Abstract
Objective
Carbon nanoparticle (CNP)-guided sentinel lymph node biopsy (SLNB) has been extensively adopted as a cost-effective and highly efficient method for tracing malignant tumors except for those associated with vulvar cancer. The current study aimed to validate the feasibility and efficacy of CNPs in tracking sentinel lymph nodes (SLNs) in patients with early vulvar cancer.
Methods
We retrospectively reviewed patients with vulvar cancer at our institution from January 2016 to April 2022 who were pathologically diagnosed and underwent SLNB or inguinofemoral lymphadenectomy (IFLND). CNPs were the only lymphatic tracer used in SLNB. Patient demographics, perioperative outcomes and follow-up results, including overall survival (OS) and progression-free survival (PFS), were compared between the SLNB and IFLND groups.
Results
Data from 52 patients were collected and investigated. Forty groins of 22 patients who underwent SLNB with CNP tracing were included. Black-stained SLNs were detected in 32 groins of 19 patients, and the rates of CNP detection by patient and by groin were 86.4 % and 80 %, respectively. Patients who underwent SLNB had better perioperative outcomes than those who underwent IFLND in certain aspects (groin drainage rate: 41.2 % and 80 %, respectively, p < 0.05; daily drainage volume (ml): 12.49 and 36.4, respectively, p < 0.05; and inguinal wound healing rate: 100 % and 80 %, respectively, p < 0.05). The results of survival analysis indicated similar prognoses for node-negative patients who underwent CNP-guided SLNB or IFLND.
Conclusions
Sentinel lymph node mapping with CNPs in vulvar cancer is feasible and demonstrates considerable biosecurity. With a satisfactory SLN detection rate achieved expediently, CNPs are a promising lymphatic tracer worthy of further utilization in vulvar cancer and could be an alternative option to canonical tracers.
1. Introduction
Inguinal lymph node metastasis has been widely accepted as a negative prognostic factor for genitourinary malignant diseases, including vulvar cancer [1] and penile cancer [2], and plays a decisive role in their treatment. Due to the high incidence of inguinal lymph node metastasis in penile cancer, up to 25 % of individuals with impalpable lymph nodes may have micrometastases, and the European Association of Urology (EAU) recommends removal of the primary lesion and inguinal lymph nodes to improve survival [3]. In early vulvar cancer (IB-II), inguinofemoral lymphadenectomy (IFLND) followed by postoperative adjuvant therapy as needed after radical partial vulvectomy is considered the standard treatment [4]. Nevertheless, patients with vulvar cancer in the early stage who underwent IFLND had a high risk of postoperative morbidity, which correspondingly impacted quality of life [[5], [6], [7]]. Sentinel lymph node biopsy (SLNB), which reduces surgical complication rates while maintaining accurate surgical staging results, has emerged as an alternative technique for surgically staging early-stage gynecological cancers such as endometrial, cervical and vulvar cancers [[8], [9], [10]]. In early vulvar cancer, SLNB presented high accuracy in identifying metastatic lymph nodes [11,12]. Compared with IFLND, SLNB has demonstrated a reduction in the occurrence of lymphedema, wound infection and dehiscence while not compromising groin recurrence rates or survival rates [10,13]. In the present study, we focused on the effective management of regional lymph nodes in patients with vulvar cancer.
The findings of the GROINSS-V and GOG-173 trials revealed less commonly occurring short-term and long-term surgical morbidities, such as poor wound healing, cellulitis and lymphedema, in the SLNB group. In parallel, no significant differences were observed in groin recurrence, 3-year overall survival (OS) or progression-free survival (PFS) between SLNB and IFLND patients [10,11]. Supported by the aforementioned results and an increasing number of clinical trials, SLNB in patients with early vulvar cancer has been recommended by the National Comprehensive Center Network (NCCN) Guidelines as an alternative surgical option for IFLND since 2016 [[14], [15], [16], [17]].
Lymphatic tracers play an essential role in SLNB, and their efficacy, safety, acceptability and accessibility should be considered when choosing the appropriate tracer. Technetium-99 m (99mTc) nanocolloid combined with blue dye is the canonical approach for SLN mapping recommended by the NCCN guidelines [4]; however, its limitations, such as radioactive pollution and allergic reactions, as well as complex preoperative preparations, can affect both the patients and medical workers. The application of either canonical tracers or newly developed indocyanine green (ICG) in SLNB depends on highly expensive imaging technology. An efficient and cost-effective lymphatic tracer that spares patients from complicated preoperative procedures is highly desirable.
In this study, for the first time, we introduced a novel tracer, carbon nanoparticles (CNPs), which have been applied in other malignant diseases, for use in treating vulvar cancer. After being intraoperatively injected around the tumor, the CNPs accumulate in the lymph nodes through the lymphatic vessels and stain the nodes black independent of any imaging technique [18]. Studies on the viability of CNPs in gastric, thyroid, breast and cervical cancers have shown considerable SLN detection rates, as well as high sensitivity and negative predictive value [[19], [20], [21], [22]]. In addition to their ability to serve as a tracer independent of auxiliary devices, the real-time staining and nonradioactivity of CNPs make them an effective option for patients with early vulvar cancer, as they outperform ICG and radiocolloids because of their easy and convenient identification. The application of CNPs in vulvar cancer is a field worthy of intensive research. The aim of this study was to explore the feasibility and validity of CNPs in evaluating the SLN detection rate in patients with early vulvar cancer.
2. Materials and methods
2.1. Patients
We retrospectively retrieved the electronic database and collected the medical records of patients who were pathologically diagnosed with vulvar cancer and underwent SLNB or IFLND at the Department of Obstetrics and Gynecology from January 2016 to April 2022. The experimental protocol was endorsed by the Ethics Committee of the First Affiliated Hospital, Army Medical University, PLA. Approval No. (B) KY2022185. The work was conducted in accordance with the STROCSS criteria.
In this retrospective study, patients who met the following criteria were included: diagnosis of unifocal vulvar cancer (T1b/2, squamous cell carcinoma, less than 4 cm in diameter, with a depth of invasion greater than 1 mm) and no suspicious inguinofemoral lymph nodes indicated by physical or imaging examination. The exclusion criteria were as follows: incomplete medical records, loss to follow-up, a history of gynecological cancer, severe underlying disease of the kidney, cardiac or pulmonary disease, previous severe allergic disorders, and complications from other malignant tumors.
In total, 52 patients were included in the current study, among whom 17 underwent sentinel lymph node biopsy and 35 underwent inguinofemoral lymphadenectomy. The median follow-up time of the 46 patients with negative sentinel or inguinal lymph nodes was 25 months (range, 4–71 months; date of final analysis: August 30, 2022).
The carbon nanoparticle detection rate was calculated by counting the black-stained lymph nodes in patients and groins. Operating time, blood loss, hospital stay, drainage time, groin drainage rate, daily drainage volume, inguinal wound healing rate, inguinal recovery time, perineal wound healing rate and postoperative complications were recorded and compared between the two groups of patients (SLNB vs. IFLND). Survival outcomes, such as overall survival and progression-free survival, were obtained by telephone follow-up.
2.2. Surgical procedure
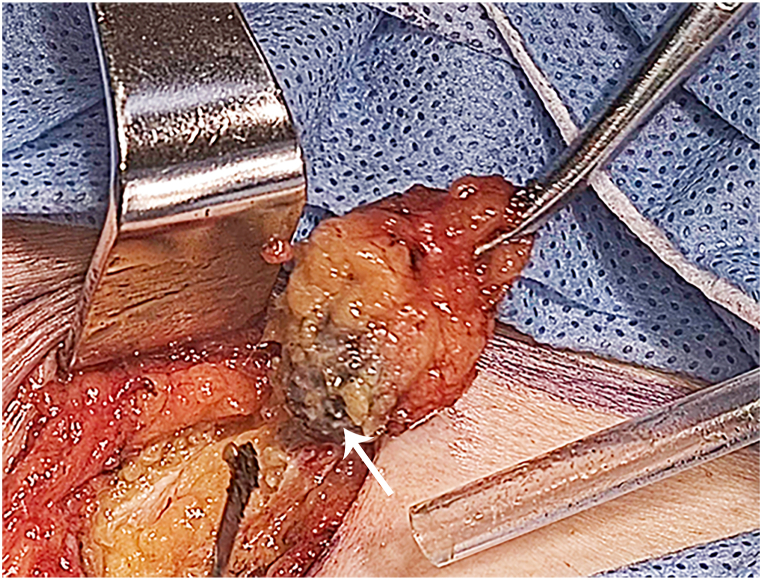
Twenty-two patients intended to undergo SLNB using CNPs as the lymphatic tracer. One milliliter of CNPs (China Food and Drug Administration approval H20041829, Lai Mei Pharmaceutical Co., Chongqing, China) was diluted with 3 ml of saline. Then, 0.5 ml of the dilution was administered intracutaneously at four injection sites at 2, 5, 7, and 10 o'clock separately around the tumor 15 min prior to surgery. After incision of the inguinofemoral skin and dissection of fatty tissue, black-stained lymph nodes could be identified by visual inspection (Fig. 1). Unilateral or bilateral SLNB was performed according to international guidelines, followed by excision of the primary vulvar tumor. Resected SLNs were sent for frozen sectioning if they were highly suspected of metastasis such that direct inguinofemoral lymphadenectomy could be performed if necessary. Patients with metastatic lymph nodes substantiated by frozen sectioning or for whom sentinel lymph nodes could not be detected underwent inguinofemoral lymphadenectomy, which was identical to the procedure performed in 30 women who chose IFLND at the start of surgery. All surgeries were performed by a team of gynecologic oncologists with many years of experience. Ultrastaging was performed as the final pathology when the routine histopathological examination results of the SLNB were negative. Postoperative therapy was conducted according to individual pathological stages and risk factors.
Fig. 1.
Black-stained sentinel lymph node tracked by carbon nanoparticles (marked by the white arrow).
2.3. Statistical analysis
Analyses were performed using Prism 9.0 software (GraphPad Software, San Diego, CA, USA) and SPSS software, version 26 (SPSS Inc, Chicago, IL). The χ2 test was adopted to analyze the differences in patient characteristics. Certain parameters, such as age and body mass index (BMI), were analyzed using the unpaired t-test. Survival analysis was conducted using the Kaplan‒Meier method. P < 0.05 was considered significant.
3. Results
3.1. Patient characteristics
From January 2016 to May 2022, fifty-two patients were enrolled in the present study according to the following inclusion criteria: 17 patients underwent SLNB, and the remaining 35 patients underwent IFLND. The baseline characteristics of the patients in the two groups are listed in Table 1. The median age, BMI, FIGO stage, tumor location, tumor diameter, depth of invasion and lymphovascular space invasion were not significantly different between patients in the two groups.
Table 1.
Patient characteristics.
| Patients' characteristics | SLNB (n = 17) | IFLND (n = 35) | p value |
|---|---|---|---|
| Median age, year (range) | 60 (48) | 55 (50) | 0.904 |
| Median body mass index, kg/m2 (range) | 24.67 (12) | 23.44 (22.1) | 0.459 |
| FIGO stage, n (%) | 0.809 | ||
| IA | 3 (17.7) | 3 (8.6) | |
| IB | 10 (58.8) | 21 (60) | |
| II | 2 (11.8) | 4 (11.4) | |
| IIIA | 2 (11.8) | 4 (11.4) | |
| IIIB | 0 | 2 (5.7) | |
| IIIC | 0 | 1 (2.9) | |
| Tumor grading, n (%) | 0.173 | ||
| 1 | 4 (23.5) | 5 (14.3) | |
| 2 | 6 (35.3) | 22 (62.9) | |
| 3 | 7 (41.2) | 8 (22.9) | |
| Negative surgical margin, n (%) | 16 (94.1) | 34 (97.1) | 0.595 |
| Depth of invasion | 0.214 | ||
| ≤ 5.0 mm | 14 (82.4) | 23 (65.7) | |
| > 5.0 mm | 3 (17.6) | 12 (34.3) | |
| Lymphovascular space invasion, n (%) | 1 (5.9) | 4 (11.4) | 0.525 |
| Location of primary tumora, n (%) | 0.506 | ||
| Midline | 14 (82.4) | 25 (71.4) | |
| Lateral | 3 (17.7) | 10 (28.6) | |
| Median tumor diameter, cm (range) | 2.5 (2.1) | 3 (2.5) | 0.256 |
| Adjuvant therapy, n (%) | 0.640 | ||
| Radiotherapy | 4 (23.5) | 8 (22.9) | |
| Chemotherapy | 2 (11.8) | 9 (25.7) |
Abbreviation: SLNB, sentinel lymph node biopsy; IFLND, inguinofemoral lymphadenectomy; CNP, carbon nanoparticle; LVSI, lymphovascular space invasion.
A lateral tumor was defined as a tumor that had a median margin located ≥1 cm from the midline. A midline tumor was defined as a tumor that had a median margin located <1 cm from the midline.
3.2. Detection rate of carbon nanoparticles in sentinel lymphatic mapping
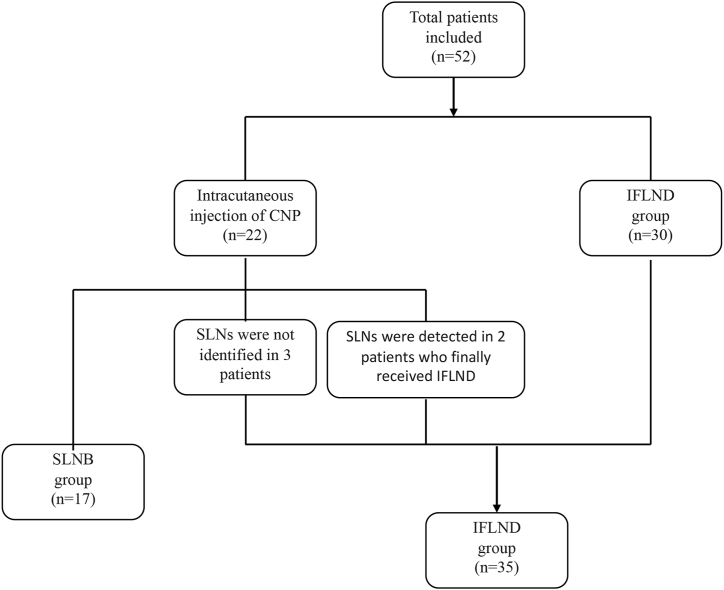
A total of 22 eligible patients with vulvar cancer who underwent sentinel lymph node biopsy were included. Carbon nanoparticles were administered peritumorally via intracutaneous injection. Black-stained lymph nodes were identified in thirty-two groins of nineteen patients in total (40 groins were excised in total from the 22 patients). The CNP detection rates by person and by groin were 86.4 % and 80 %, respectively. No allergic reactions were observed in any of the patients who received intracutaneous injections of CNPs. Among the 19 patients, SLNB was consequently performed on 17 patients, 11 patients for bilateral SLN biopsy and six patients for unilateral SLN biopsy. Additionally, one patient underwent bilateral IFLND following intraoperative frozen section confirmation of metastatic SLN. In another patient, black-stained lymph nodes were not detected initially in the bilateral groin until IFLND was performed. A flowchart of the surgical process is shown in Fig. 2.
Fig. 2.
Flow chart of patients who underwent SLNB or IFLND.
3.3. Perioperative outcomes between SLNB and IFLND
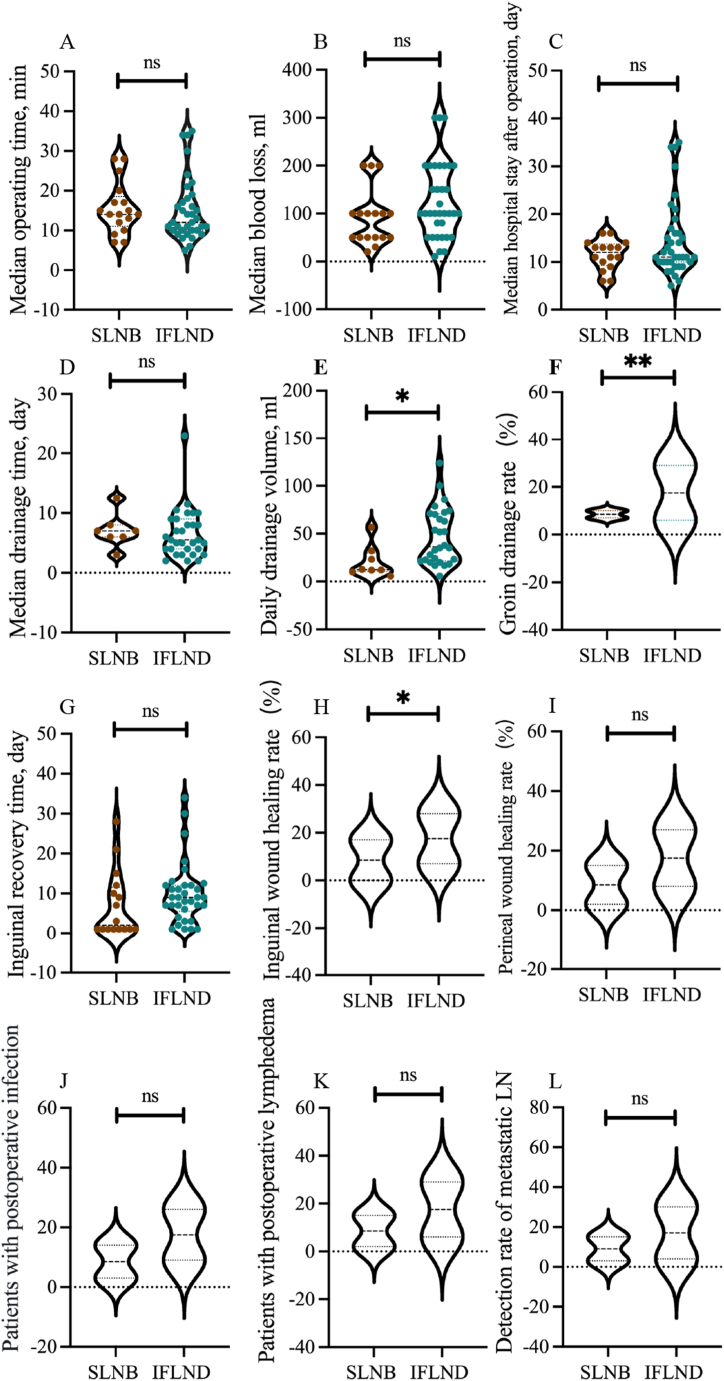
The perioperative outcomes are presented in Fig. 3. No prominent differences were observed in terms of operating time, blood loss, hospital stay, drainage time, inguinal recovery time, perineal wound healing rate or postoperative complications (Fig. 3A–D, G, I–K). The median operating time of the SLNB patients was slightly shorter than that of the IFLND patients; however, the difference was not significant, likely due to the small sample size and the start-up stage of CNP application. Similarly, we attributed the longer median hospital stay and drainage time of the SLNB patients to the sample size and marked individual variance. Remarkably, the daily drainage volume (ml) and groin drainage rate of the SLNB group were significantly lower than those of the IFLND group (12.5 vs. 36.4, p < 0.05; 41.2 % vs. 80 %, p < 0.05), and the inguinal wound healing rate was greater in the SLNB group (100 % vs. 80 %, p < 0.05), which underlines the advantage of the minimally invasive SLNB procedure (Fig. 3E, F, H). Theoretically, the inguinal recovery time and postoperative complication rate of the SLNB group should have been lower than those of the IFLND group as demonstrated in numerous studies; however, this was not the case in our study. In addition to the sample size, possible explanations for this finding, such as differences in surgical approaches, should be considered. A portion of the inguinofemoral lymphadenectomies (72.2 %) were performed laparoscopically, and heterogeneity caused by different surgical procedures might be one of the reasons for the unsatisfactory results of our study. In the SLNB group, micrometastases and macrometastases were found in three groins, whereas IFLND revealed metastases in 4 groins. The positive metastasis detection rates of the two surgical procedures were 16.7 % and 11.8 %, respectively (Fig. 3L); however, the difference between groups was not statistically significant (p = 0.682).
Fig. 3.
Perioperative outcomes of SLNB and IFLND; A. Median operating time (min) of the two groups; B. Median blood loss (ml) of the two groups; C. Median hospital stay after operation (day) of the two groups; D. Median drainage time (day) of the two groups; E. Daily drainage volume (ml) of the two groups; F. Groin drainage rate (%) of the two groups; G. Inguinal recovery time (day) of the two groups; H. Inguinal wound healing rate (%) of the two groups; I. Perineal wound healing rate (%) of the two groups; J. Postoperative infection rate (%) of the two groups; K. Postoperative lymphedema rate (%) of the two groups; L. Detection rate of the metastatic LN (lymph node).
3.4. Survival outcomes of patients with negative lymph nodes in the two groups
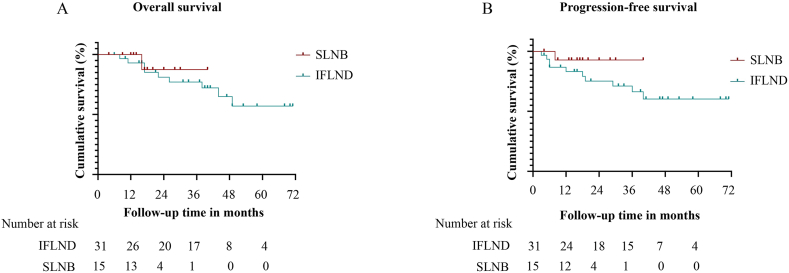
The median follow-up time of the 46 patients with negative sentinel or inguinal lymph nodes was 25 months (range, 4–71 months). In the SLNB group, groin recurrence was observed in one patient 6 months after the operation, and another patient died of cerebral hemorrhage. In the IFLND group, 10 patients experienced disease progression, three experienced groin recurrence, five experienced local recurrence, and two experienced distant metastasis; of these, nine died of disease. The recurrence rate and mortality could not be directly compared due to the inconsistent follow-up time between the two groups. Fig. 4 shows the overall and progression-free survival curves for patients without positive sentinel or inguinal lymph nodes in the two groups. No significant difference was observed (HR 0.545, 95 % CI [0.104 − 2.844], p = 0.527 for OS (overall survival) (Fig. 4A); HR 0.311, 95 % CI [0.078 − 1.231], p = 0.244 for PFS (progression-free survival)) (Fig. 4B), but SLNB seemed to be associated with a better prognosis.
Fig. 4.
Follow-up of patients with negative lymph nodes in the two groups; A. Kaplan–Meier curves of the different groups in relation to OS; B. Kaplan–Meier curves of the different groups in relation to PFS.
4. Discussion
To our knowledge, this is the first clinical study demonstrating the application of CNPs in SLNB for treatment of early vulvar cancer. CNP-guided SLN mapping was feasible with no incidence of side effects. Moreover, a fair detection rate of 86 % was achieved with CNP guidance alone. Among the perioperative outcomes of patients who underwent SLNB and IFLND, a lower grain drainage rate and daily drainage volume and a superior inguinal wound healing rate were achieved in the SLNB group. Furthermore, no significant difference in OS or PFS was observed in node-negative patients between the two groups in our investigation, which is consistent with results of a former study that investigated the safety of SLNB in vulvar cancer [23].
Sentinel lymph node biopsy, which has been applied in vulvar cancer since 1994 [24], is generally guided by radioactive tracers and blue dyes [10]. As summarized by a meta-analysis, the SLN detection rate in vulvar cancer was 56–75 % for blue stain alone, 85 % for 99mTc alone, and 87 % for 99mTc combined with blue dye [25]. Recently, data showed that ICG performed as well as 99mTc alone in SLNB for vulvar cancer, with a detection rate of 87.5 % [26]. Although a satisfactory effect has been exhibited by canonical tracers, the following deficiencies pose challenges to clinical workers: (a) side effects such as allergic reactions; (b) a blue-stained surgical field and time constraint of blue dye delivery; (c) radioactive contamination of 99mTc for both patients and surgeons; (d) fluorescent leakage of ICG after excision of the first SLN and (d) high dependence on costly devices. In this study, we highlighted the potential of CNPs as promising tracers free from complicated preoperative processes or dependence on auxiliary equipment.
CNPs have previously been confirmed as a respectable lymphatic tracer in gastric, thyroid, breast and cervical cancers, with SLN detection rates ranging from 74.7 % to 99.59 % [[19], [20], [21], [22]]. As reported in papillary thyroid cancer, CNPs contribute to reflecting the metastatic condition of the central neck and have shown potential in protecting the parathyroid glands, in addition to detecting lymph nodes [27]. For breast cancer, a recent systematic review enrolling 33 studies showed that the pooled sensitivity and specificity of CNPs in sentinel lymph node biopsy were 0.93 and 0.99, respectively, supporting the notion that CNPs could be exploited to identify true-positive patients with SLN metastases and exclude false negatives [28]. In addition, CNPs can even reveal the status of SLNs following neoadjuvant chemotherapy (NAC) and are expected to help replace axillary lymph node dissection following NAC in breast cancer patients [29].
In the present study, an encouraging CNP-based SLN detection rate (86 %) was obtained, suggesting its feasibility and quality as an SLN tracer for vulvar cancer. Due to their molecular diameter and lymphatic tropism, CNPs are transported to lymphatic vessels after being engulfed by macrophages and accumulate in the lymph nodes, resulting in black staining of the nodes in a manner that can be detected with the naked eye [19]. Because they are unable to enter capillaries, side effects were not observed in our study or in previous reports, which lays the foundation for their biosecurity. The biological properties of CNPs account for the shortcomings of canonical SLN tracers in the following aspects: (a) no allergic reactions were observed or reported; (b) intraoperative real-time staining dispensed with the need for complex preoperative preparation or imaging techniques; (c) the surgical field remained clear, as only the lymph nodes were stained; (d) patients and surgeons are protected from radiation exposure; (e) the economic burden of patients may be alleviated; (f) surgical planning can be more flexible and (g) they have been extensively applied in breast, gastric, thyroid, and in recent years, gynecological cancers such as cervical and endometrial cancers. Whether CNPs outperform traditional tracers as an alternative option for tracking SLNs should be verified in a comparative cohort study.
The intention of conducting SLNB for vulvar cancer patients with negative lymph nodes is to reduce postoperative morbidities and improve patient prognosis [10,13]. Here, we compared the perioperative outcomes and tracked the survival of node-negative patients. Neither OS nor PFS significantly differed between the two groups, which is consistent with the conclusions of previous studies [10,13], but neither the median OS nor PFS was reached in our research due to the short follow-up time. Notably, the consistency of the survival outcomes of CNP- and other accredited tracer-guided SLNBs might suggest that CNPs can accurately identify true sentinel lymph nodes.
Our study revealed a satisfactory detection rate of carbon nanoparticles and equivalent survival outcomes for patients who underwent CNP-guided SLNB. However, the statistical authenticity of our study is limited by its retrospective nature, small sample size and the relatively short and inconsistent follow-up time for the two groups. The CNP injection protocol was based on the empirical intradermal injection of canonical tracers in vulvar cancer. Approaches to the appropriate use of CNPs remain to be explored. A prospective study with a large sample size, i.e., a well-designed multicenter cohort study, is required for a comprehensive analysis of the efficacy of CNPs. Whether CNPs outperform other tracers will be demonstrated by comparison with canonical tracers. Moreover, the negative predictive value, false-negative rate, and sensitivity of CNPs for the detection of vulvar cancer will be validated in a subsequent study.
5. Conclusion
This is the first clinical study concentrating on the feasibility and validity of CNPs in tracking SLNs for early vulvar cancer. Results confirmed the detection efficiency of CNPs in identifying “true sentinel lymph nodes” from the uncompromised survival of patients who underwent CNP-guided SLNB. In short, independent of an imaging device, a credible SLN detection rate was achieved conveniently and with considerable biosecurity, indicating that CNPs are worthy of further utilization in vulvar cancer.
Ethics statement
The experimental protocol was approved by the Ethics Committee of the First Affiliated Hospital, Army Medical University. Approval No. (B) KY2022185.
Funding statement
None.
Additional information
No additional information is available for this paper.
Data availability statement
The data will be provided upon reasonable request.
CRediT authorship contribution statement
Jiahong Jiang: Writing – original draft, Formal analysis, Data curation. Shuai Tang: Supervision, Methodology, Data curation, Conceptualization. Yudi Li: Supervision, Methodology, Investigation. Yin Chen: Investigation, Formal analysis, Data curation. Xiaoxia Chen: Supervision, Formal analysis, Data curation. Maorui Jiang: Supervision, Data curation. Li Deng: Writing – review & editing, Supervision, Methodology, Conceptualization. Yanzhou Wang: Writing – review & editing, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Li Deng, Email: denglitmmu@163.com.
Yanzhou Wang, Email: w.y.z@foxmail.com.
References
- 1.Woelber L., Mahner S., Voelker K., et al. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009;29(2):545–552. [PubMed] [Google Scholar]
- 2.Srinivas V., Morse M.J., Herr H.W., Sogani P.C., Whitmore W.F., Jr. Penile cancer: relation of extent of nodal metastasis to survival. J. Urol. 1987;137(5):880–882. doi: 10.1016/s0022-5347(17)44281-9. [DOI] [PubMed] [Google Scholar]
- 3.Sachdeva A., McGuinness L., Zapala L., et al. Management of lymph node-positive penile cancer: a systematic review. Eur. Urol. 2023;85(3):257–273. doi: 10.1016/j.eururo.2023.04.018. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . NCCN; Fort Washington: 2022. NCCN Clinical Practice Guidelines in Oncology: Vulvar Cancer (Squamous Cell Carcinoma) (Version 1.2022)[EB/OL]http://www.nccn.org/ 2022-10-07. [Google Scholar]
- 5.Burger M.P., Hollema H., Emanuels A.G., Krans M., Pras E., Bouma J. The importance of the groin node status for the survival of T1 and T2 vulval carcinoma patients. Gynecol. Oncol. 1995;57(3):327–334. doi: 10.1006/gyno.1995.1151. [DOI] [PubMed] [Google Scholar]
- 6.Hacker N.F., Leuchter R.S., Berek J.S., Castaldo T.W., Lagasse L.D. Radical vulvectomy and bilateral inguinal lymphadenectomy through separate groin incisions. Obstet. Gynecol. 1981;58(5):574–579. [PubMed] [Google Scholar]
- 7.Bell J.G., Lea J.S., Reid G.C. Complete groin lymphadenectomy with preservation of the fascia lata in the treatment of vulvar carcinoma. Gynecol. Oncol. 2000;77(2):314–318. doi: 10.1006/gyno.2000.5790. [DOI] [PubMed] [Google Scholar]
- 8.Agusti N., Viveros-Carreno D., Grillo-Ardila C., et al. Sentinel lymph node detection in early-stage ovarian cancer: a systematic review and meta-analysis. Int. J. Gynecol. Cancer. 2023;33(10):1493–1501. doi: 10.1136/ijgc-2023-004572. [DOI] [PubMed] [Google Scholar]
- 9.Wess B., Kohler C., Plaikner A., et al. Comparative study using indocyanine green and patent blue dye for sentinel lymph node biopsy in patients with early-stage cervical cancer. Int. J. Gynecol. Cancer. 2024;34(5):675–680. doi: 10.1136/ijgc-2023-005206. [DOI] [PubMed] [Google Scholar]
- 10.Van der Zee A.G., Oonk M.H., De Hullu J.A., et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2008;26(6):884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 11.Levenback C.F., Ali S., Coleman R.L., et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2012;30(31):3786–3791. doi: 10.1200/JCO.2011.41.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebruers J., Elst L., Baldewijns M., et al. Accuracy of dynamic sentinel lymph node biopsy for inguinal lymph node staging in cN0 penile cancer. EJNMMI Res. 2023;13(1):62. doi: 10.1186/s13550-023-01013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Te Grootenhuis N.C., van der Zee A.G., van Doorn H.C., et al. Sentinel nodes in vulvar cancer: long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol. Oncol. 2016;140(1):8–14. doi: 10.1016/j.ygyno.2015.09.077. [DOI] [PubMed] [Google Scholar]
- 14.Woelber L., Eulenburg C., Grimm D., et al. The risk of contralateral non-sentinel metastasis in patients with primary vulvar cancer and unilaterally positive sentinel node. Ann. Surg Oncol. 2016;23(8):2508–2514. doi: 10.1245/s10434-016-5114-6. [DOI] [PubMed] [Google Scholar]
- 15.Cham S., Chen L., Burke W.M., et al. Utilization and outcomes of sentinel lymph node biopsy for vulvar cancer. Obstet. Gynecol. 2016;128(4):754–760. doi: 10.1097/AOG.0000000000001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network . NCCN; Fort Washington: 2016. NCCN Clinical Practice Guidelines in Oncology: Vulvar Cancer (Squamous Cell Carcinoma) (Version 1.2016)[EB/OL]http://www.nccn.org/ 2016-01-14. [Google Scholar]
- 17.Rottmann M., Beck T., Burges A., et al. Trends in surgery and outcomes of squamous cell vulvar cancer patients over a 16-year period (1998-2013): a population-based analysis. J. Cancer Res. Clin. Oncol. 2016;142(6):1331–1341. doi: 10.1007/s00432-016-2135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Huang Y., Yang C., et al. Application of a carbon nanoparticle suspension for sentinel lymph node mapping in patients with early breast cancer: a retrospective cohort study. World J. Surg. Oncol. 2018;16(1):112. doi: 10.1186/s12957-018-1414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ya X., Qian W., Huiqing L., et al. Role of carbon nanoparticle suspension in sentinel lymph node biopsy for early-stage cervical cancer: a prospective study. BJOG. 2021;128(5):890–898. doi: 10.1111/1471-0528.16504. [DOI] [PubMed] [Google Scholar]
- 20.Yang S.X., Wei W.S., Jiang Q.H., et al. Analysis of 246 sentinel lymph node biopsies of patients with clinical primary breast cancer by application of carbon nanoparticle suspension. J. Obstet. Gynaecol. Res. 2018;44(6):1150–1157. doi: 10.1111/jog.13635. [DOI] [PubMed] [Google Scholar]
- 21.Yu W., Xu G., Sun J., Zhong N. Carbon nanoparticles guide contralateral central neck dissection in patients with papillary thyroid cancer. Oncol. Lett. 2018;16(1):447–452. doi: 10.3892/ol.2018.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong Q., Wang Y., Wang J.J., et al. [Application of lymph node labeling with carbon nanoparticles by preoperative endoscopic subserosal injection in laparoscopic radical gastrectomy] Zhonghua Yixue Zazhi. 2017;97(2):123–126. doi: 10.3760/cma.j.issn.0376-2491.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Klapdor R., Hillemanns P., Wolber L., et al. Outcome after sentinel lymph node dissection in vulvar cancer: a subgroup analysis of the AGO-CaRE-1 study. Ann. Surg Oncol. 2017;24(5):1314–1321. doi: 10.1245/s10434-016-5687-0. [DOI] [PubMed] [Google Scholar]
- 24.Virarkar M., Vulasala S.S., Daoud T., Javadi S., Lall C., Bhosale P. Vulvar cancer: 2021 revised FIGO staging system and the role of imaging. Cancers. 2022;14(9) doi: 10.3390/cancers14092264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covens A., Vella E.T., Kennedy E.B., Reade C.J., Jimenez W., Le T. Sentinel lymph node biopsy in vulvar cancer: systematic review, meta-analysis and guideline recommendations. Gynecol. Oncol. 2015;137(2):351–361. doi: 10.1016/j.ygyno.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Siegenthaler F., Imboden S., Knabben L., Mohr S., Papadia A., Mueller M.D. Exploratory study of the clinical value of near-infrared sentinel lymph node mapping with indocyanine green in vulvar cancer patients. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.652458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu W., Cao X., Xu G., et al. Potential role for carbon nanoparticles to guide central neck dissection in patients with papillary thyroid cancer. Surgery. 2016;160(3):755–761. doi: 10.1016/j.surg.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y., Li J., Chen B., et al. Sentinel lymph node biopsy mapped with carbon nanoparticle suspensions in patients with breast cancer: a systematic review and meta-analysis. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.818812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei N., Hou J., Chen J., et al. Sentinel lymph node biopsy with carbon nanoparticle suspension after neoadjuvant chemotherapy for breast cancer patients. Ann. R. Coll. Surg. Engl. 2021;103(10):752–756. doi: 10.1308/rcsann.2021.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be provided upon reasonable request.