Abstract
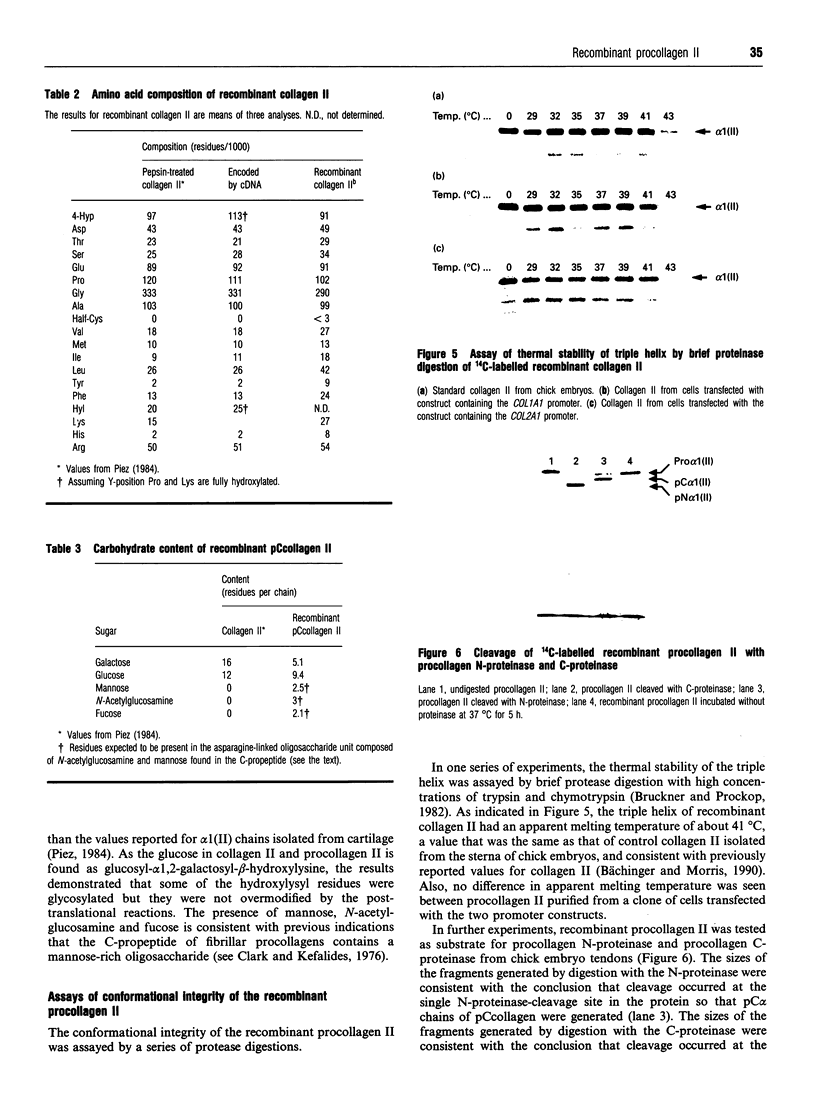
Apparently because the biosynthetic pathways involve eight or more highly specific post-translational enzymes, it has been difficult to obtain expression of genes for fibrillar collagens in recombinant systems. Here two constructs of the human gene for procollagen II (COL2A1) were prepared, one with about 0.5 kb of a promoter for a procollagen I gene (COL1A1) and the other with about 4 kb of the promoter for the procollagen II gene. The constructs, together with a neomycin-resistant gene, were transfected into a human tumour cell line (HT1080) that synthesizes the collagen IV found in basement membranes, but does not synthesize any fibrillar collagen. About two per 100 clones resistant to the neomycin analogue G418 synthesized and secreted human procollagen II. Milligram quantities of the recombinant procollagen II were readily isolated from the cultured medium. The recombinant procollagen II had the expected amino acid sequence as defined by nucleotide sequencing of mRNA-derived cDNA and the expected amino acid composition as defined by analysis of procollagen II that was converted into collagen II by digestion with procollagen N- and C-proteinases. Also, analysis of the carbohydrate content indicated that there was glycosylation of some of the hydroxylysine residues but no evidence of post-translational overmodification of the residues. In addition, the protein was shown to have a native conformation as assayed by a series of protease digestions. No essential differences were found between clones transfected with the COL2A1 gene construct containing the COL1A1 promoter and the similar construct containing the COL2A1 promoter in terms of number of clones synthesizing recombinant procollagen II and the levels of expression. With both constructs, the expression of the COL2A1 gene was closely related to copy number. The results demonstrated therefore that it is not essential to use a promoter for a gene normally expressed in a host cell in order to obtain gene copy-number-dependent expression of an exogenous collagen gene in stably transfected cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N. N., Ala-Kokko L., Knowlton R. G., Jimenez S. A., Weaver E. J., Maguire J. I., Tasman W., Prockop D. J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Hyland J., Smith C., Kivirikko K. I., Jimenez S. A., Prockop D. J. Expression of a human cartilage procollagen gene (COL2A1) in mouse 3T3 cells. J Biol Chem. 1991 Aug 5;266(22):14175–14178. [PubMed] [Google Scholar]
- Ala-Kokko L., Prockop D. J. Completion of the intron-exon structure of the gene for human type II procollagen (COL2A1): variations in the nucleotide sequences of the alleles from three chromosomes. Genomics. 1990 Nov;8(3):454–460. doi: 10.1016/0888-7543(90)90031-o. [DOI] [PubMed] [Google Scholar]
- Ala-Kokko L., Prockop D. J. Efficient procedure for preparing cosmid libraries from microgram quantities of genomic DNA fragments size fractionated by gel electrophoresis. Matrix. 1990 Oct;10(5):279–284. doi: 10.1016/s0934-8832(11)80182-4. [DOI] [PubMed] [Google Scholar]
- Anderson I. J., Goldberg R. B., Marion R. W., Upholt W. B., Tsipouras P. Spondyloepiphyseal dysplasia congenita: genetic linkage to type II collagen (COL2AI). Am J Hum Genet. 1990 May;46(5):896–901. [PMC free article] [PubMed] [Google Scholar]
- Anumula K. R., Taylor P. B. Rapid characterization of asparagine-linked oligosaccharides isolated from glycoproteins using a carbohydrate analyzer. Eur J Biochem. 1991 Jan 1;195(1):269–280. doi: 10.1111/j.1432-1033.1991.tb15703.x. [DOI] [PubMed] [Google Scholar]
- Aulthouse A. L., Beck M., Griffey E., Sanford J., Arden K., Machado M. A., Horton W. A. Expression of the human chondrocyte phenotype in vitro. In Vitro Cell Dev Biol. 1989 Jul;25(7):659–668. doi: 10.1007/BF02623638. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Gelinas R. E. DNA and chromatin structure of the human alpha 1 (I) collagen gene. J Biol Chem. 1984 Dec 10;259(23):14906–14913. [PubMed] [Google Scholar]
- Boast S., Su M. W., Ramirez F., Sanchez M., Avvedimento E. V. Functional analysis of cis-acting DNA sequences controlling transcription of the human type I collagen genes. J Biol Chem. 1990 Aug 5;265(22):13351–13356. [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P., Morris N. P. Analysis of the thermal stability of type II collagen in various solvents used for reversed-phase high performance chromatography. Matrix. 1990 Oct;10(5):331–338. doi: 10.1016/s0934-8832(11)80189-7. [DOI] [PubMed] [Google Scholar]
- Clark C. C., Kefalides N. A. Carbohydrate moieties of procollagen: incorporation of isotopically labeled mannose and glucosamine into propeptides of procollagen secreted by matrix-free chick embryo tendon cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):34–38. doi: 10.1073/pnas.73.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S., Prockop D. J. Isolation and partial characterization of the amino-terminal propeptide of type II procollagen from chick embryos. Biochemistry. 1982 Mar 30;21(7):1482–1487. doi: 10.1021/bi00536a003. [DOI] [PubMed] [Google Scholar]
- D'Alessio M., Bernard M., Pretorius P. J., de Wet W., Ramirez F., Pretorious P. J. Complete nucleotide sequence of the region encompassing the first twenty-five exons of the human pro alpha 1(I) collagen gene (COL1A1) Gene. 1988 Jul 15;67(1):105–115. doi: 10.1016/0378-1119(88)90013-3. [DOI] [PubMed] [Google Scholar]
- Elima K., Vuorio E. Expression of mRNAs for collagens and other matrix components in dedifferentiating and redifferentiating human chondrocytes in culture. FEBS Lett. 1989 Dec 4;258(2):195–198. doi: 10.1016/0014-5793(89)81651-5. [DOI] [PubMed] [Google Scholar]
- Elima K., Vuorio T., Vuorio E. Determination of the single polyadenylation site of the human pro alpha 1(II) collagen gene. Nucleic Acids Res. 1987 Nov 25;15(22):9499–9504. doi: 10.1093/nar/15.22.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francomano C. A., Liberfarb R. M., Hirose T., Maumenee I. H., Streeten E. A., Meyers D. A., Pyeritz R. E. The Stickler syndrome: evidence for close linkage to the structural gene for type II collagen. Genomics. 1987 Dec;1(4):293–296. doi: 10.1016/0888-7543(87)90027-9. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Hoffman G. G., Lee B. S. High levels of expression of full length human pro-alpha 2(V) collagen cDNA in pro-alpha 2(V)-deficient hamster cells. J Biol Chem. 1989 Dec 5;264(34):20683–20687. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hojima Y., McKenzie J. A., van der Rest M., Prockop D. J. Type I procollagen N-proteinase from chick embryo tendons. Purification of a new 500-kDa form of the enzyme and identification of the catalytically active polypeptides. J Biol Chem. 1989 Jul 5;264(19):11336–11345. [PubMed] [Google Scholar]
- Hojima Y., van der Rest M., Prockop D. J. Type I procollagen carboxyl-terminal proteinase from chick embryo tendons. Purification and characterization. J Biol Chem. 1985 Dec 15;260(29):15996–16003. [PubMed] [Google Scholar]
- Kadler K. E., Hojima Y., Prockop D. J. Collagen fibrils in vitro grow from pointed tips in the C- to N-terminal direction. Biochem J. 1990 Jun 1;268(2):339–343. doi: 10.1042/bj2680339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Genetic causes of aortic aneurysms. Unlearning at least part of what the textbooks say. J Clin Invest. 1991 Nov;88(5):1441–1444. doi: 10.1172/JCI115452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Byrne J. C., Howley P. M. A stable bovine papillomavirus hybrid plasmid that expresses a dominant selective trait. Mol Cell Biol. 1983 Nov;3(11):2110–2115. doi: 10.1128/mcb.3.11.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. L., Piez K. A. Type II collagen from lathyritic rat chondrosarcoma: preparation and in vitro fibril formation. Coll Relat Res. 1983 Mar;3(2):89–103. doi: 10.1016/s0174-173x(83)80036-3. [DOI] [PubMed] [Google Scholar]
- Olsen A. S., Geddis A. E., Prockop D. J. High levels of expression of a minigene version of the human pro alpha 1 (I) collagen gene in stably transfected mouse fibroblasts. Effects of deleting putative regulatory sequences in the first intron. J Biol Chem. 1991 Jan 15;266(2):1117–1121. [PubMed] [Google Scholar]
- Palotie A., Väisänen P., Ott J., Ryhänen L., Elima K., Vikkula M., Cheah K., Vuorio E., Peltonen L. Predisposition to familial osteoarthrosis linked to type II collagen gene. Lancet. 1989 Apr 29;1(8644):924–927. doi: 10.1016/s0140-6736(89)92507-5. [DOI] [PubMed] [Google Scholar]
- Pavlin D., Lichtler A. C., Bedalov A., Kream B. E., Harrison J. R., Thomas H. F., Gronowicz G. A., Clark S. H., Woody C. O., Rowe D. W. Differential utilization of regulatory domains within the alpha 1(I) collagen promoter in osseous and fibroblastic cells. J Cell Biol. 1992 Jan;116(1):227–236. doi: 10.1083/jcb.116.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen L., Palotie A., Hayashi T., Prockop D. J. Thermal stability of type I and type III procollagens from normal human fibroblasts and from a patient with osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1980 Jan;77(1):162–166. doi: 10.1073/pnas.77.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Myllylä R., Alitalo K., Vaheri A., Kivirikko K. I. Posttranslational modifications in the biosynthesis of type IV collagen by a human tumor cell line. Biochemistry. 1981 Dec 22;20(26):7409–7415. doi: 10.1021/bi00529a014. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi F. O., Benson-Chanda V., de Wet W. J., Sobel M. E., Tsipouras P., Ramirez F. Isolation and partial characterization of the entire human pro alpha 1(II) collagen gene. Nucleic Acids Res. 1985 Apr 11;13(7):2207–2225. doi: 10.1093/nar/13.7.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J. L., Liska D. J., Bornstein P. An upstream regulatory region mediates high-level, tissue-specific expression of the human alpha 1(I) collagen gene in transgenic mice. Mol Cell Biol. 1991 Apr;11(4):2066–2074. doi: 10.1128/mcb.11.4.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Cheah K. S., Griffin J. R., Pope F. M., Solomon E. A highly polymorphic region 3' to the human type II collagen gene. Nucleic Acids Res. 1985 Jul 11;13(13):4613–4622. doi: 10.1093/nar/13.13.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller G. E., Rimoin D. L., Murray L. W., Cohn D. H. Tandem duplication within a type II collagen gene (COL2A1) exon in an individual with spondyloepiphyseal dysplasia. Proc Natl Acad Sci U S A. 1990 May;87(10):3889–3893. doi: 10.1073/pnas.87.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre-Blanco A., Adachi E., Romanic A. M., Prockop D. J. Copolymerization of normal type I collagen with three mutated type I collagens containing substitutions of cysteine at different glycine positions in the alpha 1 (I) chain. J Biol Chem. 1992 Mar 5;267(7):4968–4973. [PubMed] [Google Scholar]
- Tryggvason K., Robey P. G., Martin G. R. Biosynthesis of type IV procollagens. Biochemistry. 1980 Apr 1;19(7):1284–1289. doi: 10.1021/bi00548a003. [DOI] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]




