Abstract
This study aimed to assess volatile impurities and ethanol content in ethanol-based hand sanitizers. A total of 31 different brands of hand sanitizers were analyzed using headspace gas chromatography-mass spectrometry to detect impurities and determine alcohol content for compliance. Volatile impurities were identified through Mass Spectrometry database analysis, and regression analysis was employed to ascertain ethanol percentage. Furthermore, a simulated toxicological analysis was conducted to evaluate the potential toxic effects associated with hand sanitizer usage.
The detected impurities primarily included ethyl acetate, benzene, acetone, and acetal, along with contaminations such as isobutanol and non-recommended alcohols. In addition, 71 % of samples contained less than the recommended 60 % v/v alcohol concentration, failing to comply with guidelines from the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). Additionally, the simulation study underscored acute and chronic toxicities primarily linked to benzene contamination.
Given that some of the studied products are imported while others are locally produced, it is imperative for consumers worldwide to be informed that certain hand sanitizers may not only be ineffective but also contain harmful residues.
Keywords: Hand sanitizer, COVID-19, Impurities, Headspace gas chromatography, Alcohol content
Highlights
-
•
Only six out of 31 samples met the compliance criteria of both the alcohol percentage and absence of impurities.
-
•
The simulation study highlights the toxicity of two impurities, i.e., benzene and 1-propanol.
-
•
Our recommendation is to increase awareness on the use of high-quality alcohol based hand sanitizers.
1. Introduction
Staring December 2019, cases of coronavirus disease inflicting the respiratory system and causing deaths were initially reported in Wuhan, China [18]. Thereafter, the number of cases started to increase noticeably not only in China but worldwide [22]. Diverse modes of transmission were reported for severe-acute-respiratory-syndrome-related coronavirus (SARS-CoV-2), including aerosol, surface contamination, direct contact, and the fecal-oral route. Respiratory droplets are predominantly the common route of coronavirus disease transmission [14]. A simple and yet effective method to decrease transmission of infections in public or in healthcare settings is hand hygiene [3]. Thus, the widespread use of hand sanitizers has emerged.
Hand sanitizers can be commonly classified into two groups: alcohol-based or alcohol free. While the first type contains one or more type of alcohol, with or without excipients, the second type does not contain alcohol [17]. The Centers for Disease Control and Prevention (CDC) has recommended the use of hand sanitizers with at least 60 % alcohol (often listed on the labels ethanol, ethyl alcohol, isopropanol, or 2-propanol). CDC [5], and opposed the use of alcohol-free sanitizers against coronavirus [13]. Consequently, in the fight against SARS-CoV-2, alcohol-based hand-sanitizers (ABHS) have emerged as a crucial tool. The most widely used alcohols in ABHS are ethanol and isopropanol (2-propanol) [7]. ABHS are usually made up of water and a variety of additional substances like emollients, moisturizers, and perfumes [19]. Two formulations have been established by the World Health Organization (WHO) based on either ethanol (80 % v/v) or isopropanol (75 % v/v) with glycerol (1.45 % v/v) and hydrogen peroxide (0.125 % v/v). WHO [32]
According to the WHO, these formulations cover a broad spectrum of microorganisms [1]. According to the United States Food and Drug Administration (US FDA), CDC, and the WHO, concentrations of 60–95 % (v/v) are suitable for eliminating microorganisms including SARS-CoV-2 [30].
Amid the widespread outbreak of Coronavirus disease, there has been a notable surge in the use of hand sanitizers by consumers. This increased demand has subsequently led to shortages in these products, prompting a rapid escalation in manufacturing by various companies. Prajapati et al. [25]. The scarcity of hand sanitizers has, at times, resulted in a rise in the prevalence of falsified alcohol-based hand sanitizers. Falsification has occurred through the illicit addition of methanol or other impurities to hand sanitizers, posing an elevated risk of accidental toxicity among users. FDA [11] Additionally, the production of hand sanitizers with an alcohol concentration below 60 % diminishes their compliance, further exacerbating concerns regarding public health and safety [11].
2. Objectives and aims
The purpose of this study is to evaluate various finished ethanol-based hand sanitizer products on the Lebanese market. This will involve 1) identifying and quantifying the volatile impurities present, 2) determining the percentage of ethanol, and 3) performing a toxicological simulation assessment of the detected impurities.
3. Materials and methods
3.1. Evaluation method
With the increase of falsified, ineffective, and possibly toxic hand sanitizers on the market, the FDA issued in the FDA guidance for hand sanitizer products a list of impurities classified as Level 1 or Level 2, relying on the toxicity of the impurities in the sanitizer, where Level 1 impurities are considered to be more toxic. Table 1 displays Level 1 and Level 2 impurities and their limit values [27].
Table 1.
FDA list of impurities and detectable concentration ranges [27].
| Compound Name | Interim Limit Listed in FDA Guidance (ppm) | Concentration Ranges for this Method (μg/mL) | ||
|---|---|---|---|---|
| Impurity | Level 1 | Methanol | NMT 630 | 15.82–791 |
| Benzene | NMT 2 | 0.044–2.2 | ||
| Acetaldehyde | NMT 50 | 1.178–58.9 | ||
| 1,1-diethoxyethane (acetal) | NMT 50 | 1.245–62.25 | ||
| Acetone | NMT 4400 | 15.8–790 | ||
| Level 2 | 1-Propanol | NMT 1000 | 16.08–804 | |
| Ethyl Acetate | NMT 2200 | 18.04–902 | ||
| 2-Butanol | NMT 6200 | 16.16–808 | ||
| Isobutanol | NMT 21700 | 16.06–803 | ||
| 1-Butanol | NMT 1000 | 16.2–810 | ||
| 3-Metyl−1-Butanol | NMT 4100 | 16.18–809 | ||
| Amyl Alcohol | NMT 4100 | 16.22–811 |
In addition, the FDA has set a “Direct Injection Gas Chromatography Mass Spectrometry (GC-MS) Method for the Detection of Listed Impurities in Hand Sanitizers” for quality assessment of hand sanitizers [10].
In this study, the authors used the qualitative and quantitative analysis of volatile impurities in ethanol-based sanitizers following the headspace GC-MS, in compliance with the FDA hand sanitizer analysis method [27].
3.2. Analytical system and conditions
Table 2 details the analytical system used along with the recommended conditions.
Table 2.
HS-GC-MS analytical conditions for analysis of impurities in hand sanitizers.
| Analytical System | |
|---|---|
| GC- MS | GCMS-QP2020 NX |
| Headspace Autosampler | HS−20 |
| Column | PoraBOND Q (25 m x 0.25 mm I.D., d.f. = 3 µm) |
| HS Parameters | |
| Oven Temperature | 85°C |
| Sample Line Temperature | 150°C |
| Transfer Line Temperature | 160°C |
| Injection Time | 1 min |
| Pressurizing Gas Pressure | 90 kPa |
| Equilibrating Time | 10 mins |
| Shaking Level | 2 |
| GC Cycle Time | 30 mins |
| GC Parameters | |
| Column temperature | 50°C |
| Injection Mode | Split mode |
| Split ratio 50 | |
| Carrier Gas | Helium |
| Gas Flow Condition | Constant linear velocity mode |
| Linear velocity 43.4 cm/s | |
| Oven Temperature Programming | 50°C (1 min) |
| → 20 °C/min to 250°C (9 mins) | |
| Column Flow | 1.19 mL/min |
| Injection Volume | 1.0 µL |
| MS Parameters | |
| Ion source temperature | 230°C |
| Interface temperature | 300°C |
| Measurement mode | Scan/SIM |
| Scan range | m/z 24–200 |
| MSD Solvent Delay | No solvent delay |
| MSD m/z Settings | Start time (0 min) |
| End Time (20 min) | |
| Acquisition mode (SIM/Scan) | |
3.3. Equipment/instrument
Among the equipment and instruments employed was the Shimadzu HS-20 headspace – GCMS- QP2020 NX machine to perform the experiments. The column used in the study was PoraBOND Q (25 m x 0.25 mm I.D., d.f. = 3 µm) purchased from Shimadzu. Solutions were measured and transferred using eppendorf Pipets and pipet tips. Conical tubes served as containers for the final diluted solutions of hand sanitizers. A 100 mL beaker was utilized to measure solvents and solutions. Finally, a 20 mL Headspace vial w/ 20 mm AL Crimp Cap & Silicone/PTFE Septa was utilized for the assessment of samples using HS-GCMS.
3.4. Chemicals
Liquid Chromatography (LC-MS) grade water (Lot No. 1714121, Code: X/0112/17) was kindly provided by Fisher SCIENTIFIC, UK. Acetonitrile >= 99.9 % (Lot. No. 1872272, Code: A/0638/17).
Absolute ethanol HPLC grade (Lot. No. 1852958, Code: E/0665DF/17) was purchased from Fisher SCIENTIFIC UK. 1-Butanol 99 %, extra pure (Lot. No. A0324323, Code: 107690025) was purchased from Acros Organics.
3.4.1. LOD/LOQ determination
The Limit of Detection (LOD) and Limit of Quantification (LOQ) were determined according to the International Conference on Harmonization (ICH) Q2(R1) guidelines. The LOD and LOQ were calculated using the following formulas:
Where σ is the standard deviation of the blank responses (noise) and S is the slope of the calibration curve obtained from the linear regression analysis of the standard concentrations. The limit of detection (LOD) was estimated to be ∼ 3 % (LOD = 3.3(Sy/S)) where Sy is the standard deviation of the response and S is the slope of the calibration curve and the limit of quantification (LOQ) was estimated to be ∼ 10 % (LOQ = 10(Sy/S)). All percentages of ethanol identified in this work were above the LOQ. (international conference on harmonisation of technical requirements for registration of pharmaceuticals for human use-2005) [15].
Spike-Recovery Experiments: To evaluate the matrix effect and confirm laboratory performance, spike-recovery experiments were conducted:
Preparation of Spiked Samples: Blank matrix samples were spiked with known concentrations of the analyte and the internal standard at low, medium, and high levels.
Sample Analysis: Spiked samples were analyzed using the headspace GC-MS method.
Recovery Calculation: Recovery was calculated as:
Evaluation Criteria: Acceptable recoveries ranged from 85 % to 115 %, per FDA guidelines (2020).
Results: Spike-recovery results indicated minimal matrix effects, with recoveries ranging from 87 % to 112 %, confirming the method's robustness and reliability. Shimadzu [27]
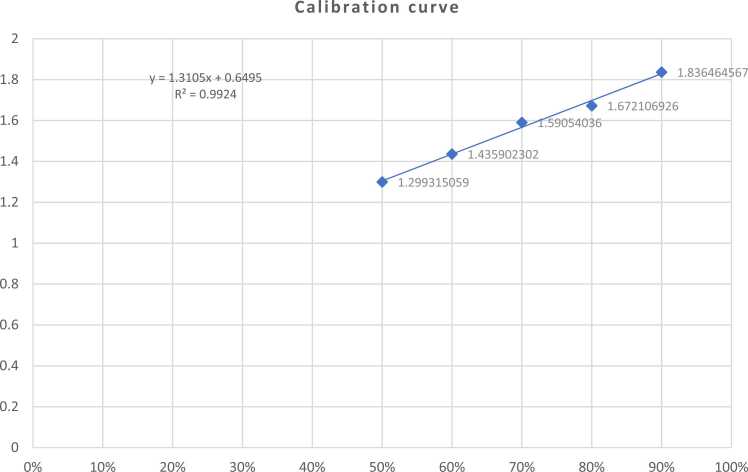
Calibration Range and Validation: The calibration curve in Fig. 3 was generated using standard solutions with concentrations ranging from 50 % to 90 % ethanol. Some reported values fall below this calibration range.
Fig. 3.
Linear regression of calibration curve of Ethanol concentration with (AS/AIS).
3.5. Hand sanitizer samples
The study focused on ethanol-based hand sanitizers available in the Lebanese market, encompassing both domestically manufactured and imported products. These hand sanitizers were procured by the School of Pharmacy at the Lebanese American University. Samples were obtained from various community and hospital pharmacies as well as supermarkets located in urban and rural areas. In total, 31 different brands of hand sanitizers were included in the study, with formulations ranging from gels to sprays; 38.7 % of the samples were sprays, while 61.2 % were gels. All samples analyzed in this research were ethanol-based.
The study spanned a two-year period from 2021 to 2022. Sample collection and analysis took place in 2022, with the products having expiry dates ranging from 2023 to 2025.
3.6. Development of headspace GC-MS method for the determination and semi-quantification of volatile impurities
A developed method was used to identify and relatively quantify the impurities present in the samples. The standards and samples were prepared in accordance with the FDA hand sanitizer analysis method. In order to prepare the diluted hand sanitizer solution, 1 mL of hand sanitizer was measured using a micropipette and topped up to 44 mL with LC-MS grade water. 90 µL of acetonitrile (surrogate standard) and 90 µL of n-butanol (internal standard) at 2 % (1620 µg/mL) were added to the dilution to reach a final volume of 45.18 mL. From this prepared mixture, 5 mL were transferred to a 20 mL headspace vial for the analysis. This method of preparation was performed for all 31 samples of hand sanitizers. The experiment was carried out in triplicate and repeated three times on consecutive days. A GCMS-QP2020 NX gas chromatograph mass spectrometer (refer to Table 2 for GC-MS detailed parameters) and GC-MS Real Time Analysis software were used for the detection (Schimadzu, Japan). The results are measured as mean +/- standard deviation. Volatile impurities were identified and semi-quantified based on internal calibration using data obtained from the mass spectrometer. The relative concentrations of the impurities were therefore determined based the internal standard concentration (1620 µg/mL). The estimation was based on the relative concentration and area under the curve of the internal standard in relation to the area under the curve of the desired impurity. A blank solution was injected (Water- LC-MS Grade) at least once at the beginning of a sequence and between samples.
3.7. Determination and relative quantification of volatile impurities
Impurities content was estimated using the following equation and reported as an average concentration range:
Where A compound represents the average Area of runs of a selected impurity. The average of the studied areas was used as a reference since each sample was run in triplicate.
1620 ug/mL represents the concentration of the internal standard employed and A butanol represents the average Area of runs of n-butanol.
In this study, the volatile impurities were identified based on mass spectrometry Nationl Institute of Standards and Technology (NIST) database and through the comparison of a peak area of an impurity in a sample chromatogram to the peak area of the internal standard n-butanol at 2 % (1620 μg/mL).
3.8. Development and validation of headspace GC-MS assay method for the determination of alcohol % in hand sanitizer samples
To determine and quantify alcohol % present in the samples, the developed method described above was adopted and validated based on a modified literature method [21]. Quantitative dilutions were performed from the stock solution to prepare standard solutions at 90 %, 80 %, 70 %, 60 %, 50 % of ethanol respectively. To obtain the ethanol percentages of 90 %, 80 %, 70 %, 60 %, 50 %, a pure ethanol solution (99.9 % w/w) was mixed with LC-MS grade water. From each dilution solution sample (Vf = 5 mL) 1 mL was poured into a 45 mL conical tube. Each dilution solution was then spiked with 90 µL of acetonitrile (surrogate standard) and 90 µL of n-butanol (internal standard) at 2 %. The final volume of 45.18 mL was obtained by adding LC-MS grade water. 5 mL of each final preparation was transferred to the headspace vial for measurement. All concentrations were measured in triplicates. A blank solution was injected (Water- LC-MS Grade) at least once at the beginning of a sequence and between samples.
A regression line was drawn between the GC peak area under curve (A) ratio of ethanol to n-butanol (y-axis) (AS /AIS) and the ethanol concentration ratio (x-axis) (CS).
4. Determination of ethanol % in hand sanitizer samples
4.1. Calibration curve for ethanol
Knowing that the hand sanitizers samples in the study were ethanol based, the calibration curve was prepared using ethanol. To improve accuracy, these tests frequently included internal standard (IS). Since ethanol elutes away from n-butanol, and the latter is not known to induce GC system deterioration, n-butanol is often used as an IS for ethanol in blood alcohol content measurements. Accordingly, the IS in this investigation was n-butanol [24].
Although acetonitrile used is also listed as an IS for ethanol in the USP method; acetonitrile elutes closer to ethanol and can lead to column/liner degradation with repeated injections; thus, acetonitrile was not selected as the IS.
The calibration curve was fitted to linear regression without pushing through zero using internal standard quantification methods.
A five-point calibration curve was derived for all concentrations listed in the table below (Table 3) with an R2 > 0.99 and an RSD ≤ 5 %.
Table 3.
Ethanol content using gas chromatography by headspace gas chromatography method with RSD.
| Ethanol Content (%) | AS1/AIS2 |
|---|---|
| 50 | 1.3 |
| 60 | 1.43 |
| 70 | 1.58 |
| 80 | 1.67 |
| 90 | 1.84 |
A regression line was generated as peak area ratios of ethanol standard solutions (AS) to n-butanol (internal standard, AIS). (AS/AIS) against the relative concentration of ethanol (CS) as shown in Fig. 3.
4.2. Ethanol percentage determination
Ethanol content was calculated using the following equation:
4.3. Toxicity assessment
To evaluate both acute and chronic toxicity, a simulation model was developed to simulate the frequent use of hand sanitizers. The authors utilized impurity relative concentration values obtained from the collected samples and simulated scenarios based on the maximum detected relative concentration values, daily exposure rates, and chronic exposure of users, following a methodology similar to that described by Boyce et al. (2017).
Specifically, the study observed that nurses often applied hand sanitizers up to 40 times during their daily shifts. For this simulation, a daily application rate of 40 applications was considered, with dosages of 0.75, 1.5, and 3 mL per dose, consistent with previous studies such as Greenaway et al. (2018).
These parameters were utilized to calculate the daily exposure of the detected volatile impurities. Subsequently, the calculated daily exposure values were employed to simulate chronic use over durations of 1, 3, and 6 months, as detailed in Table 6.
Table 6.
Daily and chronic exposure to detected impurities up to 6 months.
| Sample ID | Impurity | Daily Exposure (mg/day) |
Chronic Exposure (mg) with 3 mL/dose and 40 app/day |
||||
|---|---|---|---|---|---|---|---|
| 0.75 mL/dose | 1.5 mL/dose | 3 mL/dose | 1 month | 3 months | 6 months | ||
| 6 | 2-Methyl−1-Propanol | 20.7 | 41.4 | 82.8 | 2484 | 7452 | 14904 |
| 9 | Ethyl Acetate | 6.54 | 13.08 | 26.16 | 784.8 | 2354.4 | 4708.8 |
| 9 | 1-Propanol | 0.72 | 1.44 | 2.88 | 86.4 | 259.2 | 518.4 |
| 9 | Benzene | 0.57 | 1.14 | 2.28 | 68.4 | 205.2 | 410.4 |
| 9 | Acetone | 0.18 | 0.36 | 0.72 | 21.6 | 64.8 | 129.6 |
| 10 | Methanol | 2.19 | 4.38 | 8.76 | 262.8 | 788.4 | 1576.8 |
| 10 | 1,1-diethoxyethane | 0.3 | 0.6 | 1.2 | 36 | 108 | 216 |
| 12 | 1,1-diethoxyethane | 0.15 | 0.3 | 0.6 | 18 | 54 | 108 |
| 16 | 1,1-diethoxyethane | 0.09 | 0.18 | 0.36 | 10.8 | 32.4 | 64.8 |
| 18 | 1,1-diethoxyethane | 0.42 | 0.84 | 1.68 | 50.4 | 151.2 | 302.4 |
| 19 | 1,1-diethoxyethane | 0.06 | 0.12 | 0.24 | 7.2 | 21.6 | 43.2 |
| 20 | 1-Propanol | 5.1 | 10.2 | 20.4 | 612 | 1836 | 3672 |
| 21 | 1,1-diethoxyethane | 0.06 | 0.12 | 0.24 | 7.2 | 21.6 | 43.2 |
| 30 | 1-Propanol | 51.6 | 103.2 | 206.4 | 6192 | 18576 | 37152 |
A total of 10/ 31 (32 %) samples contain impurities
Samples that are compliant for alcohol content and include no impurities: A total number of 6/31(19.35 %) samples are fully compliant: samples 1,2,5,7,24 and 26
5. Results
Thirty-one samples of hand sanitizers from the Lebanese market underwent testing for impurities. Surprisingly, only 19.35 % of the tested hand sanitizers met the criteria for compliance with both the alcohol percentage recommended by the CDC guidelines and the absence of impurities listed in the FDA guidance.
5.1. Determination and relative quantification of volatile impurities
Analysis was conducted using head space GC-MS. 32 % of the samples contained impurities as detailed in Table 4. Seven different impurities were detected in the hand sanitizer samples: 2-methyl-1-propanol, ethyl acetate, 1-Propanol, benzene, acetone, methanol, 1,1-diethoxyethane (Acetal). The sample IDs containing the impurities were: 6, 9, 10, 12, 16, 18, 19, 20, 21, 30.
Table 4.
Impurities detected in hand sanitizers samples with chromatographic results and estimated concentration levels.
| Sample ID | Impurity | Ret. Time Min* | Average Area of Runs a.u.* |
Average area % | Average SI* | Concentration (ug/mL) |
|---|---|---|---|---|---|---|
| 6 | 2-methyl−1- propanol | 8.68 | 143841350 | 11.03 | 97 | ∼ 665–690 |
| 9 | Ethyl Acetate | 7.21 | 45494639 | 11.47 | 99 | ∼ 215 – 218 |
| 1-Propanol | 7.47 | 4673404 | 1.18 | 98 | ∼ 21 – 24 | |
| Benzene | 11.71 | 3403431 | 0.86 | 97 | ∼ 16 – 19++ | |
| Acetone | 12.54 | 1103226 | 0.28 | 97 | ∼ 5 – 6 | |
| 10 | Methanol | 3.87 | 14745922 | 1.97 | 98 | ∼ 69 – 73 |
| 1,1-diethoxyethane | 11.84 | 1819445 | 0.23 | 96 | ∼ 8 – 10 | |
| 12 | 1,1-diethoxyethane | 11.85 | 870397 | 0.12 | 96 | ∼ 4–5 |
| 16 | 1,1-diethoxyethane | 11.85 | 540876 | 0.06 | 94 | ∼ 2–3 |
| 18 | 1,1-diethoxyethane | 11.85 | 2854351 | 0.26 | 96 | ∼ 11–14 |
| 19 | 1,1-diethoxyethane | 11.19 | 253495 | 0.02 | 96 | ∼ 1–2 |
| 20 | 1-Propanol | 7.47 | 31523599 | 3.74 | 99 | ∼ 150 – 170 |
| 21 | 1,1-diethoxyethane | 11.85 | 242509 | 0.03 | 93 | ∼ 1–2 |
| 30 | 1-Propanol | 7.37 | 353794471 | 30.8 | 95 | ∼ 1660 – 1720++ |
* Min: minutes, a.u.: arbitrary unit, SI: International System of Units
++: Impurities with levels detected above FDA concentrations limits
In sample 6, 2-methyl-1-propanol that is a level 2 impurity was detected with an average area percentage of 11.03 % and a concentration ranging between ∼665–690 ug/mL, within the FDA concentration limit.
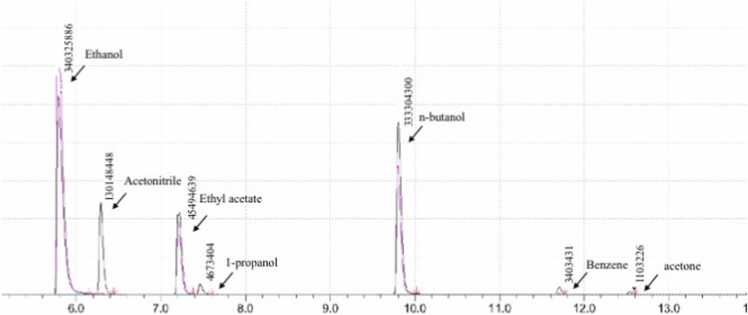
Sample 9 included the highest number of impurities with four impurities detected: ethyl acetate, 1-Propanol, benzene, and acetone with an average area percentage of 11.47 %, 1.18 %, 0.86 %, and 0.28 % respectively. The impurities identified in this sample belonged to both levels 1 and 2.
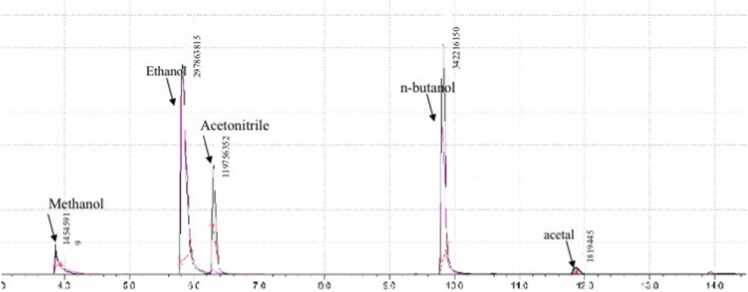
In sample 10, two impurities were detected classified as level 1: methanol and 1,1- diethoxyethane (acetal) with an area percentage of 1.97 % and 0.23 %, respectively.
Samples 12,16, 18, 19, and 21 contained 1,1-diethoxyethane (acetal) as the sole impurity. Acetal belongs to level 1 impurities with the respective area percentages of: 0.12 %, 0.06 %, 0.26 %, 0.02 %, and 0.03 %.
1-propanol was also detected impurity in samples 20 and 30 with an area percentage of 3.74 % and 30.8 %, respectively. 1,1-diethoxyethane (Acetal) was the most detected impurity, it was identified in six different samples. The second most detected impurity in this study was 1-propanol, it was found in three samples. The rest of the detected impurities were recognized in one sample only.
Fig. 1, Fig. 2 show the chromatograph peaks of the detected impurities in two different samples (9 and 10).
Fig. 1.
Chromatograph of Sample 9 showing peaks detected.
Fig. 2.
Chromatograph of Sample 10 showing peaks detected.
6. Determination of ethanol % in hand sanitizer samples
6.1. Ethanol percentage determination
A total of 31 samples were analyzed for their alcohol content. Of the 31 samples analyzed, only 29 % of the samples were compliant with the CDC recommendations (ethanol ≥ 60 %) (Table 3), while the other samples comprised ethanol in a lower percentage. Most of the samples (35.48 %) had an ethanol percentage between 20 % and 40 %, while 32.25 % of the samples contained ethanol in a percentage of 40–60 %. 3.22 % of the samples demonstrated an ethanol percentage range between 1 % and 20 %.
In Table 3, the values of ethanol percentage were depicted based on the linear equation. Where the area ratio represented the area of ethanol divided by the area of butanol since it is the internal standard.
6.2. Toxicity assessment
Multiple samples were collected from the market and tested for volatile impurities. Table 4 provides a listing of the sample IDs and the detected impurities within the respective sample. The minimum and maximum relative concentrations for each impurity in the different runs of each sample are also provided in Table 4.
Table includes the simulation values for chronic exposure over a period of 1, 3, and 6 months. The scenario is based on 40 applications per day and a 3 mL/dose; however, the extent of accumulated concentration over time could not be calculated.
7. Discussion
Toxic volatile impurities were detected in ethanol-based hand sanitizers finished products available on the Lebanese market. As well, ethanol content was shown to be below the recommended value in most studied samples. Non-compliance with international guidance accounted for ∼81 % of locally manufactured and/ or imported hand sanitizers considering both alcohol content and absence of impurities.
7.1. Detected Impurities and a summary on their risks on health care
Isobutanol (2-methyl-1-propanol) was detected as an impurity (Table 4). Isobutyl alcohol is a colorless liquid with a sweet, musty smell. It is utilized as a solvent and in the production of other compounds (Brownstein). Isobutanol's mutagenic or carcinogenic effects have only been studied in a few cases, and it has been shown to have tumorigenic and teratogenic effects in animal studies. Isobutanol has also been shown to have reproductive impacts in animal models. Tse et al. [29].
1-Propanol was identified as another impurity (Table 4). The major use of 1-Propanol is as a solvent and it is considered one of the most important industrial alcohols [8]. Exposure to n-propanol via skin contact, inhalation, or oral consumption has been shown to be harmless to animals and human; however, at doses exceeding occupational exposure, developmental and reproductive problems have been found. Mild erythema and cutaneous absorption into the circulatory system can occur after repeated use [29]. In terms of pharmacokinetics, isopropanol is identical to ethanol, but it has a higher narcotic/intoxicating impact due to central nervous system depression (CNS). Isopropanol-related deaths are uncommon, yet increased exposure can cause altered sensorium, hypotension, hypothermia, and cardiac collapse [29]. Hand sanitizer contaminated with 1-propanol might irritate the skin (or eyes, if exposed) [11]. Furthermore, isopropanol can be converted to acetone during exposure, and acetone accumulation over time could contribute to extended activity and toxicity. Tse et al. [29].
Benzene was identified as an impurity in several samples with a high percentage of the area under the curve (AUC) (Table 4). Benzene can be used to produce absolute ethanol. Benzene is a recognized as human carcinogen and mutagen that can enter the body through inhalation, and oral absorption, as well as cutaneous absorption [29]. Acute (short-term) inhalation exposure to benzene in humans can produce drowsiness, dizziness, headaches, eye, skin, and respiratory tract irritation, as well as unconsciousness at high doses (EPA). [9] Drowsiness, tremors, headaches, vomiting, irritability, convulsions, irregular heartbeat, and mortality are all common symptoms [28].
Any alcohol (ethanol) or iso propyl alcohol (IPA) containing more than 630 ppm methanol (Table 4) is in violation of the FDA temporary guideline and could be regarded as proof of contamination or substitution. Under the Federal Food, Drug, and Cosmetic Act (FD&C Act), hand sanitizers containing methanol-contaminated alcohol (ethanol) or IPA are considered adulterated. Such alcohol (ethanol) or IPA substance should be disposed of, and the company should report the material and its source to the FDA [6].
The synthetic alcohols available are very expensive. To get over these problems, some manufactures instead use toxic methyl alcohol as a replacement (sold at half the price). Unscrupulous manufactures could use branded container packing for marketers to sell spurious methanol-based sanitizers [11].
Acetal was the most prevalent impurity (Table 4); it was detected in 6 of the analyzed samples. The addition of two moles of ethanol and one mole of acetaldehyde to an acid catalyst produces 1,1-diethoxyethane (acetal) and water. In the presence of ethanol and acidic catalysts, reversible conversion of acetal to acetaldehyde can also occur. Acetal has been used as a solvent, as a chemical synthesis intermediary for protecting the carbonyl group in ketones and aldehydes, and as a fragrance ingredient [4].
According to recent research, acetaldehyde is a probable human carcinogen and the primary molecule that is involved in fetal alcohol spectrum disorder [29]. Acetaldehyde is a hazardous and suspected carcinogen (the International Agency for Research on Cancer classifies it as Group 2B).
Ethyl acetate, another impurity that was detected (Table 4). Ethyl acetate is an industrial solvent that is formed by an ester of ethanol and acetic acid (e.g. paints, plasticizers, denaturant, etc.). Although moderate toxicity has been documented by intraperitoneal, subcutaneous, and oral routes, acute toxicity is uncommon. Ethyl acetate vapors (400 ppm) can cause eye, nose, and throat irritation, as well as headaches, nausea, vomiting, lethargy, and unconsciousness [29].
Low-quality raw materials may contribute to undesirable impurity profiles in the final product, which may contain impurities such as benzene, acetaldehyde, acetal and ethyl acetate.
7.2. Ethanol percentage compliance
Alcohol Based Hand Sanitizers (ABHS) solutions with ethanol concentrations of less than 60 % provide inadequate antisepsis, putting users at risk of infection. This is made worse by the false sense of security and trust that unwary people often have in the products they utilize [1]. Of the analyzed samples 71 % contained an ethanol percentage lower than 60 % which reflects a very low percentage of compliance (Table 5). The precise assessment of ABHS alcohol (active ingredient) level is a critical quality control test that can also serve as a surrogate for compliance. Cohen et al. discovered that in order to manufacture alcohol that matches FDA impurity limitations, non-traditional high purity ethanol manufacturing plants may require infrastructural and process improvements [1]. To get the final required concentration, one should check the alcohol content and make the appropriate volume modifications. Controlling the alcohol concentration of the final solution can be done with an alcoholmeter [31].
Table 5.
Ethanol percentage present in hand sanitizer samples and compliance to CDC recommendations.
| Sample | Area ratio (A ethanol/ A butanol) |
Ethanol % based on linear equation |
Compliance to CDC recommendations | Sample | Area ratio (A ethanol/ A butanol) |
Ethanol % based on linear equation |
Compliance to CDC recommendations |
|---|---|---|---|---|---|---|---|
| 1 | 1.63 | 75 ± 1 | Yes | 17 | 1.41 | 58 ± 1 | No |
| 2 | 1.45 | 61±1 | Yes | 18 | 1.45 | 60.8 ± 0.9 | Yes |
| 3 | 1.41 | 57.7 ± 0.8 | No | 19 | 1.45 | 61.4 ± 0.5 | Yes |
| 4 | 1.41 | 57.8 ± 0.7 | No | 20 | 1.37 | 55 ± 1 | No |
| 5 | 1.5 | 64.6 ± 0.8 | Yes | 21 | 1 | 26.7 ± 0.4 | No |
| 6 | 1.48 | 64± 2 | Yes | 22 | 1.15 | 38 ± 1 | No |
| 7 | 1.44 | 60 ± 1 | Yes | 23 | 0.98 | 25.0 ± 0.7 | No |
| 8 | 1.36 | 54.2 ± 0.9 | No | 24 | 1.53 | 66.8 ± 0.9 | Yes |
| 9 | 0.99 | 26.3 ± 0.8 | No | 25 | 1.1 | 34.2 ± 0.7 | No |
| 10 | 0.88 | 18 ± 1 | No | 26 | 1.61 | 73.4 ± 0.9 | Yes |
| 11 | 1.16 | 39 ± 1 | No | 27 | 1 | 26.6 ± 0.9 | No |
| 12 | 1.13 | 36.6 ± 0.7 | No | 28 | 1.3 | 50.0 ± 0.6 | No |
| 13 | 1.08 | 33 ± 1 | No | 29 | 1.21 | 42.4 ± 0.9 | No |
| 14 | 1.36 | 54.4 ± 0.5 | No | 30 | 1.4 | 57 ± 1 | No |
| 15 | 1.16 | 39 ± 1 | No | 31 | 1.14 | 37.6 ± 0.6 | No |
| 16 | 1.19 | 41 ±1 | No |
A total of 9/ 31 (29 %) samples are compliant with CDC recommendations for alcohol content
7.3. Comparison with other studies
No studies on sanitizers were conducted in Lebanon, and results of a sample of similar studies investigated elsewhere are discussed hereafter.
A study was conducted in Bangladesh on 200 different hand sanitizers; 28 samples were found to be contaminated with methanol and 7 samples contained only methanol [16]. Another study conducted on 10 samples of alcohol based hand sanitizers; 20 % of samples were found to include methanol, and not all samples ingredients matched with their labels’. Alam et al. [2].
To a worrisome extent, a study reports numerous recall of formulations of hand sanitizers in both the United States and Canada for exceeding the limits of several key impurities. Shahbazi-Raz et al. [26].
Additionally, FDA issued a warning letter addressing acetal and acetaldehyde impurities in hand sanitizers for their harmful effects, and withdrawal of hand sanitizers were in effect in 2023 [12].
7.4. Toxicity assessment highlights
The simulation study highlights the toxicity of two impurities, i.e., benzene and 1-propanol (Table 6). Both substances were detected in levels above the allowable limits available in hand sanitizers following WHO and FDA guidance [11], [32] (Table 4).
Benzene is absorbed rapidly and extensively after inhalation and ingestion. It is absorbed less extensively through intact skin; however, percutaneous absorption may contribute to total body burden. Benzene is a carcinogen in humans. There may be no safe level of exposure to a carcinogen so all contacts should be reduced to the lowest possible. When skin contact occurs, we may be overexposed than in the case of that of air [23].
In addition to the acute toxic effects that benzene can cause such as skin irritation, erythema, a burning sensation, and in more severe cases, edema and even blistering, studies have shown benzene chronic toxic effects are more serious. Medical Management Guidelines for Benzene [20] Because benzene is a lipid solvent, it degreases the skin, particularly after prolonged or repeated contact with the liquid. Repeated exposure to benzene may cause a blood disorder (i.e., aplastic anemia and pancytopenia) and cancer of blood-forming cells (i.e., leukemia). Aplastic anemia and leukemia have been reported in some workers exposed repeatedly to benzene over long periods of time. Chronic exposure may be more serious for children because of their potential longer latency period. Medical Management Guidelines for Benzene [20]
Hematologic neoplasms such as acute myelogenous leukemia have been documented to occur with chronic exposures as low as 10 ppm benzene. Other neoplasms have been documented in animal models. Medical Management Guidelines for Benzene [20]
The study simulation results show our exposure to a high concentration of benzene upon the use of ABHS over long term use (Table 6). Since benzene is absorbed via skin exposure, the risk of metabolites associated toxicities is worrisome. Thus, our recommendation is to increase awareness on the use of high quality ABHS that have a good quality control assessing impurities in particular benzene contamination.
As for 1- Propanol, data on the absorption rate following repeated dermal exposures to 1-Propanol are lacking. Thus, the study simulation results are limited to the descriptive values.
7.5. Limitation
Sanitizers’ frequent users are shown to be exposed to high levels of impurities over a period of time. Although evidence exists and shows that most impurities are absorbed via skin contact, metabolized and toxic residues might accumulate, there is a lack of data on the accumulation extent and relative concentration, and this was a limitation for the study authors to compute.
Thus, further studies are required to assess the residual plasma concentration of the impurities and their toxic metabolites upon acute and chronic usage. As well, a quantitative accurate assessment is required in the analysis of hand sanitizers.
7.6. Ethanol concentration
The calibration curve in Fig. 3 was generated using standard solutions with concentrations ranging from 50 % to 90 % ethanol. Some reported values fall below this calibration range.
The method's validation did not initially include concentrations below the calibration range. Therefore, the accuracy and precision of these lower values have not been rigorously validated.
While the primary focus of the study was within the validated range, any values reported below the calibration range should be interpreted with caution. This represents a limitation of the current study and highlights the need for further validation to ensure reliability at lower concentrations.
8. Conclusion
Hand washing with water and soap is considered the gold standard for hand hygiene and reducing the spread of infectious diseases. Nevertheless, hand sanitizers (also known as hand antiseptic or hand rub) are indicated in the absence of water and soap. Lebanon, like many other nations across the world, has facilitated legislation to make it simpler for local enterprises to quickly create alcohol-based hand sanitizers in response to the massive increase in demand for hand sanitizers during the SARS-CoV-2 outbreak. However, people manufacturing hand sanitizers should still adhere to WHO and FDA rules and avoid using low-quality alcohol that may include harmful chemicals.
We are not aware of any study conducted in Lebanon, the present study shows that only 29 % of the samples were compliant with the CDC recommendations (ethanol content higher than 60 %), and 10 hand sanitizer samples out of 31 were found to contain impurities listed in the FDA guidance including: 2-methyl-1-propanol, ethyl acetate, 1-Propanol, benzene, acetone, methanol, 1,1-diethoxyethane (Acetal). Consequently, hand sanitizers that are fully compliant for effective impurities alcohol content and devoid of accounted for 19.35 % of all samples.
The contaminants that were found and quantified in most of the ABHRs, specifically ethanol based, suggest that potential acute toxicity is low whereas repeated doses on chronic basis in particular for ABHS containing benzene can pose a harmful health risk on users. Thus, owing to the high availability of ABHR on the market, testing should be done on a regular basis to verify product compliance and customer safety.
Funding
This work was supported and funded by the Lebanese American University; SOP-2021.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgment
The authors would like to thank Drs. Stephanie Mehanna and Cynthia Hageh for their assistance in the lab work.
Institutional review board statement
This study did not require ethical approval.
Informed consent statement
Not applicable.
Handling Editor: Prof. L.H. Lash
Contributor Information
Yolande Saab, Email: ysaab@lau.edu.lb.
Rebecca Zgheib, Email: rebecca.zgheib@lau.edu.lb.
Zahi Nakad, Email: zahi.nakad@lau.edu.lb.
Rony S. Khnayzer, Email: rony.khnayzer@lau.edu.lb.
Data availability
No data was used for the research described in the article.
References
- 1.Abuga K., Nyamweya N. Alcohol-based hand sanitizers in COVID-19 prevention: a multidimensional perspective. Pharm. J. Pharm. Educ. Pract. 2021;9(1) doi: 10.3390/pharmacy9010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam S., Rahat Md.M.R., Upoma N.J., Halder C., Moulick S.P., Islam Md.M., Liu W., Habib A. Assessment of quality of commercial hand sanitizers using Fourier transform infrared spectroscopy and gas chromatography. MethodsX. 2023;11 doi: 10.1016/j.mex.2023.102274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzyood M., Jackson D., Aveyard H., Brooke J. COVID-19 reinforces the importance of handwashing. J. Clin. Nurs. 2020;29(15–16):2760–2761. doi: 10.1111/jocn.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capeletti M.R., Balzano L., de la Puente G., Laborde M., Sedran U. Synthesis of acetal (1,1-diethoxyethane) from ethanol and acetaldehyde over acidic catalysts. Appl. Catal. A Gen. 2000;198(1–2):L1–L4. doi: 10.1016/S0926-860X(99)00502-5. [DOI] [Google Scholar]
- 5.CDC. 2020. COVID-19 and Your Health. CDC. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting- sick/hand-sanitizer.html.
- 6.CDER. 2020. Temporary policy for preparation of certain alcohol-based hand sanitizer products during the public health emergency (COVID-19). FDA, Center for Drug Evaluation and Research (CDER). resource.nlm.nih.gov/9918231203506676.
- 7.Dhama K., Patel S.K., Kumar R., Masand R., Rana J., Yatoo MohdI., Tiwari R., Sharun K., Mohapatra R.K., Natesan S., et al. The role of disinfectants and sanitizers during COVID-19 pandemic: advantages and deleterious effects on humans and the environment. Environ. Sci. Pollut. Res. 2021;28(26):34211–34228. doi: 10.1007/s11356-021-14429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EPA. 2005. Inert Reassessment - n-Propanol; CAS# 71-23-8. Environmental Protection Agency. epa.gov/sites/default/files/2015-04/documents/propanol.pdf.
- 9.EPA.Benzene CASRN 71–43-2 | DTXSID3039242 | IRIS | US EPA, ORD. EPA. [accessed 2021 Nov 11]. https?/cfpub.epa.gov/ncea/iris2/chemicallanding.cfm?substance_nmbr=276.
- 10.FDA. 2020. Direct Injection Gas Chromatography Mass Spectrometry (GC-MS) Method for the Detection of Listed Impurities in Hand Sanitizers. FDA, US Food & Drug Administration. [accessed 2022 Sep 14]. fda.gov/media/141501/download.
- 11.FDA, 2022. FDA updates on hand sanitizers consumers should not use. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-hand-sanitizers-consumers-should-not-use.
- 12.FDA Warning Letter: Acetaldehyde and Acetal Impurities in Hand Sanitizers. 2023. https://www.gmp-compliance.org/gmp-news/fda-warning-letter-acetaldehyde-and-acetal-impurities-in-hand-sanitizers.
- 13.Gold N.A., Mirza T.M., Avva U. 2021. Alcohol Sanitizer. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK513254/. [PubMed]
- 14.Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ICH-2005. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use-2005. [https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf]. [DOI] [PMC free article] [PubMed]
- 16.Islam M., Shahin Ahmed K., Karim R., Nath B.D., Prosad Moulick S., Islam R., Mahmudul Hassan S.Md, Hossain H., Moniruzzaman M., Jahan M.S., et al. Alcohol-based hand sanitizers amid COVID-19: chemical formulation, analysis, safety. ChemistrySelect. 2022;7(45) doi: 10.1002/slct.202203290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing J.L.J., Pei Yi T., Bose R.J.C., McCarthy J.R., Tharmalingam N., Madheswaran T. Hand sanitizers: a review on formulation aspects, adverse effects, and regulations. IJERPH. 2020;17(9):3326. doi: 10.3390/ijerph17093326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keni R., Alexander A., Nayak P.G., Mudgal J., Nandakumar K. COVID-19: emergence, spread, possible treatments, and global burden. Front Public Health. 2020;8:216. doi: 10.3389/fpubh.2020.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie R.A., Zhou S.S., Macinga D.R. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Am. J. Infect. Control. 2021;49(3):401–402. doi: 10.1016/j.ajic.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medical Management Guidelines for Benzene. 2015. https://wwwn.cdc.gov/TSP/MMG/MMGDetails.aspx?mmgid=35&toxid=14.
- 21.Mohammed A.H., Mohammed A.K., Kamar F.H., Abbas A.A., Nechifor G. Determination of ethanol in fermented broth by headspace gas chromatography using capillary column. Rev. Chim. 2018;69(11):2969–2972. doi: 10.37358/RC.18.11.6664. [DOI] [Google Scholar]
- 22.Muralidar S., Ambi S.V., Sekaran S., Krishnan U.M. The emergence of COVID-19 as a global pandemic: understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie. 2020;179:85–100. doi: 10.1016/j.biochi.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NJ Health. 2015. Hazardous Substance Fact Sheet, Common Name: Benzene. New Jersey Department of Health. [accessed 2022 Sep 18]. nj.gov/health/eoh/rtkweb/documents/0.197.pdf.
- 24.Paulo Amorim de Lacerda J., Souza de Oliveira S., Marcante A. A rapid and effective method for determination of ethanol content in hand sanitizers (Alcohol gel) Rev. IPT TECNOLOGIA E INOVAÇÃO. 2020;4(14) doi: 10.34033/2526-5830-v4n14-4. [accessed 2022 Sep 18]. http://revista.ipt.br/index.php/revistaIPT/article/view/118. [DOI] [Google Scholar]
- 25.Prajapati P., Desai H., Chandarana C. Hand sanitizers as a preventive measure in COVID-19 pandemic, its characteristics, and harmful effects: a review. J. Egypt Public Health Assoc. 2022;97(1):6. doi: 10.1186/s42506-021-00094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahbazi-Raz F., Egbuta M.A., Aremu B.R., Mashhadi N., Tucci P., Binder J., Trant J.F. How impurities responsible for recalls emerge in hand sanitizers. RSC Sustain. 2024;2(3):701–709. doi: 10.1039/D3SU00286A. [DOI] [Google Scholar]
- 27.Shimadzu. 2021. Analysis of Impurities in Alcohol-Based Hand Sanitizers by GC-MS. Shimadzu. [accessed 2022 Sep 14]. shimadzu.com/an/literature/an_m315_en.html.
- 28.Snyder R., Witz G., Goldstein B.D. The toxicology of benzene. Environ. Health Perspect. 1993;100:293–306. doi: 10.1289/ehp.93100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tse T.J., Purdy S.K., Shen J., Nelson F.B., Mustafa R., Wiens D.J., Reaney M.J.T. Toxicology of alcohol-based hand rubs formulated with technical-grade ethanol. Toxicol. Rep. 2021;8:785–792. doi: 10.1016/j.toxrep.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villa C., Russo E. Hydrogels in hand sanitizers. Materials. 2021;14(7):1577. doi: 10.3390/ma14071577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. 2009. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care is Safer Care. WHO. https://www.ncbi.nlm.nih.gov/books/NBK144054/. [PubMed]
- 32.WHO-2010. Guide to Local Production: WHO-recommended Handrub Formulations. 〈https://iris.who.int/bitstream/handle/10665/332005/WHO-IER-PSP-2010.5-eng.pdf〉.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.