Abstract
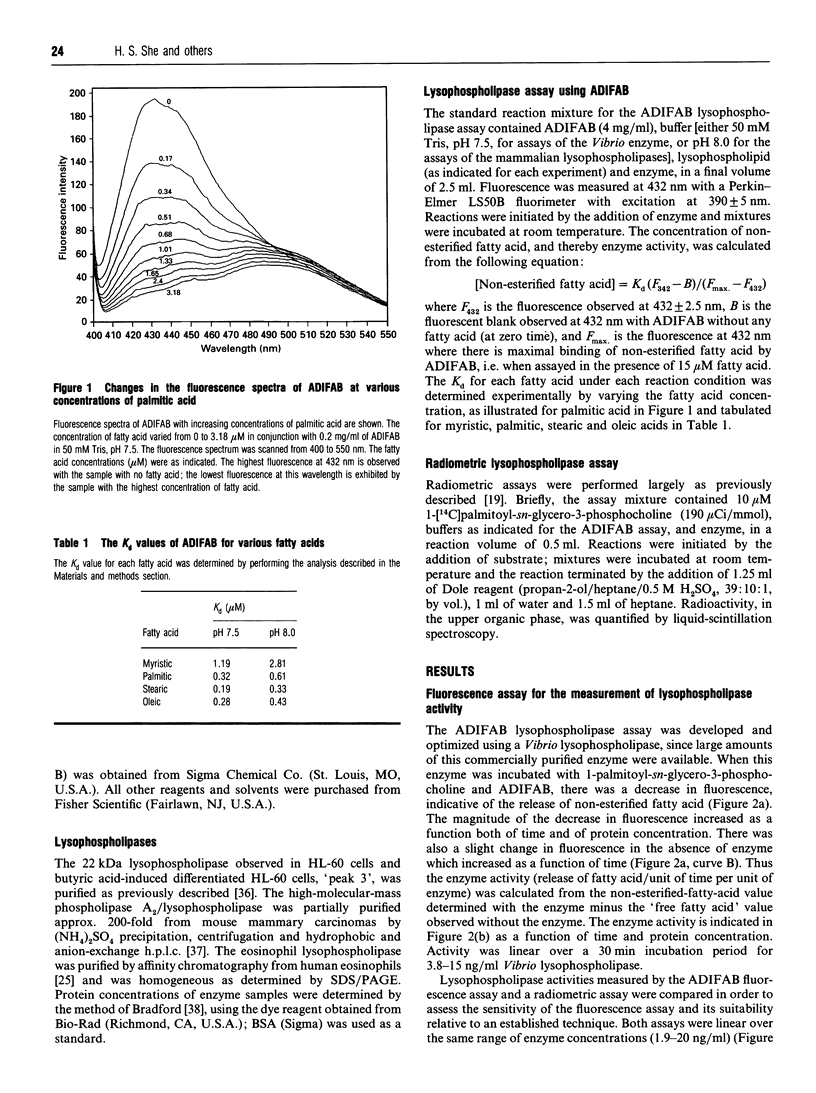
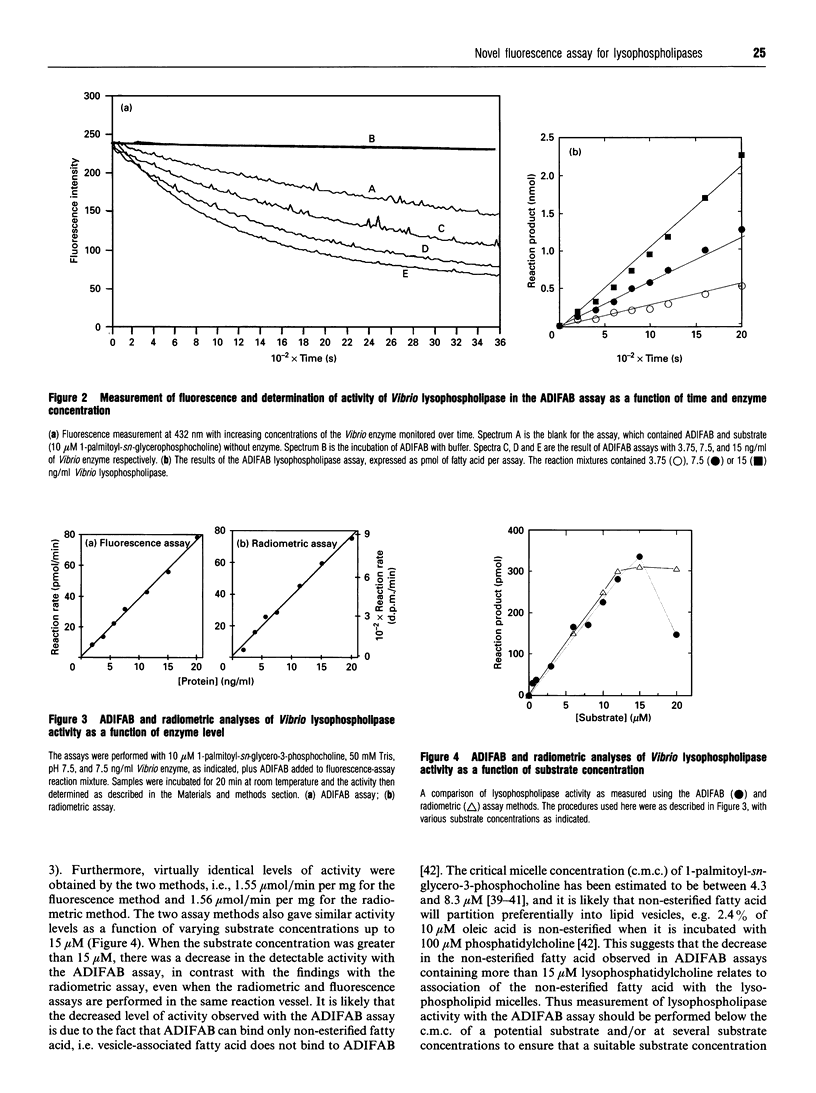
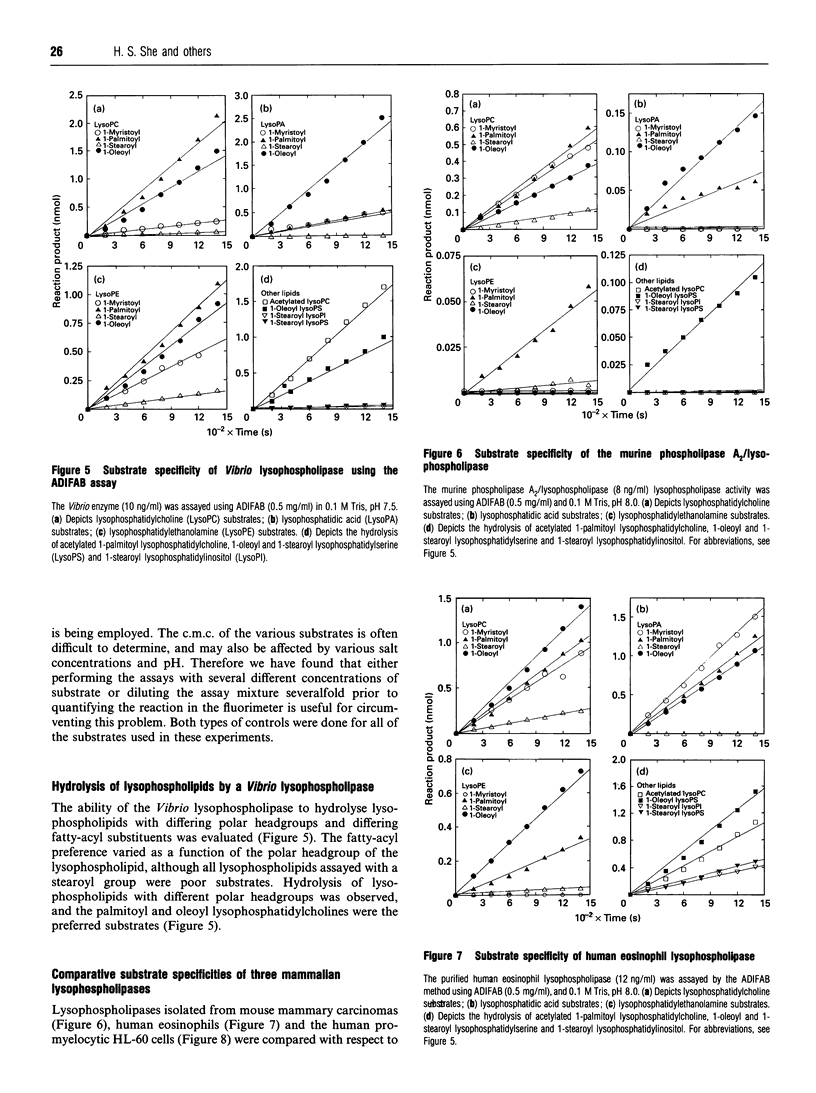
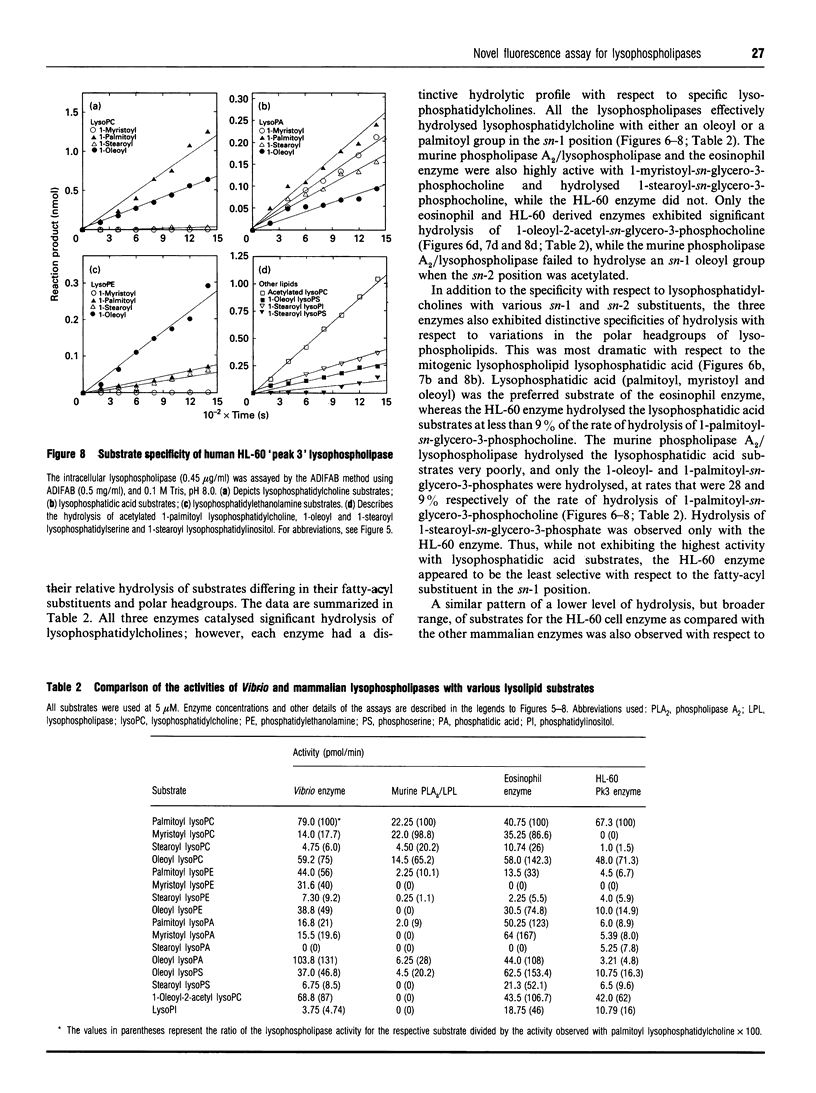
A novel fluorescence assay for quantifying lysophospholipase activity is described which utilizes a commercially available acrylodated intestinal fatty-acid-binding protein (ADIFAB) and non-radiolabelled substrate. Quantification of enzyme activity is based on the decrease in ADIFAB fluorescence at 432 nm in the presence of nanomolar concentrations of non-esterified ('free') fatty acids. Lysophospholipase activity measured by the ADIFAB assay and a conventional radiometric assay yield comparable results and have comparable levels of sensitivity (approximately 10 pmol/min per ml). The ADIFAB assay has the advantageous features of continuous monitoring of enzyme activity and the availability of a broad range of potential substrates, because non-radiolabelled lysophospholipids can be employed in the assay. The hydrolytic activities of four lysophospholipases were determined, including a bacterial secreted phospholipase A2/lysophospholipase, the human-eosinophil-secreted lysophospholipase, a human intracellular lysophospholipase (peak 3) isolated from HL-60 cells and a high-molecular-mass cytosolic phospholipase A2/lysophospholipase from a mouse mammary carcinoma. Each of these enzymes was found to have a distinctive hydrolytic profile as determined by an array of lysophospholipids differing in their polar headgroups and sn-1 fatty-acyl substituents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anel A., Richieri G. V., Kleinfeld A. M. Membrane partition of fatty acids and inhibition of T cell function. Biochemistry. 1993 Jan 19;32(2):530–536. doi: 10.1021/bi00053a018. [DOI] [PubMed] [Google Scholar]
- Asaoka Y., Oka M., Yoshida K., Sasaki Y., Nishizuka Y. Role of lysophosphatidylcholine in T-lymphocyte activation: involvement of phospholipase A2 in signal transduction through protein kinase C. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6447–6451. doi: 10.1073/pnas.89.14.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Flavahan N. A. Lysophosphatidylcholine modifies G protein-dependent signaling in porcine endothelial cells. Am J Physiol. 1993 Mar;264(3 Pt 2):H722–H727. doi: 10.1152/ajpheart.1993.264.3.H722. [DOI] [PubMed] [Google Scholar]
- Garsetti D. E., Ozgür L. E., Steiner M. R., Egan R. W., Clark M. A. Isolation and characterization of three lysophospholipases from the murine macrophage cell line WEHI 265.1. Biochim Biophys Acta. 1992 Dec 2;1165(2):229–238. doi: 10.1016/0005-2760(92)90191-w. [DOI] [PubMed] [Google Scholar]
- Garsetti D., Holtsberg F., Steiner M. R., Egan R. W., Clark M. A. Butyric acid-induced differentiation of HL-60 cells increases the expression of a single lysophospholipase. Biochem J. 1992 Dec 15;288(Pt 3):831–837. doi: 10.1042/bj2880831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I., Ward P. A., Varani J. Lysophosphatides enhance superoxide responses of stimulated human neutrophils. Inflammation. 1989 Apr;13(2):163–174. doi: 10.1007/BF00924787. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Purification of a high-molecular-mass form of phospholipase A2 from rat kidney activated at physiological calcium concentrations. Biochem J. 1990 Oct 1;271(1):37–43. doi: 10.1042/bj2710037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. W., Drisdel R. C., Sobel B. E. Rabbit myocardial lysophospholipase-transacylase. Purification, characterization, and inhibition by endogenous cardiac amphiphiles. J Biol Chem. 1983 Dec 25;258(24):15165–15172. [PubMed] [Google Scholar]
- Gross R. W., Sobel B. E. Rabbit myocardial cytosolic lysophospholipase. Purification, characterization, and competitive inhibition by L-palmitoyl carnitine. J Biol Chem. 1983 Apr 25;258(8):5221–5226. [PubMed] [Google Scholar]
- Hall P. A., Laubach H. E. Stimulation of leukocyte lysophospholipase activity by noninfectious agents. Proc Soc Exp Biol Med. 1991 Sep;197(4):435–440. doi: 10.3181/00379727-197-43279. [DOI] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Horigome K., Hayakawa M., Inoue K., Nojima S. Purification and characterization of phospholipase A2 released from rat platelets. J Biochem. 1987 Mar;101(3):625–631. doi: 10.1093/jb/101.3.625. [DOI] [PubMed] [Google Scholar]
- Igarashi Y., Kitamura K., Zhou Q. H., Hakomori S. A role of lyso-phosphatidylcholine in GM3-dependent inhibition of epidermal growth factor receptor autophosphorylation in A431 plasma membranes. Biochem Biophys Res Commun. 1990 Oct 15;172(1):77–84. doi: 10.1016/s0006-291x(05)80175-5. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Roberts E. F., Manetta J., Putnam J. E. The Ca2(+)-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J Biol Chem. 1991 Mar 15;266(8):5268–5272. [PubMed] [Google Scholar]
- Langton S. R., Cesareo S. D. Helicobacter pylori associated phospholipase A2 activity: a factor in peptic ulcer production? J Clin Pathol. 1992 Mar;45(3):221–224. doi: 10.1136/jcp.45.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach H. E., Hall P. A. Lysophospholipase activity of Ascaris suum-induced mouse peritoneal neutrophils and eosinophils. Microb Pathog. 1991 May;10(5):333–341. doi: 10.1016/0882-4010(91)90078-o. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Görlich J. K., Rustenbeck I. Regulation of transmembrane ion transport by reaction products of phospholipase A2. I. Effects of lysophospholipids on mitochondrial Ca2+ transport. Biochim Biophys Acta. 1989 Jun 26;982(1):140–146. doi: 10.1016/0005-2736(89)90184-3. [DOI] [PubMed] [Google Scholar]
- Leslie C. C. Kinetic properties of a high molecular mass arachidonoyl-hydrolyzing phospholipase A2 that exhibits lysophospholipase activity. J Biol Chem. 1991 Jun 15;266(17):11366–11371. [PubMed] [Google Scholar]
- Marsh D., King M. D. Prediction of the critical micelle concentrations of mono- and di-acyl phospholipids. Chem Phys Lipids. 1986 Dec 31;42(4):271–277. doi: 10.1016/0009-3084(86)90086-1. [DOI] [PubMed] [Google Scholar]
- Mock T., Man R. Y. Mechanism of lysophosphatidylcholine accumulation in the ischemic canine heart. Lipids. 1990 Jul;25(7):357–362. doi: 10.1007/BF02537977. [DOI] [PubMed] [Google Scholar]
- Ngwenya B. Z., Foster D. M. Enhancement of antibody production by lysophosphatidylcholine and alkylglycerol. Proc Soc Exp Biol Med. 1991 Jan;196(1):69–75. doi: 10.3181/00379727-196-43165. [DOI] [PubMed] [Google Scholar]
- Oishi K., Raynor R. L., Charp P. A., Kuo J. F. Regulation of protein kinase C by lysophospholipids. Potential role in signal transduction. J Biol Chem. 1988 May 15;263(14):6865–6871. [PubMed] [Google Scholar]
- Oliver J. D., Colwell R. R. Extractable lipids of gram-negative marine bacteria: phospholipid composition. J Bacteriol. 1973 Jun;114(3):897–908. doi: 10.1128/jb.114.3.897-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pind S., Kuksis A. Further characterization of a novel phospholipase B (phospholipase A2--lysophospholipase) from intestinal brush-border membranes. Biochem Cell Biol. 1991 May-Jun;69(5-6):346–357. doi: 10.1139/o91-054. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richieri G. V., Ogata R. T., Kleinfeld A. M. A fluorescently labeled intestinal fatty acid binding protein. Interactions with fatty acids and its use in monitoring free fatty acids. J Biol Chem. 1992 Nov 25;267(33):23495–23501. [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rustenbeck I., Lenzen S. Effect of lysophospholipids, arachidonic acid and other fatty acids on regulation of Ca2+ transport in permeabilized pancreatic islets. Cell Calcium. 1992 Apr;13(4):193–202. doi: 10.1016/0143-4160(92)90007-f. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Asaoka Y., Nishizuka Y. Potentiation of diacylglycerol-induced activation of protein kinase C by lysophospholipids. Subspecies difference. FEBS Lett. 1993 Mar 29;320(1):47–51. doi: 10.1016/0014-5793(93)81655-j. [DOI] [PubMed] [Google Scholar]
- Shinoda S., Matsuoka H., Tsuchie T., Miyoshi S., Yamamoto S., Taniguchi H., Mizuguchi Y. Purification and characterization of a lecithin-dependent haemolysin from Escherichia coli transformed by a Vibrio parahaemolyticus gene. J Gen Microbiol. 1991 Dec;137(12):2705–2711. doi: 10.1099/00221287-137-12-2705. [DOI] [PubMed] [Google Scholar]
- Stafford R. E., Fanni T., Dennis E. A. Interfacial properties and critical micelle concentration of lysophospholipids. Biochemistry. 1989 Jun 13;28(12):5113–5120. doi: 10.1021/bi00438a031. [DOI] [PubMed] [Google Scholar]
- Stafford R. E., Zhang Y. Y., Deems R. A., Dennis E. A. Kinetic analysis and substrate specificity of a lysophospholipase from the macrophage-like cell line P388D1. Biochim Biophys Acta. 1993 Mar 17;1167(1):43–48. doi: 10.1016/0005-2760(93)90215-u. [DOI] [PubMed] [Google Scholar]
- Steiner M. R., Bomalaski J. S., Clark M. A. Responses of purified phospholipases A2 to phospholipase A2 activating protein (PLAP) and melittin. Biochim Biophys Acta. 1993 Feb 10;1166(1):124–130. doi: 10.1016/0005-2760(93)90292-h. [DOI] [PubMed] [Google Scholar]
- Stoll L. L., Spector A. A. Lysophosphatidylcholine causes cGMP-dependent verapamil-sensitive Ca2+ influx in vascular smooth muscle cells. Am J Physiol. 1993 Apr;264(4 Pt 1):C885–C893. doi: 10.1152/ajpcell.1993.264.4.C885. [DOI] [PubMed] [Google Scholar]
- Triggiani M., Schleimer R. P., Warner J. A., Chilton F. H. Differential synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine and platelet-activating factor by human inflammatory cells. J Immunol. 1991 Jul 15;147(2):660–666. [PubMed] [Google Scholar]
- Weller P. F., Bach D. S., Austen K. F. Biochemical characterization of human eosinophil Charcot-Leyden crystal protein (lysophospholipase). J Biol Chem. 1984 Dec 25;259(24):15100–15105. [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Yu L., Dennis E. A. Thio-based phospholipase assay. Methods Enzymol. 1991;197:65–75. doi: 10.1016/0076-6879(91)97133-j. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y., Deems R. A., Dennis E. A. Lysophospholipases I and II from P388D1 macrophage-like cell line. Methods Enzymol. 1991;197:456–468. doi: 10.1016/0076-6879(91)97171-t. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y., Dennis E. A. Purification and characterization of a lysophospholipase from a macrophage-like cell line P388D1. J Biol Chem. 1988 Jul 15;263(20):9965–9972. [PubMed] [Google Scholar]
- Zhou Z., Tenen D. G., Dvorak A. M., Ackerman S. J. The gene for human eosinophil Charcot-Leyden crystal protein directs expression of lysophospholipase activity and spontaneous crystallization in transiently transfected COS cells. J Leukoc Biol. 1992 Dec;52(6):588–595. doi: 10.1002/jlb.52.6.588. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch H., de Jong J. G., Aarsman A. J. Lysophospholipases from bovine liver. Methods Enzymol. 1991;197:468–475. doi: 10.1016/0076-6879(91)97172-u. [DOI] [PubMed] [Google Scholar]


