Abstract
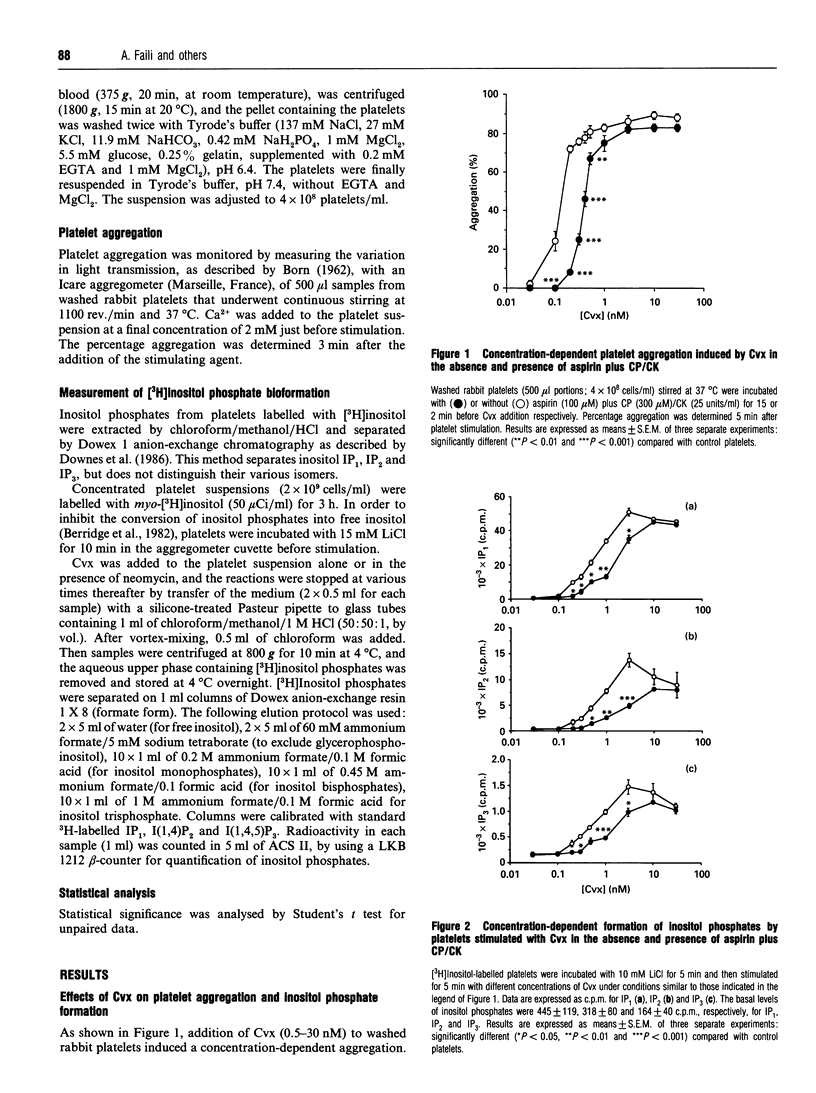
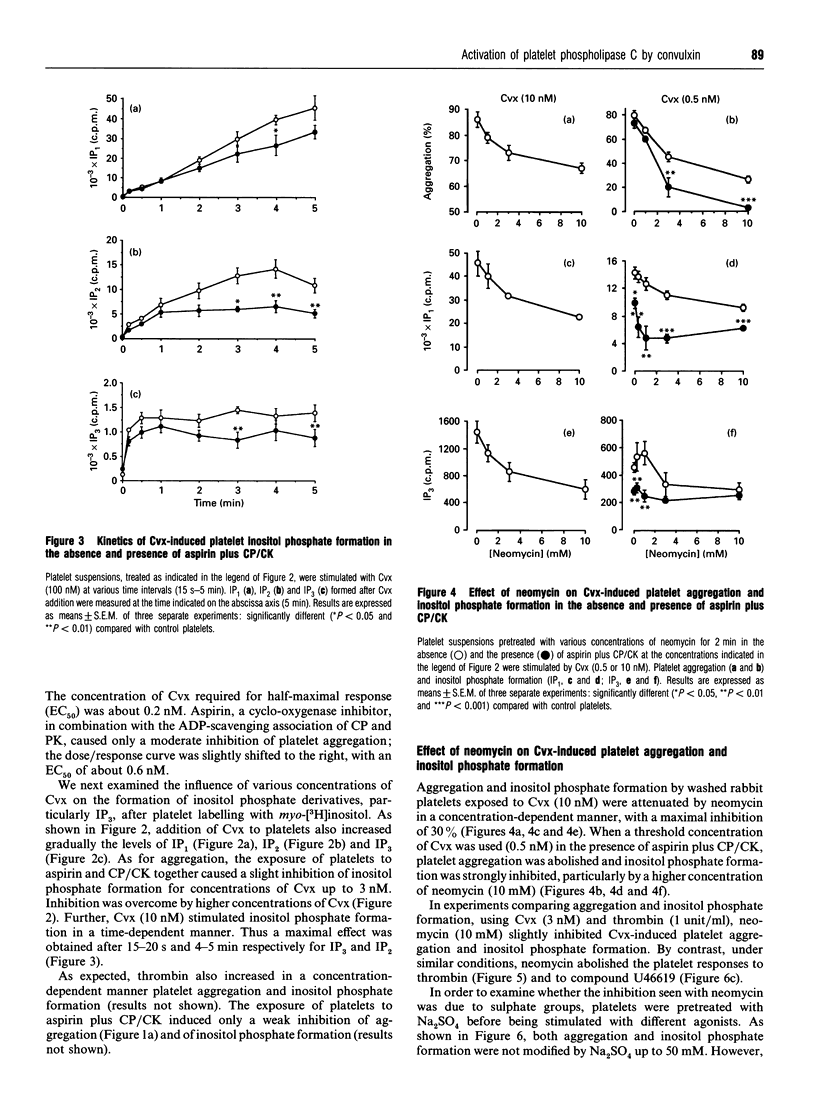
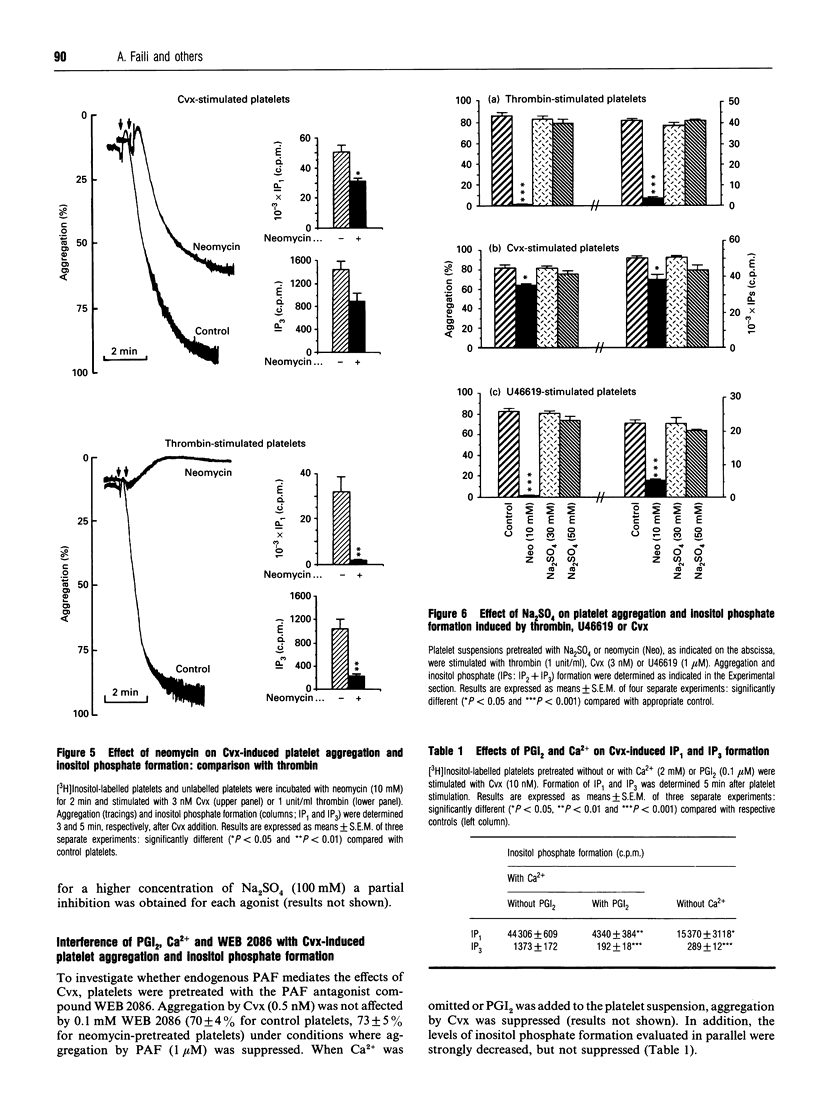
Platelet aggregation and stimulation of phosphoinositide-specific phospholipase C (PLC) by thrombin and by convulxin (Cvx), a non-enzymic snake venom glycoprotein, were compared. Cvx-stimulated production of inositol phosphates by washed platelets was independent of the cyclo-oxygenase pathway, formation of platelet-activating factor and ADP release, but prostacyclin (prostaglandin I2), a stimulator of cyclic AMP formation, suppressed its effects on platelet and PLC activation. Kinetic analysis showed that inositol 1,4,5-trisphosphate formation reached its maximal value 15 s after platelet stimulation with Cvx and persisted for at least 5 min. Neomycin sulphate (10 mM), which complexes phosphatidylinositol 4-phosphate and phosphatidyl-inositol 4,5-bisphosphate, decreased the production of inositol phosphates, partially prevented platelet aggregation induced by a high concentration of Cvx (10 nM) and abolished both platelet aggregation and inositol phosphate formation induced by thrombin (2 units/ml) and by a stable prostaglandin H2 analogue, U46619 (1 microM). In contrast with neomycin sulphate, Na2SO4 had no significant effect against all agonists tested. It is concluded that platelet activation by Cvx is partially mediated by PLC and involves other mechanisms as well.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Casals-Stenzel J., Muacevic G., Weber K. H. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther. 1987 Jun;241(3):974–981. [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Beattie L. L., Park J., Israels S. J., McNicol A., Lint D., Cragoe E. J., Jr A role for protein kinase C in the membrane fusion necessary for platelet granule secretion. Blood. 1989 Nov 15;74(7):2405–2413. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Davies T., Lynham J. A., McClenaghan M. D. Regulation of blood platelet function by cyclic nucleotides. Adv Cyclic Nucleotide Res. 1978;9:533–552. [PubMed] [Google Scholar]
- Hatmi M., Randon J., Faili A., Vargaftig B. B. Carrageenan-induced activation of human platelets is dependent on the phospholipase C pathway. Br J Haematol. 1993 Feb;83(2):270–275. doi: 10.1111/j.1365-2141.1993.tb08282.x. [DOI] [PubMed] [Google Scholar]
- Herrmann E., Jakobs K. H. Stimulation and inhibition of human platelet membrane high-affinity GTPase by neomycin. FEBS Lett. 1988 Feb 29;229(1):49–53. doi: 10.1016/0014-5793(88)80795-6. [DOI] [PubMed] [Google Scholar]
- Marlas G. Isolation and characterization of the alpha and beta subunits of the platelet-activating glycoprotein from the venom of Crotalus durissus cascavella. Biochimie. 1985 Dec;67(12):1231–1239. doi: 10.1016/s0300-9084(85)80132-2. [DOI] [PubMed] [Google Scholar]
- Marlas G., Joseph D., Huet C. I--isolation and electron microscope studies of a potent platelet-aggregating glycoprotein from the venom of Crotalus durissus cascavella. Biochimie. 1983 Jul;65(7):405–416. doi: 10.1016/s0300-9084(83)80060-1. [DOI] [PubMed] [Google Scholar]
- Mills D. C., Smith J. B. The control of platelet responsiveness by agents that influence cyclic AMP metabolism. Ann N Y Acad Sci. 1972 Oct 27;201:391–399. doi: 10.1111/j.1749-6632.1972.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Morrison W. J., Shukla S. D. Antagonism of platelet activating factor receptor binding and stimulated phosphoinositide-specific phospholipase C in rabbit platelets. J Pharmacol Exp Ther. 1989 Sep;250(3):831–835. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Polascik T., Godfrey P. P., Watson S. P. Neomycin cannot be used as a selective inhibitor of inositol phospholipid hydrolysis in intact or semi-permeabilized human platelets. Aminoglycosides activate semi-permeabilized platelets. Biochem J. 1987 May 1;243(3):815–819. doi: 10.1042/bj2430815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Franceschi J., Brazil O. V. Convulxin, a new toxin from the venom of the South American rattlesnake Crotalus durissus terrificus. Toxicon. 1981;19(6):875–887. doi: 10.1016/0041-0101(81)90085-4. [DOI] [PubMed] [Google Scholar]
- Reid D. G., Gajjar K. A proton and carbon 13 nuclear magnetic resonance study of neomycin B and its interactions with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1987 Jun 15;262(17):7967–7972. [PubMed] [Google Scholar]
- Sano-Martins I. S., Daimon T. Electron microscopic cytochemistry on the distribution of wheat germ agglutinin receptor on the platelet plasma membrane after treatment with convulxin isolated from Crotalus durissus terrificus venom. Toxicon. 1992 Feb;30(2):141–150. doi: 10.1016/0041-0101(92)90467-j. [DOI] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Siess W., Boehlig B., Weber P. C., Lapetina E. G. Prostaglandin endoperoxide analogues stimulate phospholipase C and protein phosphorylation during platelet shape change. Blood. 1985 May;65(5):1141–1148. [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Neomycin inhibits inositol phosphate formation in human platelets stimulated by thrombin but not other agonists. FEBS Lett. 1986 Oct 20;207(1):53–57. doi: 10.1016/0014-5793(86)80011-4. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tysnes O. B., Johanessen E., Steen V. M. Neomycin does not interfere with the inositol phospholipid metabolism, but blocks binding of alpha-thrombin to intact human platelets. Biochem J. 1991 Jan 1;273(Pt 1):241–243. doi: 10.1042/bj2730241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargaftig B. B., Joseph D., Wal F., Marlas G., Chignard M., Chevance L. G. Convulxin-induced activation of intact and of thrombin-degranulated rabbit platelets: specific crossed desensitisation with collagen. Eur J Pharmacol. 1983 Aug 19;92(1-2):57–68. doi: 10.1016/0014-2999(83)90108-5. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Prado-Franceschi J., Chignard M., Lefort J., Marlas G. Activation of guinea-pig platelets induced by convulxin, a substance extracted from the venom of Crotalus durissus cascavella. Eur J Pharmacol. 1980 Dec 19;68(4):451–464. doi: 10.1016/0014-2999(80)90420-3. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Reep B., McConnell R. T., Lapetina E. G. Collagen stimulates [3H]inositol trisphosphate formation in indomethacin-treated human platelets. Biochem J. 1985 Mar 15;226(3):831–837. doi: 10.1042/bj2260831. [DOI] [PMC free article] [PubMed] [Google Scholar]


