Abstract
Oxidized Organic Aerosol (OOA), a major component of fine atmospheric particles, impacts climate and human health. Previous experiments and atmospheric models emphasize the importance of nocturnal OOA formation from NO3· oxidation of biogenic VOCs. This seasonal study extends the understanding by showing that nocturnal oxidation of biomass-burning emissions can account for up to half of total OOA production in fall and winter. It is the first to distinguish nocturnal OOA characteristics from daytime OOA across all seasons using bulk aerosol measurements. Summer observations of nocturnal OOA align well with regional chemistry transport model predictions, but discrepancies in other seasons reveal a common model deficiency in representing biomass-burning emissions and their nocturnal oxidation. This study underscores the significance of near-ground nocturnal OOA production, proposes a method to differentiate it using bulk aerosol measurements, and suggests model optimization strategies. These findings enhance the understanding and prediction of nighttime OOA formation.
Subject terms: Atmospheric chemistry, Atmospheric chemistry
Introduction
Secondary organic aerosol (SOA) contributes substantially to atmospheric fine particles1–3, thus understanding SOA formation is essential to determining the effect of aerosols on climate4 and human health5. SOA is formed through the atmospheric oxidation of volatile organic compounds (VOCs) emitted from both biogenic and anthropogenic sources6. However, the exact formation and evolution process of SOA in the atmosphere is still uncertain and hence limits the predictability of aerosol concentrations and therefore their effects7.
In field studies, the concentration of oxidized organic aerosol (OOA)1,7 resolved by receptor models8,9 from the measurement of total organic aerosols (OA) by instruments like the aerosol mass spectrometer (AMS), are commonly used to show the OA contribution from secondary sources differing from primary emissions. Receptor models can further divide OOA into subtypes. Subsequently, the chemical evolution of ambient OA can be analyzed using changes in the properties of the resolved OOA subtypes with regard to volatility1,10,11 and oxidation degree12–14.
Nocturnal oxidation of biogenic VOCs has been shown to form significant OOA in chamber experiments15,16. Global chemistry transport models predict that OOA formation from nighttime oxidation of biogenic VOCs by the nitrate radical (NO3·) accounts for 5% to 21% of the global SOA production17,18. However, in the interpretation of field study results, the formation and aging of OOA are still considered to be driven by mainly photochemistry1,19, even though unexplained concentration increases of OOA during the night have been observed in many ground-based field studies9,20–23.
In addition, in the study by Kiendler-Scharr et al. 24, NO3-initiated oxidation of biogenic VOCs during nighttime has been shown to be the major source of significant particulate organic nitrate in Europe and has been estimated to be a ubiquitous and important contributor to submicron aerosol mass on the continental scale. However, in that study, the source of organic nitrate observed during cold seasons remained unresolved, suggesting a significant gap in the understanding of nocturnal particle formation. This knowledge gap may also be a contributing factor to why current chemical transport models systematically underpredict the observed OOA concentrations during winter, especially in Europe25. A recent chamber study by Kodros et al. 26 found that OOA was rapidly formed through NO3· oxidation of organic compounds emitted from biomass-burning. Moreover, in their study, the mass of OOA formed from the oxidation process was comparable to the amount of organic aerosol emitted directly from biomass-burning. However, such a large OOA source especially in wintertime still lacks direct evidence from field studies.
Biomass-burning emissions are globally increasing due to more frequent wildfire activity in a warming climate27,28. In addition, it has been reported that biomass-burning, such as residential heating, is increasing e.g. in Europe29 contributing significantly to particulate pollution in densely populated areas during winter30. Also, due to the energy crisis triggered by recent military conflicts, there is a shift from using fossil fuels for heating systems towards using more electric or biomass-fuel alternatives in Europe31. Therefore, fresh and chemically aged aerosol from biomass-burning sources may gain further importance in the future.
During the Jülich Atmospheric Chemistry Project (JULIAC) campaign32,33 (Supplementary Note 1), the concentrations of atmospheric components, including oxidants, trace gases, and the chemical composition of submicron aerosol (Supplementary Fig. 1), were measured in the atmospheric simulation chamber SAPHIR34,35 in Jülich. The chamber was continuously flowed with ambient air sampled from a height of 50 m extending well above the canopy and buildings in the vicinity of the chamber. The air had a residence time of 1 h in the chamber. By utilizing the comprehensive dataset and combining it with the analysis of the chemical composition of aerosols by a receptor model, Positive Matrix Factorization (PMF; Methods), we investigated the seasonality of the nocturnal formation of OOA. This study showed that the nocturnal oxidation of organic species is as important as their photo-oxidation for OOA production throughout all seasons. The characteristics of the chemical composition and the diurnal changes in concentration of nocturnal OOA determined from our measurements can be used for identifying nocturnal OOA in other field studies. Furthermore, a comparison between the observed OOA concentrations attributed to nighttime oxidation and results from the European Air pollution Dispersion–Inverse Model (EURAD-IM, Methods), reveal that the pathway of nocturnal oxidation is not well represented in the EURAD-IM model during all seasons except summer.
Results and Discussion
Ambient observation of large OOA formation from nocturnal chemistry
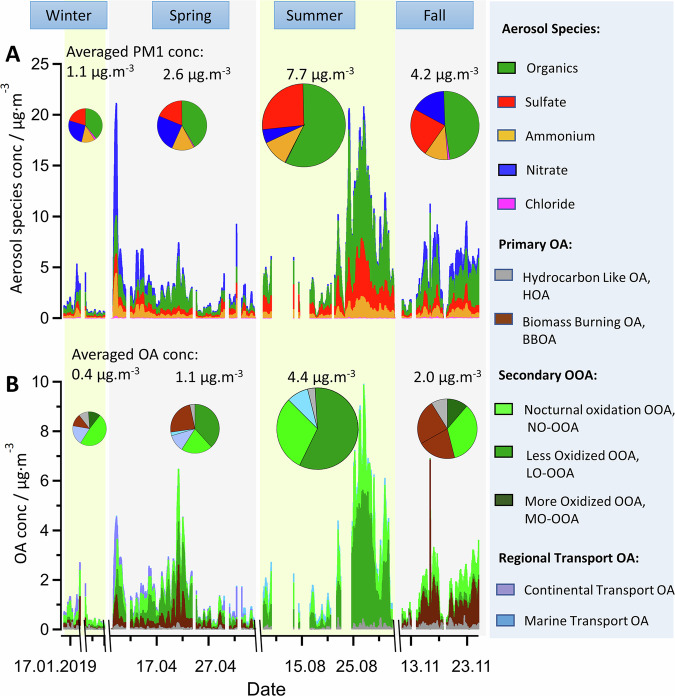
The chemical composition of non-refractory submicron particles, mainly aerosol constituents (organics, nitrate, sulfate, chloride, and ammonium) was measured by a high-resolution time-of-flight aerosol mass spectrometer (HR-ToF-AMS) during four intensive JULIAC episodes in each season in 2019 (Fig. 1). Overall, organic compounds were found to be the major components of measured submicron aerosol throughout the year accounting for 40% to 60% of the total aerosol mass in this study. The PMF analysis attributed the measured organic aerosol to (1) direct emissions (primary organic aerosol) of traffic exhaust (HOA) and biomass-burning (BBOA), (2) formation from oxidation products (OOA), and (3) long-range regional transport (Supplementary Figs. 2–5). In the PMF analysis, OOA is typically resolved into two subtypes that differ in their volatility and degree of oxidation12,14,36 and both are considered to originate mainly from photochemical activities with maximal concentrations during daytime. However, in this study, in addition to the two common subtypes of OOA from photo-oxidation, less-oxidized OOA (LO-OOA), and more-oxidized OOA (MO-OOA), a third subtype of OOA was resolved. The third OOA subtype is shown to mainly originate from nocturnal oxidation and is therefore labeled as nocturnal oxidation OOA (NO-OOA) in this work. The PMF analysis of only aerosol organics concentrations is commonly applied but fails to differentiate between OOA formed from nighttime oxidation and photo-oxidation. The reason is that, in bulk aerosol measurements, the mass spectrum of OOA becomes increasingly similar with higher levels of oxidation, regardless of whether the oxidation occurs during daytime or nighttime. Therefore, the PMF analysis was applied including both the nitrate and organics of aerosol (Methods). This allows us to distinguish between the types of OOA, as a larger nitrate fraction (NO+ + NO2+ fragments in the mass spectrum) (Fig. 2A) and a higher nitrogen-to-carbon ratio (N:C, Supplementary Fig. 7) is obtained for NO-OOA than for OOA from photo-oxidation.
Fig. 1. Seasonal overview of sources of organic aerosol and their contribution to submicron aerosol mass.
Time series of aerosol concentrations and average values during the four seasons of the JULIAC campaign for (A) the chemical composition of submicron aerosol, and (B) directly emitted organic aerosol (primary OA), secondary organic aerosol formed from oxidation processes (OOA) and organic aerosol from regional transport obtained from a PMF analysis of the measured mass spectrum of organic aerosol.
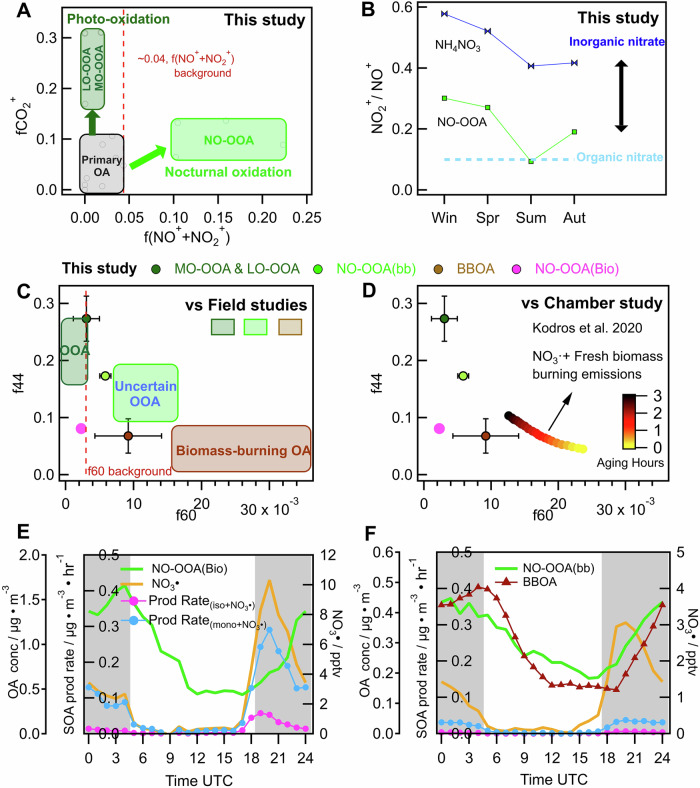
Fig. 2. Analysis of chemical characteristics and formation mechanism of nocturnal OOA.
A Directly emitted primary OA, OOA formed from photo-oxidation (LO-OOA and MO-OOA) and nocturnal oxidation processes (NO-OOA) resolved by the PMF analysis of particulate nitrate and organic aerosol measurements during the JULIAC campaign. The ratio of the ion mass signals of the fragments CO2+ (fCO2+) vs the sum of NO+ and NO2+ (f(NO+ + NO2+)) normalized to the total ion mass signal intensity is used to show the distribution of nitrate-containing OA factors. These OA factors fall in fCO2+ vs f(NO+ + NO2+) space and are represented by gray circles and categorized by the grey, dark green, and light green rectangles respectively, with a detailed graph in Supplementary Fig. 25. The value of f(NO+ + NO2+) (<0.04) of primary OA and photo-oxidation OOA is marked as background by a red dashed line. B The ratio of nitrate fragments (NO2+/NO+) in NO-OOA across seasons is shown by green markers with line, while blue markers with line represent the ratio of inorganic ammonium nitrate particles (NH4NO3). The blue dashed line represents the reference ratio of pure organic nitrate24. C The chemical composition, characterized mass spectrum characteristics of f44 vs f60, is used to compare the averaged PMF factors from this study (shown as circle with error bar) to those from previous field studies9,20,52,53 (depicted as rectangles). These studies all identified an unexplained nocturnal OOA, daytime OOA, and primary BBOA, highlighted by light green, dark green, and brown rectangles, respectively. Detailed factor positions are provided in Supplementary Fig. 25. The background value of f60 (~0.3%) for atmospheric OA87,88 is marked by a red dashed line. Additionally, (D) compares the averaged OA factors obtained in this study to the OA evolution during the dark oxidation of biomass-burning emission by NO3· in a chamber study87,88, illustrated with dotted line color-coded by aging time. The seasonal averaged diurnal pattern of the mass concentration of the NO-OOA, BBOA, NO3·, and SOA production rate of the NO3· reaction with monoterpenes and isoprene are shown for summer (E) and spring (F). The grey areas represent nighttime.
In our study, the exact mass spectrum of NO-OOA measured by the AMS instrument varied between the different seasons (Supplementary Fig. 7) due to different dominant precursors being mainly biogenic VOCs in summer and being compounds emitted from biomass-burning in the other seasons. Therefore, NO-OOA was designated as NO-OOA(Bio) for summer and NO-OOA(bb) for the other seasons (Fig. 3A). The NO-OOA(Bio) has a lower degree of oxidation, indicated by an elemental oxygen to carbon ratio (O:C) of 0.39. Additionally, it shows a low intensity of the characteristic ion mass signal for levoglucosan at mass-to-charge ratio (m/z) 60, which is only 0.2%. In contrast, NO-OOA(bb) shows a higher oxidation degree, with an O:C ratio ranging from 0.76 to 0.91 and a higher intensity ratio of the signal at m/z 60 of 0.5–0.7%. Additionally, the concentrations of both NO-OOA(Bio) and NO-OOA(bb) in the different seasons show consistently a peak value during nighttime hours. This nocturnal peak well explains the observed increase in the overall oxidation degree of the submicron organic aerosols (OA) at night (Supplementary Fig. 8). In our study, NO-OOA constituted 20% to 50% of the total submicron OA mass with the highest average concentrations of 1.3 μg m−3 in summer. During this period, concentrations of NO3· were also at their peak (derived from measured dinitrogen pentoxide (N2O5) and nitrogen dioxide (NO2), see Supplementary Fig. 1). This concurrent seasonal peak supports that NO-OOA formation is driven by NO3· chemistry. During summer, NO-OOA contributed around 30% of the overall mass of OOA produced. This is slightly higher than the value of 5% to 21% predicted in previous global model studies17,18. In contrast, during winter and fall, NO-OOA became a major part of OOA, demonstrating the importance of nocturnal chemistry for ambient OOA formation and aging during cold seasons at this semi-rural site.
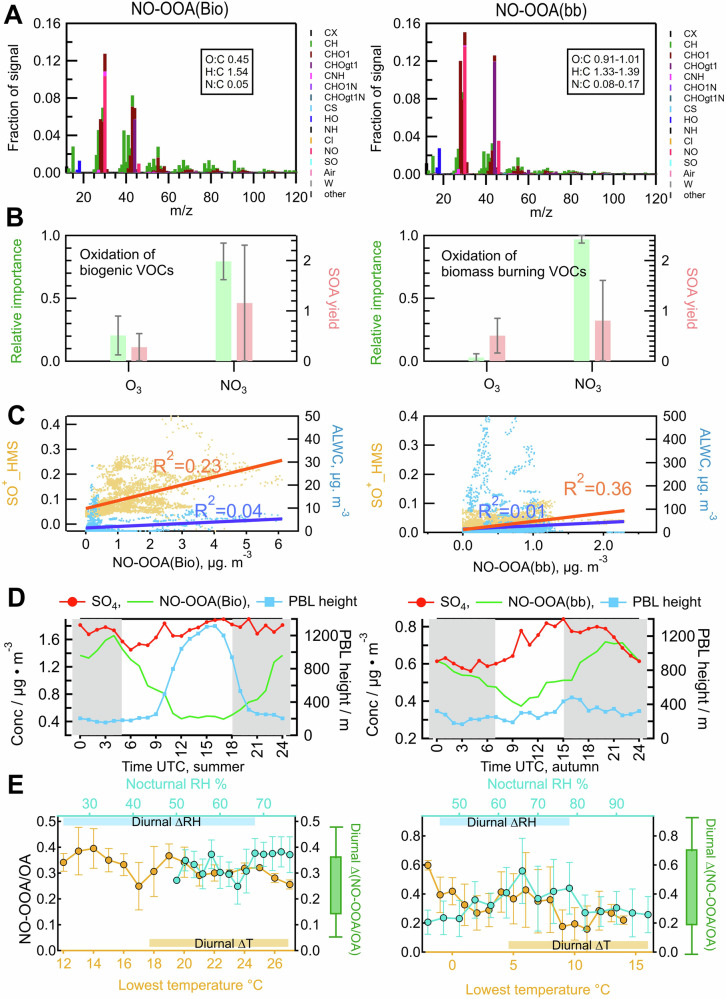
Fig. 3. Analysis of atmospheric layer development, phase-partitioning, and chemical reactions on the enhancement of nocturnal OOA.
A The ion mass spectrum of NO-OOA(Bio) for summer and an averaged spectrum of NO-OOA(bb) for the other seasons (detailed spectra in the Supplementary Fig. 7) obtained by the PMF analysis of measurements of aerosol nitrate and organics. B Competition between NO3 and O3 oxidation of VOCs (label as relative importance (green)) and the comparison of secondary organic aerosol (SOA) yield of biogenic VOCs (BVOCs) (isoprene, α-pinene, β-pinene, and limonene) and biomass-burning volatile organic compounds (bbVOCs) (furan, naphthalene) by different oxidants (NO3 and O3) (red), with the median values represented by the bars and the range of variability indicated by the error bars. (Supplementary Table 2). C Correlation of NO-OOA(Bio) and NO-OOA (bb) with the SO+ fragment originating from HMS and aerosol liquid water content (ALWC). D Averaged diurnal variations of the sulfate (SO4) aerosol mass concentrations and observed NO-OOA obtained by the PMF analysis, as well as the PBL height obtained from the EURAD-IM in summer and autumn during the JULIAC campaign. Plots showing results for the other seasons can be found in the Supplementary Fig. 18. E The variation of mass ratio of NO-OOA (Bio) to total OA in summer, and NO-OOA (bb) to total OA in the other seasons as a function of the minimum nocturnal temperature and corresponding relative humidity during these periods. Data is restricted to the period ±2 hours of the minimum temperature. The averaged diurnal variations of temperature, RH, and the ratio of NO-OOA/OA are also displayed in the plot by yellow, blue, and green bars, respectively.
Nocturnal OOA formation via the NO3-initiated oxidation of precursors in different seasons
Previous studies have reported that NO3-initiated oxidation in the atmosphere is commonly accompanied by a significant enhancement of particulate organic nitrate concentrations24,37. In this study, NO-OOA resolved from the PMF analysis of measured aerosol organics and nitrates (Supplementary Figs. 9–12), contained high concentrations of organic nitrates (Methods). This is shown by the molar ratio of the ion fragments NO2+ to NO+ in NO-OOA being lower than in ammonium nitrate (Fig. 2B), demonstrating that NO3-initiated nocturnal oxidation significantly contributed to the enhancement of NO-OOA.
The analysis of the competition between NO3· and O3 (Methods) shows that NO3· was the dominant oxidant in the night in this study (Fig. 3B). To estimate the potential effect of aqueous phase chemistry on the formation of NO-OOA, the ion mass signal of the sulfate fragment (SO+) presumably from hydroxymethanesulfonate (HMS), a tracer for liquid phase chemistry38, was calculated by ion fragmentation method39. The time series of NO-OOA showed a lack of correlation with both the sulfate fragments from particulate HMS and the aerosol liquid water content (ALWC)40,41 for most of the observations (Fig. 3C). In addition, aqueous chemistry was unlikely important in this study, as the aerosol contained little water (ALWC < 10 μg/m3) for most of the time in this campaign (Supplementary Table 3). Only during the cold season, NO-OOA concentrations weakly correlated with sulfate fragments from particulate HMS (R2 = 0.36), and a concurrent increase in the ALWC and the concentration ratio of NO-OOA(bb)/BBOA was also observed (Supplementary Fig. 13). Therefore, a small contribution of aqueous and heterogeneous reactions of NO3· and N2O542 to NO-OOA cannot be completely excluded during the cold seasons (winter, spring, and fall).
In winter, spring, and fall, the high concentrations of NO-OOA(bb) observed were produced mainly from the NO3· oxidation of biomass-burning emissions. This is evident, as NO-OOA(bb) correlated with primary organic aerosol emitted by biomass-burning emissions (BBOA, R2 0.48–0.62), as well as with gas-phase tracers for biomass-burning such as furan (R2 0.32–0.49) and CO (R2 0.44–0.75), and the characteristic ion mass signal (mass to charge ratio, m/z 60, C2H4O2+, R2 0.68–0.82) from levoglucosan, which is regarded as a tracer for biomass-burning in particles (Supplementary Table 6). Moreover, the changes in the OA composition measured in a chamber study26 of fresh biomass-burning emissions oxidized by NO3· (Fig. 2D) showed a decrease of the ion mass signal at m/z 60 accompanied by an increase of the ion mass signal at m/z 44 (an indicator for aerosol aging, mainly CO2+) with increasing aging. The same behavior is also observed in this study for BBOA and NO-OOA(bb) (Fig. 2D), which further supports that biomass burning is the precursor of NO-OOA(bb). In addition, the OOA produced in the chamber study showed a similar mass spectrum as observed in NO-OOA(bb) in the JULIAC campaign, characterized by a high linear correlation coefficient of R2 = 0.94 and a theta angle43 of θ = 14.0° (Supplementary Fig. 14). In addition, the diurnal variations and concentrations of BBOA and NO-OOA(bb) are very similar in this study (Fig. 2F), suggesting a common source. This is consistent with the model prediction in Kodros et al.26, where ~60–70% of OA related to biomass-burning emissions were found to be affected by NO3· nighttime chemistry. It is important to note that boundary layer dynamics can also affect the diurnal distribution of OOA, as discussed in the next section.
In summer, the formation of NO-OOA(Bio) was dominated by the NO3-initiated nocturnal oxidation of biogenic VOCs, especially monoterpenes. This is supported by the similarity of the chemical composition (R2 = 0.64–0.71, θ = 27.8°–34.3°) of NO-OOA(Bio) observed in this study and the OOA produced from the NO3-initiated oxidation of monoterpenes (β-pinene and limonene) in a previous chamber experiment44 (Supplementary Fig. 14). In addition, the aerosol production rates calculated from the rate constant of NO3· reactions with isoprene and monoterpenes and the aerosol yields (Methods) support this conclusion, as the NO3· oxidation of monoterpenes gave a higher SOA production rate than isoprene (Fig. 2E). The calculated total SOA concentration produced via NO3· oxidation of monoterpenes during the night (~1.7 µg m−3) was even comparable to the average nocturnal enhancement of NO-OOA(Bio) (~1.2 µg m−3). Overall, these findings demonstrate that the NO3-initiated oxidation of monoterpenes was the main contributor to the enhancement of nocturnal OOA during the JULIAC campaign in the summer.
Non-dominant role of atmospheric layer development and phase partitioning in nocturnal OOA
In addition to the chemical production, organic aerosol concentrations near the ground can be affected by vertical mixing during the development of atmospheric layers. Specifically at night, the vertical mixing is often poor. In this campaign, the sampling point was at a height of 50-m, which was above the surface layer ( 30 m height) for most of the time and, therefore, located in the nocturnal boundary layer (Methods and Supplementary Fig. 15). The vertical distribution of SOA formed from NO3· oxidation (NO3-SOA) during the JULIAC campaign (Supplementary Figure 16) was simulated by the regional chemistry transport model EURAD-IM (Methods) to estimate the vertical mixing of particles. The simulation shows a clear nocturnal increase of NO3-SOA concentrations at the ground, indicating a significant SOA production from nighttime chemistry rather than an accumulation of particles. In addition, the weak correlation (R2, 0.12–0.22) between the planetary boundary layer (PBL) height and the modeled NO3-SOA concentrations from NO3· oxidation at a height around 50 m, further confirms that particulate accumulation in the nocturnal boundary layer was not a driving factor for the observed increase of nocturnal OOA. This conclusion is further supported by a much smaller enhancement of low-volatile particulate sulfate measured by the HR-ToF-AMS instrument during the night when NO-OOA increased as observed in all seasons (Fig. 3D).
Furthermore, phase partitioning of pre-existing semi-volatile components in the gas phase driven by the changes in diurnal temperature and relative humidity (RH), also could promote the nocturnal formation of OA45. Aerosol bulk nitrate measured by the HR-ToF-AMS instrument is commonly used as a tracer for the volatile aerosol components, based on its volatility46 and atmospheric lifetime (~7.6 days)47. A weak correlation between NO-OOA and aerosol bulk nitrate (R2, 0.28, Supplementary Table 6) was observed during the JULIAC campaign. In addition, the variation in the mass fraction of NO-OOA to total OA (NO-OOA/OA), as a function of the lowest night-time temperatures and corresponding RH (Fig. 3E), demonstrates the effect of phase-partitioning in NO-OOA concentration. No consistent increase in NO-OOA/OA with a rise in nocturnal RH was observed, suggesting a weak or negligible RH-dependent gas-particle partitioning48. Meanwhile, a slight increase in NO-OOA/OA with a decrease in temperature was found, but that increase can be attributed to the phase-partitioning of both pre-existing volatile compounds and fresh volatile products from nocturnal chemistry. Despite this, the increase in NO-OOA/OA corresponding to average diurnal temperature variation does not reach half of the overall average diurnal change in NO-OOA/OA. In addition, when temperatures drop below 0 °C, an increase in biomass burning emissions was observed (Supplementary Fig. 17), likely due to increased residential heating. This could also explain the stronger temperature-dependent increase in NO-OOA(bb)/OA during the colder seasons. Therefore, we concluded that the phase partitioning of pre-existing volatile compounds may contribute to the nocturnal enhancement of NO-OOA, but it is not a dominant factor.
During the day, the concentrations of NO-OOA decreased due to the combined effects of dilution by vertical mixing during the development of the PBL, evaporation of volatile compounds due to the temperature increase, and aerosol aging by photo-oxidation processes. On most days, aerosol sulfate increased in the morning (Fig. 3D), indicating the mixing of air masses with sulfate-rich aerosol from the residual layer into the newly formed PBL. This vertical mixing during the daytime PBL formation can also dilute the NO-OOA concentration observed near the ground. The concurrent decrease of the aerosol nitrate and NO-OOA concentrations during daytime was accompanied by an increase in the more-oxidized OOA concentrations (Supplementary Fig. 19), implying a combined effect of evaporation of volatile compounds and aerosol aging.
Significant organic aerosol formation via nocturnal oxidation is ubiquitous
Unexplained significant enhancements of OOA during nighttime have been frequently observed in previous field studies9,13,21,22,38,49, indicating the ubiquity of nocturnal oxidation in the atmosphere. Potential OOA formation from nocturnal oxidation might have been underestimated and potentially subsumed into other OA types such as BBOA, OOA from photo-oxidation, or OOA subtypes with uncertain origin in the PMF analyses (Supplementary Table 4). The comparison of aerosol mass spectra observed in previous studies13,20,21,50,51 and NO-OOA(bb) or NO-OOA(Bio) shows that OOA types from unspecific sources had similar compositions as NO-OOA(bb) (R2 = 0.81-0.94, θ = 13.7°-24.1°) or NO-OOA(Bio) (R2 = 0.78–0.96, θ = 14.3°–27.7°) derived in this work (Supplementary Figs. 20 and 21). In some of the previous studies9,20,52,53, which resolved both primary BBOA and unspecific nocturnal OOA, the aerosol composition with regard to the ratio of the integrated ion mass signal at m/z 44 and m/z 60 to the total ion mass signal (f44 and f60) shows a similar chemical composition and mass spectrum characteristics compared to the nocturnal oxidation of biomass-burning emission observed in this study (Fig. 2C and Supplementary Fig. 25). Hence, our results indicate that significant nocturnal OOA formation is ubiquitous. Additionally, the main characteristics of OOA formed from nocturnal oxidation at different seasons determined in this study could help identifying and quantifying the nocturnal OOA production in future observations.
Model prediction of SOA from NO3· oxidation across Europe
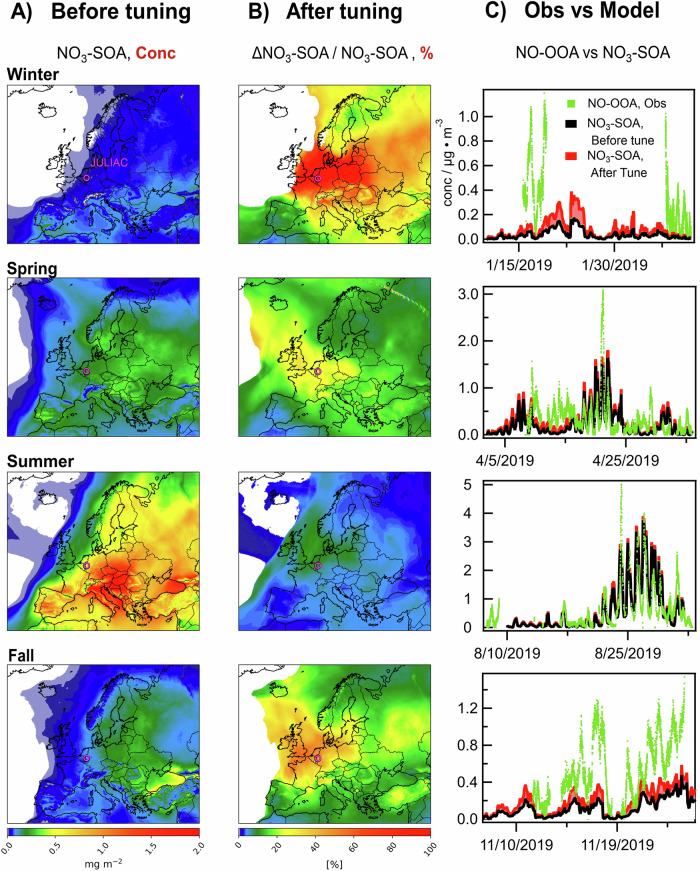
The SOA formed from NO3· oxidation (NO3-SOA) over Europe was calculated by the EURAD-IM (Methods). In the model, the concentration of NO3-SOA is calculated based on known reaction kinetics and SOA yields of organic compounds. The EURAD-IM predicted the highest NO3-SOA concentrations in central and southern Europe, particularly at low altitudes (Supplementary Fig. 16), showing the ubiquity of nocturnal oxidation at the near-ground levels across Europe (Fig. 4A), mainly during summer. At the JULIAC measurement site, the time series of observed NO-OOA concentrations and NO3-SOA concentrations calculated by the EURAD-IM show a similar behavior in all seasons (R2 ranging from 0.50 to 0.86). During summer, when biogenic VOCs emissions were high and NO3· oxidation of biogenic VOCs was the dominant source for nocturnal OOA formation as determined from the observations, the agreement between model predictions and measurements was strongest and characterized by similar shapes of the time series and comparable concentrations (Fig. 4C). Therefore, our measurements give evidence that the nocturnal formation of SOA from the oxidation of biogenic emissions is well represented in the EURAD-IM.
Fig. 4. Model prediction and optimization of SOA from NO3· oxidation across Europe.
A The seasonally averaged and vertically integrated column density of secondary organic aerosol from NO3· oxidation (NO3-SOA) across Europe predicted by the EURAD-IM model, and (B) their relative increase of vertically integrated column density for optimized emissions and aerosol yields (ΔNO3-SOA/ NO3-SOA). Panel C showed a comparison of the concentrations of secondary organic aerosol from nighttime oxidation in the observations (NO-OOA) and the EURAD-IM (NO3-SOA) before and after optimization at around 50-m height at the JULIAC site (marked by the red circle in A and B).
In contrast, in spring, fall, and winter when measurements showed that the oxidation of biomass-burning emissions was the dominant path for the nighttime formation of OOA, concentrations of NO3-SOA calculated by the EURAD-IM were 2 to 10 times lower than the measurements. This discrepancy indicates that this formation pathway is not well represented in the EURAD-IM. In the model, most of the NO3-SOA is produced from the NO3· oxidation of isoprene and monoterpenes (Supplementary Fig. 22). VOCs from biomass-burning emissions such as phenolic compounds are not included in the emission inventory used in most models54, although the NO3· oxidation of these compounds is potentially significantly contributing to the nighttime formation of SOA42,55. Moreover, compared to the results of recent laboratory studies56,57, a quite low SOA yield ( ~ 0.02) of oxidation products of VOCs from biomass burning is implemented in the aerosol dynamics module of the EURAD-IM. Model sensitivity runs (Methods, Supplementary Fig. 23) show that including emissions of three phenolic compounds typical for residential heating and implementing a lower limit SOA yield of 0.3, increases the modeled NO3-SOA concentrations by about a factor of two in all areas in Europe during the cold seasons (Fig. 4B). This shows that the underestimation of biomass-burning in the model could explain the discrepancy between observed and modeled SOA formed from nighttime chemistry during the cold season. This underestimation is likely present in most chemistry transport models. Therefore, revised emission inventories and SOA yields are recommended to ensure an accurate representation of these processes in models.
In this work, the seasonal characteristics and the chemical composition of aerosols from the NO3-initiated oxidation of organic compounds were determined for the first time. Results can be widely applied in the analysis of field studies to distinguish nocturnal organic aerosol using a PMF analysis. The ubiquity of the aerosol formation from nocturnal oxidation across Europe is shown by calculations using the chemistry transport model EURAD-IM. Measurements in this study also show that the NO3· oxidation of organic compounds from biomass burning was the dominant source for the formation of secondary aerosol in the cold seasons, but this source is not well represented in current chemical transport models. Considering the future increase in biomass-burning emissions, the nocturnal oxidation of these compounds is expected to gain in importance. Therefore, further studies are required to accurately represent secondary aerosol formation from nocturnal chemistry in models.
Methods
The JULIAC campaign
The JULIAC campaign (Supplementary Note 1) took place at the semi-rural site on the campus of the Forschungszentrum Jülich (50.91 N, 6.41E), North Rhine-Westphalia, Germany from January to November 2019. During the campaign, ambient air was continuously sampled through an inlet mounted on a 50m-high tower and injected into the atmosphere simulation chamber SAPHIR34,35,58. At night, the sampling height was mostly above the nocturnal surface layer (averaged height of 30 m). Air masses observed during the JULIAC campaign could have been affected by anthropogenic emissions from the nearby city Jülich with industry (distance <5 km) and by biogenic emissions from a nearby forest (distance <1 km) (Supplementary Fig. 24). The inlet was above the canopy height of the surrounding forest. A comprehensive set of instruments (Supplementary Note 2) was used to analyze the air in the SAPHIR chamber. The well-mixed air in the chamber ensured that all instruments observed the same air composition.
Positive Matrix Factorization (PMF) of non-refractory aerosols measured by the HR-ToF-AMS
The chemical composition of non-refractory submicron aerosols was measured by HR-ToF-AMS. The ion mass signals were analyzed using a receptor model, PMF, in order to attribute sources of aerosols (Supplementary Note 3). PMF is a mathematical technique to treat bilinear unmixing problems59 and has been extensively applied in aerosol source apportionment studies1,8,12,60,61. In this study, the software Source Finder (SoFi Pro 8.0.3.1) was used to analyze the contributions of the different sources to the aerosol62,63. The optimal solutions of the PMF analysis were defined based on the residuals, factor features (e.g., tracer ions, diurnal pattern), and the interpretability of the factor’s time series with tracer quantities (VOCs, radicals, photolysis frequencies, wind directions, wind speeds, etc.). The determined source factors for the organic fraction of the aerosol from one season were constrained and taken as prior factors for the PMF analyses when the nitrate fraction was included. A potential artificial bias introduced by including the nitrate fraction in the PMF analysis can be excluded, as the PMF results with and without including the nitrate fraction were similar (Supplementary Table 5). The robustness of the PMF results was explored by a statistical analysis of 200 bootstrap runs performed by constrained PMF analysis with the random a-values method (Supplementary Figure 6). The elemental ratios for all factors were calculated based on the improved ambient method by Canagaratna et al. 64.
Calculations of NO3· concentrations, loss of VOC by oxidation with NO3· and O3 and SOA production rate of NO3· oxidation
Nitrate radical (NO3·) concentrations were calculated from measured N2O5 and NO2 concentrations using their thermal equilibrium65. The competition between the oxidation of VOC by NO3· and O3 (denoted relative importance) is used to describe the significance of NO3· for the oxidation of one species of VOCs (marked as species i)13. The value of averaged nighttime (UTC 18:00- 5:00, Day+1) NO3·, O3, and VOC concentrations, and calculated temperature-dependent reaction rate constants (Supplementary Table 1, NIST kinetics database https://kinetics.nist.gov/kinetics/KineticsSearchForm.jsp) were used for calculation.
| 1 |
A 10% SOA yield for the NO3·oxidation products of isoprene was used based on previous studies giving SOA yields between 2% and 15%15,37,66. The SOA yield for NO3· oxidation products of monoterpenes significantly varies for different monoterpene species (Supplementary Table 2). Assuming that α-pinene (SOA yield, 0.7–25%)67–69 was the most abundant monoterpene, followed by β-pinene (SOA yield, 5–55%)13,16,44 and limonene (44–231%)44,70, a lower limit for the SOA yield of 20% for NO3· oxidation products of monoterpenes was used in this work.
Calculations of the fraction of particulate organic nitrate
The fraction of particulate organic nitrate was determined from the relative ion mass signals of the NO2+ and NO+ fragments (Rmeasured) detected by the HR-ToF-AMS instrument following the fragment pattern approach24,71:
| 2 |
The ratio of NO2+ to NO+ ion mass signals for pure inorganic nitrate (Rcalib) was determined from calibration measurements of the HR-ToF-AMS instrument with ammonium nitrate particles. In this study, a ratio of 0.1 for pure organic nitrate (ROrgNO3)24 was used to calculate the concentrations of particulate organic nitrate.
Estimation of atmospheric layer heights
In this study, the heights of near-ground atmospheric layers were determined from the vertical profile of the potential temperature (). The potential temperature was calculated from the ambient temperature measurements at different heights between 2 m and 120 m on a tower close to the measurement site (Supplementary Fig. 15). A positive change in the potential temperature with height implies a stable atmosphere, whereas a negative change indicates an unstable or well-mixed atmosphere72,73.
The EURAD-IM
The regional chemistry transport model EURAD-IM (European Air pollution Dispersion–Inverse Model)74–76 was used to simulate atmospheric trace gas and aerosol concentrations in Europe during the JULIAC campaign. The dynamics within the EURAD-IM simulations are driven by meteorological forecasts using the Weather Research and Forecasting Model (WRF Version 3.7)77. Boundary conditions were extracted from the CAMS global reanalysis EAC478 for atmospheric constituents and the ERA5 reanalysis for meteorology79. The EURAD-IM includes anthropogenic as well as biogenic emissions of trace gases and aerosols. Anthropogenic emissions80 represent 2011 data for NH3, CO, NOx, SOx, NMVOCs, PM10, and PM2.5. Emissions of biogenic VOCs were calculated by the Model of Emissions of Gases and Aerosols from nature (MEGAN) V2.181. In EURAD-IM, the aerosol dynamics are simulated by the Modal Aerosol Dynamics Model for Europe (MADE)82 with the Secondary ORGanic Aerosol Model (SORGAM)83. EURAD-IM simulations with an improved representation of the NO3· oxidation of biogenic VOCs in the SOA module84 gave good agreement with measurements of organic nitrate in Europe24. The EURAD-IM was applied with a 9 × 9 km² horizontal resolution and 23 vertical terrain-following layers up to 100 hPa. A spin-up of 5 days for each period was used. Two model sensitivity analyses were performed: (1) the primary emissions of three types of phenolic compounds (phenol, catechol, and cresols) emitted from residential heating were included with an estimated emission ratio of 2.54 ppt ppb−1 normalized to CO emitted from residential heating85; (2) the SOA yield from the oxidation of phenolic compounds (by both OH· and NO3· oxidation) was increased from ~0.02 to ~0.356,57.
Supplementary information
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (SARLEP grant agreement No. 681529) and from the European Commission (EC) under the European Union’s Horizon 2020 research and innovation program (Eurochamp 2020 grant agreement No.730997). The authors gratefully acknowledge the Earth System Modelling Project (ESM) for funding this work by providing computing time on the ESM partition of the supercomputer JUWELS86 at the Jülich Supercomputing Centre (JSC).
Author contributions
Conception or design of the work: Lu Liu, Thorsten Hohaus, and Astrid Kiendler-Scharr. Data collection: Lu Liu, Thorsten Hohaus, Ralf Tillmann, Hendrik Fuchs, Stefanie Andres, Birger Bohn, Frank Holland, Franz Rohrer, Vaishali Vardhan, Benjamin Winter, Sergej Wedel, Anna Novelli, and Andreas Hofzumahaus. Data analysis and interpretation: Lu Liu, Thorsten Hohaus, Astrid Kiendler-Scharr, Philipp Franke, Anne C. Lange, Zhaofeng Tan, Vlassis Karydis, Birger Bohn, Quanfu He, Andreas Wahner, Modelling: Philipp Franke, and Anne C. Lange, Drafting the article: Lu Liu. Critical revision of the article: Thorsten Hohaus, Astrid Kiendler-Scharr, and Hendrik Fuchs.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data used in this study are available from the Jülich DATA platform (10.26165/JUELICH-DATA/TPPXNL).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: Astrid Kiendler-Scharr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41612-024-00747-6.
References
- 1.Jimenez, J. L. et al. Evolution of organic aerosols in the atmosphere. Science326, 1525–1529 (2009). 10.1126/science.1180353 [DOI] [PubMed] [Google Scholar]
- 2.Zhang, Q. et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 34, L13801 (2007). 10.1029/2007GL029979 [DOI] [Google Scholar]
- 3.Hallquist, M. et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys.9, 5155–5236 (2009). 10.5194/acp-9-5155-2009 [DOI] [Google Scholar]
- 4.Mahowald, N. Aerosol indirect effect on biogeochemical cycles and climate. Science334, 794–796 (2011). 10.1126/science.1207374 [DOI] [PubMed] [Google Scholar]
- 5.Nel, A. Air pollution-related illness: effects of particles. Science308, 804–806 (2005). 10.1126/science.1108752 [DOI] [PubMed] [Google Scholar]
- 6.Ziemann, P. J. & Atkinson, R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chem. Soc. Rev.41, 6582–6605 (2012). 10.1039/c2cs35122f [DOI] [PubMed] [Google Scholar]
- 7.Hodzic, A. et al. Rethinking the global secondary organic aerosol (SOA) budget: stronger production, faster removal, shorter lifetime. Atmos. Chem. Phys.16, 7917–7941 (2016). 10.5194/acp-16-7917-2016 [DOI] [Google Scholar]
- 8.Zhang, Q. et al. Understanding atmospheric organic aerosols via factor analysis of aerosol mass spectrometry: a review. Anal. Bioanal. Chem.401, 3045–3067 (2011). 10.1007/s00216-011-5355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crippa, M. et al. Wintertime aerosol chemical composition and source apportionment of the organic fraction in the metropolitan area of Paris. Atmos. Chem. Phys.13, 961–981 (2013). 10.5194/acp-13-961-2013 [DOI] [Google Scholar]
- 10.Mohr, C. et al. Identification and quantification of organic aerosol from cooking and other sources in Barcelona using aerosol mass spectrometer data. Atmos. Chem. Phys.12, 1649–1665 (2012). 10.5194/acp-12-1649-2012 [DOI] [Google Scholar]
- 11.Canonaco, F., Slowik, J. G., Baltensperger, U. & Prévôt, A. S. H. Seasonal differences in oxygenated organic aerosol composition: implications for emissions sources and factor analysis. Atmos. Chem. Phys.15, 6993–7002 (2015). 10.5194/acp-15-6993-2015 [DOI] [Google Scholar]
- 12.Sun, Y. L. et al. Factor analysis of combined organic and inorganic aerosol mass spectra from high resolution aerosol mass spectrometer measurements. Atmos. Chem. Phys.12, 8537–8551 (2012). 10.5194/acp-12-8537-2012 [DOI] [Google Scholar]
- 13.Xu, L. et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. Proc. Natl Acad. Sci. USA112, E4506–E4507 (2015). 10.1073/pnas.1417609112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, G. et al. Time-dependent source apportionment of submicron organic aerosol for a rural site in an alpine valley using a rolling positive matrix factorisation (PMF) window. Atmos. Chem. Phys.21, 15081–15101 (2021). 10.5194/acp-21-15081-2021 [DOI] [Google Scholar]
- 15.Ng, N. L. et al. Secondary organic aerosol (SOA) formation from reaction of isoprene with nitrate radicals (NO3). Atmos. Chem. Phys.8, 4117–4140 (2008). 10.5194/acp-8-4117-2008 [DOI] [Google Scholar]
- 16.Fry, J. L. et al. Organic nitrate and secondary organic aerosol yield from NO3 oxidation of β-pinene evaluated using a gas-phase kinetics/aerosol partitioning model. Atmos. Chem. Phys.9, 1431–1449 (2009). 10.5194/acp-9-1431-2009 [DOI] [Google Scholar]
- 17.Hoyle, C. R., Berntsen, T., Myhre, G. & Isaksen, I. S. A. Secondary organic aerosol in the global aerosol - chemical transport model Oslo CTM2. Atmos. Chem. Phys.7, 5675–5694 (2007). 10.5194/acp-7-5675-2007 [DOI] [Google Scholar]
- 18.Pye, H. O. T., Chan, A. W. H., Barkley, M. P. & Seinfeld, J. H. Global modeling of organic aerosol: the importance of reactive nitrogen (NOx and NO3). Atmos. Chem. Phys.10, 11261–11276 (2010). 10.5194/acp-10-11261-2010 [DOI] [Google Scholar]
- 19.Zhang, Y. et al. Six-year source apportionment of submicron organic aerosols from near-continuous highly time-resolved measurements at SIRTA (Paris area, France). Atmos. Chem. Phys.19, 14755–14776 (2019). 10.5194/acp-19-14755-2019 [DOI] [Google Scholar]
- 20.Saarikoski, S. et al. Chemical characterization of springtime submicrometer aerosol in Po Valley, Italy. Atmos. Chem. Phys.12, 8401–8421 (2012). 10.5194/acp-12-8401-2012 [DOI] [Google Scholar]
- 21.Florou, K. et al. The contribution of wood burning and other pollution sources to wintertime organic aerosol levels in two Greek cities. Atmos. Chem. Phys.17, 3145–3163 (2017). 10.5194/acp-17-3145-2017 [DOI] [Google Scholar]
- 22.Cheng, Y., Ma, Y. & Hu, D. Tracer-based source apportioning of atmospheric organic carbon and the influence of anthropogenic emissions on secondary organic aerosol formation in Hong Kong. Atmos. Chem. Phys.21, 10589–10608 (2021). 10.5194/acp-21-10589-2021 [DOI] [Google Scholar]
- 23.Huang, W. et al. Chemical characterization of highly functionalized organonitrates contributing to night-time organic aerosol mass loadings and particle growth. Environ. Sci. Technol.53, 1165–1174 (2019). 10.1021/acs.est.8b05826 [DOI] [PubMed] [Google Scholar]
- 24.Kiendler-Scharr, A. et al. Ubiquity of organic nitrates from nighttime chemistry in the European submicron aerosol. Geophys. Res. Lett.43, 7735–7744 (2016). 10.1002/2016GL069239 [DOI] [Google Scholar]
- 25.Tsimpidi, A. P., Karydis, V. A., Pandis, S. N. & Lelieveld, J. Global combustion sources of organic aerosols: model comparison with 84 AMS factor-analysis data sets. Atmos. Chem. Phys.16, 8939–8962 (2016). 10.5194/acp-16-8939-2016 [DOI] [Google Scholar]
- 26.Kodros, J. K. et al. Rapid dark aging of biomass burning as an overlooked source of oxidized organic aerosol. Proc. Natl Acad. Sci. USA117, 33028–33033 (2020). 10.1073/pnas.2010365117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson, D. et al. Global increase in wildfire potential from compound fire weather and drought. npj Clim. Atmos. Sci.5, 23 (2022). 10.1038/s41612-022-00248-4 [DOI] [Google Scholar]
- 28.Tian, J., Chen, X., Cao, Y. & Chen, F. Satellite observational evidence of contrasting changes in northern Eurasian wildfires from 2003 to 2020. Remote Sens.14, 4180 (2022). 10.3390/rs14174180 [DOI] [Google Scholar]
- 29.Bertelsen, N. & Vad Mathiesen, B. EU-28 residential heat supply and consumption: historical development and status. Energies13, 1894 (2020). 10.3390/en13081894 [DOI] [Google Scholar]
- 30.Mishra, S. et al. Rapid night-time nanoparticle growth in Delhi driven by biomass-burning emissions. Nat. Geosci.16, 224–230 (2023). 10.1038/s41561-023-01138-x [DOI] [Google Scholar]
- 31.Steffen, B. & Patt, A. A historical turning point? Early evidence on how the Russia-Ukraine war changes public support for clean energy policies. Energy Res. Soc. Sci.91, 102758 (2022). 10.1016/j.erss.2022.102758 [DOI] [Google Scholar]
- 32.Tan, Z. et al. Seasonal variation of nitryl chloride and its relation to gas-phase precursors during the JULIAC campaign in Germany. Atmos. Chem. Phys. Discuss.2022, 1–30 (2022). [Google Scholar]
- 33.Cho, C. et al. Experimental chemical budgets of OH, HO2 and RO2 radicals in rural air in West-Germany during the JULIAC campaign 2019. EGUsphere2022, 1–51 (2022). [Google Scholar]
- 34.Bohn, B. & Zilken, H. Model-aided radiometric determination of photolysis frequencies in a sunlit atmosphere simulation chamber. Atmos. Chem. Phys.5, 191–206 (2005). 10.5194/acp-5-191-2005 [DOI] [Google Scholar]
- 35.Bohn, B., Rohrer, F., Brauers, T. & Wahner, A. Actinometric measurements of NO2 photolysis frequencies in the atmosphere simulation chamber SAPHIR. Atmos. Chem. Phys.5, 493–503 (2005). 10.5194/acp-5-493-2005 [DOI] [Google Scholar]
- 36.Hildebrandt, L. et al. Aged organic aerosol in the Eastern Mediterranean: the Finokalia Aerosol Measurement Experiment-2008. Atmos. Chem. Phys.10, 4167–4186 (2010). 10.5194/acp-10-4167-2010 [DOI] [Google Scholar]
- 37.Brownwood, B. et al. Gas-particle partitioning and SOA yields of organonitrate products from NO3-initiated oxidation of isoprene under varied chemical regimes. ACS Earth Space Chem.5, 785–800 (2021). 10.1021/acsearthspacechem.0c00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilardoni, S. et al. Direct observation of aqueous secondary organic aerosol from biomass-burning emissions. Proc. Natl Acad. Sci. USA113, 10013–10018 (2016). 10.1073/pnas.1602212113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge, X. L., Zhang, Q., Sun, Y. L., Ruehl, C. R. & Setyan, A. Effect of aqueous-phase processing on aerosol chemistry and size distributions in Fresno, California, during wintertime. Environ. Chem.9, 221–235 (2012). 10.1071/EN11168 [DOI] [Google Scholar]
- 40.Fountoukis, C. et al. Thermodynamic characterization of Mexico City aerosol during MILAGRO 2006. Atmos. Chem. Phys.9, 2141–2156 (2009). 10.5194/acp-9-2141-2009 [DOI] [Google Scholar]
- 41.Nowak, J. B. et al. Analysis of urban gas phase ammonia measurements from the 2002 Atlanta Aerosol Nucleation and Real-Time Characterization Experiment (ANARChE). J. Geophys. Res. Atmos. 111, D17308 (2006). 10.1029/2006JD007113 [DOI] [Google Scholar]
- 42.Li, C. et al. Formation of secondary brown carbon in biomass burning aerosol proxies through NO3 radical reactions. Environ. Sci. Technol.54, 1395–1405 (2020). 10.1021/acs.est.9b05641 [DOI] [PubMed] [Google Scholar]
- 43.Kostenidou, E., Lee, B.-H., Engelhart, G. J., Pierce, J. R. & Pandis, S. N. Mass spectra deconvolution of low, medium, and high volatility biogenic secondary organic aerosol. Environ. Sci. Technol.43, 4884–4889 (2009). 10.1021/es803676g [DOI] [PubMed] [Google Scholar]
- 44.Boyd, C. M., Nah, T., Xu, L., Berkemeier, T. & Ng, N. L. Secondary Organic Aerosol (SOA) from nitrate radical oxidation of monoterpenes: effects of temperature, dilution, and humidity on aerosol formation, mixing, and evaporation. Environ. Sci. Technol.51, 7831–7841 (2017). 10.1021/acs.est.7b01460 [DOI] [PubMed] [Google Scholar]
- 45.Hao, L. et al. Combined effects of boundary layer dynamics and atmospheric chemistry on aerosol composition during new particle formation periods. Atmos. Chem. Phys.18, 17705–17716 (2018). 10.5194/acp-18-17705-2018 [DOI] [Google Scholar]
- 46.Huffman, J. A. et al. Chemically-resolved aerosol volatility measurements from two megacity field studies. Atmos. Chem. Phys.9, 7161–7182 (2009). 10.5194/acp-9-7161-2009 [DOI] [Google Scholar]
- 47.Pye, H. O. T. et al. Effect of changes in climate and emissions on future sulfate-nitrate-ammonium aerosol levels in the United States. J. Geophys. Res.: Atmos. 114, D01205 (2009). [Google Scholar]
- 48.Zuend, A., Marcolli, C., Peter, T. & Seinfeld, J. H. Computation of liquid-liquid equilibria and phase stabilities: implications for RH-dependent gas/particle partitioning of organic-inorganic aerosols. Atmos. Chem. Phys.10, 7795–7820 (2010). 10.5194/acp-10-7795-2010 [DOI] [Google Scholar]
- 49.Li, Y. J., Lee, B. P., Su, L., Fung, J. C. H. & Chan, C. K. Seasonal characteristics of fine particulate matter (PM) based on high-resolution time-of-flight aerosol mass spectrometric (HR-ToF-AMS) measurements at the HKUST Supersite in Hong Kong. Atmos. Chem. Phys.15, 37–53 (2015). 10.5194/acp-15-37-2015 [DOI] [Google Scholar]
- 50.Chen, Q. et al. Submicron particle mass concentrations and sources in the Amazonian wet season (AMAZE-08). Atmos. Chem. Phys.15, 3687–3701 (2015). 10.5194/acp-15-3687-2015 [DOI] [Google Scholar]
- 51.Paglione, M. et al. Identification of humic-like substances (HULIS) in oxygenated organic aerosols using NMR and AMS factor analyses and liquid chromatographic techniques. Atmos. Chem. Phys.14, 25–45 (2014). 10.5194/acp-14-25-2014 [DOI] [Google Scholar]
- 52.Thamban, N. M. et al. Evolution of aerosol size and composition in the indo-gangetic plain: size-resolved analysis of high-resolution aerosol mass spectra. ACS Earth Space Chem.3, 823–832 (2019). 10.1021/acsearthspacechem.8b00207 [DOI] [Google Scholar]
- 53.Zhang, Y. J. et al. Insights into characteristics, sources, and evolution of submicron aerosols during harvest seasons in the Yangtze River delta region, China. Atmos. Chem. Phys.15, 1331–1349 (2015). 10.5194/acp-15-1331-2015 [DOI] [Google Scholar]
- 54.Carter, T. S. et al. An improved representation of fire non-methane organic gases (NMOGs) in models: emissions to reactivity. Atmos. Chem. Phys.22, 12093–12111 (2022). 10.5194/acp-22-12093-2022 [DOI] [Google Scholar]
- 55.Decker, Z. C. J. et al. Nighttime and daytime dark oxidation chemistry in wildfire plumes: an observation and model analysis of FIREX-AQ aircraft data. Atmos. Chem. Phys.21, 16293–16317 (2021). 10.5194/acp-21-16293-2021 [DOI] [Google Scholar]
- 56.Finewax, Z., de Gouw, J. A. & Ziemann, P. J. Identification and quantification of 4-nitrocatechol formed from OH and NO3 radical-initiated reactions of catechol in air in the presence of NOx: implications for secondary organic aerosol formation from biomass burning. Environ. Sci. Technol.52, 1981–1989 (2018). 10.1021/acs.est.7b05864 [DOI] [PubMed] [Google Scholar]
- 57.Mayorga, R. J., Zhao, Z. & Zhang, H. Formation of secondary organic aerosol from nitrate radical oxidation of phenolic VOCs: implications for nitration mechanisms and brown carbon formation. Atmos. Environ.244, 117910 (2021). 10.1016/j.atmosenv.2020.117910 [DOI] [Google Scholar]
- 58.Rohrer, F. et al. Characterisation of the photolytic HONO-source in the atmosphere simulation chamber SAPHIR. Atmos. Chem. Phys.5, 2189–2201 (2005). 10.5194/acp-5-2189-2005 [DOI] [Google Scholar]
- 59.Paatero, P. & Tapper, U. Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics5, 111–126 (1994). 10.1002/env.3170050203 [DOI] [Google Scholar]
- 60.Crippa, M. et al. Organic aerosol components derived from 25 AMS data sets across Europe using a consistent ME-2 based source apportionment approach. Atmos. Chem. Phys.14, 6159–6176 (2014). 10.5194/acp-14-6159-2014 [DOI] [Google Scholar]
- 61.Dai, Q. et al. Seasonal differences in formation processes of oxidized organic aerosol near Houston, TX. Atmos. Chem. Phys.19, 9641–9661 (2019). 10.5194/acp-19-9641-2019 [DOI] [Google Scholar]
- 62.Canonaco, F. et al. SoFi, an IGOR-based interface for the efficient use of the generalized multilinear engine (ME-2) for the source apportionment: ME-2 application to aerosol mass spectrometer data. Atmos. Meas. Tech.6, 3649–3661 (2013). 10.5194/amt-6-3649-2013 [DOI] [Google Scholar]
- 63.Paatero, P. The multilinear engine: a table-driven, least squares program for solving multilinear problems, including the n-way parallel factor analysis model. J. Comput. Graph. Stat.8, 854–888 (1999). [Google Scholar]
- 64.Canagaratna, M. R. et al. Elemental ratio measurements of organic compounds using aerosol mass spectrometry: characterization, improved calibration, and implications. Atmos. Chem. Phys.15, 253–272 (2015). 10.5194/acp-15-253-2015 [DOI] [Google Scholar]
- 65.Brown, S. S. et al. Nitrogen oxides in the nocturnal boundary layer: simultaneous in situ measurements of NO3, N2O5, NO2, NO, and O3. J. Geophys. Res. Atmos.108, n/a–n/a (2003). 10.1029/2002JD002917 [DOI] [Google Scholar]
- 66.Rollins, A. W. et al. Isoprene oxidation by nitrate radical: alkyl nitrate and secondary organic aerosol yields. Atmos. Chem. Phys.9, 6685–6703 (2009). 10.5194/acp-9-6685-2009 [DOI] [Google Scholar]
- 67.Hallquist, M., Wängberg, I., Ljungström, E., Barnes, I. & Becker, K. Aerosol and product yields from NO3 radical-initiated oxidation of selected monoterpenes. Environ. Sci. Technol.33, 553–559 (1999). 10.1021/es980292s [DOI] [Google Scholar]
- 68.Mutzel, A. et al. Importance of secondary organic aerosol formation of α-pinene, limonene, and m-cresol comparing day- and nighttime radical chemistry. Atmos. Chem. Phys.21, 8479–8498 (2021). 10.5194/acp-21-8479-2021 [DOI] [Google Scholar]
- 69.Day, D. A. et al. Secondary organic aerosol mass yields from NO3 oxidation of α-pinene And Δ-carene: effect of RO2 radical fate. J. Phys. Chem. A126, 7309–7330 (2022). 10.1021/acs.jpca.2c04419 [DOI] [PubMed] [Google Scholar]
- 70.Fry, J. L. et al. Secondary organic aerosol formation and organic nitrate yield from NO3 oxidation of biogenic hydrocarbons. Environ. Sci. Technol.48, 11944–11953 (2014). 10.1021/es502204x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kulmala, M. et al. General overview: European Integrated project on Aerosol Cloud Climate and Air Quality interactions (EUCAARI) - integrating aerosol research from nano to global scales. Atmos. Chem. Phys.11, 13061–13143 (2011). 10.5194/acp-11-13061-2011 [DOI] [Google Scholar]
- 72.Moore, D. J. T. Isentropic Analysis and Interpretation: Isentropic Analysis Techniques, Basic Concepts (National Weather Service Training Center, 1999).
- 73.Batchvarova, E. & Gryning, S.-E. An applied model for the height of the daytime mixed layer and the entrainment zone. Bound.-Layer. Meteorol.71, 311–323 (1994). 10.1007/BF00713744 [DOI] [Google Scholar]
- 74.Hass, H., Jakobs, H. & Memmesheimer, M. Analysis of a regional model (EURAD) near surface gas concentration predictions using observations from networks. Meteorol. Atmos. Phys.57, 173–200 (1995). 10.1007/BF01044160 [DOI] [Google Scholar]
- 75.Memmesheimer, M. et al. Long-term simulations of particulate matter in Europe on different scales using sequential nesting of a regional model. Int. J. Environ. Pollut.22, 108–132 (2004). 10.1504/IJEP.2004.005530 [DOI] [Google Scholar]
- 76.Elbern, H., Strunk, A., Schmidt, H. & Talagrand, O. Emission rate and chemical state estimation by 4-dimensional variational inversion. Atmos. Chem. Phys.7, 3749–3769 (2007). 10.5194/acp-7-3749-2007 [DOI] [Google Scholar]
- 77.Skamarock, W. C. et al. A description of the advanced research WRF version 3. UCAR27, 3–27 (2008). [Google Scholar]
- 78.Inness, A. et al. The CAMS reanalysis of atmospheric composition. Atmos. Chem. Phys.19, 3515–3556 (2019). 10.5194/acp-19-3515-2019 [DOI] [Google Scholar]
- 79.Hersbach, H. et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc.146, 1999–2049 (2020). 10.1002/qj.3803 [DOI] [Google Scholar]
- 80.Kuenen, J. J. P., Visschedijk, A. J. H., Jozwicka, M. & Denier van der Gon, H. A. C. TNO-MACC_II emission inventory; a multi-year (2003–2009) consistent high-resolution European emission inventory for air quality modelling. Atmos. Chem. Phys.14, 10963–10976 (2014). 10.5194/acp-14-10963-2014 [DOI] [Google Scholar]
- 81.Guenther, A. B. et al. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model Dev.5, 1471–1492 (2012). 10.5194/gmd-5-1471-2012 [DOI] [Google Scholar]
- 82.Ackermann, I. J., Hass, H., Schell, B. & Binkowski, F. S. Regional modelling of particulate matter with MADE. Environ. Manag. Health10, 201–208 (1999). 10.1108/09566169910276012 [DOI] [Google Scholar]
- 83.Schell, B., Ackermann, I. J., Hass, H., Binkowski, F. S. & Ebel, A. Modeling the formation of secondary organic aerosol within a comprehensive air quality model system. J. Geophys. Res.106, 28275–28293 (2001). 10.1029/2001JD000384 [DOI] [Google Scholar]
- 84.Li, Y. P. et al. Updated aerosol module and its application to simulate secondary organic aerosols during IMPACT campaign May 2008. Atmos. Chem. Phys.13, 6289–6304 (2013). 10.5194/acp-13-6289-2013 [DOI] [Google Scholar]
- 85.Palm, B. B. et al. Quantification of organic aerosol and brown carbon evolution in fresh wildfire plumes. Proc. Natl Acad. Sci. USA117, 29469–29477 (2020). 10.1073/pnas.2012218117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alvarez, D. JUWELS cluster and booster: exascale pathfinder with modular supercomputing architecture at juelich supercomputing Centre. J. Large-scale Res. Facilities JLSRF7, A183–A183 (2021). 10.17815/jlsrf-7-183 [DOI] [Google Scholar]
- 87.Aiken, A. C. et al. Mexico City aerosol analysis during MILAGRO using high resolution aerosol mass spectrometry at the urban supersite (T0) – Part 1: Fine particle composition and organic source apportionment. Atmos. Chem. Phys.9, 6633–6653 (2009). 10.5194/acp-9-6633-2009 [DOI] [Google Scholar]
- 88.Cubison, M. J. et al. Effects of aging on organic aerosol from open biomass burning smoke in aircraft and laboratory studies. Atmos. Chem. Phys.11, 12049–12064 (2011). 10.5194/acp-11-12049-2011 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available from the Jülich DATA platform (10.26165/JUELICH-DATA/TPPXNL).