Abstract
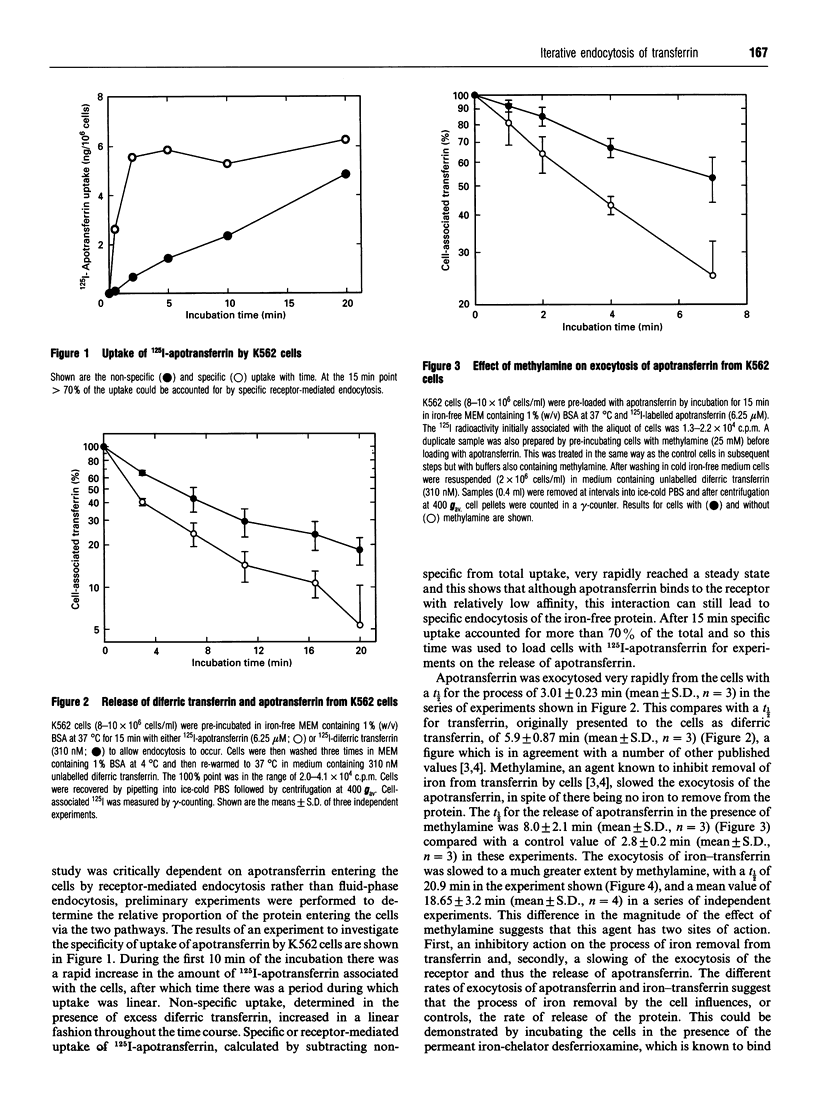
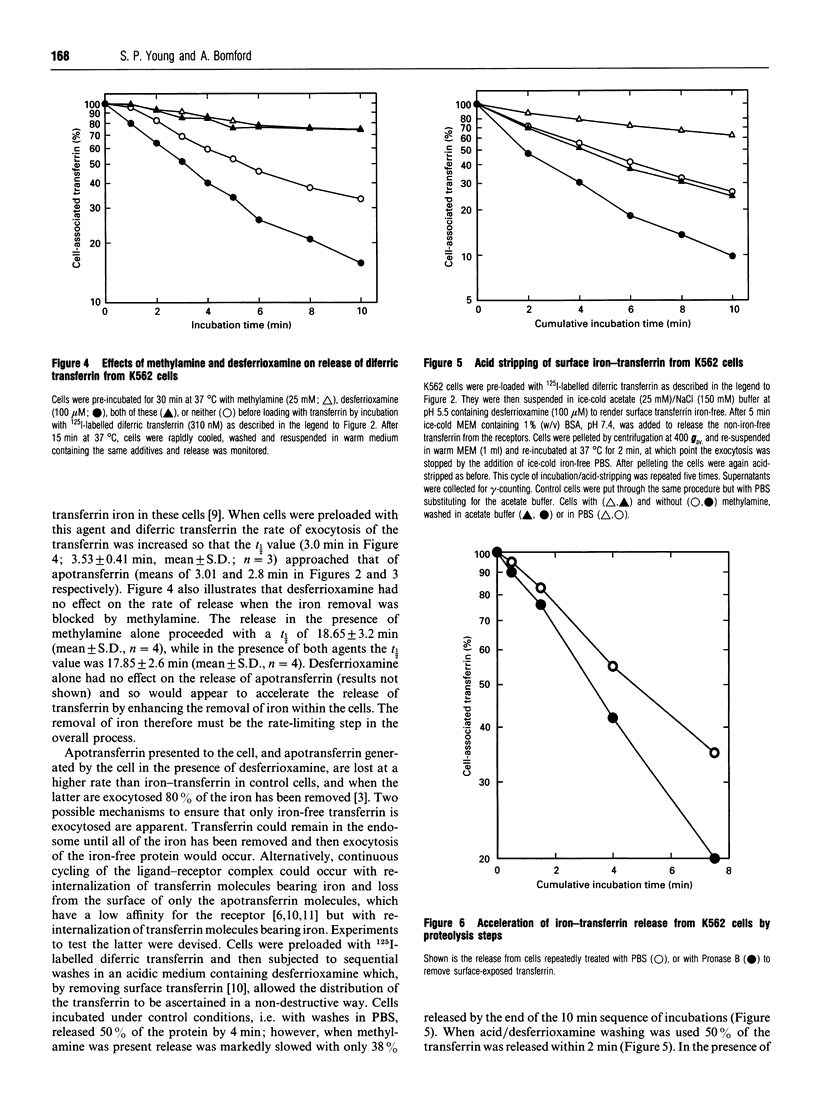
The effect of iron on the exocytosis of transferrin by K562 cells was studied by first allowing the cells to endocytose apotransferrin or diferric transferrin. Subsequent release of the apotransferrin was very rapid with a t 1/2 of 3.01 min, compared with 5.5 min for diferric transferrin. Release of apotransferrin was slowed by the weak base methylamine, t 1/2 8.0 min, but the effect of this agent was substantially greater when iron-transferrin was used, t 1/2 18.65 min, suggesting that methylamine affects both iron removal and receptor recycling. Release of iron-transferrin could be accelerated to a rate comparable with that of apotransferrin by addition of the permeant iron-chelator desferrioxamine. The difference in the rates of release of different forms of the protein could be explained by the re-endocytosis of the iron-rich protein, a process detected by the accelerated release of transferrin when the cells were washed in medium at pH 5.5 containing an iron-chelator or treated with a protease-containing medium to digest transferrin accessible at the cell surface. It appears that in cells incubated under control conditions, re-endocytosis of transferrin, which is incompletely depleted of iron, occurs and that a transferrin molecule may make two passes through the cell before all the iron is removed. This mechanism helps to explain why very little iron-transferrin is released from cells and why the efficiency of the iron uptake process is so high.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bali P. K., Zak O., Aisen P. A new role for the transferrin receptor in the release of iron from transferrin. Biochemistry. 1991 Jan 15;30(2):324–328. doi: 10.1021/bi00216a003. [DOI] [PubMed] [Google Scholar]
- Bomford A., Young S. P., Williams R. Release of iron from the two iron-binding sites of transferrin by cultured human cells: modulation by methylamine. Biochemistry. 1985 Jul 2;24(14):3472–3478. doi: 10.1021/bi00335a013. [DOI] [PubMed] [Google Scholar]
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford J., Bridges K., Ashwell G., Klausner R. D. Intracellular dissociation of receptor-bound asialoglycoproteins in cultured hepatocytes. A pH-mediated nonlysosomal event. J Biol Chem. 1983 Mar 10;258(5):3191–3197. [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. The mechanism of iron exchange between synthetic iron chelators and rabbit reticulocytes. Biochim Biophys Acta. 1974 Nov 27;373(1):84–99. doi: 10.1016/0005-2736(74)90108-4. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Klausner R. D., van Renswoude J., Kempf C., Rao K., Bateman J. L., Robbins A. R. Failure to release iron from transferrin in a Chinese hamster ovary cell mutant pleiotropically defective in endocytosis. J Cell Biol. 1984 Mar;98(3):1098–1101. doi: 10.1083/jcb.98.3.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub R., Schneider Y. J., Octave J. N., Trouet A., Crichton R. R. Cellular pharmacology of deferrioxamine B and derivatives in cultured rat hepatocytes in relation to iron mobilization. Biochem Pharmacol. 1985 Apr 15;34(8):1175–1183. doi: 10.1016/0006-2952(85)90492-7. [DOI] [PubMed] [Google Scholar]
- Makey D. G., Seal U. S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976 Nov 26;453(1):250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Princiotto J. V., Zapolski E. J. Difference between the two iron-binding sites of transferrin. Nature. 1975 May 1;255(5503):87–88. doi: 10.1038/255087a0. [DOI] [PubMed] [Google Scholar]
- Roberts S., Bomford A. Chelation of transferrin iron by desferrioxamine in K562 cells. The partition of iron between ferrioxamine and ferritin. Biochem J. 1988 Sep 15;254(3):869–875. doi: 10.1042/bj2540869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Bolognesi A., Fridovich S. E. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in hepatoma cells. J Cell Biol. 1984 Feb;98(2):732–738. doi: 10.1083/jcb.98.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe D. M., Jesurum A., Murphy R. F. Absence of Na+,K(+)-ATPase regulation of endosomal acidification in K562 erythroleukemia cells. Analysis via inhibition of transferrin recycling by low temperatures. J Biol Chem. 1991 Feb 25;266(6):3469–3474. [PubMed] [Google Scholar]
- Sipe D. M., Murphy R. F. Binding to cellular receptors results in increased iron release from transferrin at mildly acidic pH. J Biol Chem. 1991 May 5;266(13):8002–8007. [PubMed] [Google Scholar]
- Stein B. S., Sussman H. H. Demonstration of two distinct transferrin receptor recycling pathways and transferrin-independent receptor internalization in K562 cells. J Biol Chem. 1986 Aug 5;261(22):10319–10331. [PubMed] [Google Scholar]
- Tycko B., Keith C. H., Maxfield F. R. Rapid acidification of endocytic vesicles containing asialoglycoprotein in cells of a human hepatoma line. J Cell Biol. 1983 Dec;97(6):1762–1776. doi: 10.1083/jcb.97.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985 Feb;100(2):633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. P., Bomford A., Williams R. The effect of the iron saturation of transferrin on its binding and uptake by rabbit reticulocytes. Biochem J. 1984 Apr 15;219(2):505–510. doi: 10.1042/bj2190505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]


