Abstract
Background
People with low levels of vitamin D and its metabolites are at increased risk for osteoporotic fractures. We wished to ascertain levels of vitamin D in a representative sample of adult western Canadians, to help assess the level of risk. We evaluated the prevalence of vitamin D insufficiency, defined as 25-hydroxyvitamin D [25(OH)D] less than 40 nmol/L, and seasonal variations in 25(OH)D, parathyroid hormone and related biochemical indices in a community-dwelling population of healthy Canadians living in Calgary (lati- tude 51°07'N).
Methods
During calendar year 1999, we collected fasting overnight blood samples every 3 months from 60 men and 128 women (age range 27 to 89 years) who had volunteered to participate in another study. We used commercial radioimmunoassay kits to measure calciotrophic hormones and other biochemical indices. Regression models for longitudinal data were used to assess the effect of season and other potential predictors on individual parameters.
Results
For a total of 64 participants (34%), vitamin D insufficiency, defined as 25(OH)D less than 40 nmol/L, was recorded at least once out of the 4 sampling times. After adjustment for age, body mass index and holiday travel, we observed the anticipated rise in serum 25(OH)D from a mean of 57.3 (standard deviation [SD] 21.3) nmol/L in the winter to 62.9 (SD 28.8) nmol/L in spring (p = 0.001) and 71.6 (SD 23.6) nmol/L in summer (p < 0.001), with a subsequent decline to 52.9 (SD 17.2) nmol/L in the fall (p = 0.008). The anticipated inverse relation between 25(OH)D and parathyroid hormone was not consistently observed: after adjustment for age, sex, body mass index and serum calcium, serum levels of parathyroid hormone did decrease significantly, from 39.5 (SD 18.8) ng/L in winter to 36.3 (SD 17.8) ng/L in summer (p = 0.001), but they continued to decline to 34.5 (SD 17.3) ng/L in the fall (p < 0.001). There was no association between 25(OH)D and parathyroid hormone (p = 0.21).
Interpretation
We documented a high prevalence of vitamin D insufficiency, which warrants consideration of dietary vitamin D supplementation.
Vitamin D deficiency has long been recognized as a cause of rickets in children and osteomalacia in adults. More recent is the awareness of a preclinical phase of vitamin D deficiency, known as vitamin D insufficiency, which increases the risk of osteoporotic fractures.1,2,3 Low levels of vitamin D metabolites are associated with malabsorption of calcium, which results in bone loss.4,5 Vitamin D can be obtained through the diet or it is synthesized in the skin after exposure to the sun. However, because few foods provide a natural source of vitamin D6 and because fortification of foods with vitamin D is often unreliable,7,8 skin synthesis is thought to constitute the major source. People living in countries at higher latitudes are more prone to seasonal vitamin D insufficiency because wintertime sunlight does not promote conversion of the vitamin D precursor in the skin.9 Levels of vitamin D and its main circulating metabolite, 25-hydroxy vitamin D [25(OH)D], are under the predominant influence of solar ultraviolet B radiation (wavelength 290 to 315 nm). Webb and colleagues9 have shown that in Boston (latitude 42°N), sun irradiation is inadequate to generate previtamin D in vitro from November through February; in Edmonton (latitude 53°30́N), this period extends from October through March.
The vitamin D hormone system and parathyroid hormone are the principal regulators of serum concentrations of calcium and therefore influence skeletal calcium reserves. Because of the health risks associated with low levels of vitamin D, our objective was to determine patterns of seasonal variation in 25(OH)D, as well as the prevalence of vitamin D insufficiency, defined as 25(OH)D less than 40 nmol/L,10 in a population of healthy men and women living in western Canada. Although 1,25-dihydroxy vitamin D [1,25-(OH)2D] is the most potent form of vitamin D, 25(OH)D is the main circulating metabolite of vitamin D and is considered the correct functional indicator of vitamin D stores in humans.11,12 We also examined associations between 25(OH)D and serum concentrations of 1,25-(OH)2D, parathyroid hormone and other related biochemical markers.
Methods
Participants for this study were recruited from participants in the Canadian Multicentre Osteoporosis Study (CaMOS). Details of the design and methods for CaMOS have been described elsewhere.13 In brief, CaMOS involves a population-based, randomly selected group of approximately 9000 Canadians 25 years of age and older, distributed across 9 study centres. The number of participants at each centre reflects relative population size at that location, and age and sex distribution (approximately 30% men) is similar for all centres. Those invited to participate in the vitamin D study were randomly selected (by a computerized random selection process) from the Calgary cohort of CaMOS (n = 1065) in age and sex proportions corresponding to those of the main study. Of 463 people contacted by telephone, 204 subjects, ranging from 27 to 89 years of age, agreed to participate. All participants were healthy and ambulatory and were dwelling in the community. Exclusion criteria were vitamin D deficiency [serum 25(OH)D below 25 nmol/L] on prestudy screening or daily consumption of vitamin D supplements providing more than 200 IU daily. Participants were instructed to not exceed this intake for the duration of the study. The University of Calgary Conjoint Medical Research Ethics Board approved the study, and all participants provided informed written consent.
During calendar year 1999, each participant came to the centre for testing 4 times, at 3-month intervals (in February or March, May or June, August or September, and November or December). Blood samples were collected between 7:30 am and 10:30 am after an overnight fast. Height and weight were measured with a stadiometer and balance scale respectively after participants had removed their shoes. Body mass index was calculated as weight divided by the square of height (kilograms per square metre). At each visit, participants were interviewed to determine holiday travel of at least 1 day at a latitude below 40°N and vitamin D supplementation up to 200 IU/day. Dietary calcium intake, smoking status, alcohol intake, self-reported ethnicity and menopausal status (for women) were obtained from the CaMOS baseline questionnaires that had been administered 2 years previously. Dietary calcium intake was estimated by means of an abbreviated food frequency questionnaire administered by a skilled technician, who used food models to help participants estimate portion sizes. Serum levels of total calcium, inorganic phosphate, alkaline phosphatase and creatinine (to check for renal insufficiency) were measured with a Roche Hitachi autoanalyzer (Roche Diagnostics, Laval, Que.) on the same day. Blood samples for 25(OH)D, 1,25-(OH)2D, parathyroid hormone and osteocalcin were frozen at -80°C and analyzed in batches within the next month. Both 25(OH)D and 1,25-(OH)2D were measured in 2-step procedures with commercial immunoradiometric assay kits (DiaSorin Inc., Stillwater, Minn.). These assays involved a preliminary extraction and purification, followed by addition of antibody and tracer specific for the 2 metabolites of interest. The intra- and inter-assay coefficients of variation were 11.7% to 12.5% and 9.4% to 11.0% respectively for 25(OH)D and 10.5% to 13.1% and 9.2% to 10.8% respectively for 1,25-(OH)2D. Intact serum parathyroid hormone and osteocalcin were measured by a one-step commercial immunoradiometric assay (DiaSorin Inc.). The intra- and inter-assay coefficients of variation were 2.4% to 3.6% and 3.4% to 4.9% respectively for parathyroid hormone and 4.5% to 6.3% and 7.1% to 9.5% respectively for osteocalcin.
Data are presented as means (and standard deviation [SD]) unless otherwise noted. Log transformations of body mass index, 25(OH)D, 1,25-(OH)2D, parathyroid hormone and osteocalcin were used in the regression analysis to correct for right-skewed distributions, but the data are presented as means (and SD) for ease of interpretation. Regression models for longitudinal data14 were used to assess seasonal variations in biochemical indices and other potential predictors. Separate models were created for 25(OH)D, 1,25-(OH)2D, parathyroid hormone, total calcium, inorganic phosphate, alkaline phosphatase and osteocalcin. Interaction variables were created to assess possible effect modification between age and BMI, as well as all other covariates that changed with season (e.g., holiday travel or other biochemical markers). Nonsignificant predictors were removed from the final model. A p value less than 0.05 (2-sided test) was considered statistically significant.
Results
Of the 204 subjects recruited, 188 completed the study. Three women were excluded before the study began because their serum level of 25(OH)D was less than 25 nmol/L. An additional 13 participants dropped out during the study for various reasons: 3 women began taking vitamin D supplements greater than 200 IU/day, 4 women and 1 man could not fulfill the time commitment, 3 men and 1 woman did not show up on test days, and 1 woman died.
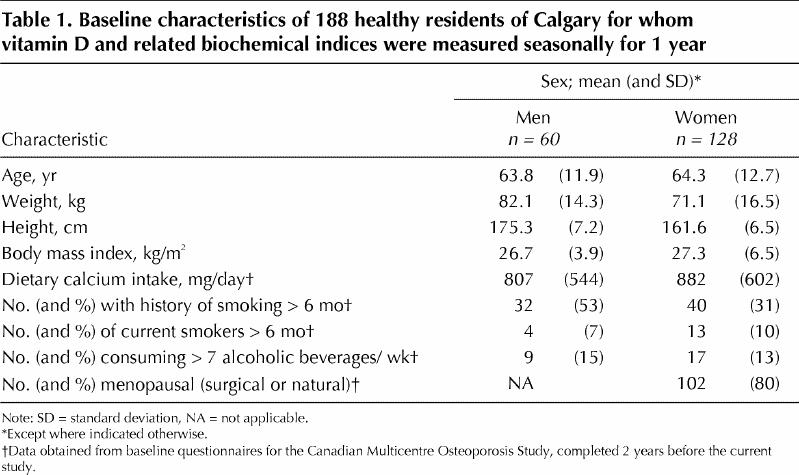
There were no significant differences between men and women in terms of age or BMI (p = 0.52) (Table 1). Of the 188 subjects who completed the study, 3 had identified themselves on entry to CaMOS as members of an ethnic minority; the other 185 subjects did not identify themselves as members of an ethnic minority.
Table 1
Seasonal changes in calciotrophic hormones and related biochemical markers are presented in Table 2; the changes in 25(OH)D, 1,25-(OH)2D and parathyroid hormone are presented graphically in Fig. 1. Other significant predictors in the seasonal regression model for each biochemical marker are summarized in Table 3.
Table 2
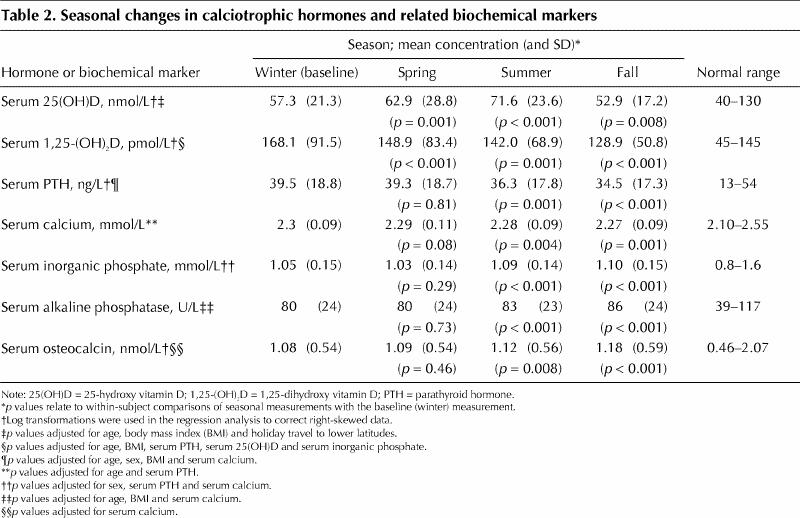
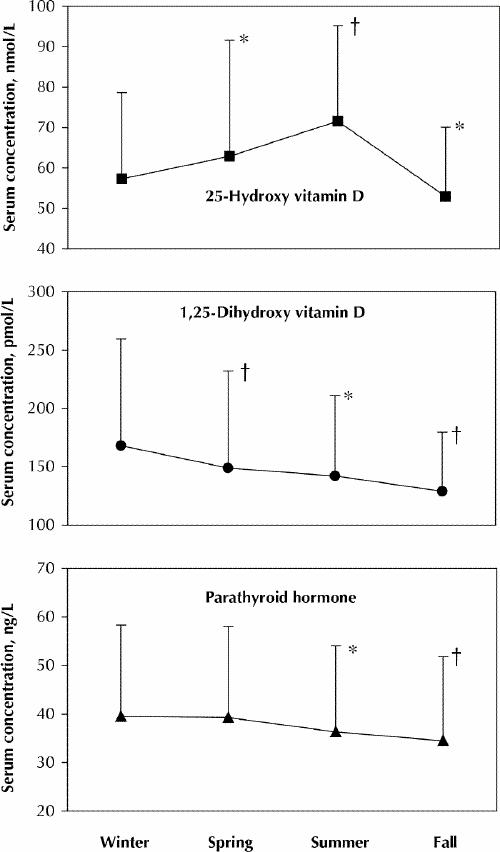
Fig. 1: Mean seasonal values for 25-hydroxy vitamin D, parathyroid hormone and 1,25-dihydroxy vitamin D. The vertical lines represent standard deviation of the means. The p values refer to within-subject comparisons of seasonal measurements with the baseline (winter) measurement (*p < 0.01; †p < 0.001) as determined by longitudinal regression models adjusted for other significant predictors (see Table 2).
Table 3
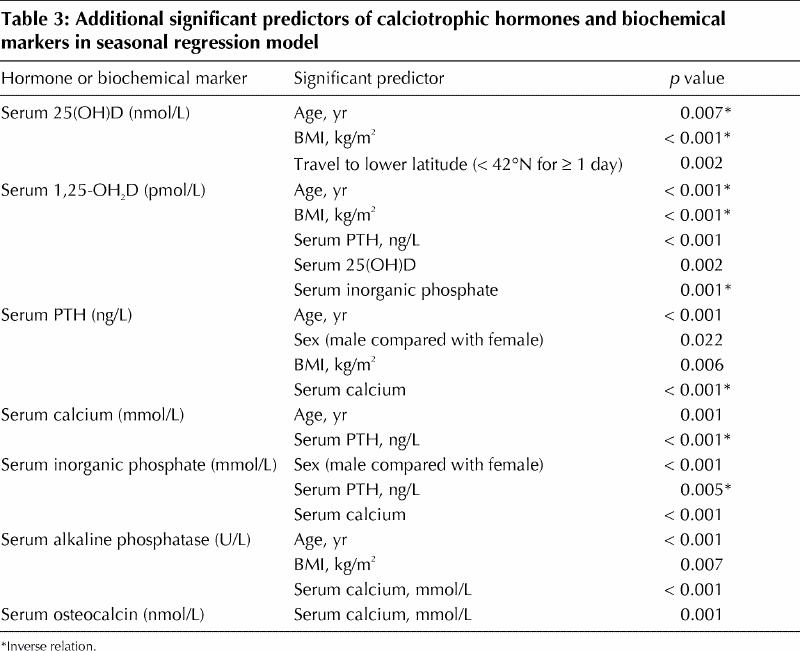
As anticipated, there was a significant rise in serum 25(OH)D during spring (May or June) and summer (August or September) relative to winter values (February or March) (Table 2). In the fall (November or December), 25(OH)D levels declined to significantly lower levels than those observed during the winter months (Table 2). There were also several other independent predictors of 25(OH)D: both increasing age and increasing BMI were associated with significant decreases in 25(OH)D, whereas travel to lower latitudes (below 42°N) for at least 1 day was associated with significant increases in 25(OH)D (Table 3). Because individual travel to lower latitudes was not consistent for each season, we tested for, but did not find, any interactions between travel and season. This apparent lack of significant interactions may be influenced in part by the relatively low number of people who travelled to lower latitudes: 22 participants (12%) during the winter, 16 (8%) during the spring, 1 (1%) in the summer and 12 (6%) in the fall. Also worth noting is that sex (p = 0.61) and low-level vitamin D supplementation (p = 0.28) were not significant predictors of 25(OH)D.
1,25-(OH)2D decreased significantly from above the normal range in winter to close to or within the normal range in spring, summer and fall (Table 2). In addition to being associated with season, lower 1,25-(OH)2D levels were associated with increasing age and BMI (Table 3). Finally, the complex regulation of 1,25-(OH)2D was supported by the biochemical parameters significant in the model: both 25(OH)D and parathyroid hormone were positively associated with 1,25-(OH)2D, whereas inorganic phosphate was negatively associated with this metabolite (Table 3).
In contrast to the trends for 25(OH)D, serum levels of parathyroid hormone decreased significantly in the summer and in the fall, so that the anticipated inverse relation between 25(OH)D and parathyroid hormone was not consistently observed (Table 2). In addition to season, age, sex, BMI and serum calcium were significant independent predictors of parathyroid hormone levels (Table 3). 25(OH)D was not a significant predictor of parathyroid hormone (p = 0.21).
In conjunction with the declines in parathyroid hormone levels in the summer and fall, serum inorganic phosphate increased during the same seasons (Table 2). Serum levels of alkaline phosphatase and osteocalcin also increased in these 2 seasons (Table 2). The latter were unusual and unexpected findings, because these markers are expected to decrease with decreases in parathyroid hormone. Although the changes in inorganic phosphate, alkaline phosphatase and osteocalcin during the summer and fall were statistically significant, the mean values were all within the normal reference ranges (Table 2).
The prevalence of vitamin D insufficiency according to several different thresholds (less than 40, less than 50 and less than 80 nmol/L) is shown in Fig. 2. The prevalence of vitamin D insufficiency was generally lowest in the summer and highest in the fall. A total of 64 (34%) of the participants had 25(OH)D levels less than 40 nmol/L for at least one point during the year, 115 (61%) had levels less than 50 nmol/L for at least one point, and 182 (97%) had levels less than 80 nmol/L for at least one point.
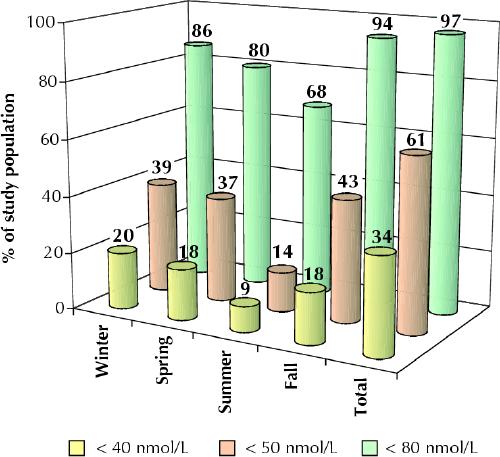
Fig. 2: Prevalence of vitamin D insufficiency, defined in terms of serum level of 25-hydroxy vitamin D. The data for “total” represent the percentages of subjects with vitamin D insufficiency at least once during the year.
Interpretation
We documented seasonal variations in levels of 25(OH)D and a high prevalence of vitamin D insufficiency during fall and winter in an ambulatory population of healthy western Canadians situated at 51°07́N. Although seasonal variations in serum 25(OH)D in healthy populations have been documented for several European countries and the United States,15 studies of the seasonal effects on vitamin D status in Canadians have been limited to elderly people living in institutions,16,17,18 low-income elderly people,19 and young women.20 In accord with the in vitro evidence of Webb and colleagues9 that, at a latitude of 53°N, the skin does not produce any precursors for vitamin D3 (the form generated by exposure to ultraviolet radiation) from October through March, we found that levels of 25(OH)D in late fall (virtually all measurements were obtained during November) were similar to those obtained in winter. However, we were surprised that the serum levels of 25(OH)D in the fall were significantly lower than the winter levels, given the relatively small absolute difference (4.4 nmol/L) between the 2 seasons (Table 2).
Seasonal changes in serum 25(OH)D levels may reflect synthesis of both vitamin D3 and 25(OH)D. Gascon-Barré and associates21 demonstrated an increase in expression of the gene for vitamin D3-25 hydroxylase (CYP27A) during spring and summer, with corresponding increases in serum 25(OH)D. These results were corroborated by Lehmann and collaborators,22 who demonstrated that both vitamin D3 and exposure to ultraviolet B radiation led to increases in expression of D3-25 hydroxylase in keratinocytes.
This study also confirmed the importance of age as a major determinant of vitamin D status. Regardless of season, increasing age was associated with lower levels of 25(OH)D and 1,25-(OH)2D. The cause of the age-related decline in 1,25-(OH)2D is multifactorial. Several theories, including a decrease in renal 1α-hydroxylase activity with age,23 compounded by decreased renal function,4 and decreased availability of 25(OH)D as a substrate,24 have all been implicated in the age-related decline of 1,25-(OH)2D. Declining levels of 25(OH)D with age have been attributed to impaired vitamin D absorption from the intestine,25 as well as a decline in the concentration of the vitamin D precursors that are normally stored in the skin.26
After adjusting for season, age and travel to lower latitudes, we found that BMI was inversely related to serum 25(OH)D. Such a relation has also been observed in postmenopausal women,27 elderly people,1,28 and younger obese subjects.29 Need and associates27 suggested that the inverse relation between 25(OH)D and BMI is due a larger body pool of vitamin D and 25(OH)D or slower saturation and mobilization of these compounds from adipose tissue (or both).
Serum 25(OH)D is our best available laboratory aid for diagnosing frank vitamin D deficiency, which causes rickets in children and osteomalacia in adults. Vitamin D deficiency is typically associated with 25(OH)D levels below 25 mmol/L.30 However, identification of inadequate levels of vitamin D for optimal bone health — vitamin D “insufficiency” — is likely of greater clinical importance. The most commonly used definition of vitamin D insufficiency is the lower limit of the normal range for the 25(OH)D assay (40 mmol/L).10 However, recent investigations have suggested that the threshold for vitamin D insufficiency should be the 25(OH)D level below which parathyroid hormone secretion begins to rise. The authors of these studies have proposed that the cutoff value for vitamin D insufficiency may be as high as 80 mmol/L.31,32,33 In support of a higher threshold, it has been noted that serum 25(OH)D is lower in people who have experienced fractures than in controls.34,35 Center and colleagues36 showed that subjects with a 25(OH)D level less than 68 nmol/L had a 4-fold greater risk of major fracture over an 8-year period. In patients with osteoporosis this risk was increased 19-fold.
On the basis of the most conservative definition of insufficiency (less than 40 nmol/L), 34% of our subjects had vitamin D insufficiency at at least one point during the year (Fig. 2). If the proposed 80 mmol/L threshold is used, virtually all participants (97%) would be assessed as having had vitamin D insufficiency at least once during the year. Raising the threshold by only 10 mmol/L, to 50 mmol/L, nearly doubles the proportion of our study population who would be classified as having vitamin D insufficiency (to 61%).
Despite the high prevalence of vitamin D insufficiency in this population and appropriate seasonal changes, we found no relation between parathyroid hormone and serum 25(OH)D. Only a small proportion (10% to 17%) of participants had parathyroid hormone levels above the normal range of the DiaSorin assay (greater than 54 pg/mL) at any point. Similar to other studies,37,38 we observed an increase in parathyroid hormone with age, and women had higher levels of this hormone than men. In addition, parathyroid hormone increased with increasing BMI. The seasonal declines in parathyroid hormone, which lagged behind seasonal increases in 25(OH)D by one season, may be the result of previously established vitamin D stores in body fat.39 Another possible reason for the lack of an association between parathyroid hormone and 25(OH)D is that other factors, particularly calcium consumption, may have served to keep serum parathyroid hormone levels low, even during times of 25(OH)D insufficiency. Both Riggs and collaborators40 and Storm and associates41 reported wintertime suppression of parathyroid hormone with calcium supplementation (without dietary vitamin D supplementation) in postmenopausal women. In both of these studies, mean daily calcium intake was well over 1500 mg in each of the intervention groups. Storm and associates41 also reported a significant inverse relation between parathyroid hormone and 25(OH)D only when calcium consumption was less than 800 mg/day, which suggests a threshold dose of calcium for suppression of parathyroid hormone. An important limitation of our study is that we did not assess participants' dietary calcium during the study. Although the subjects had all reported relatively low calcium intake on entry into CaMOS 2 years earlier (Table 1), they probably increased their calcium intake after entering the study, as a result of their participation in an “osteoporosis study.”
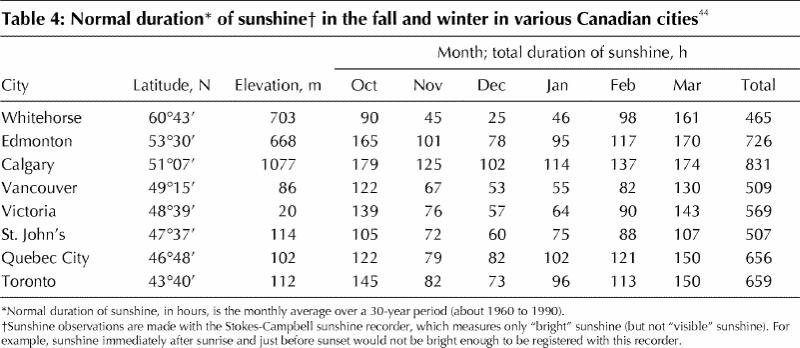
This study has several other limitations that must be considered when generalizing the results to other Canadian populations. The study sample was predominately white (except for 3 individuals), which does not accurately reflect more diverse ethnic communities such as those in Vancouver and Toronto. However, given that people with greater skin pigmentation have less circulating vitamin D3 than those with lighter skin tone,42,43 it is likely that a larger proportion of dark-skinned participants would have reduced the mean serum 25(OH)D levels throughout the year. Other factors unique to the Calgary population might have led to an underestimation of the seasonal variations of 25(OH)D in the Canadian population as a whole. Calgary receives more hours of sunshine per year than any other Canadian city, particularly in the fall and winter (Table 4).44 Although sunshine hours do not accurately indicate how much time people spend in the sun, several studies have reported a direct relation between meteorologically measured sunshine hours and levels of 25(OH)D.27,31 In addition, at an elevation of 1077 m, Calgary residents are situated at a considerably higher altitude than most other Canadians (Table 4). Rigel and colleagues45 have demonstrated increases in ultraviolet B radiation of approximately 8% to 10% with every 300 m increase in elevation. Given that participants in this study lived in a region with more sunshine hours and higher elevation than most other Canadian locations, it is likely that the seasonal decreases observed in late fall and winter underestimate those of other Canadian populations. These factors also suggest that the observed prevalence of seasonal vitamin D insufficiency in a Calgary population may be a conservative estimate of vitamin D insufficiency among other Canadians.
Table 4
In conclusion, we documented marked seasonal effects on vitamin D levels in a community-dwelling sample of Canadians. These results support recommendations for clinicians recently outlined by Heaney46 regarding assessment of vitamin D metabolism. According to Heaney, the reference values provided by manufacturers of 25(OH)D measurement kits should be revised. Experts suggest that optimal levels of 25(OH)D should be somewhere above 80 nmol/L (32 ng/mL).31,46 Furthermore, we do not recommend using elevated serum levels of parathyroid hormone as a surrogate marker for vitamin D insufficiency, because parathyroid hormone is modulated by several other factors, particularly calcium intake. However, despite these expert suggestions, routine measurement of 25(OH)D is probably not justified.46 Given that almost every person in our sample had serum 25(OH)D levels below 80 nmol/L at one point in the year, and that more than one-third of subjects had levels below the most conservative definition of vitamin D insufficiency, our findings support a recommendation for more aggressive vitamin D supplementation, particularly for elderly people and especially during the fall and winter months. The current dietary recommendation for vitamin D in Canada and the United States is 200 IU/day for adults (600 IU/day for those over the age of 70),47 although Vieth48 has argued that the adult requirement should be at least 800 IU/day. Our study suggests a need to review Canada's recommendation for vitamin D nutrition.
β See related article page 1541
Acknowledgments
We are indebted to the volunteers of the Calgary cohort of the Canadian Multicentre Osteoporosis Study (CaMOS) for their enthusiastic participation. We wish to thank Howard Cheng who performed the assays for 25- hydroxyvitamin D, 1,25-dihydroxy vitamin D, parathyroid hormone and osteocalcin. This study was funded by the Health Research Fund of the Alberta Heritage Foundation for Medical Research. At the time of this study, CaMOS was supported by the Medical Research Council of Canada, Health Canada (National Health Research and Development Program), Dairy Farmers of Canada, the Osteoporosis Society of Canada, Merck Frosst Canada, Eli Lilly Canada, and Procter and Gamble Pharmaceuticals (Canada).
Footnotes
This article has been peer reviewed.
Contributors: Ms. Rucker assisted with data collection and performed the data entry, cleanup and analysis, as well as contributing to the initial writing of the manuscript. Jane Allan was responsible for coordinating the project, and Dr. Fick contributed to the statistical methods and the interpretation of the data. Dr. Hanley was the principal investigator responsible for project conception, study design, grant application and supervision of the writing of the final manuscript. All authors contributed to reviewing and making revisions to the manuscript.
Competing interests: None declared.
Correspondence to: Dr. David A. Hanley, 3330 Hospital Dr. NW, Calgary AB T2N 4N1; fax 403 270-0979; dahanley@ucalgary.ca
References
- 1.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670-6. [DOI] [PubMed]
- 2.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 1992;327:1637-42. [DOI] [PubMed]
- 3.Lips P, Netelenbos JC, Jongen MJ, van Ginkel FC, Althius AL, van Schaik CL, et al. Histomorphometric profile and vitamin D status in patients with femoral neck fracture. Metab Bone Dis Relat Res 1982;4:85-93. [DOI] [PubMed]
- 4.Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest 1979;64:729-36. [DOI] [PMC free article] [PubMed]
- 5.Riggs BL, Melton LJ. Involutional osteoporosis. N Engl J Med 1986; 314: 1676-86. [DOI] [PubMed]
- 6.Holick MF. Vitamin D requirements for humans of all ages: new increased requirements for women and men 50 years and older. Osteoporos Int 1998;8:S24-9. [DOI] [PubMed]
- 7.Faulkner H, Hussein A, Foran M, Szijarto L. A survey of vitamin A and D contents of fortified fluid milk in Ontario. J Dairy Sci 2000;83:1210-6. [DOI] [PubMed]
- 8.Holick MF, Shao Q, Liu WW, Chen TC. The vitamin D content of fortified milk and infant formula. N Engl J Med 1992;326:1178-81. [DOI] [PubMed]
- 9.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373-8. [DOI] [PubMed]
- 10.Need AG, Horowitz M, Morris HA, Nordin BC. Vitamin D status: effects on parathyroid hormone and 1,25- dihydroxyvitamin D in postmenopausal women. Am J Clin Nutr 2000;71:1577-81. [DOI] [PubMed]
- 11.Holick MF. The use and interpretation of assays for vitamin D and its metabolites. J Nutr 1990;120(Suppl 11):1464-9. [DOI] [PubMed]
- 12.Heaney RP. Lessons for nutritional science from vitamin D. Am J Clin Nutr 1999; 69:825-6. [DOI] [PubMed]
- 13.Tenenhouse A, Joseph L, Kreiger N, Poliquin S, Murray TM, Blondeau L, et al. Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 2000;11:897-904. [DOI] [PubMed]
- 14.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121-30. [PubMed]
- 15.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med 1992;93:69-77. [DOI] [PubMed]
- 16.Liu BA, Gordon M, Labranche JM, Murray TM, Vieth R, Shear NH. Seasonal prevalence of vitamin D deficiency in institutionalized older adults. J Am Geriatr Soc 1997;45:598-603. [DOI] [PubMed]
- 17.Kendler DL, Uhrin Z, Dian L, Liu A. Seasonal changes in serum vitamin D in a geriatric residential care population [abstract]. Bone 1998;23:S493.
- 18.Fraher LJ, Caveney AN, Hodsman AB. Vitamin D status of elderly institutionalized Canadians: effect of season and oral vitamin D2 on circulating 1,25-(OH)2D [abstract]. J Bone Min Res 1990;5(Suppl 5):S133.
- 19.Delvin EE, Imbach A, Copti M. Vitamin D nutritional status and related biochemical indices in an autonomous elderly population. Am J Clin Nutr 1988; 48: 373-8. [DOI] [PubMed]
- 20.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr 2001;55:1091-7. [DOI] [PubMed]
- 21.Gascon-Barré M, Demers C, Ghrab O, Theodoropoulos C, Lapointe R, Jones G, et al. Expression of CYP27A, a gene encoding a vitamin D-25 hydroxylase in human liver and kidney. Clin Endocrinol (Oxf) 2001;54:107-15. [DOI] [PubMed]
- 22.Lehmann B, Tiebel O, Meurer M. Expression of vitamin D3 25-hydroxylase (CYP27) mRNA after induction by vitamin D3 or UVB radiation in keratinocytes of human skin equivalents — a preliminary study. Arch Dermatol Res 1999;291:507-10. [DOI] [PubMed]
- 23.Slovik DM, Adams JS, Neer RM, Holick MF, Potts JT Jr. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med 1981;305:372-4. [DOI] [PubMed]
- 24.Bouillon RA, Auwerx JH, Lissens WD, Pelemans WK. Vitamin D status in the elderly: seasonal substrate deficiency causes 1,25-dihydroxycholecalciferol deficiency. Am J Clin Nutr 1987;45:755-63. [DOI] [PubMed]
- 25.Clemens TL, Zhou XY, Myles M, Endres D, Lindsay R. Serum vitamin D2 and vitamin D3 metabolite concentrations and absorption of vitamin D2 in elderly subjects. J Clin Endocrinol Metab 1986;63:656-60. [DOI] [PubMed]
- 26.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 1985;76:1536-8. [DOI] [PMC free article] [PubMed]
- 27.Need AG, Morris HA, Horowitz M, Nordin C. Effects of skin thickness, age, body fat, and sunlight on serum 25- hydroxyvitamin D. Am J Clin Nutr 1993; 58: 882-5. [DOI] [PubMed]
- 28.Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, et al. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr 1997;66:929-36. [DOI] [PubMed]
- 29.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest 1985;76:370-3. [DOI] [PMC free article] [PubMed]
- 30.Holick MF. Vitamin D: photobiology, metabolism, mechanism of action and clinical application. In: Favus MJ, editor. Primer on the metabolic bone diseases and mineral metabolism. 4th ed. Philadelphia: Lippincott, Williams and Wilkins, 1999. p. 92-8.
- 31.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 1997;7:439-43. [DOI] [PubMed]
- 32.Guillemant J, Taupin P, Le HT, Taright N, Allemandou A, Peres G, Guillemant S. Vitamin D status during puberty in French healthy male adolescents. Osteoporos Int 1999;10:222-5. [DOI] [PubMed]
- 33.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med 1991;115:505-12. [DOI] [PubMed]
- 34.Lips P, van Ginkel FC, Jongen MJ, Rubertus F, van der Vijgh WJ, Netelenbos JC. Determinants of vitamin D status in patients with hip fracture and in elderly control subjects. Am J Clin Nutr 1987;46:1005-10. [DOI] [PubMed]
- 35.Baker MR, McDonnell H, Peacock M, Nordin BE. Plasma 25-hydroxy vitamin D concentrations in patients with fractures of the femoral neck. BMJ 1979;1:589. [DOI] [PMC free article] [PubMed]
- 36.Center JR, Nguyen TV, Sambrook PN, Eisman JA. Hormonal and biochemical parameters and osteoporotic fractures in elderly men. J Bone Miner Res 2000; 15:1405-11. [DOI] [PubMed]
- 37.Gallagher JC, Kinyamu HK, Fowler SE, Dawson-Hughes B, Dalsky GP, Sherman SS. Calciotropic hormones and bone markers in the elderly. J Bone Miner Res 1998;13:475-82. [DOI] [PubMed]
- 38.Marcus R, Madvig P, Young G. Age-related changes in parathyroid hormone and parathyroid hormone action in normal humans. J Clin Endocrinol Metab 1984; 58:223-30. [DOI] [PubMed]
- 39.Rosenstreich SJ, Rich C, Volwiler W. Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J Clin Invest 1971;50:679-87. [DOI] [PMC free article] [PubMed]
- 40.Riggs BL, O'Fallon WM, Muhs J, O'Connor MK, Kumar R, Melton LJ 3rd. Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res 1998; 13:168-74. [DOI] [PubMed]
- 41.Storm D, Eslin R, Porter ES, Musgrave K, Vereault D, Patton C, et al. Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo-controlled trial. J Clin Endocrinol Metab 1998;83:3817-25. [DOI] [PubMed]
- 42.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr 1998;67:1232-6. [DOI] [PubMed]
- 43.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982;1:74-6. [DOI] [PubMed]
- 44.Environment Canada. Canadian climate normals. Available: www.msc-smc.ec.gc.ca/climate/climate_normals/index_e.cfm (accessed 2002 Apr 21).
- 45.Rigel DS, Rigel EG, Rigel AC. Effects of altitude and latitude on ambient UVB radiation. J Am Acad Dermatol 1999;40:114-6. [DOI] [PubMed]
- 46.Heaney RP. Vitamin D: How much do we need, and how much is too much? Osteoporos Int 2000;11:553-5. [DOI] [PubMed]
- 47.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes: calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington: National Academy Press; 1997. [PubMed]
- 48.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999;69:842-56. [DOI] [PubMed]


