Abstract
Banana fruit is a highly consumed and widely cultivated world food crop that generates plenty of waste globally. In this work, the phytochemical, nutritional, scavenging and therapeutic potentials of banana peel (BP) extracts were compared before and after fermentation. Halophilic fungi (Alternaria alternata, Pleosporaceae spp., Fusarium culmorum) were used in fermentation media designated as fermented banana peel FBP1, FBP2, and FBP3, respectively. Phytochemical coumarins, terpenoids, tannins, saponins, quinones, flavonoids, alkaloids, carbohydrates, proteins and steroids were found in all extracts while anthraquinone was identified in BP extracts only. Fermented extracts showed less quantity of Carbohydrate, compared to BP (477.1 ± 28.93 mg/g). Fermentation influenced the protein concentration as FBP1 showed a maximum protein of 56.9 ± 8.91 mg/g. Decreased quantities of Total Phenolic Contents (TPC), Total Flavonoid contents (TFC), and Vitamin C were noted in fermented products. The BP contained TPC (18 ± 2.59 mg GAE/g), TFC (20.5 ± 2.11 mg QE/g), carotenoid (1.03 ± 0.19 mg/g) and vitamin C (33.46 ± 2.63 mg/L). For BP, high antioxidant activity was observed, IC50 values of DPPH scavenging and FRAP assay were 2.01 ± 0.06 mg/mL and 12.81 ± 0.03 mg/mL, respectively. All the extracts were potentially active against the Salmonella typhi, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli BP extract showed high antibacterial activity than the fermented products. Among all the above, S. aureus showed high sensitivity to BP and FBP2 with 26.33 ± 2.49 and 26.33 ± 0.97 mm zone of inhibition and S. typhi was highly inhibited by BP and FBP1 with 26.26 ± 1.77 and 26.66 ± 2.63 mm. BP was highly active against K. pneumoniae and P. aeruginosa with 31.33 ± 1.74 and 32.33 ± 1.59 mm zone of inhibition and E. coli was sensitive to FBP2 with 25.7 ± 2.33 mm zone, respectively. The BP extract possessed potent antifungal activity against Mucor mucedo (84 %), Aspergillus niger (72 %) and Aspergillus flavus (83 %), which was higher than the fermented products. The antileishmanial assay was undertaken for all extracts against promastigotes of Leishmania major, BP showed good activity IC50 = 0.763 ± 0.01 mg/g. In the anti-inflammatory assays the BP showed lowest IC50 values by protein denaturing (0.612 ± 0.01), proteinase inhibitory (0.502 ± 0.01) and blood hemolysis assay (0.515 ± 0.01 mg/g). The minimum concentration indicated that BP was highly potent in response to antileishmanial and inflammation activity.
Keywords: Fermentation, Waste recovery, Functional food, Halophilic fungi, Food security
1. Introduction
The growing demand for food with eliminating environmental consequences of disposing of food processing wastes has led to their long-term consideration as a subject of treatment, mitigation, and prevention [1]. According to The State of Food and Agriculture (2019) report published by the Food and Agriculture Organization (FAO), 14 % of food is lost or wasted after it has grown and before reaching retailers [2]. Now food wastes serve as a rich source of nutraceuticals and address the challenge of feeding the world's rapidly expanding population in the twenty-first century. Among all these food waste banana account for 220 tonnes byproduct per hectare annually [3].
Banana (Musa spp.), is an important crop grown in tropical regions of Southeast Asia, Latin America and Africa. Banana waste, which is predominantly made up of lignocellulosic material includes leaves, peels and stems [4]. Banana peel is rich in dietary fibers, nutrients, phytochemicals and bioactive compounds that has been known for several biological activities such as antioxidant, antimicrobial, hypoglycemic, antihypertensive and anti-inflammatory potential [5,6]. Its significant outcome benefits the pharmaceutical and food industries [7]. Historically, the banana peel has been prescribed to treat menstrual cramps, burns, anemia, diarrhea, abscesses, coughing, snakebite and heavy menstrual flow [8]. Banana peel was reported to contain gallic acid, catechin [9], furthermore, gallocatechin was found more abundant in the peel than in the pulp [10]. Consuming foods rich in antioxidants reduces the risk of illnesses linked to oxidative stress [11]. Antioxidant concentration in banana peel increased with the ripening of fruit and decreased once it was fully ripened [12]. β-sitosterol, palmitic acid, malic acid and succinic acid identified in green banana peel extract were found to be effective against S. aureus, B. subtilis, B. cereus and E. coli [13]. According to Aboul-Enein et al. [14], tannins found in banana peels have antibacterial potential against E. coli, S. aureus and P. aeruginosa. The flavonoids included in banana peels also have an anti-tumor effect through cell cycle arrest, stimulation of apoptosis, and suppression of ROS-scavenging enzyme activity. Ferulic acid is a key component of banana peels and it also inhibits the development of tumors, the synthesis of a growth factor for vascular endothelial cells and the initiation of nitric oxide synthase, which stops melanogenesis [15]. However, banana peel is thrown away in the environment untreated. It contains a considerable amount of nutrients and anti-nutrients. As a result, it may be utilized as organic fertilizer and animal feed [8].
Fermentation is a metabolic process that involves yeast, bacteria and molds that break down complex organic chemicals into their simpler counterparts [16]. Reducing sugar is used by microorganisms as a growth substrate and is transformed into alcohol and other byproducts. Fermentation transforms the biochemical and nutritional qualities of raw food material by decreasing antinutrients, generating vitamin B and synthesizing other value-added metabolites [17]. Fungal diversity is often higher under extreme environments. The production of antibiotics and other desirable bioactive compounds can be better studied in halophilic fungal species [18]. A. alternata, Pleosporaceae spp. and F. culmorum were previously cultured in a hypersaline environment to obtain extracellular antioxidants [19,20].
There is a lack of information regarding how fermentation by halophilic fungi affects the phytochemical composition and biological activity of banana peel. This study aims to determine the potential use of halophilic fungi for food enhancement. The fungal species were used for the comparative analysis of fermentation on banana peel extracts. The fermentation impact was evaluated on the phytoconstituents, biological, and antioxidant activities on fermented and non-fermented banana peel.
2. Materials and methods
2.1. Sample collection and preparation
Bananas (Musa Spp.) were obtained from the local market in Quetta City, Pakistan. The banana was identified by the botanist Dr. Zareen Gul department of Botany University of Balochistan Quetta. Specimen of the banana peel were stored in the Food Microbiology and Bioprocess Technology laboratory department of microbiology University Balochistan. The peels were removed, washed with running tap water, and dried in the shade at room temperature and controlled humidity. The dried peels were finely ground with an electrical grinder and placed in desiccators for further uses.
2.2. Inoculum preparation
The preserved culture of three halophilic fungi, namely Alternaria alternate (MH282516), Pleosporaceae spp. (MH282510) and Fusarium culmorum (MH282513) were acquired from Food Microbiology and Bioprocess Technology Laboratory Department of Microbiology, University of Balochistan Quetta. The cultures were refreshed in Sabroud Dextrose Broth with 10 % NaCl for 72 h at 37 °C. The cultures were re-grown on potato dextrose agar for further use.
2.3. Extraction of Banana peel extract by maceration
For analysis of bioactive compounds, 200 g of banana peels powder was soaked in 2 L of absolute ethanol with 1:10 for three days, following standard procedure [19]. The process was carried out in the dark to avoid light exposure, with frequent shaking. The ethanolic mixture was filtered through Whatman filter paper No.1. The extracts were dried by rotary evaporator for further analysis. The extraction yield (weight of extract/weight of dry banana peel) was 7 ± 2 %.
2.4. Solid state fermentation
An amount of 15 g dried banana peel powder was added to 30 mL of salt medium containing the following per 500 mL: 50 g NaCl, 0.15 g urea, 0.37 g peptone, 0.7 g (NH4)2SO4, 0.15 g MgSO4.7H2O, 1.00 g K2HPO4, 0.20 g CaCl2.2H2O, 2.5 mg FeSO4⋅7H2O, 0.7 mg ZnSO4, 0.8 mg MnSO4.5H2O, 1.00 mg CoCl2.6H2O in each flask as described by Ref. [21]. Each flask was then sterilized at 121 °C for 15 min. Each fungal species inoculum (5 % v/v) previously prepared from fresh culture on the same media was added to the sterilized fresh medium and fermented at 37 °C for 7 days. The fungal biomass was removed from the semisolid processed fermented media, and 150 mL absolute ethanol were added to the fermented media and kept for three days, the liquid contents were then evaporated with the help of rotary evaporator. The dry solid paste from fermented and unfermented extracts were then analyzed for different phytochemicals and bioactive compounds presence and was also assessed for their chemical and biological potential. The fermented samples extracts were named FBP1 (A. alternata), FBP2 (Pleosporaceae spp.) and FBP3 (F. culmorum), respectively. All assessments were conducted in triplicates.
2.5. Phytochemical analysis
The phytochemical constituents including carbohydrates, cardiac glycosides, tannins, steroids, terpenoids, flavonoids, saponins, coumarins, quinones, anthraquinones, and alkaloids in fermented and unfermented banana peel extracts were qualitatively determined following [22].
2.6. Total carbohydrate estimation
Total carbohydrates concentration was determined in BP, FBP1, FBP2 and FBP3 extracts using the phenol-sulphuric acid reagent method [23]. An amount of 1 mL of tested extract was dissolved in a solution possessing 0.05 mL of phenol (80 %) and 5 mL Conc. H2SO4. The distinctive yellow-orange color was observed after incubating the solution in water bath for 10–20 min at 25–30 °C. The T60 UV-VIS Spectrophotometer was used to measure the absorbance at 510 nm with deionized water used as the blank and glucose as the standard. The carbohydrate concentration was expressed in mg/g.
2.7. Total protein analysis
The Lowry's method was used to analyze proteins in unfermented and fermented banana peel extracts banana [24]. In each test tube, 1 mL of extract, 8 mL of reagent 1 (48 mL Na2CO3 (2 %), 1 mL KNaC4H4O6.4H2O (1 %) and 1 mL CuSO4.5H2O (0.5 %) were added. The mixture was then incubated for 15 min after which 1 mL of freshly prepared reagent 2 (2 mL Folin-Ciocalteu (FC) reagent and 4 mL water) was rapidly added. The mixture was re-incubated for 30 min in the dark. Bovine serum albumin (BSA) was taken as standard to plot calibration curve. Deionized water was taken as blank in this assay. The absorbance of incubated extract mixtures was measured at 660 nm and the total protein content was calculated in terms of mg/g for each extract.
2.8. Total phenolic content (TPC) determination
The TPC of BP, FBP1, FBP2 and FBP3 extracts was determined using the Folin Ciocalteu reagent method following [25]. In brief, 0.4 mL of FC reagent and 1 mL of extract were mixed and left to react. Following a 10 min incubation period, 1.2 mL of Na2CO3 (20 % w/v) was added and the mixture was then incubated for 30 min at 40 °C. The T60 UV-VIS spectrophotometer was used to measure the absorbance at 765 nm. A calibration curve was constructed using gallic acid (0.0625–1 mg/mL).
2.9. Total flavonoids content determination
The TFC in fermented and unfermented banana peel extracts was assessed using the AlCl3 colorimetric method as described by Ref. [26]. In brief, 500 μL of each extract was treated with 2.35 mL distilled water and 150 μL of NaNO2 solution (5 %). After the reaction mixture had been incubating, 150 μL of AlCl3.6H2O (10 %) was added. An amount of 0.5 mL of (1 M) NaOH was added after incubation period of 5 min absorbance was measured at 516 nm using spectrophotometer for quantification. In order to construct the calibration curve, quercetin concentrations ranging from 0.0625 to 1 mg/mL were utilized. The results were expressed in mg quercetin equivalent (QE) per gram.
2.10. Total carotenoids determination
Each extract (BP, FBP1, FBP2 and FBP3) was homogenized for 1 min at 1000 rpm in 100 % acetone (50 mL/g). After another 10 min centrifugation at 2500 rpm, the mixture was filtered using Whatman filter paper No. 1. The chlorophyll a and b concentrations were determined by measuring the absorbance of supernatant at 470 nm, 662 nm and 646 nm, respectively. Total amount of these pigments was calculated using the Lichtentaler and Wellburn formula [27].
| C a = 1 1.75A662 - 2.35A645, C b = 18.61A645 - 3.96A662 |
| C x + c = (1000A470 - 2.27Ca - 81.4Cb)/270 |
Ca = Chlorophyll a, Cb = Chlorophyll b, (C x + c) = Total quantity of carotenoids.
2.11. Vitamin C analysis
The vitamin C analysis of BP, FBP1, FBP2 and FBP3 Extract was performed using the procedure outlined by Ref. [28]. A solution containing 70 % ethanol, methanol and water in a 1:1:1 ratio was added to the dry banana peel powder and its crude fermented extract. The mixture was filtered after 60 min. Then an amount 1 mL of 0.5 % starch solution was added to 20 mL of filtrates. The extracts were titrated against 0.005 mol/L of iodine solution. At the end of the titration, a dark blue-black tint was observed due to starch-iodine reaction.
2.12. Antioxidant activity
2.12.1. 1,1-Diphenyl-2-picryl-hydrazil (DPPH) method
DPPH stable radical was employed to measure the antioxidant activity of the of fermented and unfermented banana peel extracts using the standard protocol [19]. A mixture of 100 mL of 100 % ethanol and 0.0039432 g of DPPH was used to make 0.1 mM DPPH solution. Subsequently, 50 μL of each extract was mixed in 0.5 mL of DPPH (0.1 mM), and then allowed to incubate for 30 min at 37 °C to reach a steady state. At 517 nm, de-colorization occurred, and DPPH inhibition was calculated using the following equation:
| % Scavenging rate = (A1-A2)/A1 X 100 |
A1 = (DPPH) absorbance, A2 = extract.
2.13. Ferric reducing antioxidant power (FRAP) method
The antioxidant capacity of extracts, was analyzed following the method described by Ref. [29]. The 2,4,6-tripyridyl-s-triazine in 40 mM HCl, 20 mM FeCl3.6H2O and 300 mM acetate buffer were aggregated in a ratio of 1:1:10 at 37 °C to synthesize the FRAP reagent. Then 5 mL of each extract was mixed in 3.995 mL of freshly prepared FRAP reagent. The blue color developed after 30 min of 37 °C incubation, the absorbance was taken at 593 nm. A standard curve was plotted using various concentrations of FeSO4.7H2O at different concentrations.
2.14. Antibacterial activity determination
Well diffusion method was used to determine the antibacterial activity of BP, FBP1, FBP2, FBP3 extracts [22]. The target bacterial strains (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, and Staphylococcus aureus) were cultured in sterile nutrient broth for 24 h at 37 °C. Petri plates with sterile Muller Hinton Agar were used to evaluate antibacterial activity. Extracts with a concentration of 1 mg/mL diluted in Doxycycline and dimethyl sulfoxide (DMSO) were loaded into 6 mm wells made in agar plated using sterile cork borer. The culture plates were then kept for 24 h incubation at 37 °C for 24 h. DMSO were served as positive and negative controls, respectively. The results were interpreted based on the diameter in millimeters (mm) of the clean zone surrounding the wells.
2.15. Antifungal activity
The antifungal properties of BP, FBP1, FBP2 and FBP3 were assessed using agar mix method [30]. Briefly, in 35 mL of sterile potato dextrose agar, each extract (2 mg/mL dissolved in 1 mL DMSO) was added and mixed carefully before being poured into petri plates. Wells were made in the agar and filled with fresh fungal inoculum (Aspergillus flavus, Aspergillus niger, Mucor mucedo) and incubated at 37 °C for 72 h. Each media plate containing fungal strains was used as positive control. Fluconazole was taken as a standard antifungal drug. The linear fungal growth was measured in millimeter in comparison to positive control and the percent inhibition was calculated using the subsequent equation.:
| % Inhibition = (100 - linear growth in test /linear growth in control) X 100 |
2.16. In-vitro antileishmanial assay
2.16.1. Parasite culture
Leishmania major promastigotes that had previously been isolated and preserved in culture were obtained from the Food Microbiology and Bioprocess Technology Laboratory. In biphasic Novy-MacNeal-Nicolle (NNN) culture medium containing streptomycin and penicillin, the promastigotes were refreshed.
2.16.2. Antileishmanial assay
The antileishmanial assay for fermented and unfermented banana peel extracts was carried out using the methodology previously described [31] with some minor modifications. Promastigotes in the logarithmic phase were utilized in the present study at a concentration of 1 × 106 cells/mL. The BP and its fermented extracts were evaluated against L. major in culture using a 96-well plate. A stock solution (1 mg/mL) prepared from the test extract was dissolved into DMSO. All extracts (BP, BP1, BP2, BP3) were serially diluted at altered concentrations (1 mg/mL - 0.0625 mg/mL). Then 50 mL log-phase culture promastigote mixture was poured into all wells of 96-well plate along with 10 mL of each extract dilutions and incubated for 72 h at 37 °C. Glucantime, a recommended antileishmanial drug, was used as standard drug in the study, while media with organism as positive control and DMSO was taken as negative control. After incubation, 1 mL DMSO and 20 mL NBT solution (5 mg/mL in phosphate buffer, pH 7.2) was poured into all wells. The NBT solution was used to validate the percentage of mortality of extracts and drug. The absorbance at 630 nm was measured using an ELISA reader and the IC50 value was calculated via linear regression method. The cell viability percentage was calculated by given formula:
| Cell viability percentage = (A630 of test extract/A630 of control) X 100 |
| Inhibition percentage = 100 - percentage viability |
2.17. Anti-inflammatory activity
2.17.1. Effect on protein denaturation
The procedure for protein denaturation was carried out according to the guidelines provided by Ref. [32]. To summarize, a mixture of 1 % bovine albumin, 4.78 mL of phosphate buffer saline (PBS, pH 6.4) and 0.02 mL of extract was to make 5 mL reaction mixture. This mixture was heated for 5 min at 70 °C after being incubated in a water bath for 15 min at 37 °C. The turbidity was then measured at 660 nm using T60 UV-VIS spectrophotometer after cooling. Phosphate Buffer Saline (PBS) was used as a control. The percentage of protein denaturation was calculated using the formula given below:
| Percent Inhibition = (1 - A2/A1) × 100 |
A1 = Control absorbance, A2 = Test sample absorbance.
2.17.2. Proteinase inhibitory activity
The ability of the BP FBP1, FBP2 and FBP3 extracts to inhibit proteinase was assessed following [33]. In brief, 0.06 mg of trypsin, 1 mL of 20 mM Tris-HCl buffer (pH 7.4) and 1 mL of extract was all included in the reaction solution of 2 mL. The solution was incubated at 37 °C for 5 min. Then, 1 mL of casein (0.8 % w/v) was added, and the mixture was further incubated for 20 min. The reaction was stopped by adding 2 mL of 70 % HClO4 to the mixture. After centrifugation, the absorbance of supernatant was measured at 210 nm against a buffer blank after centrifuging the mixture. A phosphate buffer solution (PBS) operated as the control. The percentage inhibition was calculated, using following formula:
| Proteinase Percentage Inhibition = (1 − A2/A1) × 100 |
A1 = Control absorbance, A2 = Test sample absorbance.
2.17.3. Heat-induced hemolysis assay
The blood was collected and treated using the method recommended by Ref. [34] with minor alterations. Following the protocol, the blood was obtained from a healthy individual and treated with Na2C2O4, then centrifuged for 5 min at 2500 rpm to avoid coagulation. The resulting mixture was washed with normal saline through three repetitions of process. The volume of blood was then adjusted to re-form a 10 % (v/v) suspension in an isotonic buffer solution (PBS) (10 mM sodium phosphate buffer, pH 7.4). The buffer solution (g/L) was prepared from NaH2PO4.2H2O (0.2 g), Na2HPO4 (1.15 g) and NaCl (0.1 g).
The heat-induced hemolysis activity was carried out as per the protocol described by Ref. [35] with minor modifications. The blood cell suspension 0.05 mL was mixed with 2.95 mL of phosphate buffer and 0.05 mL of each extract with the pH 7.4. The mixture was set in water bath for 20 min at 54 °C. Following incubation, the centrifugation of mixture was done for 3 min at 2500 rpm and supernatant was separated. The absorbance of supernatant was measured on spectrophotometer at 540 nm. PBS and aspirin were used as control and standard drug, respectively.
The inhibition percentage was calculated via the formula given below:
| Inhibition percentage of Hemolysis = 100 × (A1-A2)/A1 |
A1 = Control absorbance, A2 = Sample absorbance.
2.17.3.1. Statistical analysis
The experiments were done in triplicates, the results are shown as mean and standard deviation (±SD). The one-way analysis of variance (ANOVA) and Tukey tests were performed utilizing the SPSS statistical software package (SPSS, version 23.0) significant differences (p < 0.05). For IC50 estimation, data analysis was carried out using GraphPad Prism® version 9 (San Diego, US).
3. Results
3.1. Qualitative analysis of bioactive compounds
The qualitative phytochemical analysis of BP, FP1, FBP2 and FBP3 extracts revealed the presence of alkaloids, protein, carbohydrates, flavonoids, quinone, saponins, steroids, tannins, coumarins and terpenoids. Cardiac glycoside and phlobatannins were absent in all extracts, while anthraquinone were only found in BP (Table 1).
Table 1.
Phytochemical analysis of BP, FBP1, FBP2 and FBP3 extracts.
| Phytochemicals | BP | FBP1 | FBP2 | FBP3 |
|---|---|---|---|---|
| Anthraquinone | +ve | - ve | - ve | - ve |
| Quinone | + ve | + ve | + ve | + ve |
| Carbohydrates | + ve | + ve | + ve | + ve |
| Cardiac glycosides | - ve | - ve | - ve | - ve |
| Steroids | + ve | + ve | + ve | + ve |
| Coumarins | + ve | + ve | + ve | + ve |
| Flavonoids | + ve | + ve | + ve | + ve |
| Phlobatannins | - ve | - ve | - ve | - ve |
| Alkaloids | + ve | + ve | + ve | + ve |
| Tannins | + ve | + ve | + ve | + ve |
| Protein | + ve | + ve | + ve | + ve |
| Saponins | + ve | + ve | + ve | + ve |
| Terpenoids | + ve | + ve | + ve | + ve |
Note: banana peel extract (BP), fermented banana peel 1 extract (FBP1), fermented banana peel 2 extract (FBP2), and fermented banana peel 3 extract (FBP3) Positive = +ve, means present, Negative = -ve, means absent.
3.2. Total carbohydrate and protein analysis
The total carbohydrate and protein content of the fermented and unfermented BP were estimated. Compared to fermented extracts (FBP1, FPB2 and FBP3), BP was found with significantly high carbohydrate content (477.1 ± 28.93 mg/g). Carbohydrate contents of FBP2, FBP1 and FBP3 were 346.1 ± 28.4 mg/g, 318.8 ± 4.85 mg/g and 233.7 ± 13.25 mg/g, respectively. On the other hand, the analysis for protein contents revealed that FBP1 had the high protein content56.9 ± 8.91 mg/g, whereas BP had lower concentration of protein at 47.7 ± 3.74 mg/g (Table 2).
Table 2.
Total carbohydrate and protein contents in BP, FBP1, FBP2 and FBP3 extracts.
| Extracts | Total Carbohydrate (mg/g) | Total Protein (mg/g) |
|---|---|---|
| BP | 477.1 ± 28.93a | 47.7 ± 3.74a |
| FBP1 | 318.8 ± 4.85b | 56.9 ± 8.91a |
| FBP2 | 346.1 ± 28.4b | 51.6 ± 6.78a |
| FBP3 | 233.7 ± 13.25c | 53.4 ± 9.21a |
Note: Results are expressed in mean ± Standard deviation.
3.2.1. Total phenolic and flavonoid content
A significantly high TPC of 18 ± 2.59 mg GAE/g was observed in BP extracts, followed by FBP3 with a TPC of 16 ± 1.25 mg GAE/g, while the lowest TPC was observed in FBP1 at 14.6 ± 0.46 mg GAE/g. Similarly, high TFC of 20.5 ± 2.11 mg QE/g was found in BP, which decreased after the fermentation (Table 3). The TFC of FBP1, FBP3 and FBP2 extracts were measured at 1.52 ± 0.02 mg QE/g, 1.62 ± 0.09 mg QE/g and 3.75 ± 0.1 mg QE/g, respectively.
Table 3.
Total phenolic and flavonoid contents of BP, FBP1, FBP2 and FBP3 extracts.
| Extracts | Total Flavonoid Content (mg QE/g) dw | Total Phenolic Content (mg GAE/g) dw |
|---|---|---|
| BP | 20.5 ± 2.11a | 18 ± 2.59a |
| FBP1 | 1.52 ± 0.02b | 14.6 ± 0.46a |
| FBP2 | 3.75 ± 0.1b | 14.7 ± 1.04a |
| FBP3 | 1.62 ± 0.09b | 16.0 ± 1.25a |
3.3. Carotenoids and Vitamin C content
The quantitative analysis of all extracts revealed that FBP3 had the highest concentration of total carotenoids (1.46 ± 0.32 mg/g), followed by BP (1.03 ± 0.19 mg/g), FBP1 (0.88 ± 0.14 mg/g) and FBP2 (0.76 ± 0.44 mg/g). The vitamin C content was lower in FBP1 (28.46 ± 2.5 mg/L), compared to FBP2 (31.40 ± 2.22 mg/L) and FBP3 (33.16 ± 3.07 mg/L) (Table 4). BP was found to contain high vitamin C content (33.46 ± 2.63 mg/L).
Table 4.
Total carotenoids and vitamin C contents of BP, FBP1, FBP2 and FBP3 extracts.
| Extracts | Total Carotenoids mg/g | Vitamin C mg/L |
|---|---|---|
| BP | 1.03 ± 0.19ab | 33.46 ± 2.63a |
| FBP1 | 0.88 ± 0.14ab | 28.46 ± 2.5a |
| FBP2 | 0.76 ± 0.44b | 31.40 ± 2.22a |
| FBP3 | 1.46 ± 0.32a | 33.16 ± 3.07a |
3.4. Antioxidant activity by DPPH and FRAP
The 50 % proportion of radical scavenging activity was indicated by the IC50 value. Table 5 showed the amount of extract needed to neutralize 50 % of free radical [36]. There is an inverse correlation between IC50 values and scavenging activity. Decreasing IC50 values results in a higher amount of scavenging potential. In this study, the IC50 values for DPPH ranged from 2.01 ± 0.06 to 16.25 ± 0.24 mg/mL, as shown in cT. The BP extract demonstrated the highest antioxidant activity with an IC50 value of 2.01 ± 0.06 mg/mL, followed by those for FBP1 and FBP3 at 9.33 ± 0.07 mg/mL and 13.87 ± 0.1 mg/mL, respectively. FBP2 exhibited the lowest antioxidant activity, with an IC50 value of 16.25 ± 0.24 mg/mL. The IC50 values for FRAP was in the range of 12.81 ± 0.03 to 32.33 ± 2.43 mg/mL, BP showed good activity compared to FBP1, FBP2 and FBP3.
Table 5.
DPPH and FRAP IC50 values of BP, FBP1, FBP2 and FBP3 extracts.
| Extracts | DPPH |
FRAP |
|---|---|---|
| IC50 (mg/mL) | ||
| BP | 2.01 ± 0.06d | 12.81 ± 0.03b |
| FBP1 | 9.33 ± 0.07c | 31.55 ± 2.44a |
| FBP2 | 16.25 ± 0.24a | 27.8 ± 1.68a |
| FBP3 | 13.87 ± 0.1b | 32.33 ± 2.43a |
| Ascorbic acid | 0.77 ± 0.1e | – |
| FeSO4 | – | 0.68 ± 0.02c |
Note: Ascorbic acid and FeSO4 = standard.
3.5. Antibacterial activity
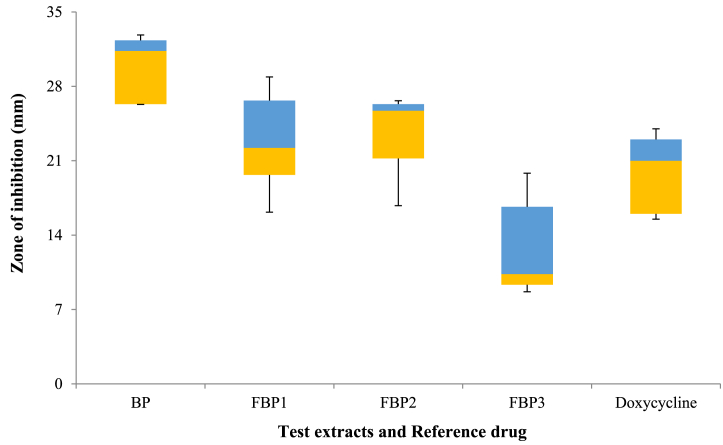
The antibacterial properties of extracts were evaluated by measuring the inhibition zones around wells. DMSO and doxycycline served as negative and positive controls, respectively. The inhibition zone of the BP extract against S. aureus, S. typhi, K. pneumonia, P. aeruginosa and E. coli was 26.33 ± 2.49 mm, 26.26 ± 1.77 mm, 31.33 ± 1.74 mm, 32.33 ± 1.59 mm and 21.33 ± 1.67 mm, respectively. The FBP2 extract exhibited comparatively higher inhibitory activity against S. aureus (26.33 ± 0.97 mm), E. coli (25.7 ± 2.33 mm), S. typhi (21.21 ± 1.71 mm), K. pneumonia (12.33 ± 3.10 mm), and P. aeruginosa (9.66 ± 0.59 mm). The FBP1 extract resulted in inhibition zones of 19.66 ± 1.80 mm, 26.66 ± 2.63 mm, 12.66 ± 2.39 mm, 7.5 ± 0.86 mm, and 22.21 ± 0.70 mm against S. aureus, S. typhi, K. pneumonia, P. aeruginosa, and E. coli, respectively (Fig. 1). Meanwhile, the FBP3 extract exhibited relatively low inhibitory effects against all bacteria, with S. typhi and P. aeruginosa demonstrating high resistance, displaying inhibition zones of 7.66 ± 3.67 mm and 8 ± 1.26 mm, respectively (Table 6)
Fig. 1.
The antibacterial activity of BP, FBP1, FBP2, FBP3 extracts and reference drug doxycycline.
Table 6.
Antibacterial activity of BP, FBP1, FBP2 and FBP3 extracts with inhibitory zones.
| Extracts | Diameter of zone of inhibition excluding well size (mm) |
||||
|---|---|---|---|---|---|
| S. aureus | S. typhi | K. pneumoniae | P. aeruginosa | E. coli | |
| BP | 26.33 ± 2.49 | 26.26 ± 1.77 | 31.33 ± 1.74 | 32.33 ± 1.59 | 21.33 ± 1.67 |
| FBP1 | 19.66 ± 1.80 | 26.66 ± 2.63 | 12.66 ± 2.39 | 7.5 ± 0.86 | 22.21 ± 0.70 |
| FBP2 | 26.33 ± 0.97 | 21.21 ± 1.71 | 12.33 ± 3.10 | 9.66 ± 0.59 | 25.7 ± 2.33 |
| FBP3 | 16.66 ± 1.67 | 7.66 ± 3.67 | 10.33 ± 1.24 | 8 ± 1.26 | 9.33 ± 0.56 |
| Doxycycline | 14 ± 0.74 | 15 ± 0.98 | 21 ± 1.54 | 16 ± 1.72 | 23 ± 1.09 |
Note: Doxycycline = Antibacterial drug used as control.
3.6. Antifungal activity
The fungal growth inhibition potential of the unfermented banana peel extracts against A. flavus (83 %), M. mucedo (84 %) and A. niger (72 %) revealed its broad-spectrum antifungal activity. Whereas the antifungal activities of the fermented banana peel showed a significant decrease, which shows the inverse effect to the fermentation on the antifungal activities (Table 7).
Table 7.
Antifungal activity of BP, FBP1, FBP2 and FBP3 extracts against targeted fungi.
| Extract | Percent (%) inhibition |
||
|---|---|---|---|
| M. mucedo | A. niger | A. flavus | |
| BP | 84 ± 2.01a | 72 ± 1.39b | 83 ± 0.50a |
| FBP1 | 54 ± 0.98b | 38 ± 1.45c | 60 ± 0.30b |
| FBP2 | 46 ± 0.65c | 13 ± 1.22d | 20 ± 0.43c |
| FBP3 | 54 ± 1.76b | 40 ± 0.98c | 15 ± 0.67c |
| Fluconazole | 82 ± 0.68a | 85 ± 2.13a | 84 ± 1.77a |
Note: Fluconazole = Antifungal drug used as control.
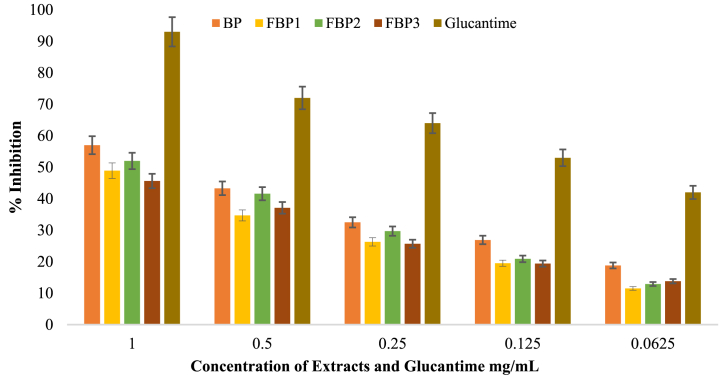
3.7. Antileishmanial activity estimation
Antileishmanial test was conducted to evaluate the antileishmanial activity of BP and its fermented FBP1, FBP2, and FBP3 extracts. The test involved using standard and extract concentrations ranging from 0.0625 to 1 mg/mL. The findings show that BP extract has high activity with an IC50 value of 0.763 ± 0.01 mg/mL, outperforming FBP2 (0.862 ± 0.04 mg/mL), FBP1 (0.980 ± 0.01 mg/mL) and FBP3 extracts (1.050 ± 0.02 mg/mL) (Table 8). The BP extracts had the highest activity, followed by FBP2, FBP1 and FBP3 in that order (Fig. 2).
Table 8.
Estimated IC50 values of BP, FBP1, FBP2 and FBP3 against promastigotes.
| Extracts | IC50 (mg/mL) |
|---|---|
| BP | 0.763 ± 0.01d |
| FBP1 | 0.980 ± 0.01b |
| FBP2 | 0.862 ± 0.04c |
| FBP3 | 1.050 ± 0.02a |
| Glucantime | 0.087 ± 0.004e |
Note: Glucantime = Antiprotozoal drug used as control.
Fig. 2.
Antileishmanial potential of unfermented banana peel (BP), and fermented banana peel (FBP1, FBP2, FBP3) extracts against promastigotes (L. major).
3.8. Estimation of anti-inflammatory activity
3.8.1. Inhibition of protein denaturation analysis
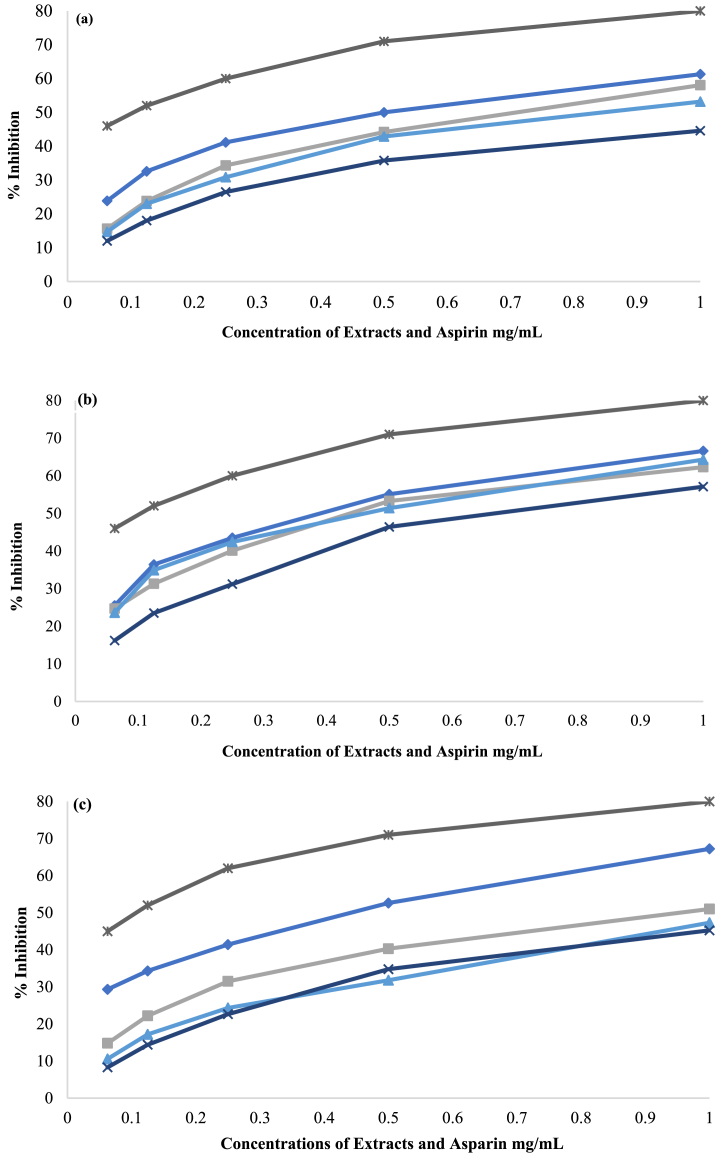
The concentration-dependent ability of BP and its fermented extracts to reduce protein denaturation is shown in Fig. 3a. When comparing BP, FBP1, FBP2 and FBP3, BP was found to be significantly more effective. The IC50 value for BP was (0.612 ± 0.01 mg/mL), followed by the IC50 values of FBP1, FBP2 and FBP3 that were (0.738 ± 0.02 mg/g), (0.831 ± 0.04 mg/g) and (1.077 ± 0.02 mg/g), respectively. The proportion of inhibition, based on benchmark data analysis, was follows: FBP3 < FBP2 < FBP1 < BP. Additionally, aspirin (IC50 = 0.043 ± 0.004 mg/g) was found to perform substantially better than the others, as shown in Table 9.
Fig. 3.
(a) Protein denaturation assay of BP, FBP1, FBP2 and FBP3 extracts (b) Proteinase inhibitory assay of BP, FBP1, FBP2 and FBP3 extracts (c) Heat-induced hemolysis assay of BP, FBP1, FBP2 and FBP3 extracts. (♦) represents BP, (■) represents FBP1, (▲) represents FBP2, ( × ) represents FBP3 and (⁕) represents anti-inflammatory and anti-platelet drug used as control (Aspirin).
Table 9.
IC50 concentration of anti-inflammatory assay conducted on BP, FBP1, FBP2 and FBP3 extracts.
| Extracts ID | Inhibition of Protein Denaturation |
Proteinase Inhibitory Activity |
Heat-Induced Hemolysis |
|---|---|---|---|
| IC50 (mg/mL) | |||
| BP | 0.612 ± 0.01d | 0.502 ± 0.01b | 0.515 ± 0.01c |
| FBP1 | 0.738 ± 0.02c | 0.586 ± 0.01b | 0.868 ± 0.01b |
| FBP2 | 0.831 ± 0.04b | 0.563 ± 0.01b | 1.037 ± 0.01a |
| FBP3 | 1.077 ± 0.02a | 0.984 ± 0.01a | 1.045 ± 0.003a |
| Aspirin | 0.043 ± 0.004e | 0.065 ± 0.004c | 0.041 ± 0.003d |
Note: Aspirin = Anti-inflammatory and anti-platelet drug used as control.
3.8.2. Proteinase inhibitory activity
The anti-inflammatory effects of BP, FBP1, FBP2, and FBP3 extracts were assessed using a proteinase inhibitory assay. The extracts were tested at concentrations ranging from 0.0625 to 1 mg/mL for each extract. The outcomes showed that maximum activity was seen at a concentration of 1 mg/mL and that the inhibition increased as concentration increased (Fig. 3b). The lowest IC50 (0.502 ± 0.01 mg/g) was observed with BP, indicating its strong anti-inflammatory activity. The relative IC50 values for FBP2, FBP1 and FBP3 were 0.563 ± 0.01 mg/g, 0.586 ± 0.01 mg/g and 0.984 ± 0.01 mg/g, respectively. in summary, the anti-inflammatory activity of the extracts can be ranked as follows: FBP3 < FBP1 < FBP2 < BP (Table 9).
3.8.3. Heat-induced hemolysis assessment
The anti-inflammatory effect of BP, FBP1, FBP2, and FBP3 extracts was tested using the heat-induced hemolysis method. The % inhibition was analyzed at concentrations ranging from 0.0625 to 1 mg/g. Each extract showed its inhibitory action in a concentrated manner (Fig. 3c). The BP extract had significantly higher hemolysis inhibition values (0.515 ± 0.01 mg/mL) than FBP1 (0.868 ± 0.01 mg/mL), FBP2 (1.037 ± 0.01 mg/mL) and FBP3 (1.045 ± 0.003 mg/mL). Aspirin exhibited the highest % inhibition followed by BP, FBP1, FBP2 and then FBP3 in that order.
4. Discussion
It has been reported that fermented products possess a variety of health-promoting compounds, such as organic acids, alcohol, or other antimicrobial substances that prevent the growth of bacteria causing food spoilage. Fermentation also enhance fragrances appearance and texture of food and it has functional and economic value [37]. A recent study has shown the nutritional, bioactive, radical scavenging and biological potential of banana peel, as well as the effect of fermentation on various contents. The study concluded that all extracts contain flavonoids, quinone, alkaloids, carbohydrates, saponins, protein, steroids, tannins, coumarins and terpenoids with anthraquinones being identified only in BP. The presence of these phytochemicals in banana peel aligns with previous study conducted by Ref. [38]. The research conducted by Ref. [39] stated that banana peel is an inexpensive source of phytochemicals, but the quantity may vary depending on ripening stages, weather, soil type, fertilizer, and climatic variations. The identification of phytochemicals may lay the foundation for the development of novel drugs. These phytoconstituents show potential against several cancers, heart disease and neurological disorders [40].
The standard procedure described was used to estimate total carbohydrates and protein. The current study presented that protein and carbohydrate were found in BP, FBP1, FBP2 and FBP3. The investigated results showed that protein concentration increased in fermented extracts while carbohydrate concentration decreased after fermentation. The current research on the concentration of protein and carbohydrates is consistent with [41]. Plant fibers degradation led to a decrease in the quantity of carbohydrates in fermented extracts, while an increase in protein content in fermented extracts may stimulate the production of extracellular enzymes synthesized by microbes [42].
Phenolics are linked to the biological, nutritive, and sensory qualities of fruits and vegetables. phenolic compounds present in banana peel has antibacterial, spasmodic, antitumor, anti-inflammatory and antidepressant properties [9]. These bioactive molecules also regulate cellular proliferation, growth and metabolism [43]. Phenolic chemicals correlate with plants' ability to produce antioxidants [44]. Bastola et al. [44], estimated the total phenolic content through Lowry's method using.
According to Oguntoyinbo et al. [45], fermented banana peel extract has a phenolic concentration of 29.13 ± 0.15 mg GAE/g, whereas unfermented extract has 31.36 ± 0.152 mg GAE/g. The quantitative results of our investigation are in contrast to past findings indicating the phenolic content reduced during fermentation. Similarly, the results of the current analysis showed that the amount of total flavonoids in the ethanolic extract of banana peel was 20.5 ± 2.11 mg QE/g, which was higher than the figure reported in a previously study [14], they determined that the amount of total flavonoids in the extract was 18.52 mg QE/g.
It has been previously reported that, the TPC in fermented banana peel, caused by yeast, was 4.15 ± 0.046 % [46]. Adebo and Gabriela [47] determined that, the total phenolic concentration reduced after fermentation. It was anticipated that polyphenols would degrade after fermentation, leading to decrease in total phenolics [48]. These variation in phenolic compounds are result by differences in the species of Musa, the stages of ripening, the type of microbes used for fermentation, growth, and environmental factors [46].
Carotenoids are reactive oxygen species which combat free radicals and are thought of as the first step in treating cardiac issues [49]. The pro-vitamin A, anti-obesity and anti-tumor properties of carotenoids are also effective [50]. It has been reported that Banana peel has total carotenoids in the range of 7760 – 10633 µg/100 g, whereas, R. mucilaginosa UANL001 fermented banana peels was reported with 279 ± 24 µg/g of carotenoids [51,52]. Comparing the results of the current study to earlier research, it was found that banana peel and its fermented extracts are correspondingly rich in carotenoids.
Water-soluble vitamin C, commonly referred to as ascorbic acid, is essential for treating and preventing AIDS, infertility, scurvy and the common cold. Additionally, it aids in the hydroxylation and oxidation of organic compounds in mammalian metabolism [53]. Vitamin C, an antioxidant, is found in banana peel [54]. It has been reported that the vitamin C concentration in cauliflower xylem decreases after fermentation [55]. Despite the difference in the FBP1 of the current study from previously published research, the concentration in FBP1 and FBP2 were found to be correlated.
In the food and pharmaceutical industries, antioxidants have enormous potential to reduce free radicals. The extracts antioxidant components deliver active electrons to snare free radicals [19,56]. Natural antioxidants are more in demand due to the anti-carcinogenic effects of synthetic antioxidants. Halophilic fungi have a high capability for scavenging free radicals. The ability of extracts to neutralize free radicals was examined using synthetic FRAP and DPPH reagents. Total phenols and carotenoids are linked to FRAP by donating electrons and converting Fe3+ into Fe2+, according to published research, whereas high flavonoid content considerably scavenges radicals in the DPPH activity [57]. Banana peels DPPH% inhibition with an IC50 value of 180.33 g/mL was reported by Ref. [40]. Banana peel has higher antioxidant potential than banana pulp, according to research by Ref. [58]. The DPPH % inhibition of A. alternata, Pleosporaceae spp. and F. culmorum were determined by Ref. [18] with respective values of 71 %, 74 % and 93.4 %. Özen and Demirtaş [59] mentioned in their findings, that banana peel ethanolic extract exhibited more antioxidant activity compared to previously been reported.
Agar well diffusion was used to assess the anti-bacterial capability of each test extract. Clear zone below 10 mm were classified as weak, moderate between 10 and 16 mm, and potent over 16 mm [60]. The A. alternata extract produced the maximum zone of inhibition against K. pneumonia, which was measured at 5.04 ± 0.29 mm [61] while the F. culmorum showed moderate anti-septic potential to inhibit the growth of E. coli, P. aeruginosa and S. aureus [18]. The results conducted from the present study are comparatively more promising than those from earlier published. The significant antibacterial properties of fermented extracts have been connected to the bioactive chemicals produced by fungal species. These compounds suppressed nucleic acid production in bacterial cells [62]. In addition, a study by Hikal et al. [63], evaluated the antibacterial significance of banana peel extract, demonstrating potential activity against infections caused by E. coli, S. aureus, S. typhi, K. pneumoniae, and P. aeruginosa. The extracts likely had a potent antibacterial impact due to the presence of secondary metabolites such as phenolic and flavonoids, which are implicated in the suppression of nucleic acid synthesis and other metabolic activities [64].
The anti-fungal activity of the BP, FBP1, FBP2 and FBP3 extracts was determined by calculating the percentage of reserve zone beside targeted fungi. Banana peel was previously reported to have 26 mm zone of inhibition and be effective against A. niger [65]. Additionally, research shown that banana peel extract effectively prevented the growth of A. niger and A. alternata fungi compared to pineapple, cashew and orange peel. Moreover, the ethanolic extract showed potent antifungal activity against A. flavus [66]. [67] discovered that A. niger and A. flavus were not resistant to the extract of dried powered banana peel. This study contrasts with our findings while the results of this experiment supported those of earlier investigations that discovered banana peel extract has a significant inhibitory effect. Significant anti-fungal activity of banana peel extract was observed against S. boulardii but A. niger was found inactive [18]. The antifungal efficacy observed in fermented extracts contrasts with the findings from the prior study conducted by Ref. [18]. [68] evaluated the antifungal activity of infections caused by A. niger, A. flavus, and C. albicans.
Leishmaniasis is a parasitic infection that is spread by vectors and is caused by the obligatory protozoan of the genus Leishmania. The most common types of leishmaniasis are visceral, cutaneous, and mucocutaneous infections. More often ignored, the disease is most prevalent in developing nations. The moment has come to put more emphasis on natural substances such as leishmanicidal agents. The peel, leaves, fruit, juice, pulp, and seeds of banana (Musa paradisiaca), ají (Capsicum annum), naranja (Citrus sinensis), yerba mora (Solanum nigrum), tobacco (Nicotiana tabacum), lechuga (Lactuca sativa), mejorana (Mejorana hortensis), santa maría (Tanacetum parthenium), verbena (Verbena litoralis), tiatina (Escopaia educlis), matico (Aristeguietia glutinosa), and balsa (Ochroma pyramidale) have been documented for their potent antileishmanial properties (Ullah et al., 2016). As coumarins contribute to antileishmanial activity it proves that phytochemicals found in plants are responsible for biological activities [69]. The current investigation determined that coumarins, which are essential for curing leishmaniasis, are contained in BP and its fermented extracts.
Inflammation is a biotic reaction to harmful stimuli that is associated with discomfort and biological disorders, such altering membranes and causing protein denaturation [70]. Evidence in literature suggests that ongoing inflammation can result in metabolic diseases, nervous system disorders and cardiovascular problems [71]. Additionally, research has shown a strong correlation between inflammation, infections and the growth of tumors which manifest phenotypically as inflammation [72]. Anti-inflammatory drugs can help patients have a better prognosis. There are many FDA-approved anti-inflammatory drugs available that are intended to prevent inflammation, but some of them may also have anti-emetic, anti-thrombotic [73], anti-proliferative, pro-apoptotic and anti-angiogenic actions [74].
Consequently, natural medications are more reliable than synthetic ones [75]. The anti-inflammatory potential of banana peel and its fermented extracts was investigated by protein denaturation, proteinase inhibition and membrane stability. The findings for lowering inflammation were significant. For instance, using these methods, proteins lose their secondary and tertiary structures when exposed to acid, base, organic salt, or heat, leading to inflammation. It has been found that white blood cell proteinase is crucial for the development of tissue damage after inflammatory reactions [76]. According to published research, ripe peel water extract strongly inhibits nitric oxide synthesis, with an IC50 value of 6.68 + 0.34 g/mL [77]. Furthermore, the anti-inflammatory effects of terpenoids, alkaloids, flavonoids and polyphenols may all be interconnected.
5. Conclusion
Findings of this study revealed that banana peel contains a diverse array of beneficial substances such as phytochemicals, nutrients, antioxidants, and bioactive compounds. These contribute to the therapeutic potential of banana peel including antibacterial, antifungal, anti-inflammatory, and antileishmanial activities. Fermentation led to an elevation in protein content and a slight reduction in carbohydrates, TPC, TFC, total carotenoids, vitamin C, and scavenging activity. However, the fermented peel exhibited potent antimicrobial activity. This study underscores the potential use of both fermented and unfermented banana peels in the pharmaceutical sector to prevent chronic diseases and enhance food quality.
Ethics approval
The authors declare that the study requires no ethical approval, as it is an observational study.
Funding
None.
Data availability statement
A major portion of the data is included in the manuscript in the form of text, figures and tables. For more detail the corresponding author can be requested with valid reason.
CRediT authorship contribution statement
Mehnaz Hashim: Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation. Ali Akbar: Writing – review & editing, Supervision, Resources, Funding acquisition, Formal analysis, Conceptualization. Zareen Gul: Methodology, Investigation, Formal analysis. Muhammad Bilal Sadiq: Writing – review & editing, Resources, Methodology. Jahangir Khan Achakzai: Writing – review & editing, Resources. Nazir Ahmad Khan: Writing – review & editing, Resources, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
List of Abbreviation
| BP | Banana Peel | FBP2 | Fermented Banana Peel 2 |
|---|---|---|---|
| FBP1 | Fermented Banana Peel 1 | FBP3 | Fermented Banana Peel 3 |
| % | Percentage | H2SO4 | Sulphuric acid |
| DPPH | 2,2-Diphenylpicrylhydrazyl | NaOH | Sodium hydroxide |
| TPC | Total phenolic count | HCl | Hydrochloric acid |
| MgSO4.7H2O | Magnesium sulfate heptahydrate | AlCl3 | Aluminum tri chloride |
| NBT | Nitro blue tetrazolium chloride | CaCl2 | Calcium chloride |
| HClO4 | Perchloric acid | FeSO4 | Ferrous sulphtae |
| (NH4)2SO4 | Ammonium sulfate | CoCl2 | Cobalt chloride |
| ZnSO4 | Zinc sulfate | MnSO4 | Manganese sulfate |
| K2HPO4 | Potassium bi phosphate | NaNO2 | Sodium nitrite |
| GAE | Gallic acid equivalent | QE | Quercetin equivalent |
| TFC | Total phenolic content | MHA | Muller Hinton agar |
| SDA | Sabouraud dextrose agar | IC50 | Half maximal inhibitory concentration |
| mm | Millimeter | FRAP | Ferric Reducing Antioxidant Power |
References
- 1.Ingrao C., Faccilongo N., Di Gioia L., Messineo A. Food waste recovery into energy in a circular economy perspective: a comprehensive review of aspects related to plant operation and environmental assessment. J. Clean. Prod. 2018;184:869–892. [Google Scholar]
- 2.Loss F.T.F. FAO; Rome, Italy: 2022. Waste: A Triple Win Opportunity. [Google Scholar]
- 3.Hashim M., Hamid Z., Gul Z., Akbar A. Functional, nutritional and medicinal potential of banana peel. Pure Appl. Biol. 2023;12(1):470–490. [Google Scholar]
- 4.Chai S.Y., Abbasiliasi S., Lee C.K., Ibrahim T.A.T., Kadkhodaei S., Mohamed M.S., et al. Extraction of fresh banana waste juice as non-cellulosic and non-food renewable feedstock for direct lipase production. Renew. Energy. 2018;126:431–436. [Google Scholar]
- 5.Fidrianny I., Anggraeni N., Insanu M. Antioxidant properties of peels extracts from three varieties of banana (Musa sp.) grown in West Java-Indonesia. Int. Food Res. J. 2018;25(1) [Google Scholar]
- 6.Pereira M.A.F., Monteiro C.R.M., Pereira G.N., Júnior S.E.B., Zanella E., Ávila P.F., et al. Deconstruction of banana peel for carbohydrate fractionation. Bioproc. Biosyst. Eng. 2021;44(2):297–306. doi: 10.1007/s00449-020-02442-1. [DOI] [PubMed] [Google Scholar]
- 7.Rasheed H., Shehzad M., Rabail R., Kowalczewski P.Ł., Kidoń M., Jeżowski P., et al. Delving into the nutraceutical benefits of purple carrot against metabolic syndrome and cancer: a review. Appl. Sci. 2022;12(6):3170. [Google Scholar]
- 8.Pereira A., Maraschin M. Banana (Musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015;160:149–163. doi: 10.1016/j.jep.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Sidhu J.S., Zafar T.A. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf. 2018;2(4):183–188. [Google Scholar]
- 10.Adetuyi B.O., Ogundipe A.E., Ogunlana O.O., Egbuna C., Estella O.U., Mishra A.P., et al. Food and Agricultural Byproducts as Important Source of Valuable Nutraceuticals. Springer; 2022. Banana peel as a source of nutraceuticals; pp. 243–250. [Google Scholar]
- 11.Nisha P., Mini S. In vitro antioxidant and antiglycation properties of methanol extract and its different solvent fractions of Musa paradisiaca L.(Cv. Nendran) inflorescence. Int. J. Food Prop. 2014;17(2):399–409. [Google Scholar]
- 12.Vu H.T., Scarlett C.J., Vuong Q.V. Changes of phytochemicals and antioxidant capacity of banana peel during the ripening process; with and without ethylene treatment. Sci. Hortic. (Amst.) 2019;253:255–262. [Google Scholar]
- 13.Mokbel M.S., Hashinaga F. Antibacterial and antioxidant activities of banana (Musa, AAA cv. Cavendish) fruits peel. Am. J. Biochem. Biotechnol. 2005;1(3):125–131. [Google Scholar]
- 14.Aboul-Enein A.M., Salama Z.A., Gaafar A.A., Aly H.F., Abou-Elella F., Ahmed H. Identification of phenolic compounds from banana peel (Musa paradaisica L.) as antioxidant and antimicrobial agents. J. Chem. Pharmaceut. Res. 2016;8(4):46–55. [Google Scholar]
- 15.Phacharapiyangkul N., Thirapanmethee K., Sa-Ngiamsuntorn K., Panich U., Lee C.-H., Chomnawang M.T. Effect of sucrier banana peel extracts on inhibition of melanogenesis through the ERK signaling pathway. Int. J. Med. Sci. 2019;16(4):602. doi: 10.7150/ijms.32137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampen W.H. Fermentation and Biochemical Engineering Handbook. Elsevier; 2014. Nutritional requirements in fermentation processes; pp. 37–57. [Google Scholar]
- 17.Nkhata S.G., Ayua E., Kamau E.H., Shingiro J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018;6(8):2446–2458. doi: 10.1002/fsn3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan S.A., Akbar A., Aslam M., Shafee M., Samad A., Rehman F.U., et al. Biotechnologically potent halophilic fungal biodiversity from mangrove ecosystem of lasbela, balochistan. Pakistan J. Bot. 2022;54(3):1103–1112. [Google Scholar]
- 19.Akbar A., Ali I., Samiullah N.U., Khan S.A., Rehman Z., Rehman S. Functional, antioxidant, antimicrobial potential and food safety applications of curcuma longa and cuminum cyminum. Pakistan J. Bot. 2019;51(3):1129–1135. [Google Scholar]
- 20.Behlil F., Samiullah K.N., Akbar A., Tareen R.B., Achakazai A.K.K., Ali I., et al. Phytochemical screening and antioxidant activity determination of some medicinally important plants of Balochistan. Pakistan J. Bot. 2019;51(2):601–608. [Google Scholar]
- 21.Mandels M., Weber J. ACS Publications; 1969. The Production of Cellulases. [Google Scholar]
- 22.Gul Z., Akbar A., Ali I., Muhammad J., Rehman Z.U., Bano A., et al. High throughput screening for bioactive components of berberis baluchistanica ahrendt root and their functional potential assessment. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/1746116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahad F.I., Barua N., Islam M.S., Sayem S.A.J., Barua K., Uddin M.J., et al. Investigation of the pharmacological properties of Lepidagathis hyalina nees through experimental approaches. Life. 2021;11(3):180. doi: 10.3390/life11030180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizvi N.B., Aleem S., Khan M.R., Ashraf S., Busquets R. Quantitative estimation of protein in sprouts of vigna radiate (Mung Beans), lens culinaris (Lentils), and cicer arietinum (Chickpeas) by Kjeldahl and Lowry Methods. Molecules. 2022;27(3):814. doi: 10.3390/molecules27030814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luaces P., Pascual M., Pérez A.G., Sanz C. An easy-to-use procedure for the measurement of total phenolic compounds in olive fruit. Antioxidants. 2021;10(11):1656. doi: 10.3390/antiox10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nurcholis W., Putri D.N.S.b., Husnawati H., Aisyah S.I., Priosoeryanto B.P. Total flavonoid content and antioxidant activity of ethanol and ethyl acetate extracts from accessions of Amomum compactum fruits. Ann. Agric. Sci. (Cairo) 2021;66(1):58–62. [Google Scholar]
- 27.Kumar P., Ramakritinan C., Kumaraguru A. Solvent extraction and spectrophotometric determination of pigments of some algal species from the shore of puthumadam, southeast coast of India. Int. J. Oceans Oceanogr. 2010;4(1):29–34. [Google Scholar]
- 28.Satpathy L., Pradhan N., Dash D., Baral P.P., Parida S.P. Quantitative determination of vitamin C concentration of common edible food sources by redox titration using iodine solution. Letters in Applied Bioscience NanoBioSci. 2021;10:2361–2369. [Google Scholar]
- 29.Setiawan V., Phangestu S., Soetikno A.G., Arianti A., Kohar I. Rapid screening analysis of antioxidant activities in green tea products using DPPH and FRAP. Pharma. J. Indonesia. 2021;7(1):9–14. [Google Scholar]
- 30.Gul Z., Akbar A., Leghari S.K. Elucidating therapeutic and biological potential of Berberis baluchistanica Ahrendt bark, leaf, and root extracts. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaheen N., Qureshi N.A., Iqbal A., Ashraf A., Fatima H. Evaluation of safety, antileishmanial, and chemistry of ethanolic leaves extracts of seven medicinal plants: an in-vitro study. Open Chem. J. 2020;7(1) [Google Scholar]
- 32.Mikailu S., Obomate N.L., Ugochukwu O.P., Ekenna I.C. Anti-Inflammatory, Fibrinolytic and Anti-Oxidant Activities of the N-Hexane Extract of Ficus sur Forssk (Moraceae) Leaves. Haya Saudi J. Life Sci. 2022;7(2):44–50. [Google Scholar]
- 33.Alam A., Jawaid T., Alam P. In vitro antioxidant and anti-inflammatory activities of green cardamom essential oil and in silico molecular docking of its major bioactives. J. Taibah Univ. Sci. 2021;15(1):757–768. [Google Scholar]
- 34.Yesmin S., Paul A., Naz T., Rahman A., Akhter S.F., Wahed M.I.I., et al. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba) Clin. Phytosci. 2020;6(1):1–10. [Google Scholar]
- 35.Gunathilake K., Ranaweera K., Rupasinghe H. Influence of boiling, steaming and frying of selected leafy vegetables on the in vitro anti-inflammation associated biological activities. Plants. 2018;7(1):22. doi: 10.3390/plants7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paśko P., Galanty A., Zagrodzki P., Ku Y.G., Luksirikul P., Weisz M., et al. Bioactivity and cytotoxicity of different species of pitaya fruits–A comparative study with advanced chemometric analysis. Food Biosci. 2021;40 [Google Scholar]
- 37.Kiczorowski P., Kiczorowska B., Samolińska W., Szmigielski M., Winiarska-Mieczan A. Effect of fermentation of chosen vegetables on the nutrient, mineral, and biocomponent profile in human and animal nutrition. Sci. Rep. 2022;12(1):1–13. doi: 10.1038/s41598-022-17782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kibria A.A., Rahman M.M., Kar A. Extraction and evaluation of phytochemicals from banana peels and banana plants. Malaysian J. Halal Res. 2019;2(1):22–26. [Google Scholar]
- 39.Kalemba-Drożdż M., Kwiecień I., Szewczyk A., Cierniak A., Grzywacz-Kisielewska A. Fermented vinegars from apple peels, raspberries, rosehips, lavender, mint, and rose petals: the composition, antioxidant power, and genoprotective abilities in comparison to acetic macerates, decoctions, and tinctures. Antioxidants. 2020;9(11):1121. doi: 10.3390/antiox9111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durgadevi P.K.S., Saravanan A., Uma S. Antioxidant potential and antitumour activities of Nendran banana peels in breast cancer cell line. Indian J. Pharmaceut. Sci. 2019;81(3):464–473. [Google Scholar]
- 41.Ojokoh A. Effect of fermentation on the chemical composition of mango (Mangifera indica R) peels. Afri. J. Biotechnol. 2007;6(16) [Google Scholar]
- 42.Ojokoh A., Daramola M. Evaluation of lima bean flour fermented with Lactobacillus sp. as a probiotic food. Afr. J. Food Sci. 2012;6(13):352–361. [Google Scholar]
- 43.Swamy M.K., Sinniah U.R., Akhtar M. In vitro pharmacological activities and GC-MS analysis of different solvent extracts of Lantana camara leaves collected from tropical region of Malaysia. Evid. base Compl. Alternative Med. 2015;2015 doi: 10.1155/2015/506413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastola K.P., Guragain Y.N., Bhadriraju V., Vadlani P.V. Evaluation of standards and interfering compounds in the determination of phenolics by Folin-Ciocalteu assay method for effective bioprocessing of biomass. Am. J. Anal. Chem. 2017;8(6):416–431. [Google Scholar]
- 45.Oguntoyinbo O., Olumurewa J., Omoba O. Chemical composition, dietary fiber and antioxidant activity of fermented ripe banana peel flour. J. Food Stab. 2020;3(2):27–42. [Google Scholar]
- 46.Ozabor P., Ojokoh A., Wahab A., Aramide O. Effect of fermentation on the proximate and antinutrient composition of banana peels. Int. J. Biotechnol. 2020;9(2):105–117. [Google Scholar]
- 47.Adebo O.A., Gabriela Medina-Meza I. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: a mini review. Molecules. 2020;25(4):927. doi: 10.3390/molecules25040927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svanberg U., Lorri W. Fermentation and nutrient availability. Food Control. 1997;8(5–6):319–327. [Google Scholar]
- 49.Gunathilaka K., Ranaweera K., Rupasinghe H.P.V., Perera O., Jayaweera H. 2017. Response Surface Optimization of Extraction of Polyphenols and Carotenoids from Sesbania Grandiflora Leaves with Ethanol-Water System. [Google Scholar]
- 50.Kumar K.S., Bhowmik D., Duraivel S., Umadevi M. Traditional and medicinal uses of banana. J. Pharmacogn. Phytochem. 2012;1(3):51–63. [Google Scholar]
- 51.Singh B., Singh J.P., Kaur A., Singh N. Bioactive compounds in banana and their associated health benefits–A review. Food Chem. 2016;206:1–11. doi: 10.1016/j.foodchem.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 52.Torres-Alvarez D., León-Buitimea A., Albalate-Ramírez A., Rivas-García P., Hernández-Núñez E., Morones-Ramírez J.R. Conversion of banana peel into diverse valuable metabolites using an autochthonous Rhodotorula mucilaginosa strain. Microb. Cell Factories. 2022;21(1):1–12. doi: 10.1186/s12934-022-01834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pathy K. Process for preparation of vitamin C and method for determination of vitamin c in tablets. SF J. Chem. Res. 2018;2(1):2. [Google Scholar]
- 54.Widjastuti T., Hernawan E. Utilizing of banana peel (Musa sapientum) in the ration and its influence on final body weight, percentage of carcass and abdominal fat on broilers under heat stress condition. Lucrări Ştiinţifice - Ser. Zootehnie. 2012;57:104–109. [Google Scholar]
- 55.Bekhit A.E., Lingming K., Mason S.L., Zhou J., Sedcole J.R. 2013. Upgrading the Utilization of brassica Wastes: Physicochemical Properties and Sensory Evaluation of Fermented brassica Stalks. [Google Scholar]
- 56.Ravindran C., Varatharajan G.R., Rajasabapathy R., Vijayakanth S., Kumar A.H., Meena R.M. A role for antioxidants in acclimation of marine derived pathogenic fungus (NIOCC 1) to salt stress. Microb. Pathog. 2012;53(3–4):168–179. doi: 10.1016/j.micpath.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Fidrianny I., Aristya T., Hartati R. Antioxidant capacities of various leaves extracts from three species of legumes and correlation with total flavonoid, phenolic, carotenoid content. Int. J. Pharmacog. Phytochem. Res. 2015;7(3):628–634. [Google Scholar]
- 58.Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96(2):254–260. [Google Scholar]
- 59.O. Tevfik, İ. Demi̇rtas, Comparison of the chemical composition and bioactive properties of extracts prepared from the mature Turkish and Brazilian banana peels. Int. J. Chem. Tech. 5(1):67-76.
- 60.Irshad A., Khan N., Farina Y., Baloch N., Ali A., Mun L.K., et al. Synthesis, spectroscopic characterization, X-ray diffraction studies and in-vitro antibacterial activities of diorganotin (IV) derivatives with N-methyl-4-bromobenzohydroxamic acid. Inorg. Chim. Acta. 2018;469:280–287. [Google Scholar]
- 61.Kamal S.A., Hamza L.F., Hameed I.H. Antibacterial activity of secondary metabolites isolated from Alternaria alternata. Afr. J. Biotechnol. 2015;14(43):2972–2994. [Google Scholar]
- 62.Iqbal J., Abbasi B.A., Ahmad R., Mahmoodi M., Munir A., Zahra S.A., et al. Phytogenic synthesis of nickel oxide nanoparticles (NiO) using fresh leaves extract of Rhamnus triquetra (wall.) and investigation of its multiple in vitro biological potentials. Biomedica. 2020;8(5):117. doi: 10.3390/biomedicines8050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hikal W.M., Said-Al Ahl H.A. Banana peels as possible antioxidant and antimicrobial agents. Asian J. Res. Rev. Agri. 2021:137–147. [Google Scholar]
- 64.Gul Z., Akbar A., Leghari S.K. Elucidating therapeutic and biological potential of berberis baluchistanica ahrendt bark, leaf, and root extracts. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prakash B., Ch S., Melappa G., Gavimath C. Evaluation of antifungal activity of banana peel against scalp fungi. Mater. Today: Proc. 2017;4(11):11977–11983. [Google Scholar]
- 66.El Zawawy N.A. Antioxidant, antitumor, antimicrobial studies and quantitative phytochemical estimation of ethanolic extracts of selected fruit peels. Int. J. Curr. Microbiol. Appl. Sci. 2015;4(5):298–309. [Google Scholar]
- 67.Hanafy S.M., El-Shafea A., Mohamed Y., Saleh W.D., Fathy H.M. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Genet. Eng. Biotechnol. 2021;19(1):1–10. doi: 10.1186/s43141-021-00151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korni R.D., Boddepalli T., Elusuri J., Panda J. Banana Peel: a potential waste product with numerous pharmacological activities. GSC Biolog. Pharma. Sci. 2023;23(2):160–174. [Google Scholar]
- 69.Gonçalves G.A., Spillere A.R., das Neves G.M., Kagami L.P., von Poser G.L., Canto R.F.S., et al. Natural and synthetic coumarins as antileishmanial agents: a review. Eur. J. Med. Chem. 2020;203 doi: 10.1016/j.ejmech.2020.112514. [DOI] [PubMed] [Google Scholar]
- 70.Ferrero-Miliani L., Nielsen O., Andersen P., Girardin S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007;147(2):227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calder P.C., Albers R., Antoine J.-M., Blum S., Bourdet-Sicard R., Ferns G., et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009;101(S1):1–45. doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
- 72.Rayburn E.R., Ezell S.J., Zhang R. Anti-inflammatory agents for cancer therapy. Mol. Cell. Pharmacol. 2009;1(1):29. doi: 10.4255/mcpharmacol.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salman M.C., Ayhan A. Use of anti-thrombotic agents during chemotherapy for epithelial ovarian cancer. Med. Hypotheses. 2006;66(6):1179–1181. doi: 10.1016/j.mehy.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 74.Monnier Y., Zaric J., Ruegg C. Inhibition of angiogenesis by non-steroidal anti-inflammatory drugs: from the bench to the bedside and back. Curr. Drug Targets: Inflammation Allergy. 2005;4(1):31–38. doi: 10.2174/1568010053622975. [DOI] [PubMed] [Google Scholar]
- 75.Kaushik S., Kaushik S., Sharma V., Yadav J. Antiviral and therapeutic uses of medicinal plants and their derivatives against dengue viruses. Pharm. Rev. 2018;12(24) [Google Scholar]
- 76.Oguntibeju O.O. Antidiabetic, anti-Inflammatory, antibacterial, anti-helminthic, antioxidant and nutritional potential of Musa paradisiaca. Asian J. Pharmaceut. Clin. Res. 2019;12(10):9–13. [Google Scholar]
- 77.Mpharm S.R. Anti-inflammatory and antioxidant activities of extracts from Musa sapientum peel. J. Med. Assoc. Thai. 2012;95(1):S142–S146. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A major portion of the data is included in the manuscript in the form of text, figures and tables. For more detail the corresponding author can be requested with valid reason.