Graphical abstract

Keywords: Warming, Cyrinus carpio, Juvenile, Swimming behavior, Transcriptome
Highlights
-
•
Warming impacted both individual and group behavior in juvenile C. carpio.
-
•
Long-term warming had a more pronounced impact on the decline of swimming activity.
-
•
Transcriptomics revealed that warming affected cytoskeletal organization, mitochondrial regulation, and energy metabolism in brain.
-
•
Synaptic transmission and signal transduction pathways were the key signature to explain behavior changes under warming.
Abstract
Introduction
Global warming is increasing interest in how aquatic animals can adjust their physiological performance and cope with temperature changes. Therefore, understanding the behavioral changes and molecular underpinnings in fish under warming is crucial for both the individual and groups survival. This could provide experimental evidence and resource for evaluating the impact of global warming.
Objective
Three genetic families of common carp (Cyprinus carpio) were generated. These juveniles were constructed short-term (4 days) and long-term (30 days) warming groups to investigate the effects of warming on behavioral responses and to elucidate the potential underlying mechanisms of warming-driven behavior.
Methods
Behavioral tests were used to explore the effects of short- and long-term exposure to warming on the swimming behavior of C. carpio. Brain transcriptome combined with measurement of nervous system activity was used to further investigated the comprehensive neuromolecular mechanisms under warming.
Results
Long-term warming groups had a more significant impact on the decline of swimming behavior in juvenile C. carpio. Furthermore, brain comparative transcriptomic analysis combined with measurement of nervous system activity revealed that genes involved in cytoskeletal organization, mitochondrial regulation, and energy metabolism are major regulators of behavior in the juvenile under warming. Importantly, especially in the long-term warming groups, enrichment analysis of associated gene expression suggested functional alterations of synaptic transmission and signal transduction leading to swimming function impairment in the central nervous system, as revealed by behavioral tests.
Conclusions
Our study provides evidence of the neurogenomic mechanism underlying the decreased swimming activity in juvenile C. carpio under warming. These findings have important implications for understanding the impacts of climate change on aquatic ecosystems and the organisms that inhabit them.
Introduction
Global warming has become a pressing environmental issue, and its impact on freshwater ecosystems is of great concern [1]. Temperature is a key driver of ecological processes in aquatic environments, as it shapes the structure of ecosystems and the composition of biological communities [2]. Each species has an optimal temperature range for their survival and performance, and deviations from this range can have profound effects on individuals and populations. When water temperature rises, the aquatic organisms demonstrate signs of responses and stress, resulting in changes in egg hatching, development, behavior, and sex differentiation [3], [4]. Given the vital link between temperature, development, and species’ survival, the effects of global warming on aquatic communities are attracting attention. In recent decades, there has been an increase in site-specific studies, investigations at different spatial scales, and long-term monitoring efforts to understand the effects of warming, supported by biomonitoring data sets [5]. However, the genetic and behavioral effects of temperature changes, particularly in juvenile fish, remain relatively understudied among ectotherms. Therefore, it is crucial for individual and groups survival to investigate the behavioral changes and molecular underpinnings in juvenile fish under different warming temperatures. This will provide experimental evidence for comprehensively evaluating the impact of global warming.
Behavior is typically considered the first line of response for an individual experiencing environmental temperature changes [6]. Swimming behavior is the most critical functional trait in developing fish and largely determines to the success of survival, settlement, and reproductive migration [7], [8]. The relationship between swimming activity and temperature follows an approximate bell-shaped curve, where it initially increases to a maximum as the temperature rises to an optimum value and decreases at higher temperatures [9]. Each species has an optimal temperature range for physiological processes, and deviations from these optimal temperatures might dramatically affect fish health and survival. Short-term exposure (hours or days) to suboptimal temperature results in a stress response, while longer-term exposure (weeks or months) may result in an acclimatization response [10], [11]. The brain plays a key role in regulating the swimming behavior in zebrafish larval in response to heat stimuli [12]. Fish swimming behaviors are often controlled by neurotransmitters secreted from the central nervous system [13], [14], [15], [16]. There is a strong link between heat modulation and swimming behavior in the brain of zebrafish larval [12], [17]. However, the behavioral and neuromolecular mechanisms by which acute and/or chronic exposure to high temperatures affects the fish swimming behavior are largely unknown. Investigating the potential mechanisms expands the knowledge of behavioral plasticity, and provides alternative parameters for evaluating environmental temperature effects.
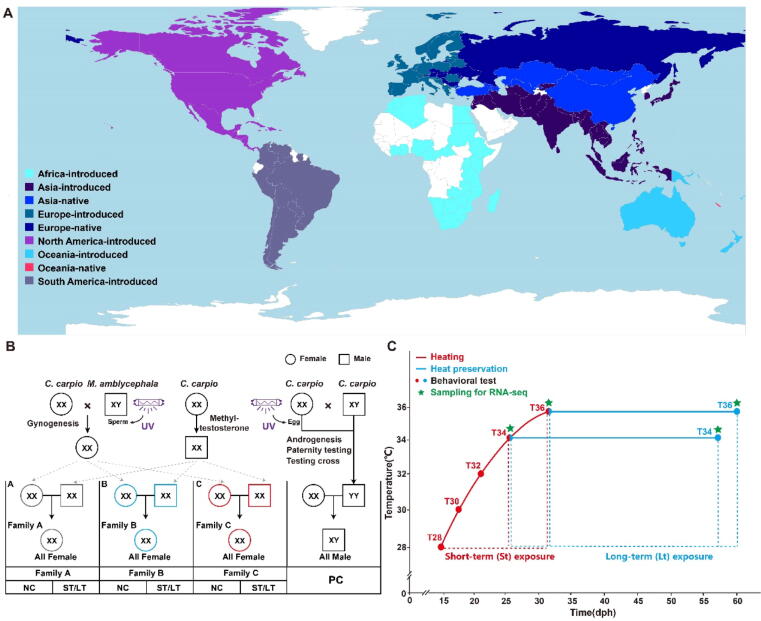
The common carp (Cyprinus carpio L., 1758) is a globally distributed freshwater fish species found in 139 countries and islands (Fig. 1A) [18]. Although it is considered highly invasive in some non-native areas like North America and Australia, carp contributes significantly to freshwater aquaculture production, accounting for approximately 10% of the total output and being economically valuable [19]. As a eurythermal species, C. carpio encounters a wide range of temperatures in its natural habitats. The optimum temperature range for the normal development of C. carpio is typically between 23 °C and 30 °C [20], [21]. However, predictive models estimate a mean water temperature rise of 2.49 °C-5.51 °C by 2080 [22], indicating that C. carpio will likely be exposed more frequently to a temperature of 34 °C or higher in the future. India carp (C. carpio L) exposure to high temperatures (34 °C and 36 °C) significantly alters the sex ratios during the transition of sex differentiation [23]. Therefore, global warming has the potential to impact fish reproduction and govern population dynamics. However, the effects of warming on the swimming behavior in juvenile C. carpio and the molecular mechanism of behavior changes is still limited.
Fig. 1.
An overview diagram of the experiment. (A) The world distribution of common carp including both introduced and native. The red star where the parents of female and male common carp were sampled. (B) A crossing scheme to obtain XX all-female and XY all-male common carp. The XX all-female parents were generated through artificial gynogenesis using ultraviolet inactivated M. amblycephala sperm [30]. On the other hand, the XX physiological male parents were produced through methyltestosterone treatment. For the establishment of three genetic families (A, B, and C), three pairs of XX female parents and XX physiological male parents were selected for artificial insemination. The XY all-male carp were generated by crossing the XX normal females with YY super-male carp which were produced by artificial androgenesis [31]. Circles represent female carps, squares represent male carps. Grey, blue, and red represent A, B, and C genetic families from different parental origins, respectively. NC stands for negative control groups and PC denotes positive control groups. A, B, and C represent three different families of female carp, respectively. ST stands for short-term warming groups; LT stands for long-term warming groups. (C) From the age of fourteen days post-hatch (dph), the fish were acclimated from the initial acclimation of 28 °C to their respective treatment temperatures (34 °C and 36 °C) by 2 °C every four days. This period is called short-term (St) warming group. The fish were maintained at two high temperatures (34 °C and 36 °C) for 30 days, called the long-term (Lt) warming group. The dot stands for the behavioral test; the asterisk stands for sampling for RNA-seq. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Recent studies have demonstrated a robust relationship between individual brain gene expression and behavioral plasticity. Changes in gene expression in response to a variety of contexts such as foraging and maturation can alter a number of cellular and neural pathways that lead to changes in behavior [24], [25]. Transcriptomics studies have emerged as valuable tools for investigating the molecular mechanisms underlying animal behavior [26], [27], [28], [29]. Therefore, alternations of brain genes expression from transcriptome data were used to demonstrate a molecular “signature” in the juvenile C. carpio brain that is robustly associated with swimming behavior under warming. This study aims to investigate the effects of warming on behavioral responses and to elucidate the potential underlying mechanisms of warming-driven behavior. Understanding the effects of temperature changes on swimming behavior and molecular responses in fish is pivotal for ecological research. These results provide valuable insights into environmental “omics” studies in juvenile fish and contribute to the development of new strategies in fish farming for comprehensively evaluating the impact of global warming.
Materials and methods
In this study, we focused on female C. carpio to avoid the influence of sex difference on swimming behavior (Fig. 1B), and constructed both short-term (St) and long-term (Lt) warming groups to investigate the swimming behavior changes and molecular underpinnings responses in brain of juvenile C. carpio (Fig. 1C). Transcriptomic data at the St and Lt warming groups in three independent genetic families of juvenile C. carpio were elucidated the behavioral changes underlying molecular underpinnings in the brain under warming.
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of Institute of Hydrobiology, Chinese Academy of Sciences, Hubei, PR China (Approval No. IHB/LL/2021039).
Genetic family generation and maintenance
All juveniles of C. carpio were sourced from the Guanqiao Experimental Station, Wuhan, China. To exclude the effects of sex differences on the swimming behavior, we selected all-female C. carpio as the experimental group (Fig. 1B). Concurrently, all-female and all-male C. carpio were used as the negative and positive control groups, respectively. The XX female parents were generated through artificial gynogenesis using ultraviolet inactivated M. amblycephala sperm [30], [31]. On the other hand, the XX male parents were produced through methyltestosterone treatment. For the establishment of three genetic families (A, B, and C), three pairs of XX female parents and XX physiological male parents were selected for artificial insemination. Eggs were collected immediately after spawning and incubated at 26 °C for seven days. The common carp fry reached the free-swimming stage five days after fertilization. At fourteen days post-hatch, 200 individuals female C. carpio from family A, B, and C were each transferred into 200 L tanks (100 × 100 × 20 cm) for temperature treatment, as well as their respective negative control (NC) groups. Meanwhile, male C. carpio were placed into the positive control (PC) groups (Fig. S1). During the entire warming, the survival rates of both the experiment and the control carps in the short-term and long-term warming groups were all above 93% (Fig. S2). The fish were fed fairy shrimp three times a day during the adaptation period, and 30 % of the total water volume was replaced daily. The tank water was maintained at a stable pH of 7.0, a water temperature of 26 °C, and nitrate, nitrite, and ammonia levels of 0 ppm (parts per million). Water quality parameters were assessed once a week to ensure consistency.
Warming and experiment design
We conducted an experiment using three families of C. carpio to investigate the impact of different temperature treatment ranging from 28 °C to 36 °C on fish swimming activity. To maintain the desired temperatures with a high level of precision (±0.5 °C), we utilized an electronic temperature controlling device (BM8036) equipped with digital sensors (SN18B20). The fish were gradually acclimated from the initial acclimation temperature of 28 °C to their designated treatment temperatures. Each temperature treatment group consisted of two replicate tanks, with an initial density of 200 fish per tank. Throughout the heating process, the tanks were checked twice daily for mortalities whose fish were immediately removed and processed. The averages of body length within each group were in the Table S1 and Fig. S3-S4.
St warming groups– A day before the start of the trial, the fish were exposed to 28 °C, which was the initial temperature for this particular treatment. The temperature in treatments, namely 28 °C, 30 °C, 32 °C, 34 °C, and 36 °C was incrementally raised by 2 °C every four days (Fig. 1C).
Lt warming groups– To assess whether long-term warming impacted on swimming activity in C. carpio, they were maintained at the two treatment temperatures: 34 ± 0.5 °C, and 36 ± 0.5 °C, for thirty days (Fig. 1C).
Constant temperature control– The temperature was consistently maintained at 28 °C (±0.5 °C) throughout the entire duration of the trial. The control group served as a reference for C. carpio under optimal temperature conditions. The temperature control was maintained ± 0.5 °C of the desired set points. There are two types of temperature control groups: PC consisting of male juveniles and NC consisting of female juveniles from genetic family A, B, and C.
Behavioral testing procedure and apparatus
The behavioral trials were conducted in a plastic tank (8 × 13 × 7 cm), which was placed inside a water bath kettle (34.4 × 34.4 × 20 cm) with a temperature conditioning system. The average body length of the juveniles C. carpio in these 10 groups was measured in the St and Lt warming groups (Table S1; Fig. S3 and S4). Six individuals with body lengths within a range of plus or minus 0.1 cm from the average were selected from each group for behavioral tests. Prior to the behavioral tests, the juveniles were acclimated in their respective water baths for at least 30 min, and then started to record behavior video for 30 min with a Basler aca1920-155uc video camera placed 30 cm above the water bath kettle. Subsequently, the behavior video recordings were uploaded onto a windows 10 computer and analyzed using the Ethovision XT 15.0 (Noldus InfoTech., Wageningen, The Netherlands) behavioral tracking software. To assess the impact of warming on behavioral responses, the typical individual and group behaviors, including five parameters such as swimming speed, mobility state, turns frequency, acceleration, and inter-individual distance, were measured unbiased in three independent genetic families of C. carpio. The St, Lt, NC, and PC groups simultaneously underwent behavioral video recording, and all were analyzed using the same parameters for unbiased behavioral data analysis. These parameters were analyzed using commercially available video-tracking systems, which have been previously used to study swimming behavior in zebrafish [32].
Data analysis of behavior
We employed EthoVision XT 15.0 for automatic video-tracking, digitizing a 10-minute segment from the 2nd to the 12th minute of the video, and then imported this segment into the software, recording the positions of the six fishes every 0.04 s. Subsequently, we calculated five activity variables using EthoVision XT 15.0 software [33]. These quantified activity variables included: (a) Velocity (cm/sec): the swimming distance per unit time in different experimental compartments. This parameter provides information on locomotor activity, hyperactivity, and anxiety levels [34]. (b) Mobility state: the mobility state was categorized into three groups (lowly mobile, mobile, and high mobile) based on the changes in detected subject pixels using predefined thresholds. Low mobility represented below 20 % mobility, mobile was between 20 % and 80 % mobility, and highly mobility indicated above 80 % mobility [33]. (c) Turns angle: the angle formed by C. carpio during changes in direction. (d) Acceleration: The maximum increase in velocity over time, representing the mean acceleration of C. carpio observed during a 10-minute movement. (e) Inter-individual distance: The distance between subjects measured from their center-point. To compare different temperature groups, we employed a Friedman’s test, followed by Wilcoxon Rank Sum tests to assess differences between regions when p < 0.05. All statistical analyses were performed in Grahpad Prism software (version 9.0.0), with the significance level set at p < 0.05. Based on these data, we evaluated the swimming activity of all groups during the same time periods.
Measurement of nervous system activity
To investigate activity of the nervous system in response to warming stimuli, samples for dopamine (DA) and serotonin (5-hydroxytryptamine, 5-HT) were collected from C. carpio exposed to high temperatures (34 °C and 36 °C) in two phases (St and Lt). For sample preparation, the brain of C. carpio was extracted and placed in a pre-weighed 1.5 mL tube. Following the addition of 500 μL of phosphate buffer (0.1 M, pH 8.0), the brains were homogenized using a plastic homogenizer. The homogenate was then centrifuged at 6000g for 15 min at 4 °C. Total protein content was quantified using a BCA protein quantification kit (Sangon Biotech, Shanghai, China). The concentrations of DA and 5-HT in supernatant were determined by using enzyme-linked immunosorbent assay (ELISA) kits (Minneapolis, Minn. and USCN Life Sciences, Wuhan, China) following the manufacturer’s instructions. The supernatant was incubated in microelisa strip plate at 37 °C for 60 min. After three washes with wash solution, 100 μL of HRP-conjugated reagent was added and incubated at 37 °C for 30 min. Following five washes with the wash solution, the 90 μL chromogen solution was added and incubated for 20 min, followed by the addition of 50 μL of stop solution. The optical density (OD) values of each well were measured at 450 nm absorbance using an MD SpectraMax M5 Microplate Reader (USA) after the addition of the stop solution.
Sample collection, library preparation and sequencing
Fish used for transcriptome analysis were euthanized using MS-222 for 5 min. A total of sixty samples (whole brain tissues) for RNA extracted using Trizol Reagent (Invitrogen, USA), comprising 20 groups (as shown in Table S2). Each group consisted of three biological replicates, with one biological replicate corresponding to one whole brain tissue. To remove genomic DNA contaminants, the extracted RNA was treated with RNase-free DNase I (Thermo Scientific, USA) at 30 °C for 30 min. The purity, quantity, and integrity of total RNA were assessed using a NanoDropND-2000 spectrophotometer (Thermo, Waltham, USA), 1.2% (w/v) agarose gel electrophoresis, and an Agilent 2100 Bioanalyzer (Agilent Technologies, Richardson, USA), respectively. For the RNA-seq library construction, RNA samples with desirable quality criteria were selected, including an RNA Integrity Number (RIN) > 8, 28S/18S > 0.7, and A260/280 values around 2.0. Each treatment group had three biological replicates, where each replicate consisted of homogeneous brain tissue. The RNA samples were divided into two groups: one for transcriptome sequencing and the other for quantitative real-time PCR to validate the reliability of the transcriptome data. Poly (A) mRNAs were isolated from the total RNAs using poly (dT) oligo-attached magnetic beads, and cDNA libraries were prepared using the TruSeq RNA Sample Preparation Kit (Illumina, USA). The resulting sixty cDNA libraries were sequenced on the Illumina Nova-seq (BGI) sequencing platform, generating 150 bp pair-end reads.
RNA-seq data analysis for differential expression and regulation pathway
A total of 277 million raw reads were subjected to filtering using the fastp version 0.20.1 software [35], resulting in 359.3 Gb of clean data. The criteria for filtering raw data are: (1) remove reads containing adapters; (2) filter out low-quality sequences (Q < 15); (3) Discard reads with an N (undetermined base information) proportion>5%. The reminder was termed as clean reads. High quality clean reads from each sample were then mapped to the 2021 version genome in C. carpio (ASM1834038v1_genomic.fna and ASM1834038v1_genomic.gtf) using the hisat2 version 2.1.0 software [36]. Subsequently, the number of counts mapped to the C. carpio genome per sample was calculated using featureCounts version 1.6.0 software [37]. To investigate the transcriptome responses of C. carpio to warming stimuli, we analyzed the number and biological functions and pathways of differentially expressed genes (DEGs) in two high temperatures (34 °C and 36 °C) and two phases (St and Lt). In the St groups, differentially expressed genes at 34 °C and 36 °C is compared to the control group. For Lt groups, they're compared to their respective short-term groups. Differential expression analysis was performed using DESeq2 [38]. DEGs were identified in the treatment groups using the Baggerly’s test on FPKMs. The original P-values from the Baggerly’s test were adjusted using Benjamini-Hochberg correction [39] to control the false discovery rate. A DEG was considered if the associated false discovery rate (FDR) was<0.05, and the absolute value of log2(fold change) was>1, serving as the cut-off criteria. For clustering analysis, the FPKM values of DEGs were used, and the Pheatmap package in R was employed. To gain insights into the functions and pathways of the DEGs, we performed Gene Ontology (GO) analysis using the ClusterProfiler package in R version 3.6.1 [40]. The threshold for significance in these analyses was set at P-adjusted value of < 0.05.
Quantitative real-time PCR (qPCR) validation of DEGs
Twelve genes are randomly chosen to validate the RNA-seq results using qPCR. Reversed cDNA was synthesized from the same total RNA used for transcriptome sequencing using the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Shanghai, China) following the manufacturer’s protocol. The synthesized cDNA, derived from 1000 ng of total RNA, was diluted five times and used as a template for qPCR analysis. Specific primers of each gene were designed using NCBI Primer-BLAST (NCBI, USA), and the primer sequences are provided in Table S3. All qPCR reactions were conducted in triplicate, and the dissociation curve analysis was performed to verify the specificity of the amplification. qPCR was carried out using a Bio-Rad CFX96 Touch Detection System (BioRad, USA) with the 2 × SYBR qPCR Master mix (Monad, China) under the following conditions: an initial denaturation at 95 °C for 5 min, followed by 39 cycles of denaturation at 95 °C for 10 s and annealing at 58 °C for 30 s. The relative fold change of gene expression was calculated by the 2-ΔΔCt method [41], with the housekeeping gene elongation factor (β-actin) used as the reference gene for expression normalization. The obtained data were subjected to statistical analysis using GraphPad Prism (version 9.0.0).
Results and discussion
No significant differences in swimming behavior among three families of C. carpio
Sex-specific differences in swimming behavior have been reported in zebrafish and Trinidadian guppies [42], [43], [44]. To exclude the effects of sex difference on the swimming behavior in C. carpio, a female juvenile was used as the experimental group, while a female and a male juvenile were used as the NC and PC reference groups, respectively (Fig. 1B). Research on individual behavior in water is well documented [5], but much less is known about the behavior of intra-family or inter-family. We constructed three independent genetic families (A, B, and C) of female juvenile to explore intra-individual variance in the swimming behavior of C. carpio (Fig. 1C). Interestingly, we found no significant differences in the swimming activity (i.e., swimming speed, mobility state, and turn frequency) among the three families (Fig. 2A–C; Fig. 3C). Therefore, the changes in swimming activity in juvenile C. carpio were much more dependent on the temperature than the spontaneous one.
Fig. 2.
The behavioral data analysis of the three genetic families of C. carpio in the St warming (28 °C, 30 °C, 32 °C, 34 °C, and 36 °C). (A) Movement velocity; (B) Percentage of visiting frequency for C. carpio exposure to different elevated temperatures with the mobility state (low mobility, mobile, and highly mobile); (C) The relative turn angle based on the heading of the Center-point; (D) Mean acceleration; (E) Inter-individual distance. ∗∗∗∗P < 1e-04, ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P ≤ 0.05 and ‘ns’ denotes P > 0.05.
Fig. 3.
The behavioral data analysis of the three genetic families of C. carpio in the Lt warming groups (34 °C and 36 °C). (A) The behavioral videos with different temperatures (34 °C and 36 °C) during treatment, respectively. (B) The heatmaps were generated by automated video tracking of C. carpio activity during the 10 min trial of the high-temperature conditions (Ethovision, Noldus). The color shows the relative dwell time in a given area (blue, low; red, high) and averaged over all fishes in a treatment group. (C) The percentage of cumulative duration of juvenile C. carpio in different mobility states after 30 days of exposure to high temperatures (34 °C and 36 °C) (D) Mean acceleration (E) Inter-individual distance. ∗∗∗∗P < 1e-04, ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P ≤ 0.05 and ‘ns’ denotes P > 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The peak temperature for swimming behavior in C. carpio observed was 32 °C
The Gill Oxygen Limitation (GOL) hypothesis proposes that fish inhabiting warm waters may experience a decrease in available oxygen due to limitations in their gill function [45]. This limitation can potentially affect their physiological processes and survival. Additionally, the Oxygen- and Capacity-limited Thermal Tolerance (OCLTT) hypothesis suggests that an animal's physiological systems may have temperature-related limitations, which can impact its ability to maintain aerobic performance as temperatures increase [46], [47]. Both the GOL and OCLTT hypotheses highlight the potential consequences of climate change-induced temperature increases on fish physiology and survival.
To investigate the effects of warming-driven on the individual behaviors of juvenile C. carpio, the swimming speed, mobility state, turn frequency, and acceleration was analyzed in this study. The swimming activity of C. carpio showed significant alterations in water temperature between 28 °C and 36 °C in the St warming groups, which is close to the “thermal performance curve” (Fig. 2; Table S4-S7; Supplementary video 1–5). The swimming speed of C. carpio at 28 °C was significantly slower compared to that at 30 °C (P = 0.0009 in family A, P = 0.051 in family B, P = 0.0357 in family C) and 32 °C (P < 0.0001 in family A, P < 0.0001 in family B, P < 0.0001 in family C), while C. carpio at 32 °C swam significantly faster compared to that at 34 °C (P < 0.0001 in family A, P = 0.2269 in family B, P < 0.0001 in family C) and 36 °C (P = 0.0616 in family A, P = 0.011 in family B, P = 0.0002 in family C) (Fig. 2A). Further, the swimming activity at 36 °C was lower than that at 34 °C (Fig. 2 A–B; D; Table S4-S6). A state of low mobility in the three families of juvenile C. carpio was maintained throughout the observation period from 28 °C to 36 °C (Fig. 2B; Table S5). The turn frequency of C. carpio was the highest at 32 °C and their head tended to skew to the left (Fig. 2C). The angle between them was calculated at the frame prior to eye convergence. These angles were predominantly in the frontal field, i.e., angles < 0.05° (Fig. 2C). To further investigate the effects of warming-driven on the group behavior of juvenile C. carpio, the inter-individual distance (IID) was tracked and calculated. The IID notably decreased from 28 °C to 32 °C, then gradually increased after 32 °C, highlighting that the IID is minimum at 32 °C (Fig. 2E; Table S7). The group behavior in juvenile C. carpio was the most active at 32 °C, demonstrating the peak temperature for the swimming activity at this temperature. Taken together, the swimming activity of C. carpio at 34 °C is similar to that at 30 °C, the activity at 36 °C parallels that at 28 °C, with the peak swimming activity notably occurring at 32 °C.
The normal development of C. carpio generally occurs within an optimum temperature range of 23 °C to 30 °C [20], [21]. However, our study revealed that the peak swimming activity of C. carpio occurs at 32 °C. A similar phenomenon of a difference between the optimal temperature for living and the temperature for peak swimming activity is observed in juvenile southern catfish [48]. The optimal living temperature for fish refers to a range within which they can engage in normal growth, reproduction, and vital life processes [49], [50]. On the other hand, the temperature for peak swimming activity is generally associated with the fish’s locomotive capabilities, efficiency in predation, and ability to evade predators [51], [52]. Our results provided new data from juvenile fish support for the conclusion that the optimal temperature for living and the temperature for peak swimming activity often differ in fish [53], [54]. Our findings suggested that the impact of global warming on fish behavior is complex, with potentially contrasting effects at different temperature ranges. Interestingly, our findings demonstrated that warming up to 32 °C can actually activate swimming behaviors, as shown in Fig. 2. However, the significant drop in swimming behavior were described when water temperatures exceed 32 °C (Fig. 2). This observation highlighted the dramatic swimming behavior drop at 34 °C and 36 °C in juvenile carp. To investigate the underlying molecular mechanisms responsible for this drop in swimming behavior, three genetic families of C. carpio both the St and Lt warming groups were sampled for brain transcriptome analysis. This will provide valuable insights into the adaptive strategies and physiological limitations of fish in coping with global warming.
Long-term warming has more significant effects on swimming behavior drop
To further investigate the impacts of long-term warming on the individual and group behaviors of juvenile C. carpio, the mobility state, acceleration, and IID were systemically analyzed. In this study, when compared to the corresponding genetic family of female juveniles in the NC group, the warming groups (34 °C and 36 °C) tended to spend most of their time in the middle zone (Fig. 3B; Supplement video 6–7). This demonstrated that warming groups were in a less anxious state compared with the NC groups. Therefore, long-term warming decreased the swimming activity in three families of juvenile C. carpio. As can be seen in Fig. 3C, the mobility state in C. carpio was significantly decreased after thirty days exposure, especially in the family C exposed to 34 °C (Table S8; P < 0.0001). After exposure to 36 °C for thirty days, the acceleration of C. carpio in the Lt group significantly decreased compared to the St group (Fig. 3D; P = 0.0356; Table S9). This tendency significantly decreased at 36 °C compared to 34 °C. The IID of C. carpio in the Lt group increased compared to the St groups (Fig. 3E; P = 0.0012 in 34 °C; P = 0.1505 in 36 °C; Table S10). Further, in the Lt and St groups, the median IID at 36 °C is lower than at 34 °C. This indicated decreased swimming activity after long-term exposure to 34 °C and 36 °C for thirty days. Therefore, long-term warming has more significant effects on swimming behavior drop compared to short-term warming (Fig. 3). This is somewhat inconsistent with a previous study, which carp acclimated to warmer temperatures exhibited greater endurance swimming capacity [55]. While there may be some differences in the specific results, these studies highlight that C. carpio exhibited changes in their swimming behavior in response to thermal acclimation. This suggests that behavioral plasticity could serve as an adaptive mechanism for fish to cope with changing environmental conditions caused by global warming. This is somewhat consistent with previous studies in zebrafish (Danio rerio) [27] and Japanese medaka (Oryzias latipes) [56]. Interestingly, the fish in the warming groups exhibited lower levels of anxiety compared to those in the NC groups, as indicated by their lower mobility and the increase IID (Fig. 3C, E). Several previous research also reported that warming temperatures can result in a reduction in anxiety-like behavior in fish [30], [57], while others have found contradictory or no inconclusive effect [58]. The relationship between temperature and anxiety in fish is complex and may not be straightforward.
A robust association between swimming behaviour and brain in C. carpio
To investigate the possible links between swimming behaviour and the center nervous system in C. carpio, variations in behavior-related physiological indicators DA and 5-HT levels in the brain exposed to St and Lt warming were measured. In the St warming groups, the levels of 5-HT and DA in the brain of C. carpio exposed to 34 °C significantly declined to 48.66% (t-test, t = 8.676, P = 0.0001, Fig. S5A) and 54.31% (t-test, t = 4.41, P = 0.0045, Fig. S5B) of that in control fish, and those exposed to 36 °C significantly decreased to 67.36% (t-test, t = 9.069, P = 0.0001, Fig. S5A) and 83.26% (t-test, t = 1.839, P = 0.1156, Fig. S5B) of that in control fish, respectively.
In the Lt warming groups, the levels of 5-HT and DA in the brain of C. carpio exposed to 34 °C significantly elevated to 271.51% (t-test, t = 7.436, P = 0.0003, Fig. S5C) and 248.74% (t-test, t = 4.496, P = 0.0041, Fig. S5D) of that in control fish, and those exposed to 36 °C significantly increased to 222.04% (t-test, t = 5.145, P = 0.0021, Fig. S5C) and 184.64% (t-test, t = 5.68, P = 0.0013, Fig. S5D), respectively. Therefore, the center nervous system in C. carpio modulate DA and 5-HT in response to St and Lt warming, indicating a strong correlation with swimming behaviour.
Brain transcriptome response to warming
The brain is known to be highly sensitive to temperature changes, and it plays a critical role in regulating the body’s stress response and thermoregulation. Previous studies have shown that warming can lead to brain injury and impair neurological function in aquatic organisms [46], [59]. To further investigate molecular mechanism underlying behaviors changes in juvenile C. carpio in response to warming, brain transcriptomes were compared among the NC, St, and Lt groups. Transcriptome sequencing was performed on sixty brain samples from two high-temperature groups (i.e., St and Lt groups in Table S2). Sixty samples yielded 277 million raw reads by RNA-seq, which were deposited in the NCBI Sequence Read Archive database with the accession numbers SRR23085892 to SRR23085951 (PRJNA923838). After filtering, approximately 359.3 Gb of clean data were generated, and the alignment rate mapping of the genome (ASM1834038v1_genomic.fna and ASM1834038v1_genomic.gtf) in C. carpio was>91% (Fig. S6; Table S11). Due to the correlation coefficients of St-34-A2 and St-36-A2, samples did not meet the requirements for biological repetition within their respective groups and were thus excluded. The remaining fifty-eight samples were used for subsequent analysis (Fig. S7). To elucidate the underlying mechanisms of swimming behavior in juvenile C. carpio under warming, two high temperature groups (34 °C and 36 °C) and two phases (St and Lt) were performed on the RNA-seq analysis for differential expression and regulation pathway. Based on the FPKM expression levels of all DEGs, the Principal Component Analysis (PCA) and hierarchical clustering analysis separated the St and Lt groups (Fig. 4A-B), evidencing the warming-up period greatly affected C. carpio brain gene expression. For the St group compared to NCs: at 34 °C, families A, B, and C had 522, 297, and 431 DEGs. At 36 °C, the numbers were 397, 851, and 389 (Fig. 4C). In the Lt group against St: at 34 °C, the DEGs for families A, B, and C were 1466, 944, and 2220; and 837, 731, 3050 at 36 °C (Fig. 4D). Further, the number of DEGs and degree of expression changes in these DEGs involved in the Lt groups was higher than in the St groups (Fig. 4C-F).
Fig. 4.
The differentially expressed genes (DEGs) analysis in the St and Lt warming groups. (A) principal component analysis; and (B) Heatmap basis on fragments per kilo base of transcript per million mapped fragments (FPKM) expression levels of all differentially expressed genes. The bar chart shows the number of DEGs in the St (C) and Lt (D) warming groups among the three families of the juvenile C. carpio. The volcano plot shows up- and down-regulated DEGs across all six groups in the St (E) and Lt (F) warming groups. An adjusted P-value < 0.01 is indicated in red, while an adjusted 0.01 ≤ P-value < 0.05 is in black. The volcano plot showed gene id or gene name top five absolute values of Log2FoldChange and P-adjust < 0.01 in each group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Twelve DEGs were randomly analyzed using qPCR to validate the reliability of the transcriptomic data (Table S12-S13). In fish, the hemoglobin components, hbbl, hbae, hba1l, and hbb2, were involved in the GO terms for “oxygen transport”, “gas transport”, and “oxygen carrier activity”. The genes expression of hbbl and hba1l were downregulated in the St warming groups, while hbae and hbb2 were significantly downregulated in the Lt warming groups. Rag1l was decreased expression in the St warming groups, which involved GO term for the “positive regulation of hemopoiesis”. Sp1 and ctral play an essential role in the metabolism, involved in the “sterol metabolic process” and “water-soluble vitamin metabolic process”, respectively. The expressions of sp1 and ctral genes were higher in the Lt warming groups than that in the St warming ones. Fold change differences in expression levels observed in RNA-seq and qPCR for these 12 genes displayed similar patterns and also showed good correlations of 0.84 (Fig. 7), confirming the reliability of the transcriptomic data.
Fig. 7.
Validation of RNA-Seq data by qPCR. In total, the expression of twelve genes was detected by qPCR (A) and RNA-seq (B). The light blue bars stand for the short-term warming groups, and the dark red bars represent the long-term warming groups. (C) Scatter plots of correlation test between qPCR and RNA-Seq results. R2 is Pearson correlation coefficient. The blue and red bars represent the distribution of 12 genes on the × and y axes, respectively, with the height of the bars indicating the level of gene expression fold change. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Potential mechanisms for decreased swimming behavior underlying warming
To explore the mechanism potentially warming-driven behavior neurogenomic states, the St and Lt groups were subjected to common and specific GO enrichment analysis (Fig. S8; Table S14–S17). In this study, the St and Lt groups both showed a significant decrease (P-value < 0.05) in the metabolism-related pathways among the three genetic families of C. carpio under warming (34 °C and 36 °C) (Fig. 5 and Fig. 6; Table S18–S29). These metabolism-associated pathways involve ATP metabolic, mitochondrial regulation and neurotransmitter metabolic processes, suggesting a disruption in cellular energy metabolism that may compromise cell physiology and brain function. Moreover, the reduction of genes expression involved in the mitochondrial transport in the St and Lt group suggests potential impairment in the positioning of mitochondria within synaptic terminals, where they regulate neurotransmission and synaptic plasticity. Additionally, the St and Lt groups have a profound impact on cytoskeleton, especially in the Lt groups showing a significantly downregulated genes involved in muscle contraction and actin cytoskeleton (Fig. 5 and Fig. 6; Table S18-S29).
Fig. 5.
Gene ontology enrichment analysis for short-term warming groups. (A) The bubble charts of enrichment results of the DEGs showing GO terms related to the five clustering (i.e., “Cellular response”, “Cytoskeleton”, “Metabolism”, “Protein”, and “Transport”). The color of the point represents the size of the P-value, and the GeneRatio of DEGs enriched in each term is represented by the size of the point. (B-D) display the upregulated and downregulated levels of DEGs enriched in these five major pathways for families A, B, and C at 34 °C, respectively. (E-G) show the upregulated and downregulated levels of DEGs from these pathways for families A, B, and C at 36 °C, respectively. The outermost circle represents the GO terms of five categories enriched with DEGs. The second outer circle represents the upregulation and downregulation of DEGs corresponding to the five major GO terms. Red represents upregulated DEGs, and blue represents downregulated DEGs. The innermost circle represents the trend of GO term towards upregulation or downregulation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Gene ontology enrichment analysis for short-term warming groups. The bubble charts of enrichment results of the DEGs showing GO terms related to the eight clustering (i.e., “Cell cycle,” “Cellular response”, “Cytoskeleton”, “Metabolism”, “Protein”, “Synapse”, “Signal transduction”, and “Transport”). The color of the point represents the size of the P-value, and the GeneRatio of DEGs enriched in each term is represented by the size of the point. (B-D) depict the patterns of upregulated and downregulated DEGs associated with these eight pathways in families A, B, and C at 34 °C, respectively. (E-G) illustrate the expression levels of DEGs within these pathways for the three families at 36 °C. The outermost circle represents the GO terms of eight categories enriched with DEGs. The second outer circle represents the upregulation and downregulation of DEGs corresponding to the eight major GO terms. Red represents upregulated DEGs, and blue represents downregulated DEGs. The innermost circle represents the trend of GO term towards upregulation or downregulation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Notably, compared to the St group, the Lt group showed descreased expression of genes associated with synapse and neurotransmitter secretion, suggesting impairment in intercellular communication associated with the modulation of signal transduction pathways (Fig. 6; Table S24-S29). Consequently, the three genetic families of juvenile C. carpio in response to warming, especially in the Lt group, determined synaptic function impairments that might be responsible for functional alterations of the central nervous system. These data indicated the induction of the classical stress response to the warming conditions and distinct neurogenimic state underlie differential impacts of short- and long-term warming in the brain.
To further investigate a link between metabolites and genes expression about behavior-related neurotransmission in the brain, C. carpio at 34 °C and 36 °C in the St and Lt warming groups were subjected to common GO terms enrichment analysis involved in the DA, and 5-HT pathways. In this study, a large number of DEGs related to DA and 5-HT in brain of C. carpio were significantly enriched in both St and Lt warming groups (Fig. S5; Table S30-S33). The detection of 5-HT and DA metabolites further support the data of comparative transcriptome (Fig. S9). These results demonstrated the important role of the serotoninergic and dopaminergic systems in regulating swimming behavior in C. carpio.
Our data provides comprehensive resources for understanding the neuromolecular mechanism underlying the impact of warming on swimming behavior in fish. Previous research has identified several genes and pathways that may be involved in temperature-dependent behavior in fish, including genes involved in the stress response, circadian rhythms, and neurotransmitter signaling pathways [17], [27], [60], [61], [62]. Interestingly, our comparative transcriptome analysis revealed a reduction in the expression of a subset of transcripts associated with synapse and neurotransmitter secretion suggesting impairment (Fig. 6; Table S24-S29). These gene cluster may provide more genes and details that were introduced in the aforementioned works. Our data contributes to a deeper understanding of the underlying mechanisms behind phenomena.
Conclusions
Our study sheds light on the potential molecular mechanisms that lead to decreased swimming activity in juvenile C. carpio under warming (Fig. 8). These changes in the central nervous system induce significant physiological effects, which in turn, influence the swimming behavior in C. carpio. These insights are critical not only for understanding the biological adaptations of C. carpio but also for predicting the broader impacts of global warming on aquatic ecosystems. Given that temperature-dependent changes in swimming activity can drastically affect the survival prospects of wild fish, our findings highlight the urgent need for more in-depth research into the ecological consequences of these physiological alterations. Future studies would aim to assess the downstream effects of altered swimming behavior on population dynamics, predator–prey interactions, and ecosystem functioning in a warming world. Ultimately, a more comprehensive understanding of these interactions will be crucial for developing strategies to mitigate the impacts of climate change on aquatic biodiversity and ecosystem health.
Fig. 8.
The schematic summary of the transcriptomic mechanism underlying behavior change in juvenile C. carpio under warming. Downregulation ↓, Upregulation ↑, and general variation ↓↑ of genes are reported in the St on the left and Lt on the right. IID: inter-individual distance.
CRediT authorship contribution statement
Yuanli Zhao: Investigation, Data curation, Visualization, Software, Writing – original draft, Writing – review & editing. Ming Duan: Funding acquisition, Data curation, Formal analysis, Resources, Writing – review & editing. Xing Lin: Data curation, Formal analysis, Validation, Resources, Software, Writing – review & editing. Weiwei Li: Formal analysis, Software. Hairong Liu: Resources. Kaifeng Meng: Data curation. Fei Liu: Conceptualization. Wei Hu: Funding acquisition, Resources. Daji Luo: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Rui Li, Yuanyuan Chen, Meidi Hu and Chaolin Jiang for their help in experimental process; Guangxin Wang and Xin Wang (The analysis and Testing Center of Institute of Hydrobiology, Chinese Academy of Sciences) for their support of instrument platform and technical. This work was supported by funds from the Strategic Priority Research Program of CAS (Grant No. XDA24010108) to DL and WH, National Natural Science Foundation of China (No. 31922085) to DL, (No. 32030113) to WH and (No. 32172955) to MD, National Key R&D Program of China (No. 2022YFB3206900) to MD, and China Postdoctoral Science Foundation on the 70th finance (Grant No. 2021M703436 to YZ).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.10.017.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Matthews H.D., Wynes S. Current global efforts are insufficient to limit warming to 1.5°C. Science. 2022;376(6600):1404–1409. doi: 10.1126/science.abo3378. [DOI] [PubMed] [Google Scholar]
- 2.Jourdan J., O'Hara R.B., Bottarin R., Huttunen K.L., Kuemmerlen M., Monteith D., et al. Effects of changing climate on European stream invertebrate communities: A long-term data analysis. Sci Total Environ. 2018;621:588–599. doi: 10.1016/j.scitotenv.2017.11.242. [DOI] [PubMed] [Google Scholar]
- 3.Breau C., Cunjak R.A., Peake S.J. Behaviour during elevated water temperatures: can physiology explain movement of juvenile Atlantic salmon to cool water? J Anim Ecol. 2011;80:844–853. doi: 10.1111/j.1365-2656.2011.01828.x. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs L., Minya D., Homoki D., Toviho O.A., Feher M., Stundl L., et al. Effect of different water temperatures on sex ratio, gonad development and production parameters of common carp (Cyprinus carpio L.) Aquac Res. 2020;51:858–862. [Google Scholar]
- 5.Bonacina L., Fasano F., Mezzanotte V., Fornaroli R. Effects of water temperature on freshwater macroinvertebrates: a systematic review. Biol Rev. 2022;98(1):191–221. doi: 10.1111/brv.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habary A., Johansen J.L., Nay T.J., Steffensen J.F., Rummer J.L. Adapt, move or die - how will tropical coral reef fishes cope with ocean warming? Glob Chang Biol. 2017;23(2):566–577. doi: 10.1111/gcb.13488. [DOI] [PubMed] [Google Scholar]
- 7.Downie A.T., Illing B., Faria A.M., Rummer J.L. Swimming performance of marine fish larvae: review of a universal trait under ecological and environmental pressure. Rev Fish Biol Fisher. 2020;30(1):93–108. [Google Scholar]
- 8.Leis J.M. Measurement of swimming ability in larval marine fishes: comparison of critical speed with in situ speed. Mar Ecol Prog Ser. 2020;650:203–215. [Google Scholar]
- 9.Colchen T., Teletchea F., Fontaine P., Pasquet A. Temperature modifies activity, inter-individual relationships and group structure in a fish. Curr Zool. 2017;63(2):175–183. doi: 10.1093/cz/zow048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie S, Schulte PM. Neuronal regeneration. In: Evans DH, Claiborne JB, Currie S, editor. The physiology of fishes. CRC Press; 2014, 257-287.
- 11.Rummer J.L., Couturier C.S., Stecyk J.A.W., Gardiner N.M., Kinch J.P., Nilsson G.E., et al. Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob Chang Biol. 2014;20(4):1055–1066. doi: 10.1111/gcb.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haesemeyer M., Robson D.N., Li J.M., Schier A.F., Engert F. A Brain-wide circuit model of heat-evoked swimming behavior in larval zebrafish. Neuron. 2018;98(4):817–831.e816. doi: 10.1016/j.neuron.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundlach M., Paolo C.D., Chen Q.Q., Majewski K., Haigis A.C., Werner I., et al. Clozapine modulation of zebrafish swimming behavior and gene expression as a case study to investigate effects of atypical drugs on aquatic organisms. Sci Total Environ. 2022;815 doi: 10.1016/j.scitotenv.2021.152621. [DOI] [PubMed] [Google Scholar]
- 14.Nasri A., Mezni A., Lafon P.A., Wahbi A., Cybedo N., Clair P., et al. Ethinylestradiol (EE2) residus from birth control pills impair nervous system development and swimming behavior of zebrafish larvae. Sci Total Environ. 2021;770:145272–1145210. doi: 10.1016/j.scitotenv.2021.145272. [DOI] [PubMed] [Google Scholar]
- 15.Tudorache C., Braake A.T., Tromp M., Slabbekoom H., Schaaf M.J.M. Behavioral and physiological indicators of stress coping styles in larval zebrafish. Stress. 2015;18(1):121–128. doi: 10.3109/10253890.2014.989205. [DOI] [PubMed] [Google Scholar]
- 16.Wang K., Wang C.J., Wang J.H., Dong Y.F., Che W.N., Li X.W. Acute toxicity of broflanilide on neurosecretory system and locomotory behavior of zebrafish (Danio rerio) Chemosphere. 2022;305 doi: 10.1016/j.chemosphere.2022.135426. [DOI] [PubMed] [Google Scholar]
- 17.Toni M., Angiulli E., Miccoli G., Cioni C., Alleva E., Frabetti F., et al. Environmental temperature variation affects brain protein expression and cognitive abilities in adult zebrafish (Danio rerio): A proteomic and behavioural study. J Proteomics. 2019:204. doi: 10.1016/j.jprot.2019.103396. [DOI] [PubMed] [Google Scholar]
- 18.Vilizzi L., Copp G.H. Global patterns and clines in the growth of common carp Cyprinus carpio. J Fish Biol. 2017;91(1):3–40. doi: 10.1111/jfb.13346. [DOI] [PubMed] [Google Scholar]
- 19.Fao . FAO; Rome: 2022. Fishery and Aquaculture Statistics. [Google Scholar]
- 20.Sapkale P.H., Singh R.K., Desai A.S. Optimal water temperature and pH for development of eggs and growth of spawn of common carp (Cyprinus carpio) J Appl Anim Res. 2011;39:339–345. [Google Scholar]
- 21.Oyugi D.O., Cucherousset J., Baker D.J., Britton R. Temperature effects on the growth and foraging of juvenile common carp Cyprinus carpio. J Therm Biol. 2012;37:89–94. [Google Scholar]
- 22.Wenger S.J., Isaak D.J., Luce C.H., Neville H.M., Fausch K.D., Dunham J.B., et al. Flow regime, temperature, and biotic interactions drive differential declines of trout species under climate change. P Natl Acad Sci U S A. 2011;108(34):14175–14180. doi: 10.1073/pnas.1103097108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas C., Chakraborty S., Munilkumar S., Gireesh-Babu P., Sawant P.B., Chadha N.K., et al. Effect of high temperature during larval and juvenile stages on masculinization of common carp (Cyprinus carpio, L) Aquaculture. 2021;530 [Google Scholar]
- 24.Baker M.R., Hofmann H.A., Wong R.Y. In: eLS. John Wiley & Sons Ltd:; Chichester: 2015. Neurogenomics of behavioural plasticity in socioecological contexts. [Google Scholar]
- 25.Wong R.Y., Lamm M.S., Godwin J. Characterizing the neurotranscriptomic states in alternative stress coping styles. BMC Genomics. 2015;16:425. doi: 10.1186/s12864-015-1626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrasekaran S., Ament S.A., Eddy J.A., Rodriguez-Zas Schatz B.R., Price N.D., Robinson G.E. Behavior-specific changes in transcriptional modules lead to distinct and predictable neurogenomics states. P Natl Acad Sci U S A. 2011;108(44):18020–18025. doi: 10.1073/pnas.1114093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott G.R., Johnston I.A. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. P Natl Acad Sci U S A. 2012;109(35):14247–14252. doi: 10.1073/pnas.1205012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu S.J., Yang H., Menon V., Lemire A.L., Wang L., Henry F.E., et al. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science. 2020;370:313. doi: 10.1126/science.abb2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young R.L., Ferkin M.H., Ockendon-Powell N.F., Orr V.N., Phelps S.M., Pogany A., et al. Conserved transcriptomic profiles underpin monogamy across vertebrates. P Natl Acad Sci U S A. 2019;116(4):1331–1336. doi: 10.1073/pnas.1813775116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao J., Zou T.M., Chen L., et al. Microsatellite analysis of different ploidy offspring of artificial gynogenesis in Cyprinus carpio. J Fish Biol. 2011;78(1):150–165. doi: 10.1111/j.1095-8649.2010.02848.x. [DOI] [PubMed] [Google Scholar]
- 31.Jiang M.Y., Wu X., Chen K., et al. Production of YY supermale and XY physiological female common carp for potential eradication of this invasive species. J World Aquac Soc. 2018;49(2):315–327. [Google Scholar]
- 32.Abozaid A., Tsang B., Gerlai R. The effects of small but abrupt change in temperature on the behavior of larval zebrafish. Physiol Behav. 2020;227 doi: 10.1016/j.physbeh.2020.113169. [DOI] [PubMed] [Google Scholar]
- 33.Noldus L., Spink A.J., Tegelenbosch R.A.J. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput. 2001;33(3):398–414. doi: 10.3758/bf03195394. [DOI] [PubMed] [Google Scholar]
- 34.Blaser R., Gerlai R. Behavioral phenotyping in zebrafish: comparison of three behavioral quantification methods. Behav Res Methods. 2006;38(3):456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 35.Chen S.C., Zhou Y.Q., Chen Y.R., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D.H., Paggi J.M., Park C.H., Bennett C.P., Salzberg S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomics features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 38.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 40.Xie C., Mao X., Huang J., Ding Y., Wu J., Dong S., et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39 doi: 10.1093/nar/gkr483. Web Server issue), W316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Ampatzis K., Dermon C.R. Sexual dimorphisms in swimming behavior, cerebral metabolic activity and adrenoceptors in adult zebrafish (Danio rerio) Behav Brain Res. 2016;312:385–393. doi: 10.1016/j.bbr.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 43.Conradsen C., McGuigan K. Sexually dimorphic morphology and swimming performance relationships in wild-type zebrafish Danio rerio. J Fish Biol. 2015;87(5):1219–1233. doi: 10.1111/jfb.12784. [DOI] [PubMed] [Google Scholar]
- 44.Gordon S.P., Chen Y.Y., Yamashita K., Bejar C., Wilshire A., Cheung V. Sex-specific genetic differences in endurance swimming of Trinidadian guppies. Ecol Evol. 2015;5(22):5318–5328. doi: 10.1002/ece3.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauly D. The relationships between gill surface area and growth performance in fish: a generalization of von Bertalanffy's theory of growth. Berichte der Dtsch Wissenchaftlichen Kommission fur Meeresforsch. 1981;28:251–282. [Google Scholar]
- 46.Pörtner H.O. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- 47.Pörtner H.O. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol. 2010;213:881–893. doi: 10.1242/jeb.037523. [DOI] [PubMed] [Google Scholar]
- 48.Zeng L.Q., Cao Z.D., Fu S.J., Peng J.L., Wang Y.X. Effect of temperature on swimming performance in juvenile southern catfish (Silurus meridionalis) Comp Biochem Phys A. 2009;153:125–130. doi: 10.1016/j.cbpa.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Crozier L.G., Hendry A.P., Lawson P.W., Quinn T.P., Mantua N.J., Battin J., et al. Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evol Appl. 2018;1(2):252–270. doi: 10.1111/j.1752-4571.2008.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niu J.Y., Huss M., Vasemägi A., Gardmark A. Decades of warming alters maturation and reproductive investment in fish. Ecosphere. 2023;14:e4381. [Google Scholar]
- 51.Nati J.J., Lindström J., Halsey L.G., Killen S.S. Is there a trade-off between peak performance and performance breadth across temperatures for aerobic scope in teleost fishes? Biol Lett. 2016;12(9):20160191. doi: 10.1098/rsbl.2016.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y.J., Tüzün N., Sentis A., Stoks R. Thermal plasticity and evolution shape predator-prey interactions differently in clear and turbid water bodies. J Anim Ecol. 2022;91(4):883–894. doi: 10.1111/1365-2656.13680. [DOI] [PubMed] [Google Scholar]
- 53.Johansen J.L., Jones G.P. Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Global Change Biol. 2011;17:2971–2979. [Google Scholar]
- 54.Johansen J.L., Messmer V., Coker D.J., Hoey A.S., Pratchett M.S. Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Global Change Biol. 2014;20:1067–1074. doi: 10.1111/gcb.12452. [DOI] [PubMed] [Google Scholar]
- 55.Wakeling J.M., Cole N.J., Kemp K.M., Johnston I.A. The biomechanics and evolutionary significance of thermal acclimation in the common carp Cyprinus carpio. Am J Physiol Regul Integr Comp Physiol. 2000;279(2):R657–R665. doi: 10.1152/ajpregu.2000.279.2.R657. [DOI] [PubMed] [Google Scholar]
- 56.Hechter D.T., Hasler C.T. Repeatability of burst swimming performance in medaka (Oryzias latipes) Fish Physiol Biochem. 2019;45:1299–1307. doi: 10.1007/s10695-019-00679-6. [DOI] [PubMed] [Google Scholar]
- 57.Luchiari A.C., Oliveira J.J. The interaction of innate and imposed colour perception: a behavioural approach. J Ethol. 2014;32:179–183. [Google Scholar]
- 58.Silva P.F., de Leaniz C.G., Freire F.A.M., Silveira V.A.M., Luchiari A.C. Different housing conditions for zebrafish: What are the effects? Behav Process. 2023;209 doi: 10.1016/j.beproc.2023.104886. [DOI] [PubMed] [Google Scholar]
- 59.Przepiura T.D.S., Herreria T., Kandalski P.K., Zaleski T., Machado C., Forgati M., et al. Metabolic responses in Antarctic Nototheniidae brains subjected to thermal stress. Brain Res. 2019;1708:126–137. doi: 10.1016/j.brainres.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Liu L.L., Zhang R., Wang X.W., Zhu H., Tian Z.H. Transcriptome analysis reveals molecular mechanisms responsive to acute cold stress in the tropical stenothermal fish tiger barb (Puntius tetrazona) BMC Genomics. 2020;21:737. doi: 10.1186/s12864-020-07139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z., Okamoto K., Hayashi Y., Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Mattson M.P., Gleichmann M., Cheng A. Mitochondria in Neuroplasticity and Neurological Disorders. Neuron. 2008;60(5):748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.