (Table 3, Supplementary Fig. 2).
4. Discussion
Both homologous boosters induced a peak humoral response two weeks after administration. Afterwards, a significant decrease of binding and neutralizing antibodies was observed and, at 6 months after the booster administration, a significant proportion of subjects fell below the positivity threshold. Nevertheless, the long-term humoral response was more sustained with the mRNA-1273 booster, with half-lives of 167, 55, and 48 days for binding, BA.1, and BA.5 neutralizing antibodies, respectively, compared to 144, 30, and 29 days for the BNT162b2 booster. Our findings confirm the waning of immunity coinciding with the emergence of variants (Fig. 1), particularly the XBB.1.5 [22]. The removal of patients who had COVID-19 infection before the booster administration did not alter the results.
Other reports corroborate the fact that the mRNA-1273 might be more immunogen compared to the BNT162b2 vaccine. Steensels et al. identified a higher humoral response using a total binding IgG assay in individuals vaccinated with the mRNA-1273 compared to those receiving BNT162b2 [15]. Adjobimey et al. found that two doses of the mRNA-1273 vaccine induced a stronger humoral response compared to the BNT162b2 vaccine by measuring binding IgG and neutralizing antibodies using an ACE-2-RBD assay [11]. Additionally, studies conducted up to 8 months after two doses, identified a more sustained humoral response in individuals having received the mRNA-1273 vaccine [12,14,23,24]. Hvidt et al. reported higher titers of binding IgG and neutralizing antibodies associated with the mRNA-1273 vaccine and also observed a decline of the response with the emergence of variants (Alpha, Beta, Delta, and Omicron) [13]. Similar observations, using binding IgG as endpoint, were made in studies involving dialysis patients [25,26]. The higher amount of mRNA and the longer interval between the first two doses of mRNA-1273 vaccine may explain this increased humoral response [15].
Accordingly, the vaccine effectiveness (VE) against symptomatic infections was higher with the mRNA-1273 vaccine compared to the BNT162b2 [[27], [28], [29], [30]]. These observations were expected since a strong correlation exists between the VE against symptomatic infections and the level of the humoral response, especially regarding the neutralizing capacity [18,[31], [32], [33]]. The association between the level of the humoral response and the development of a severe form of the disease is less established. Although some studies observed an increased VE against general hospitalization, intensive care unit hospitalization, or death [27,28,34,35], other reports were not able to establish statistical significance [29,36]. While it seems clear that neutralizing antibodies indicate protection [33,37], more long-term large-scale studies that track vaccinated participants for a longer time are necessary to determine the minimum levels of protection against serious forms of the disease.
Cellular immunity, mediated by cytotoxic CD8+ and helper CD4+ T lymphocytes that are activated earlier, may be essential for controlling infection and avoiding severe disease. Indeed, it is more and more suggested that T cell response could play an essential role in the protection against more severe disease including hospitalization or death [31,38,39]. Contrarily to neutralizing antibodies, T cells are able to recognize more epitopes in the sequence of the spike protein [31]. T cell epitopes also appear to be more stable than antibody epitopes [40].
The T cell response is therefore more robust against variants with many mutations, with more than 80 % of epitopes conserved among T cells [31,38,39,41]. However, further studies are still needed to clearly establish a link between the T cell response and the risk of severe disease development. It may be important to develop additional vaccines that specifically target T lymphocytes, especially for long-term protection.
Our study has some limitations. First, we could not accurately determine the timing of symptomatic or asymptomatic infections among participants. The detection of the onset or increase of anti-NCP antibody levels only enabled the identification of individuals who have been exposed to the virus versus those who never developed antibodies after infection. Although there was no significant difference in the number of BK infections between the two groups of participants, it is important to note that the observed differences in humoral response between the two groups could also have been influenced by the immune response to natural infection. Secondly, our study mainly concentrates on the BA.1 and BA.5 variants. However, other variants have appeared, and other updated boosters have been given. More research is needed to assess the humoral response against the most prevalent variants.
Our results extend the ones presented in the literature that mainly focus their analyses after the two first doses of mRNA vaccines and have several implications. Since there is a clear decrease of the humoral response over time, other boosters would be needed to restore the "pool” of neutralizing antibodies in favor of a lower risk of a SARS-CoV-2 infection episode. Since the humoral response decreased with the emergence of new variants (i.e., Omicron-derived), the formulation of boosters has been recently adapted to face this thread. The amount of mRNA as well as the duration between doses should also be carefully evaluated and might be directly proportional to the strength of the humoral response. This should be evaluated in the light of an acceptable rate of adverse events [42]. These studies are needed to inform vaccination strategies and public health policies. Finally, the consequences of not adhering to the recommended interval between the administration of a subsequent booster should also be studied.
Besides the reformulation of current vaccine to face the emergence of variant that escape more and more immunity, manufacturers also need to rethink their commercial assays since they are for the most part still using the protein of the wild-type SARS-CoV-2 [18,38].
5. Conclusion
Although a decrease in the humoral response was observed with both mRNA vaccines over time, a more sustained response was noted with the mRNA-1273 vaccine. Moreover, the emergence of Omicron-based variants has led to a reduced neutralizing capacity, especially considering the XBB.1.5. subvariant. Consequently, the administration of subsequent boosters would therefore be needed to restore a sufficiently high neutralizing response. These boosters have recently been adapted to counteract the increase escape of immunity of highly mutated variants. The amount of mRNA available in the vaccine might also be an interesting characteristic to work on for the development of a sustained humoral response.
Data availability statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Julien Favresse: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Marie Tré-Hardy: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Constant Gillot: Writing – review & editing, Visualization, Validation, Formal analysis, Data curation, Conceptualization. Roberto Cupaiolo: Writing – review & editing. Alain Wilmet: Writing – review & editing. Ingrid Beukinga: Writing – review & editing. Laurent Blairon: Writing – review & editing. Jean-Louis Bayart: Writing – review & editing. Mélanie Closset: Writing – review & editing. Loris Wauthier: Writing – review & editing. Julien Cabo: Writing – review & editing. Clara David: Writing – review & editing. Marc Elsen: Writing – review & editing, Validation, Supervision. Jean-Michel Dogné: Writing – review & editing. Jonathan Douxfils: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36116.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Nations Unies Session extraordinaire de l'Assemblée générale consacrée à la pandémie de maladie à coronavirus (COVID-19), les 3 et 4 décembre. 2020. https://www.un.org/fr/pga/75/events 2020.
- 2.European Commission First technology transfer of mRNA vaccines: working together to build new solutions. 2022. https://www.consilium.europa.eu/en/european-council/president
- 3.Johns Hopkins University & Medicine . 2023. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [Google Scholar]
- 4.European Centre for Disease Prevention and Control European centre for disease prevention and control - COVID-19 vaccine tracker. 2023. https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19
- 5.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden L.R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klemis V., et al. Comparative immunogenicity and reactogenicity of heterologous ChAdOx1-nCoV-19-priming and BNT162b2 or mRNA-1273-boosting with homologous COVID-19 vaccine regimens. Nat. Commun. 2022;13:4710. doi: 10.1038/s41467-022-32321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan K., et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat. Commun. 2022;13:4686. doi: 10.1038/s41467-022-32396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tre-Hardy M., et al. Assessment 2 months after the administration of a 3rd dose mRNA: a new variant-adapted vaccine is expected. J. Infect. 2022;84:e31–e33. doi: 10.1016/j.jinf.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control Country overview report: week 42 2023. 2023. https://covid19-country-overviews
- 11.Adjobimey T., et al. Comparison of IgA, IgG, and neutralizing antibody responses following immunization with Moderna, BioNTech, AstraZeneca, sputnik-V, johnson and johnson, and sinopharm's COVID-19 vaccines. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.917905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnet B., et al. Comparative T and B immune responses of four different anti-COVID-19 vaccine strategies 6 months after vaccination. J. Infect. 2022;84:e45–e47. doi: 10.1016/j.jinf.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hvidt A.K., et al. Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines. Front. Med. 2022;9 doi: 10.3389/fmed.2022.994160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano L., et al. Assessment of humoral immune response to two mRNA SARS-CoV-2 vaccines (Moderna and Pfizer) in healthcare workers fully vaccinated with and without a history of previous infection. J. Appl. Microbiol. 2022;133:1969–1974. doi: 10.1111/jam.15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sariol C., et al. Function is more reliable than quantity to follow up the humoral response to the receptor-binding domain of SARS-CoV-2-spike protein after natural infection or COVID-19 vaccination. Viruses. 2021;13(1972) doi: 10.3390/v13101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favresse J., et al. Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2) Clin. Microbiol. Infect. 2021;27:1351 e1355–e1351 e1357. doi: 10.1016/j.cmi.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favresse J., et al. Vaccine-induced binding and neutralizing antibodies against Omicron 6 months after a homologous BNT162b2 booster. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douxfils J., Gillot C., Mullier F., Favresse J. Post-SARS-CoV-2 vaccination specific antibody decrease - thresholds for determining seroprevalence and seroneutralization differ. J. Infect. 2021;83:e4–e5. doi: 10.1016/j.jinf.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillot C., Favresse J., Maloteau V., Dogne J.M., Douxfils J. Identification of SARS-CoV-2 neutralizing antibody with pseudotyped virus-based test on HEK-293t hACE2 cells. Bio Protoc. 2022;12 doi: 10.21769/BioProtoc.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favresse J., et al. Clinical performance of the Elecsys electrochemiluminescent immunoassay for the detection of SARS-CoV-2 total antibodies. Clin. Chem. 2020;66:1104–1106. doi: 10.1093/clinchem/hvaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurhade C., et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nature medicine. 2023;29:344–347. doi: 10.1038/s41591-022-02162-x. [DOI] [PubMed] [Google Scholar]
- 23.Barbeau D.J., et al. Comparative analysis of human immune responses following SARS-CoV-2 vaccination with BNT162b2, mRNA-1273, or Ad26.COV2.S. NPJ Vaccines. 2022;7:77. doi: 10.1038/s41541-022-00504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korosec C.S., et al. Long-term durability of immune responses to the BNT162b2 and mRNA-1273 vaccines based on dosage, age and sex. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-25134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser R.A., Haller M.C., Apfalter P., Kerschner H., Cejka D. Comparison of BNT162b2 (Pfizer–BioNtech) and mRNA-1273 (Moderna) SARS-CoV-2 mRNA vaccine immunogenicity in dialysis patients. Kidney Int. 2021;100:697–698. doi: 10.1016/j.kint.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau K., et al. Differences in mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 vaccine immunogenicity among patients undergoing dialysis. Canadian Medical Association Journal. 2022;194:E297–E305. doi: 10.1503/cmaj.211881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickerman B.A., et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. Veterans. N. Engl. J. Med. 2022;386:105–115. doi: 10.1056/nejmoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannou G.N., Locke E.R., Green P.K., Berry K. Comparison of Moderna versus Pfizer-BioNTech COVID-19 vaccine outcomes: a target trial emulation study in the U.S. Veterans Affairs healthcare system. eClinicalMedicine. 2022;45 doi: 10.1016/j.eclinm.2022.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islam N., Sheils N.E., Jarvis M.S., Cohen K. Comparative effectiveness over time of the mRNA-1273 (Moderna) vaccine and the BNT162b2 (Pfizer-BioNTech) vaccine. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-30059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puranik A., et al. Comparative effectiveness of mRNA-1273 and BNT162b2 against symptomatic SARS-CoV-2 infection. Med. 2022;3:28–41.e28. doi: 10.1016/j.medj.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wherry E.J., Barouch D.H. T cell immunity to COVID-19 vaccines. Science. 2022;377:821–822. doi: 10.1126/science.add2897. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert P.B., et al. A covid-19 milestone attained - a correlate of protection for vaccines. N. Engl. J. Med. 2022;387:2203–2206. doi: 10.1056/NEJMp2211314. [DOI] [PubMed] [Google Scholar]
- 33.Khoury D.S., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 34.Self W.H., et al. Comparative effectiveness of Moderna, pfizer-BioNTech, and janssen (johnson & johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions — United States, march–august 2021. MMWR. Morbidity and Mortality Weekly Report. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajema K.L., et al. Comparative effectiveness and antibody responses to Moderna and pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five veterans affairs medical centers, United States, february 1–september 30, 2021. MMWR. Morbidity and Mortality Weekly Report. 2021;70:1700–1705. doi: 10.15585/mmwr.mm7049a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulme W.J., et al. Comparative effectiveness of BNT162b2 versus mRNA-1273 covid-19 vaccine boosting in England: matched cohort study in OpenSAFELY-TPP. Bmj. 2023 doi: 10.1136/bmj-2022-072808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau E.H.Y., et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021;12:63. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippi G., Adeli K., Plebani M. Commercial immunoassays for detection of anti-SARS-CoV-2 spike and RBD antibodies: urgent call for validation against new and highly mutated variants. Clin. Chem. Lab. Med. 2021 doi: 10.1515/cclm-2021-1287. [DOI] [PubMed] [Google Scholar]
- 39.Ledford H. Killer immune cells still recognize Omicron variant. Nature. 2022;601:307. doi: 10.1038/d41586-022-00063-0. [DOI] [PubMed] [Google Scholar]
- 40.Young A. T cells in SARS-CoV-2 infection and vaccination. Ther Adv Vaccines Immunother. 2022;10 doi: 10.1177/25151355221115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keeton R., et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.