The use of fresh-frozen plasma (FFP) continues to rise,1 despite the fact that the supply of plasma derived from allogeneic blood donation is finite. Unfortunately, this product is commonly overused or inappropriately used.1
In 1997, in view of the limited supply of FFP and the shrinking donor pool, an expert working group convened by the Canadian Medical Association (CMA) published recommendations for the appropriate use of erythrocyte (red blood cells) and plasma transfusion.2 The guidelines recommended transfusion of FFP in 3 specific situations: for patients with significant coagulopathy because of acquired deficiencies of multiple coagulation factors in whom serious bleeding has occurred or for whom emergency surgery or other procedures are planned, for treatment of thrombotic thrombocytopenic purpura and for treatment of acquired single-factor deficiencies where a product containing the specific factor is ineffective or unavailable. We conducted a prospective audit to determine whether current FFP transfusion practice in 2 tertiary care teaching hospitals in London, Ont., followed these recommendations.
From June to August 1999 the following information was collected by blood bank technologists at the time FFP was requested for any adult patient: diagnosis, clinical indication (e.g., active bleeding, emergency surgery or invasive procedure) and pretransfusion coagulation result. The clinical information was obtained from the health care practitioner placing the FFP order, and the coagulation results were obtained from a computerized laboratory reporting system. The patient's chart was reviewed later if any information was missing. A hematologist (CL) applied the CMA guidelines to each transfusion request and classified the transfusion as appropriate if the criteria were completely fulfilled, as probably appropriate if the criteria were not completely fulfilled but FFP use was likely necessary in the context of the clinical scenario, or as inappropriate. Questionable scenarios (22% total cases) were judged by consensus after case reviews with two additional hematologists.
Over the 3-month study period, there were 671 FFP transfusion orders, accounting for 2372 units for 358 patients. The FFP transfusions for 2 patients were excluded because available information was insufficient to determine the appropriateness of transfusion. Furthermore, an additional 15 transfusion orders for plasmapheresis used in 2 patients with vasculitis were omitted from the analysis because there were no guidelines to judge the appropriateness of transfusion in this situation. The transfusions were judged appropriate for 167 patients (47%), probably appropriate for 31 (9%) and inappropriate for 160 (45%). The situations in which transfusion was judged inappropriate are listed in Table 1.
Table 1
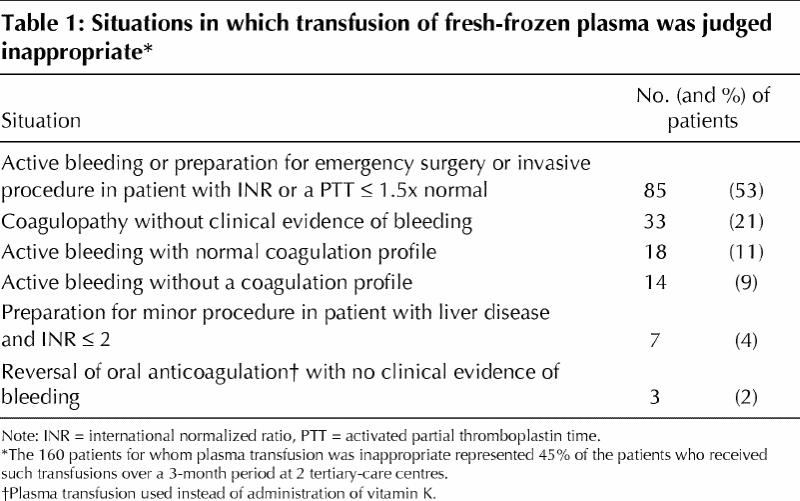
The rate of inappropriate use of FFP in this study (45%) was within the range reported in previous studies (10% to 73%).3,4,5,6 However, the results of our study are not directly comparable with the earlier results because of differences in the guidelines used. The high rate of inappropriate use reported here is due in part to our stringent application of the CMA guidelines. The strengths of this audit include the large sample size and its prospective nature. A limitation of the assessment is that the adjudication process was performed without full knowledge of each case.
The purpose of auditing transfusion use is to raise awareness and to improve the rate of appropriate use of blood products. In this study we found a high rate of inappropriate FFP use and identified the situations in which FFP was used inappropriately. We recommend adoption of a program to promote more efficient use of the limited FFP supply in all institutions. Such a program should incorporate strategies that have proven effective, such as formal education and regular utilization audits7,8,9 targeting the inappropriate indications that we identified.
Footnotes
This article has been peer reviewed.
Contributors: Dr. Luk was responsible for data analysis and preparation of the manuscript. Ms. Eckert was responsible for study design, data collection and manuscript revision. Drs. Barr and Chin-Yee contributed to study design, data interpretation and manuscript preparation.
Competing interests: None declared.
Correspondence to: Dr. Ian H. Chin-Yee, Department of Hematology, Room 2760, London Health Sciences Centre, 800 Commissioners Rd. E, London ON N6A 4G5; fax 519 685-8477; ian.chinyee@lhsc.on.ca
References
- 1.Thomson A, Contreras M, Knowles S. Blood component treatment: a retrospective audit in five major London hospitals. J Clin Pathol 1991;44(9): 734-7. [DOI] [PMC free article] [PubMed]
- 2.Expert Working Group. Guidelines for red blood cell and plasma transfusion for adults and children. CMAJ 1997;156(11 Suppl):S1-S24. Available: www.cma.ca/cmaj/vol-156/issue-11/blood/index.htm9347786
- 3.Blumberg N, Laczin J, McMican A, Heal J, Arvan D. A critical survey of fresh-frozen plasma use. Transfusion 1986;26(6):511-3. [DOI] [PubMed]
- 4.Jones HP, Jones J, Napier JA, al-Ismail S. Clinical use of FFP: results of a retrospective process and outcome audit. Transfus Med 1998;8(1):37-41. [DOI] [PubMed]
- 5.Brien WF, Butler RJ, Inwood MJ. An audit of blood component therapy in a Canadian general teaching hospital. CMAJ 1989;140(7):812-5. [PMC free article] [PubMed]
- 6.Mozes B, Epstein M, Ben Bassat I, Modan B, Halkin H. Evaluation of the appropriateness of blood and blood product transfusion using preset criteria. Transfusion 1989;29(6):473-6. [DOI] [PubMed]
- 7.Morrison JC, Sumrall DD, Chevalier SP, Robinson SV, Morrison FS, Wiser WL. The effect of provider education on blood utilization practices. Am J Obstet Gynecol 1993;169(5):1240-5. [DOI] [PubMed]
- 8.Shanberge JN. Reduction of fresh-frozen plasma use through a daily survey and education program. Transfusion 1987;27(3):226-7. [DOI] [PubMed]
- 9.Barnette RE, Fish DJ, Eisenstaedt RS. Modification of fresh-frozen plasma transfusion practices through educational intervention. Transfusion 1990; 30 (3): 253-7. [DOI] [PubMed]


