Abstract
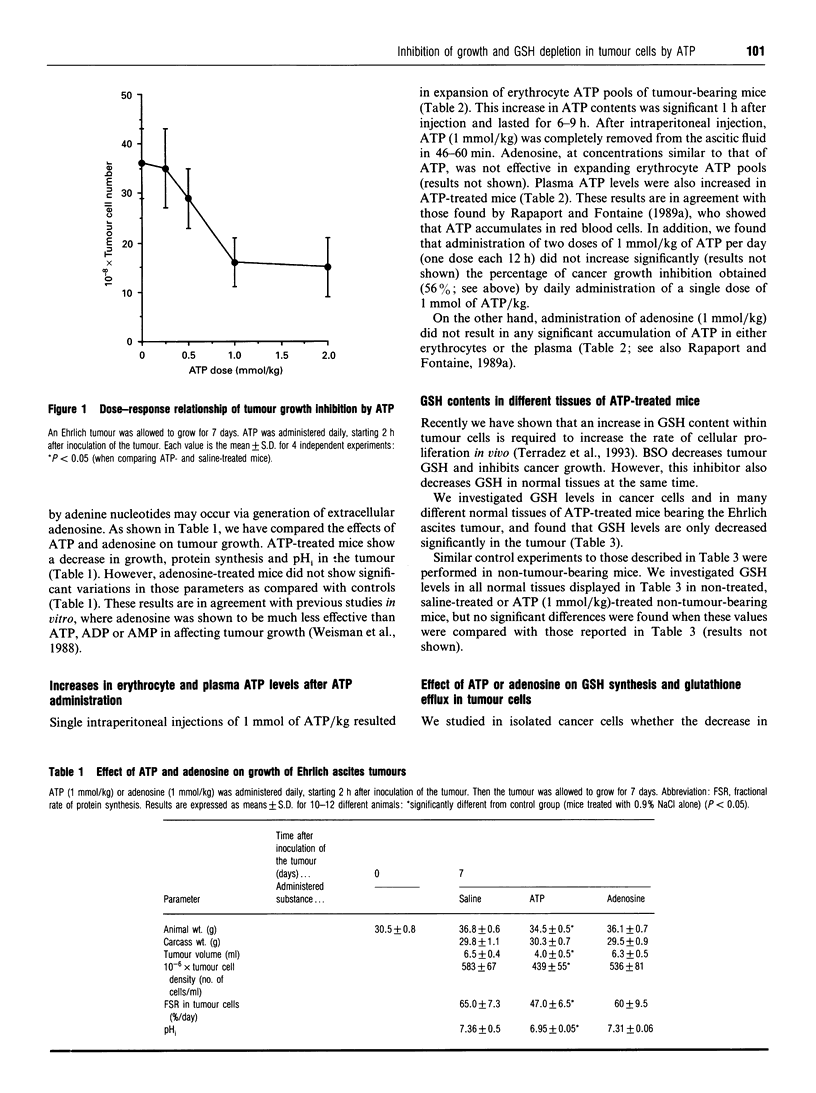
We have investigated the effect of extracellular ATP on tumour-cell proliferation and GSH levels in Ehrlich-ascites-tumour-bearing mice. After daily administration of exogenous ATP (1 mmol/kg) during 7 days, we found a 56% inhibition of tumour growth, precisely when controls show the highest rates of cell proliferation and the highest levels of GSH. This effect is accompanied by a decrease in GSH content in the tumour, but not in normal tissues. The decrease in GSH concentration within the cancer cells is associated with a decrease in gamma-glutamylcysteine synthetase activity and in protein synthesis. Growth inhibition is mediated by generation of extracellular adenosine, which subsequently increases intracellular levels of ATP and decreases intracellular levels of UTP in the cancer cells. Our results suggest that inhibition of tumour growth by ATP is due to an adenosine-dependent pyrimidine starvation effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Archer S., Juranka P. F., Ho J. H., Chan V. L. An analysis of multiple mechanisms of adenosine toxicity in baby hamster kidney cells. J Cell Physiol. 1985 Aug;124(2):226–232. doi: 10.1002/jcp.1041240209. [DOI] [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984 Oct;44(10):4224–4232. [PubMed] [Google Scholar]
- Chahwala S. B., Cantley L. C. Extracellular ATP induces ion fluxes and inhibits growth of Friend erythroleukemia cells. J Biol Chem. 1984 Nov 25;259(22):13717–13722. [PubMed] [Google Scholar]
- Chaudry I. H. Cellular mechanisms in shock and ischemia and their correction. Am J Physiol. 1983 Aug;245(2):R117–R134. doi: 10.1152/ajpregu.1983.245.2.R117. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H., Hirasawa H., Baue A. E. Effect of adenosine triphosphate-glucose administration following sepsis. J Surg Res. 1980 Oct;29(4):348–356. doi: 10.1016/0022-4804(80)90068-2. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H., Sayeed M. M., Baue A. E. Effect of adenosine triphosphate-magnesium chloride administration in shock. Surgery. 1974 Feb;75(2):220–227. [PubMed] [Google Scholar]
- Crook T. R., Souhami R. L., Whyman G. D., McLean A. E. Glutathione depletion as a determinant of sensitivity of human leukemia cells to cyclophosphamide. Cancer Res. 1986 Oct;46(10):5035–5038. [PubMed] [Google Scholar]
- Estrela J. M., Hernandez R., Terradez P., Asensi M., Puertes I. R., Viña J. Regulation of glutathione metabolism in Ehrlich ascites tumour cells. Biochem J. 1992 Aug 15;286(Pt 1):257–262. doi: 10.1042/bj2860257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins J. P., Buchanan B. J. Protection against endotoxin shock and impaired glucose homeostasis with ATP. Circ Shock. 1977;4(3):253–258. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. The role of adenosine and 2'-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee F. Y., Allalunis-Turner M. J., Siemann D. W. Depletion of tumour versus normal tissue glutathione by buthionine sulfoximine. Br J Cancer. 1987 Jul;56(1):33–38. doi: 10.1038/bjc.1987.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum C. T., Marz R., Plagemann P. G., Wohlhueter R. M. Adenosine transport and metabolism in mouse leukemia cells and in canine thymocytes and peripheral blood leukocytes. J Cell Physiol. 1979 Nov;101(2):173–200. doi: 10.1002/jcp.1041010202. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Mills G. C., Schmalstieg F. C., Trimmer K. B., Goldman A. S., Goldblum R. M. Purine metabolism in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2867–2871. doi: 10.1073/pnas.73.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. B., Cook J. A., DeGraff W., Glatstein E., Russo A. Glutathione modulation in cancer treatment: will it work? Int J Radiat Oncol Biol Phys. 1989 May;16(5):1289–1295. doi: 10.1016/0360-3016(89)90301-5. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke J. F., Dogterom P., De Bont H. J., Mulder G. J. Prolonged high intracellular free calcium concentrations induced by ATP are not immediately cytotoxic in isolated rat hepatocytes. Changes in biochemical parameters implicated in cell toxicity. Biochem J. 1989 Oct 15;263(2):347–353. doi: 10.1042/bj2630347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsse M., Egner H. J. Can nocodazole, an inhibitor of microtubule formation, be used to synchronize mammalian cells? Accumulation of cells in mitosis studied by two parametric flow cytometry using acridine orange and by DNA distribution analysis. Cell Tissue Kinet. 1984 Jan;17(1):13–23. [PubMed] [Google Scholar]
- Rapaport E. Experimental cancer therapy in mice by adenine nucleotides. Eur J Cancer Clin Oncol. 1988 Sep;24(9):1491–1497. doi: 10.1016/0277-5379(88)90340-9. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Fishman R. F., Gercel C. Growth inhibition of human tumor cells in soft-agar cultures by treatment with low levels of adenosine 5'-triphosphate. Cancer Res. 1983 Sep;43(9):4402–4406. [PubMed] [Google Scholar]
- Rapaport E., Fontaine J. Anticancer activities of adenine nucleotides in mice are mediated through expansion of erythrocyte ATP pools. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1662–1666. doi: 10.1073/pnas.86.5.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E., Fontaine J. Generation of extracellular ATP in blood and its mediated inhibition of host weight loss in tumor-bearing mice. Biochem Pharmacol. 1989 Dec 1;38(23):4261–4266. doi: 10.1016/0006-2952(89)90524-8. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Garcia-Blanco M. A., Zamecnik P. C. Regulation of DNA replication in S phase nuclei by ATP and ADP pools. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1643–1647. doi: 10.1073/pnas.76.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Seelig G. F., Meister A. Glutathione biosynthesis; gamma-glutamylcysteine synthetase from rat kidney. Methods Enzymol. 1985;113:379–390. doi: 10.1016/s0076-6879(85)13050-8. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Chou I. N. Elevation of intracellular glutathione content associated with mitogenic stimulation of quiescent fibroblasts. J Cell Physiol. 1986 Nov;129(2):193–198. doi: 10.1002/jcp.1041290210. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Seegmiller J. E. The adenosine-like effect of exogenous cyclic AMP upon nucleotide and PP-ribose-P concentrations of cultured human lymphoblasts. FEBS Lett. 1976 Jul 1;66(1):102–106. doi: 10.1016/0014-5793(76)80595-9. [DOI] [PubMed] [Google Scholar]
- Sumpio B. E., Hull M. J., Baue A. E., Chaudry I. H. Comparison of effects of ATP-MgCl2 and adenosine-MgCl2 on renal function following ischemia. Am J Physiol. 1987 Feb;252(2 Pt 2):R388–R393. doi: 10.1152/ajpregu.1987.252.2.R388. [DOI] [PubMed] [Google Scholar]
- Suthanthiran M., Anderson M. E., Sharma V. K., Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci U S A. 1990 May;87(9):3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradez P., Asensi M., Lasso de la Vega M. C., Puertes I. R., Viña J., Estrela J. M. Depletion of tumour glutathione in vivo by buthionine sulphoximine: modulation by the rate of cellular proliferation and inhibition of cancer growth. Biochem J. 1993 Jun 1;292(Pt 2):477–483. doi: 10.1042/bj2920477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G. A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J Theor Biol. 1980 Dec 7;87(3):609–621. doi: 10.1016/0022-5193(80)90239-8. [DOI] [PubMed] [Google Scholar]
- Weisman G. A., De B. K., Friedberg I., Pritchard R. S., Heppel L. A. Cellular responses to external ATP which precede an increase in nucleotide permeability in transformed cells. J Cell Physiol. 1984 May;119(2):211–219. doi: 10.1002/jcp.1041190211. [DOI] [PubMed] [Google Scholar]
- Weisman G. A., Lustig K. D., Lane E., Huang N. N., Belzer I., Friedberg I. Growth inhibition of transformed mouse fibroblasts by adenine nucleotides occurs via generation of extracellular adenosine. J Biol Chem. 1988 Sep 5;263(25):12367–12372. [PubMed] [Google Scholar]


