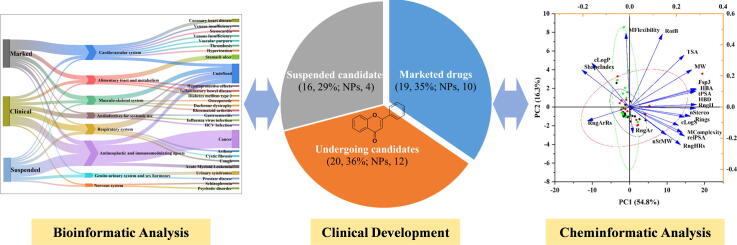
Graphical abstract
Keywords: Semi-synthetic flavonoid, Flavone, Flavonol, Flavan, Chemometrics
Highlights
-
•
This is the most systematic review of flavonoid drugs and clinical candidates to date.
-
•
19 flavonoid drugs are approved for the market, while 36 candidates in clinical phases.
-
•
52.6% of the marketed flavonoid drugs and 44.4% of clinical candidates are natural sources.
-
•
30% of the marketed flavonoid drugs are used for the treatment of cardiovascular diseases.
-
•
50% of the suspended clinical candidates are antineoplastic and immunomodulating agents.
-
•
Unexpectedly, flavonoid glycosides account for 36.8% of the marketed flavonoid drugs.
Abstract
Background
Flavonoids are one of the most important metabolites with vast structural diversity and a plethora of potential pharmacological applications, which have drawn considerable attention in the laboratory. Nevertheless, it remains uncertain how many candidates were progressed to clinical application.
Aim of Review
We carried out a critical review of natural and semi-synthetic flavonoid drugs and candidates undergoing different clinical phases worldwide by applying an adequate search method and conducted a brief cheminformatic and bioinformatic analysis. It was expected that the obtained results might narrow the screening scope and reduce the cost of drug research and development.
Key scientific Concepts of Review
To our knowledge, this is the most systematic summarization of flavonoid-based drugs and clinical candidates to date. It was found that a total of 19 flavonoid-based drugs have been approved for the market, and of these, natural flavonoids accounted for 52.6%. Besides, a total of 36 flavonoid-based clinical candidates are undergoing or suspended in different phases, and of these, natural flavonoids account for 44.4%. Thus, natural flavonoids remain the best option for finding novel agents/active templates, and when investigated in conjunction with synthetic chemicals and biologicals, they offer the potential to discover novel structures that can lead to effective agents against a variety of human diseases. Additionally, flavonoid-based marketed drugs have been successful in cardiovascular treatment, and the related drugs account for more than 30% of marketed drugs. However, the use of flavonoids as antineoplastic and immunomodulating agents is not likely for approximately 50% of the candidates suspended in the clinical stage. Interestingly, the marketed drugs covered a broader range of chemical spaces based on size, polarity, and three-dimensional structure compared to the clinical candidates. In addition, flavonoid glycosides with poor oral bioavailability account for 36.8% of the marketed drugs, and thus, they could be thoroughly investigated.
Introduction
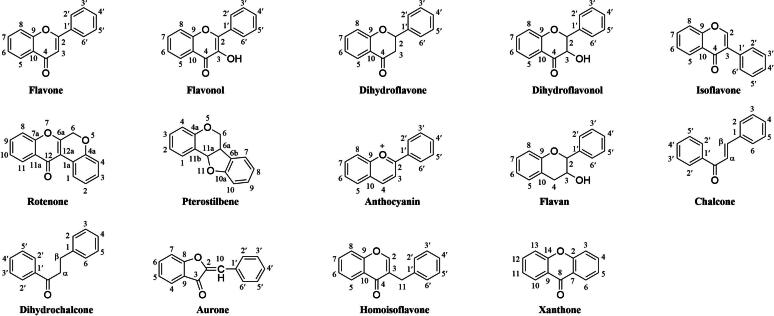
Flavonoids are a large group of natural metabolites that are especially abundant in plants. The first known use of the word ‘flavonoid’ was in 1947, and it generally referred to a large group of flavones and related analogs possessing only the C6-C3- fragment (2-phenyl chromogen ketone) [1], [2], [3]. Since 1952, flavonoids have been designated by the broader term ‘C6-C3-C6′ compounds, which have a basic 15-carbon flavone skeleton with two benzene rings (A and B) linked by a three-carbon pyran ring (C) [4]. Depending on the saturation level of the C-ring and the substitution position of the B-ring, the structure of the flavonoid compounds can be classified into 14 types of scaffolds, including flavone, flavonol, dihydroflavone, dihydroflavonol, isoflavone, rotenone, pterostilbene, anthocyanin, flavan, chalcone, dihydrochalcone, aurone, homoisoflavone, and xanthone (Fig. 1) [5], [6], [7], [8]. The different types, numbers, positions and connection methods of substituents on these ‘C6-C3-C6′ carbon skeletons produce a wide variety of structural analogs with diverse biological activities. Their properties have been used as a primary source of medicines for early drug discovery.
Fig. 1.
The 14 basic skeleton types of flavonoid-based molecules.
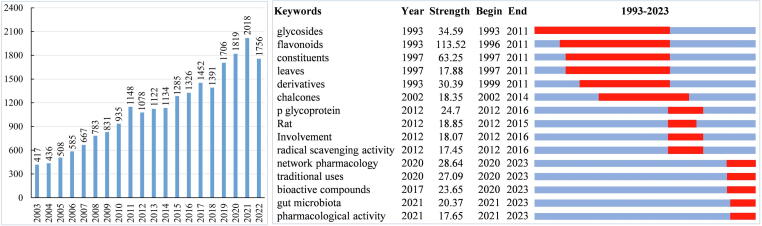
According to available statistic data, more than ten thousand different flavonoids have been reported, and a considerable number of new structures are being reported every year [9]. Herein, we conducted a literature survey on the pharmaceutical research of flavonoids in the Web of Science (WoS) Core Collection Database, which was accessed on January 11, 2023, through the Shandong University of Traditional Chinese Medicine Library [10]. The search strategy was as follows: “Topic (flavonoid), refine by Document types (article & review article) and WoS categories (Pharmacology Pharmacy & Chemistry Medicinal), and no restrictions on other conditions”. A total of 16,607 results publications on the topic of flavonoids were obtained by using the WoS literature output function and CiteSpace deduplication function. Of these publications, 14,505 were original articles, accounting for approximately 87.3 % of all retrieved publications, while 2102 were review articles, accounting for 12.7 % of all retrieved publications. The number of publications gradually increased from 1986 to 2011 and then increased rapidly from 2012 to 2022 (with an average development rate of 5.4 %) (Fig. 2A). The top keywords with the strongest citation bursts showed that more attention has been given to antioxidants and oxidative stress (Fig. 2B).
Fig. 2.
A bibliometric data of flavonoids associated to pharmaceutical studies. (Left) Annual publications from 2003 to 2022; (Right) burst detection of the top 15 keywords from 1993 to 2022.
Although there have been numerous excellent reviews that thoroughly summarize the potential therapeutic effects of flavonoid molecules on different aspects of human diseases in the past 37 years (from 1986 to 2022) [11], [12], [13], [14], [15], it remains unknown how many flavonoid analogs can be eventually developed as drug candidates and ultimately applied in clinics worldwide. As part of our ongoing investigations on natural flavonoids [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], we realized that clearly understanding the research progress of flavonoid-based drug development worldwide is imperative. Thus, our research group carried out a systematic data search through a joint professional big-data company “YAOZH” in China. Additionally, a brief cheminformatic analysis was conducted using Data Warrio and principal component analysis (PCA) [26], [27], [28]. To our knowledge, this is the most systematic summary of flavonoid drugs and clinical candidates to date.
Literature search strategy
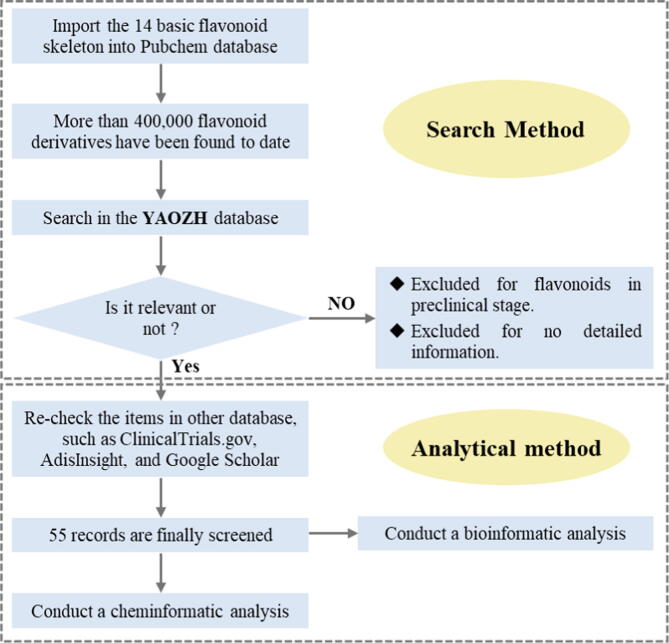
Search methods
To search the related flavonoid-based drug discovery and development thoroughly, we took the following two steps in conducting this study. First, a structured search of flavonoid-based compounds containing the 14 basic skeleton types (Fig. 1) was performed using the PubChem website (https://pubchem.ncbi.nlm.nih.gov/) through the “Draw Structure” method. After deduplication, more than 400,000 flavonoids were finally extracted. Second, key information, including the “CAS Number”, “international nonproprietary name (INN)”, and “China Approved Drug Names (CADN)” of these flavonoid-based molecules, was input into the Global Drug Database of YAOZH (https://db.yaozh.com/) to retrieve their drug development information. Notably, YAOZH is a leading open medical database in China that has a wealth of original databases and integrates data from domestic and foreign authoritative institutions to form a relatively comprehensive medical information resource, covering global drugs, drug registration, corporate reports, drug listings, and drug bids [29], [30]. The obtained results were carefully rechecked in the ClinicalTrials.gov, AdisInsight, and Google Scholar databases and are summarized in Table 1, which includes the compound name, CAS No., anatomical therapeutic chemical (ATC), therapeutic class (Indication), originator or developer, and highest phases.
Table 1.
Flavonoid-based marketed drugs (1–19) and candidates for clinical trials (or submitted to clinical trials) (20–55) undergoing or already excluded in different phases of pharmacological evaluation.
| NO. | Compound namea | CAS No. | Anatomical Therapeutic Chemical (ATC) | Therapeutic Class (Indication) | Originator or Developer b | More advanced phase of research |
|---|---|---|---|---|---|---|
| 1 | Eupatilin [36] | 22368–21-4 | Alimentary tract and metabolism | Gastric ulcer; Gastritis | Dong-A ST Co. Ltd.; Korea | Marketed |
| 2 | Diosmin [37] | 520–27-4 | Cardiovascular system | Venous lymphatic insufficiency | Servier Pharmaceutical Co., Ltd.; France | Marketed |
| 3 | Efloxate [38] | 119–41-5 | Cardiovascular system | Coronary heart disease treatment, relieve angina pectoris | Recordati S.P.A., Italy | Marketed |
| 4 | Baicalin [39], [40] | 21967–41-9 | Musculo-skeletal system | Osteoarthritis | Primus Pharmaceuticals, Inc.; United States | Marketed |
| Antiinfectives for systemic use | Chronic hepatitis B | Jiangxi Puzhong Pharmaceutical Co., Ltd.; China | Marketed | |||
| 5 | Dosmalfate [41] | 122312–55-4 | Alimentary tract and metabolism | Duodenal ulcer; esophageal dysfunction | Faes Farma Co., Ltd.; Spain | Marketed |
| 6 | Scutellarin [42] | 27740–01-8 | Cardiovascular system | Angina pectoris; stroke; coronary heart disease | Yunnan Pharmaceutical Research Institute; China | Marketed |
| 7 | Disodium flavodate [43] | 13358–62-8 | Antineoplastic and immunomodulating agents | Reduce capillary fragility and permeability | Farmaceutici Formenti S.P.A., Italy | Marketed |
| 8 | Troxerutin [44] | 7085–55-4 | Cardiovascular system | Chronic venous insufficiency | Inpharma S.A.; France | Marketed |
| 9 | Icaritin [45] | 118525–40-9 | Antineoplastic and immunomodulating agents | Liver cancer | Shenogen Pharma Group Ltd.; China | Marketed |
| 10 | Dimefline Hydrochloride [46] | 2740–04-7 | Respiratory system | Respiratory dysfunction | Recordati S.P.A., Italy | Marketed |
| 11 | Flavoxate [47] | 15301–69-6 | Genito-urinary system and sex hormones | Neuropathy; urinary incontinence; dysuria | Recordati S.P.A., Italy | Marketed |
| 12 | Hesperidin [48] | 520–26-3 | Cardiovascular system | Chronic venous insufficiency | Technological Unit of Nutrition and Health; Spain | Marketed |
| 13 | Silibinin [49], [50] | 22888–70-6 | Alimentary tract and metabolism | Liver disorders; Poisoning | Rottapharm Madaus Co., Ltd; Italy | Marketed |
| 14 | umbralisib (TGR-1202) [51] | 1532533–67-7 | Antineoplastic and immunomodulating agents | Chronic lymphocytic leukaemia | Rhizen Pharmaceuticals S.A.; Switzerland | Marketed |
| 15 | Puerarin [52] | 3681–99-0 | Cardiovascular system | Myocardial infarction | Weifang Medical University, Shandong Provincial Academy of Medical Science, etc.; China | Marketed |
| 16 | Ipriflavone [53], [54] | 35212–22-7 | Musculo-skeletal system | Osteoporosis | Takeda Pharmaceuticals Co., Ltd.; Japan | Marketed |
| 17 | Daidzein [55] | 486–66-8 | Cardiovascular system | Hypertension, coronary heart disease, cerebral thrombosis, vertigo | Liaoning Yihe Pharmaceutical Co. Ltd, China | Marketed |
| 18 | Catechin [56] | 154–23-4 | Musculo-skeletal system | Osteoarthritis | Primus Pharmaceuticals, Inc.; United States | Marketed |
| 19 | Sofalcone [57] | 64506–49-6 | Antiinfectives for systemic use | Stomach disease | Taisho Pharmaceutical Co., Ltd.; Japan | Marketed |
| 20 | Baicalein [58] | 491–67-8 | Antiinfectives for systemic use | Influenzas virus infections | CSPC Ouyi Pharmaceutical Co., Ltd.; China | Phase II |
| 21 | Wogonin [59] | 632–85-9 | Antineoplastic and immunomodulating agents | Liver cancer, breast cancer, leukemia | China Pharmaceutical University, China | Phase I |
| 22 | Alvocidib (Flavopiridol) [60], [61] | 146426–40-6 | Antineoplastic and immunomodulating agents | Acute myeloid leukaemia; Germ cell and embryonal neoplasms | Sanofi-Aventis U.S. LLC; United States | Phase II |
| 23 | TP-1287 [62] | 2044686–42-0 | Antineoplastic and immunomodulating agents | Sarcoma; Solid tumors | Tolero Pharmaceuticals Inc.; United States | Phase I |
| 24 | Voruciclib (P1446A) [63] |
1000023–04-0 | Antineoplastic and immunomodulating agents | Acute myeloid leukaemia; B-cell lymphoma | Piramal Life Sciences Co., Ltd.; India | Phase I |
| 25 | Recoflavone [64] | 203191–10-0 | Alimentary tract and metabolism | Gastritis | Dong-A St Co., Ltd.; Korea | Phase III |
| Sensory organs | Dry Eye | Dong-A St Co., Ltd.; Korea | Phase II | |||
| 26 | Oroxylin A (CPU-118) [65] | 480–11-5 | Antineoplastic and immunomodulating agents | Liver neoplasms, cellular lymphomas | China Pharmaceutical University; China | Phase I |
| 27 | Fisetin [66] | 528–48-3 | Musculo-skeletal system | Knee Osteoarthritis; Meniscus Repair | Austin V Stone; University of Kentucky; United States | Phase II |
| 28 | Quercetin [67] | 117–39-5 | Respiratory system | Chronic obstructive pulmonary disease | Internal Medicine, University of Michigan; United States | Phase II |
| 29 | Isoquercitrin [68] | 482–35-9 | Cardiovascular system | Venous thromboembolism | Quercis Pharma AG; Switzerland | Phase III |
| 30 | Pinocembrin [69] | 480–39-7 | Cardiovascular system | Ischemic stroke | CSPC ZhongQi Pharmaceutical Technology; China | Phase II |
| 31 | Naringenin [70], [71] | 93602–28-9 | Antiinfectives for systemic use | Hepatitis C Virus; HCV Infection; Chronic HCV Hepatitis C | Massachusetts General Hospital; Harvard University; United States | Phase I |
| 32 | Naringin [72] | 10236–47-2 | Respiratory system | Cough | Sun Yat-Sen University; China | Phase I |
| 33 | Tenalisib [73] | 1639417–53-0 | Antineoplastic and immunomodulating agents | Breast cancer; Chronic lymphocytic leukaemia; Non-Hodgkin's lymphoma | Rhizen Pharmaceuticals S.A.; Switzerland | Phase II |
| 34 | Genistein [74] | 446–72-0 | Respiratory system | Pulmonary fibrosis | National Institutes of Health; United States | Phase III |
| 35 | Epicatechin [75] | 490–46-0 | Musculo-skeletal system | Friedreich's ataxia | Cardero Therapeutics, Inc.; United States. | Phase II |
| 36 | Epigallocatechin [76] | 989–51-5 | Antiinfectives for systemic use | Hepatitis C virus infection | Egyptian Liver Research Institute and Hospital (ELRIAH), Egypt | Phase III |
| 37 | ABBV-2222 (GLPG2222) [77], [78] | 1918143–53-9 | Respiratory system | Cystic fibrosis | AbbVie, Inc.; United States | Phase II |
| 38 | Erteberel (LY500307) [79] | 533884–09-2 | Nervous system | Perimenopause-Related Depression | National Institute of Mental Health (NIMH); United States | Phase II |
| 39 | Elafibranor (GFT505) [80], [81] | 923978–27-2 | Alimentary tract and metabolism | Primary biliary cirrhosis | GENFIT S.A.; France | Phase III |
| 40 | Mitoflaxone (FAA) [82] | 87626–55-9 | Antineoplastic and immunomodulating agents | Cancer | Lipha Pharmaceuticals; France | Not in progress c |
| 41 | Riviciclib (P276-00) [83], [84] | 920113–02-6 | Antineoplastic and immunomodulating agents | Mantle-cell lymphoma; Solid tumors | Piramal Enterprises Limited, India | Not in progress |
| 42 | PD 98,059 [85] | 167869–21-8 | Antineoplastic and immunomodulating agents | Cancer | Pfizer Inc.; United States | Not in progress |
| 43 | Aminoflovone (AFP-464) [86], [87] | 165179–35-1 | Antineoplastic and immunomodulating agents | Breast cancer; Solid tumors | National Cancer Institute; United States | Not in progress |
| 44 | Flavodilol [88], [89] | 79619–31-1 | Cardiovascular system | Hypertension | Pennwalt Corporation; United States | Not in progress |
| 45 | Vitexin [90] | 3681–93-4 | Antineoplastic and immunomodulating agents | Radioprotective effects | Vietnam Institute of Traditional Medicine, Vietnam | Not in progress |
| 46 | Upidosin (Rec 15/2739) [91] | 152735–23-4 | Genito-urinary system and sex hormones | Benign prostatic hyperplasia | Recordati S.P.A.; Italy | Not in progress |
| 47 | Terflavoxate [92] | 86433–40-1 86433–39-8 |
Genito-urinary system and sex hormones | Urination disorders | Recordati S.P.A.; Italy | Not in progress |
| 48 | NPC-16377 [93] | 139652–86-1 | Nervous system | Neurological disorders; Psychotic disorders | Nova Pharmaceutical Corporation; United States | Not in progress |
| 49 | (±)-3′-Hydroxyfarrerol (IdB-1031) [94] | 95272–99-4 | Respiratory system | Respiratory tract disorders | Indena S.p.A.; Italy | Not in progress |
| 50 | Liquiritigenin [95] | 578–86-9 | Antineoplastic and immunomodulating agents | Menopausal hot flashes | Bionovo Inc.; United States | Not in progress |
| 51 | WS-7528 [96] | 132147–69-4 | Antineoplastic and immunomodulating agents | Cancer | Fujisawa Pharmaceutical Co. Ltd.; Japan | Not in progress |
| 52 | Silipid (IdB 1016) [97] | 134499–06-2 | Antineoplastic and immunomodulating agents | Ovarian cancer | Indena S.p.A.; Italy | Not in progress |
| 53 | Tectorigenin sodium sulfonate [98] | 807636–25-5 | Respiratory system | Acute respiratory infection and viral pneumonia | Sichuan Academy of Chinese Medicine Sciences; China | Not in progress |
| 54 | YM-26734 [99] | 144337–18-8 | Musculo-skeletal system | Rheumatoid arthritis | Yamanouchi Pharmaceutical Co. Ltd.; Japan | Not in progress |
| 55 | SU-740 [100] | 134336–72-4 | Alimentary tract and metabolism | Peptic ulcer | Taisho Pharmaceutical Co Ltd.; Japan | Not in progress |
Note: a) The compounds can be referred to by their common name, trade name, or code name; b) The research team responsible for the initial discovery or development of the pharmaceutical compounds; c) Not in progress means there are no clinical trial updates after 01/01/2015.
Analytical methods
A cheminformatic analysis was performed on the flavonoid-based drugs and clinical candidates, employing a previously established method with several modifications [31], [32], [33]. The structural and physicochemical parameters were calculated in the DataWarrior program (https://openmolecules.org/), an open-source data visualization and analysis program with embedded chemical and physical data [26], [27], including the MW (Molweight), HBD (H-Donors), HBA (H-Acceptors), cLogP [P: conc(octanol)/conc(water)], cLogS [S: water solubility in mol/L, pH = 7.5, 25℃], RotB (Rotatable Bond Count), tPSA (Topological Polar Surface Area), Fsp3 (sp3-Atom Count/Total Carbon Atom Count), RngAr (Aromatic Rings Count), TSA (Total Surface Area, SAS Approximation, van der Waals radii, 1.4A probe), relPSA (Relative Polar Surface Area, from polar and nonpolar SAS Approximation), nStereo (Stereo Center Count), nStMW (nStereo/MW), Rings (Small Rings Count), RngH (Small Ring Count with Hetero Atoms), RngHRs (RngH/Rings), RngArRs (Aromatic Ring Count/Rings), ShapeIndex (Shape Index), MFlexibility (Molecular Flexibility, low < 0.5 < high), and MComplexity (Molecular Complexity, low < 0.5 < high)) (Supplementary Material, Table S1). Finally, principal component analysis (PCA) was used to visualize the distributions of the compound sets in chemical space, which is a statistical method that allows the complete 20-dimensional dataset to be visualized on two or three unitless, orthogonal axes, each representing a linear combination of the original 20 parameters [34]. The bioinformatic analysis of these flavonoid-based drugs and clinical candidates was performed using a previously published method [35]. Drug targets were collected from the DrugBank (https://go.drugbank.com/) and TTD (https://db.idrblab.net/ttd/) databases and imported into the DAVID 6.8 database (https://david.ncifcrf.gov/) for Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis (P < 0.05). The related biological processes and signaling pathways involved in the key targets were analyzed, and the disease enrichment results were derived. The literature search was performed according to the aforementioned search criteria, and the search strategy is summarized in Fig. 3.
Fig. 3.
Flavonoid search flow in databases showing inclusion or exclusion criteria for the data found.
Drug development history
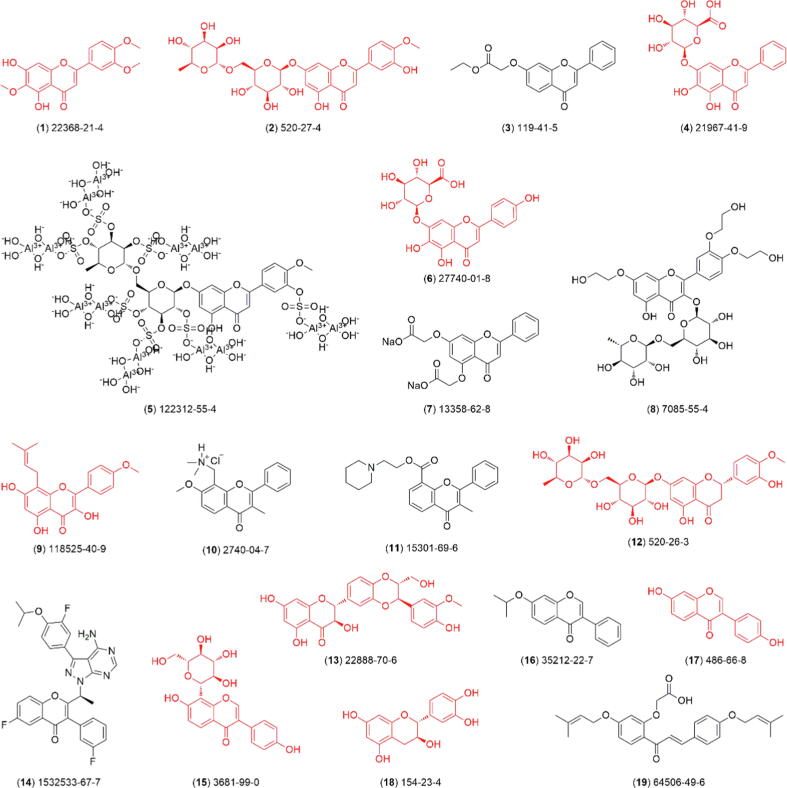
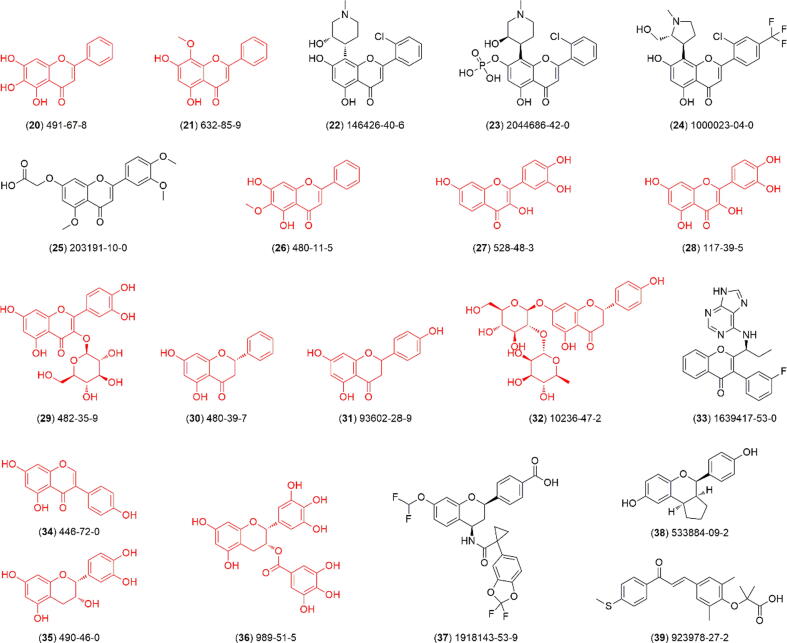
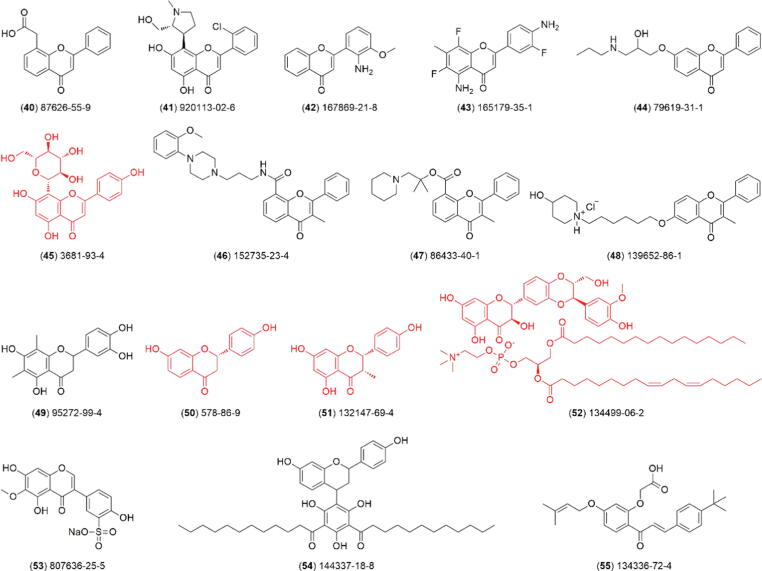
According to our retrieval strategy, a total of 55 flavonoid-based drugs and candidates are summarized in this review (Table 1). Of these, 19 have been approved for medical prescription (1–19, Fig. 4), 20 are undergoing clinical trials (20–39, Fig. 5), and 16 are not in progress in clinical research (40–55, Fig. 6).
Fig. 4.
Chemical structure of flavonoid drugs (1–19), with its CAS number, available for medical prescriptions. The ten compounds highlighted in red are of natural origin. The semi-synthetic routes for 3, 7, 8, 10, 11, 14, 16, and 19 are detailed in Scheme S1 of Supplementary Material. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Chemical structure of flavonoids (20–39) and its CAS number that are undergoing different phases of pharmacological studies. The 12 flavonoids highlighted in red are of natural origin. The semi-synthetic routes for 22–25, 33, and 37–39 are detailed in Scheme S2 of Supplementary Material. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Chemical structure of the flavonoids (40–55) that are clinical candidates suspended in different phases of pharmacological tests. The 4 compounds marked in red are of natural origin. The semi-synthetic routes for 40–44, 46–49, and 53–55 are detailed in Schemes S3 and S4 of Supplementary Material. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Flavonoid-based marketed drugs
Compound 1 (Eupatilin) is a major bioactive component of Artemisia asiatica Nakai ex Pamp. used in Korean traditional medicine that has been sold by Dong-A ST Co., Ltd. called Stillen® since 2002; it has been used for the treatment of gastric ulcer and gastritis [36]. Compound 2 (Diosmin®) was originally produced by Servier Pharmaceutical Co., Ltd. in France and is a new natural flavonoid derivative for the treatment of various hemorrhoids and chronic venous insufficiency [37]. Compound 3 (Recordil® 1956, efloxate, coronary vasodilator) and compound 10 (Remeflin® 1962, dimefline, respiratory analeptic) are registered and commercialized as new original drugs in numerous countries by Recordati S.P.A [38], [46]. In 1973, this corporation also developed a urinary anti-spasmodic compound, compound 11 (Genurin®/Urispas® and other brands, flavoxate). It was first synthesized in 1960 and developed in Italy before receiving FDA approval in the USA. It has been extensively used to treat urinary tract disorders induced by smooth muscle spasms, such as frequency of voiding, urgency to void, urine loss, suprapubic pain, pain during voiding and nocturnal voiding [47]. Primus Pharmaceuticals, Inc. of the United States previously developed an FDA-regulated medical flavonoid food product (Flavocoxid®) that is mainly composed of compounds 4 (baicalin) and 18 (catechin) and was used to treat knee osteoarthritis [39], [56]. Jiangxi Puzhong Pharmaceutical Co., Ltd. of China also developed baicalin capsules for the treatment of chronic hepatitis B [40]. Compound 5 (Dosmaltate®) was developed and commercialized by Faes Farma Co., Ltd. for the treatment of duodenal ulcers and esophageal dysfunction in 2000 [41]. Compound 6 (scutellarin®), the major component of a Chinese traditional medicine, Erigeron breviscapus (Vant.) Hand.-Mazz., was developed for the treatment of angina pectoris, stroke, and coronary heart disease by Yunnan Pharmaceutical Research Institute of China in the 1980 s [42]. Compound 7 (disodium flavodate, Pericel®, LIRCA) is a synthetic hydrosoluble derivative of natural flavonoids that was developed by Farmaceutici Formenti S.P.A. for reducing capiliary fragility and permeability [43]. Compound 8 (Troxerutin®) is a hemisynthetic flavonoid derivative of rutin that was developed by the Inpharma S.A. for the treatment of chronic venous insufficiency [44]. Compound 9 (Icaritin®, SNG-162) is a natural component isolated from Epimedium brevicornu Maxim, which was developed by Shenogen Pharma Group Ltd. of China. This drug targets the estrogen receptor subtype ER-α36 to treat liver cancer and was approved for listing on January 10, 2022 [45]. Compound 12 (hesperidin®), a dihydroflavone glycoside rich in citrus fruits, was developed by the Technological Unit of Nutrition and Health of Spain as a new drug able to reduce several CVD (cardiovascular disease) risk factors. It was found to have glucose-lowering and anti-inflammatory effects in diabetic disease models, to have dyslipidemia-, atherosclerosis-, and obesity-preventing effects in CVDs and obese models, and to have antihypertensive and antioxidant effects in hypertensive disease models [48]. Compound 13 (Silibinin®) is a low-toxicity natural dihydroflavonol that was first isolated from Silybum marianum. It is considered the main bioactive component of silymarin, which was previously developed for the treatment of liver disorders and poisoning by Rottapharm Madaus Co., Ltd. of Italy [49], [50]. Compound 14 (Umbralisib®, TGR-1202) was previously developed as an inhibitor of phosphatidylinositol 3-kinase delta (PI3K6) and casein kinase 1 epsilon (CKle) by TG Therapeutics for treating various hematological malignancies. Two years ago (2021), this compound received its first approval in the United States for treating adults with relapsed or refractory marginal zone lymphoma (MZL) [51]. Compound 15 (Puerarin injection®) was jointly developed by Changwei Medical College (Weifang Medical University) from 1981 to 1985. It can significantly reduce the myocardial oxygen consumption index, effectively limit the myocardial infarction area, and reduce the myocardial infarction expansion rate, thereby significantly reducing the hospital mortality rate (6.6 % and 16.2 % in the administration and control groups, respectively) [52]. Compound 16 (Ipriflavone®) was first synthesized by Chinoin Pharmaceutical and Chemical Works Ltd. Hungary, and it could prevent the decrease in bone mass and mineral content in experimental animal models. In 1983, this compound was developed as a therapeutic drug for the treatment of osteoporosis in Japan. [53], [54]. Compound 17 (daidzein) is the major bioactive ingredient of the nonsteroidal estrogens isolated from the traditional Chinese medicine Puerariae Radix, which was developed by Liaoning Yihe Pharmaceutical Co., Ltd., China, for treating hypertension, coronary heart disease, cerebral thrombosis, and vertigo [55]. Compound 19 (Sofalcone®) is characterized as a synthetic isopropyl flavonoid derived from the natural component “sophoradin”. Taisho Pharmaceutical Co., Ltd. of Japan previously developed it as an anti-gastric ulcer agent for increasing the amount of mucosal prostaglandin (PG) [57].
Flavonoid-based clinical candidates that are undergoing
In addition to the above approved drugs, a total of 20 flavonoid-based drug candidates are undergoing different clinical phases. Compound 20 (Baicalein) is a natural flavone identified from Scutellaria baicalensis Georgi, a commonly used traditional Chinese medicine. It could improve the symptoms of COVID-19 through various mechanisms and has been advanced to Phase II clinical development by CSPC Ouyi Pharmaceutical Co., Ltd. of China [58]. Compound 21 (wogonin) is also a natural flavone from the roots of S. baicalensis Georgi. Researchers from China Pharmaceutical University found that it could selectively kill cancer with low toxicity to normal cells at concentrations that are lethal to tumor cells. Currently, this compound has been approved for Phase I/II clinical studies by the China Food and Drug Administration (CFDA) [59]. Compound 22 (alvocidib) was developed as an inhibitor of cyclin-dependent kinase 9 (CDK9) for treating acute myeloid leukemia and has currently progressed in a Phase II study (NCT02520011). It is also being evaluated in a Phase I clinical study evaluating the maximum tolerated dose, safety and clinical activity (NCT03298984, NCT03593915). In addition, it is being evaluated in a Phase I study in patients with relapsed or refractory acute myeloid leukemia in combination with venetoclax (NCT03441555) [60], [61]. Compound 23 (TP-1287) was also developed as an investigational oral CDK9 inhibitor and has entered into a Phase I study in patients with advanced solid tumors (NCT03604783). When enzymatically cleaved, it can yield the parent drug alvocidib and showed better favorable oral bioavailability in preclinical models. [62]. Compound 24 (P1446A05) was previously synthesized as a selective CDK 4/6 inhibitor for killing multiple BRAF mutant and wild-type cell lines and is currently being evaluated in a Phase I study registered by Piramal Life Sciences Co., Ltd. of India [63]. Compound 25 (Recoavone, DA-6034) was first developed by Dong-A ST Co., Ltd. A Phase III clinical trial for this compound was previously completed (NCT01813812) in Korea for treating gastritis, and a Phase II trial (NCT01670357) for treating dry eye syndrome has also been completed. It could enhance the migration of gastric epithelial cells by activating mTOR and S6K1 downstream of PI3K [64]. Presently, there exists a dearth of information pertaining to the availability of DA-6034 within the South Korean market. However, the 2013 annual report of Dong-A Pharmaceutical reveals that Phase III clinical trials for DA-6034, specifically targeting gastritis indications, have been successfully concluded. Furthermore, the company's official website released a quarterly report in the fourth quarter of 2014, affirming the completion of Phase III trials for DA-6034 gastritis indications and projecting its imminent launch in 2015. Regrettably, the drug in question is conspicuously absent from the company's second quarter report of 2015. Compound 26 (Oroxylin) is also a natural flavone from S. baicalensis Georgi and has a reliable anticancer effect on human primary hepatocellular carcinoma cells with low toxicity. The research group of China Pharmaceutical University is currently developing this compound into a novel differentiation inducer agent for hepatoma [65]. Compound 27 (fisetin) suppressed osteoclastogenesis through the inhibition of RANKL-mediated ROS production by Nrf2-mediated upregulation of Phase II antioxidant enzymes. It was previously developed by Austin V Stone for treating knee osteoarthritis and has now entered the Phase II clinical stage [66]. Compound 28 (quercetin) is a natural flavonol and has a potential therapeutic effect on chronic obstructive pulmonary disease (COPD). Researchers from the University of Michigan found that quercetin was safely tolerated up to 2000 mg/day as assessed by lung function, blood profile and COPD assessment test questionnaire [67]. Compound 29 (isoquercetin, Kinisoquin™) is also a natural active pharmaceutical constituent and was developed as an antithrombotic agent with a significantly lower risk of adverse events than existing preparations. Quercis Pharma AG of Switzerland previously initiated two Phase III clinical studies for the prevention of venous thromboembolism (VTE) in pancreatic cancer and glioblastoma patients [68]. Compound 30 (pinocembrin) was found to have a potent protective effect on the neurovascular unit, which could induce the relaxation of rat aortic rings through an endothelium-dependent and endothelium-independent pathway. Additionally, pinocembrin could improve rat cognitive impairments induced by chronic cerebral hypoperfusion. It is promising that pinocembrin could be developed for treating ischemic stroke and has been advanced to Phase I clinical development by CSPC ZhongQi Pharmaceutical Technology of China [69]. Researchers from the Massachusetts General Hospital Center previously found that nontoxic amounts of naringenin (compound 31) reduced hepatitis C virus secretion in infected cells by 80 %. Injections of naringenin effectively reduced circulating VLDL (very low-density lipoprotein) levels in mouse plasma without toxicity [70], [71]. Compound 32 (Naringin) exhibited anti-AHR (airway hyperresponsiveness) and antitussive effects on chronic cigarette smoke exposure-induced chronic bronchitis in guinea pigs and possesses novel therapeutic potential in the treatment of chronic bronchitis, which is currently being investigated in a Phase I clinical study by Sun Yat-Sen University of China [72]. Compound 33 (tenalisib, RP6530) could modulate the tumor microenvironment, resulting in the reprogramming of tumor-associated macrophages from a protumor M2 phenotype to an antitumor M1 phenotype and a marked reduction in angiogenesis in preclinical models. It was developed as a selective PI3Kδ/g inhibitor with relapsed/refractory lymphoid malignancies in Phase I studies by Rhizen Pharmaceuticals S.A. of Switzerland [73]. Compound 34 (genistein) is a soy isoflavone that can induce the reversal of fibrosis and lung inflammation during pulmonary hypertension by mediating estrogen receptor β. It has progressed into a Phase III clinical study for treating pulmonary fibrosis by the National Institutes of Health (NIH) of the United States [74]. Compound 35 (epicatechin) could induce mitochondrial biogenesis and antioxidant metabolism in muscle fibers and neurons. A Phase II clinical study revealed that it was well tolerated over 24 weeks at up to 150 mg/d. Improvements in cardiac structure and function were observed in a subset of subjects with Friedreich's ataxia without statistically significant improvements in primary outcomes [75]. Researchers from the Egyptian Liver Research Institute and Hospital previously sponsored a Phase III open-label study for compound 36 (EGCG) and found that its incorporation interferes with viral entry mechanisms against HCV (hepatitic C virus) and in turn enhances efficacy and prevents relapse compared to the standard of care. Additionally, its anti-hemolytic and anti-fibrotic activities may improve the safety and tolerability of the therapy [76]. Compound 37 (ABBV-2222) was first developed by AbbVie, Inc. of the United States and is currently being evaluated in Phase II clinical trials as a C1-corrector for treating cystic fibrosis. In a twofold Phase Ia clinical trial, a high dose of ABBV-2222 markedly decreased sweat Cl concentrations in patients heterozygous for F508del [77], [78]. Synthetic compound 38 (LY500307) was previously developed as a highly potent and selective ERβ agonist. It showed a 12-fold higher affinity for ERβ than ERα and exhibited 32-fold more functional potency. The National Institute of Mental Health of the United States previously sponsored a Phase I clinical trial (NCT03689543) for improving estradiol-withdrawal-induced mood symptoms in women with past perimenopausal depression [79]. Compound 39 (elafibranor) was synthesized as a novel agonist of peroxisome proliferator-activated receptor-α and -δ. GENFIT S.A. of France previously sponsored an international, randomized, double-blind, Phase II clinical trial (NCT03124108). They found that it was generally safe and well tolerated. It significantly reduced the levels of alkaline phosphatase (ALP), composite endpoints of bilirubin and ALP, and other markers of disease activity in patients with primary biliary cholangitis and an incomplete response to ursodeoxycholic acid[80], [81].
Flavonoid-based clinical candidates that are suspended
In addition to the above marketed drugs and clinical candidates, 16 flavonoid-based candidates have also been suspended in clinical phases; that is, no recent clinical trials have been updated after 01/01/2015. For example, compound 40 (also named FAA, NSC 347512, LM 975, and flavone acetic acid) is a flavone synthesized by Lipha Pharmaceuticals of France. Its novel structure and unusual spectrum of activity in preclinical solid tumor models led to its introduction into clinical trials in the United States of America [82]. Compound 41 (raviciclib) is an inhibitor of several cyclin-dependent kinase (CDK) isoforms. Piramal Enterprises Limited of India previously sponsored a Phase II clinical study of P276-00 in patients with relapsed or refractory mantle cell lymphoma. Although relatively well tolerated, no desired pharmacological effects were observed for flavonoid 41 [83], [84]. Compound 42 (PD 098059) was previously identified as a noncompetitive inhibitor of mitogen-activated protein kinase kinase-1 (MAPKK1) using a MAP kinase cascade assay comprising unphosphorylated MAPKK1 and unphosphorylated MAP kinase and monitoring phosphorylation of myelin basic protein [85]. Compound 43 (AFP-464) was a 5-aminoflavone derivative originally designed to inhibit the growth of the estrogen receptor-expressing MCF7 breast cancer cell line. The National Cancer Institute of the United States previously developed a Phase I clinical study of a once-weekly aminoflavone prodrug (AFP464) in solid tumor patients (NCT00348699) [86], [87]. Compound 44 (flavodilol) is a new orally effective antihypertensive agent developed by Pennwalt Corporation of the United States. It extensively depleted catecholamines and serotonin in the heart tissue of normotensive and spontaneously hypertensive rats and has been previously advanced to Phase II clinical development [88], [89]. Compound 45 (Vitexina) is derived from Vigna radiata (L.) Wilczek, which is widely used in Vietnam for detoxification. It was developed as a potent antioxidant radioprotective agent and introduced into a series of experimental studies conducted by the research team of the Vietnam Institute of Traditional Medicine [90]. Compound 46 (Rec 15/2739) showed excellent in vivo urethral selectivity in dogs, inhibiting prostatic urethral contractions at doses much lower than those required to lower basal blood pressure or induce orthostatic hypotension. For this, Recordati S.p.A. choose it for clinical development as a novel α-1 adrenoceptor antagonist [91]. Additionally, Recordati S.p.A. previously investigated the antispasmodic effect of compound 47 (terflavoxate), a flavone derivative with spasmolytic properties in the urinary tract, by comparing it to the most common drugs utilized in the therapy of overactive detrusor [92]. Compound 48 (NPC16377) was first developed by Nova Pharmaceutical Corporation of the United States as a structurally novel and highly selective σ receptor ligand with low affinity for other neurotransmitter receptors. It was found that it exhibits a pharmacological profile similar to that previously ascribed to atypical antipsychotic drugs [93]. Compound 49 (IdB 1031) was considered as a good inhibitor of microsomal lipid peroxidation. Indena S.p.A. of Italy previously developed it as a clinical candidate for treating peroxidative damage associated with the induction of inflammatory responses and specifically with activation of a respiratory burst of leucocytes [94]. Compound 50 (liquiritigenin), a natural bioactive ingredient from Glycyrrhizae uralensis Fisch, could activate multiple ER (estrogen receptor) regulatory elements and native target genes of ER but not ER. ER selectivity was due to the selective recruitment of the coactivator steroid receptor coactivator-2 to target genes. Bionovo Inc. of the United States had previously tried to develop it as an antineoplastic and immunomodulating agent for treating menopausal hot flashes [95]. Compound 51 (WS-7528) was first extracted from cultured broth of Streptomyces sp. No. 7528. It could induce the growth of the estrogen-dependent cell line MCF-7 and showed weak anti-inflammatory activity on carrageenin rat paw edema. It has previously progressed to clinical development as an antineoplastic and immunomodulating agent by Fujisawa Pharmaceutical Co., Ltd. of Japan [96]. Compound 52 (IdB 1016) is a complex of silybin (the main active component of silymarin) and phosphatidylcholine in a molar ratio of 1:1 and was previously developed by Indena S.p.A. of Italy. This compound possesses greater oral bioavailability and therefore greater pharmacological activity than pure silybin or silymarin [97]. Compound 53 (tectorigenin sodium sulfonate) is a novel semisynthetic clinical candidate originating from the flavonoid active ingredients from Belamcanda chinensis (L) DC, which was developed for treating acute respiratory infections and viral pneumonia by the Sichuan Academy of Chinese Medicine Sciences (CN 1594308A). It had previously entered the clinical phase in China (No. 2006L00092) [98]. Compound 54 (YM-26734) was developed as a potent and competitive inhibitor of extracellular phospholipase A2 (PLA2), with selectivity for group II PLA2, by Yamanouchi Pharmaceutical Co. Ltd. Of Japan. It was found that the inhibition of group II enzyme activity could suppress inflammatory responses to 12-O-tetradecanoylphorbol-13-acetate and carragenin [99]. A research group from Taisho Pharmaceutical Co Ltd. previously synthesized a new chalcone derivative (compound 55, SU-740) with potent antiulcer activity. However, the aqueous solubility of SU-740 is very low, thus limiting the dosage form design and future clinical application [100].
Therapeutic class
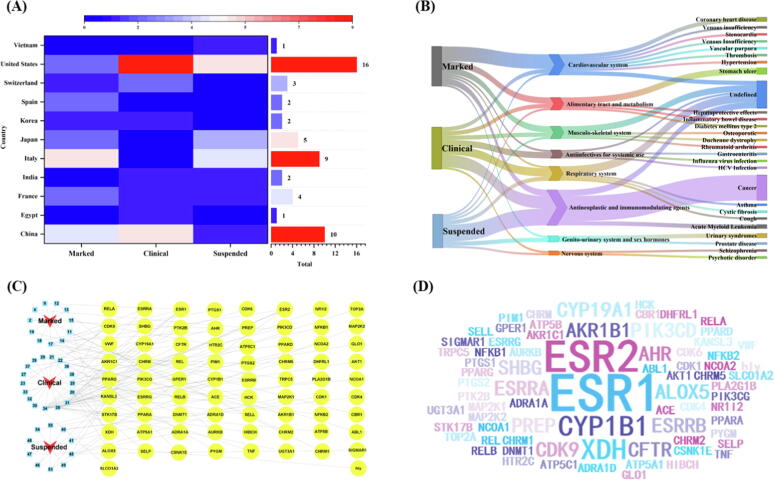
It was found that the United States, China, and Italy are the first, second and third countries, respectively, with the greatest number of flavonoid-based marketed drugs and clinical candidates. Specifically, Italy developed the largest number of marked flavonoid drugs, while the United States has the largest number of clinical candidates with the majority of international cooperation partners. The candidates of China undergoing different clinical phases are also worthy of expectation (Fig. 7A). The flavonoid-based drugs and clinical candidates were further classified based on the Anatomical Therapeutic Chemical (ATC) system adopted by World Health Organization (WHO) (Fig. 7B). In the ATC system, substances are grouped according to the organ or physiological system on which they act, based on their chemical, therapeutic or pharmacological properties. It was found that all these drugs and candidates are distributed in eight categories, including alimentary tract and metabolism, cardiovascular system, anti-infectives for systemic use, antineoplastic and immunomodulating agents, respiratory system, genitourinary system and sex hormones, musculoskeletal system, anti-infectives for systemic use, and nervous system. Of these, the class of antineoplastic and immunomodulating agents account for the largest proportion of compounds (30.9 %), and this was followed by the cardiovascular system (18.2 %). In terms of the 19 marketed drugs alone, the cardiovascular system was the most common class (36.8 %), while antineoplastic and immunomodulating agents accounted for only 15.8 % of the compounds. Notably, antineoplastic and immunomodulating agents are the most common class (50 %) among the 16 suspended clinical candidates. That is, flavonoid-based drugs have been successful in cardiovascular treatment but are not ideal for the development of anticancer drugs.
Fig. 7.
General statistics of flavonoid-based drug discovery and development. (A) The distribution of originator or developer countries. (B) Sankey diagram of ATC classification. (C) Sankey diagram of drug-target-pathway for 55 flavonoid-based marketed drugs and clinical candidates. (D) Word cloud for 55 flavonoid-based marketed drugs and clinical candidates.
A brief bioinformatic analysis showed that 55 flavonoid-based marketed drugs and clinical molecules might act on 74 key targets collected from the DrugBank and TTD databases; of these targets, ESR1, ESR2, CYP1B1, and XDH were present at the highest frequency (Fig. 7C and 7D). Most flavonoid-based molecules correspond to a single target, but a few correspond to multiple targets, such as compounds 34 (genistein) and 28 (quercetin). Performing GO and KEGG functional enrichment analyses on the target genes can shed light on their involvement in various pathological conditions such as cancers, infectious diseases, endocrine and metabolic diseases, cardiovascular diseases, among others (Supplementary Material, Figure S1). The bioinformatic analysis results were consistent with the results of the ATC system analyses.
Structural features
Structurally, the 19 marked flavonoid-based drugs could be classified as seven flavones (1–7), two flavonols (8 and 9), two 3-methylflavones (10 and 11), one dihydroflavone (12), one dihydroflavonol (13), four isoflavones (14–17), one flavan (18), and one chalcone (19). More specifically, compounds 4 and 6 are two glycosides with a glucuronic acid group, while 2, 5, 8, and 12 possess an α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranose moiety. Compound 5 is a salt of aluminum sulfate with a large molecular weight of m/z 2133.65, while compound 7 is a sodium carbonate with a small molecular weight of m/z 414.03. Compound 5 could be synthesized from hesperidin by dehydrogenation, followed by sulfonation to the intermediate aluminum free dosimarate, which is then combined with basic aluminum chloride to form an aluminum salt complex of diosmin sulfate. The structures of compounds 10, 11, and 14 have at least one nitrogen heteroatom. Furthermore, compound 10 is a kind of quaternary ammonium salt, while compounds 11 and 14 were nonsalts with a nitrogen heterocycle unit.
The 20 ongoing clinical flavonoid-based candidates are composed of seven flavones (20–26), three flavonols (27–29), three dihydroflavones (30–32), two isoflavones (33 and 34), four flavanones (35–38), and one chalcone (39). Compared to the above drugs, these candidates contain more heteroatoms in their structures (22–24, 33, 37, and 39). For instance, a piperidine ring was located at C-8 and a chlorine at the C-2 positions of both compounds 22 and 23, and the C7-OH of compound 23 was substituted by a phosphate group. Compared to the structure of 22, the substituent group at the C-8 position of 24 was a tetrahydrofuran ring, and the C4-H was replaced by a trifluoromethyl group. In the structure of compound 33, a purine group was linked at the C-2 side chain to form an ammonia bond, and a fluorine was substituted at the C-3′ position. There are four fluorine and nitrogen atoms in the structure of compound 37, while a sulfur atom is located in the structure of compound 39. In addition to the above structural features, compounds 29 and 32 could be further classified as two glycosides. The former is a glucopyranoside, while the latter possesses a moiety of α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranose.
The 16 suspended (not in progress) flavonoid-based candidates contain six flavones (40–45), three 3-methylflavones (46–48), two dihydroflavones (49, 50), one 3-methyldihydroflavone (51), one dihydroflavonol (52), one isoflavone (53), one flavan (54), and one chalcone (55). The structures of such compounds (41–44, 46–48, 52, and 53) have the most heteroatoms in comparison with those of flavonoid-based drugs and clinical candidates. For instance, a tetrahydrofuran ring and a chlorine atom were located at the C-8 and C-2′ positions of compound 41, respectively, while an amino group was substituted at the C-2′ position of compound 42. The structure of compound 43 contains three fluorine atoms at the C-6, C-8, and C-3′ positions and two amino groups located at the C-5 and C-4′ positions. Compound 44 was produced by a dehydration reaction of 3-(propylamino)propane-1,2-diol and C7-OH. There is a piperazine ring linked at the C-8 position of compound 46 in the form of amide bonds, while a piperidine ring is located at the C-8 position of compound 47 in the form of an ester bond. There is also a piperidine ring located at the C-6 side chain in the structure of compound 48, which exists in the form of quaternary ammonium salts. Compound 52 is a mixture of silibinin (13) and phosphatidylcholine. The most interesting thing about the structure of compound 53 is the C-3′ position substituted by a sodium sulfonate group.
Cheminformatic analysis
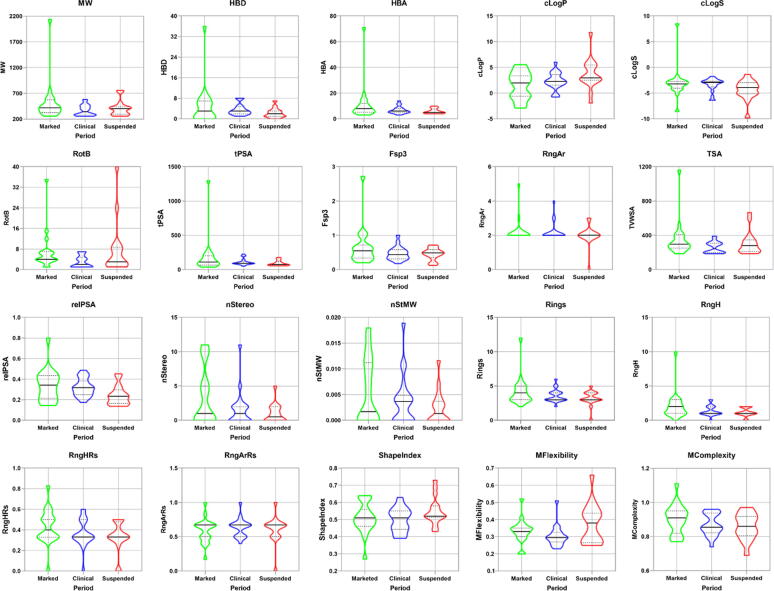
The cheminformatic analysis was conducted using methods published by Professor Derek S. Tan with several changes on structural and physicochemical parameters [31], [32], [33]. The parameters were computed using the DataWarrior software and subsequently analyzed and visualized as a violin map using the Origin software (Fig. 8 and Supplementary Material, Table S1). The parameter distribution of clinical drugs is relatively concentrated, and marketed and suspended drugs have somewhat obvious discrete values. The marketed drugs had higher median values and scope compared to suspended drugs for multiple parameters, including H-acceptors (HBA), rotatable bonds (RotB), solubility (cLogS), total surface area (TSA), and ring counts (Rings, RngH, and RngHRs). In parameters normalized for molecular size, the marketed drugs also had higher sp3 content (Fsp3) and relative polar surface area (relPSA). Conversely, the suspended drugs had higher hydrophobicity (cLogP) and fewer aromatic rings (RngAr) than the marketed drugs. Clinical drugs have higher solubility (cLogS) and stereochemical density (nStMW) than marketed and suspended drugs. Notably, the marketed drugs had the highest molecular complexity (MComplexity), while the suspended drugs had the highest molecular flexibility (MFlexibility).
Fig. 8.
Results of the cheminformatic analysis on structural and physicochemical properties of flavonoid-based marketed drugs (1–4 and 6–19) and clinical candidates (20–51 and 53–55).
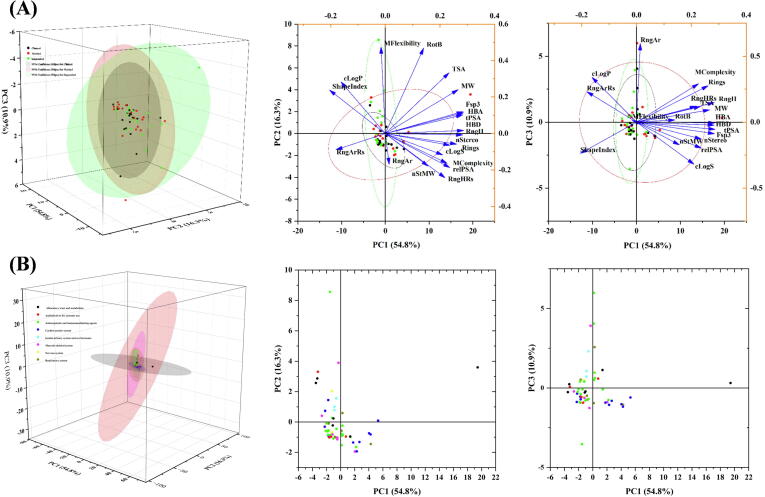
We next used principal component analysis (PCA) to visualize the distributions of the compound sets in chemical space (Fig. 9A). PCA showed that the first three principal components retained 82 % of the variance in the total dataset, and > 90 % of the variance was included in the first five main components. The load diagram showed that the parameters related to molecular size (HBA, HBD, tPSA, Fsp3, and RngH) were highly correlated in the positive direction of the PC1 axis, and the HBA along the PC1 axis was the strongest. ShapeIndex significantly contributes to the negative variance of PC1. RotB and MFlexibility contribute more significantly to the conflict of the positive direction of PC2, while RngHRs have a greater contribution to negative suggestions. RngAr greatly influences the positive direction of PC3, while cLogS greatly influences the negative direction. The PCA results showed that the extension direction of the chemical space of the clinical drugs and approved drugs is highly similar, but the extension direction of the chemical space of the suspended drugs is markedly different from that of the clinical drugs and approved drugs. Compared with approved drugs, current clinical research drugs occupy less chemical space. The positive range of marketed drugs going deep into PC1 is more remarkable, consistent with the results of a more extensive range of molecular weights of marketed drugs. The positive upward extension range of the suspended drug PC2 is larger. The suspended drug 52 extends to the positive range of PC2 (to-8), and its molecular flexibility (RotB and MFlexibility) parameters are the largest.
Fig. 9.
Results of the PCA and loading plots analysis on structural and physicochemical parameters of flavonoid-based marketed drugs (1–4 and 6–19) and clinical candidates (20–51 and 53–55).
The biological activity of a compound is influenced by its physical and chemical parameters. In this study, we conducted a PCA analysis (Fig. 9B) to explore the relationship between chemical parameters and pharmacological activity. The analysis revealed that the classes of antineoplastic and immunomodulating agents, as well as cardiovascular drugs, had the highest representation with 17 and 10 unique structures, respectively. The antineoplastic and immunomodulating agents were predominantly concentrated in the negative direction of the PC2 axis, while their distribution range was wide along the PC1 and PC3 axes. Cardiovascular drug compounds are distributed across all four quadrants of both the PC1 vs. PC2 axes. However, in the direction of PC3, there is a predominant concentration in the negative direction, indicating favorable hydrophilic properties. Antiinfectives for systemic use drugs exhibit the widest range of chemical space, which aligns with the diverse biological targets these drugs address. The alimentary tract and metabolism also exhibit a wide range along the PC1 axis due to the large size of compound 5. The genito-urinary system and sex hormones are positively distributed along the PC2 axis and demonstrate high CLogP values.
Conclusion and perspectives
Flavonoids are important natural metabolites with vast structural diversity and a plethora of interesting pharmacological effects, which have drawn considerable attention in pharmaceutical research and development. The latest studies of natural and semi-synthetic flavonoid drugs used as therapeutic agents have not been systematically summarized. Therefore, we carried out a systematic summarization of flavonoid-based drugs and candidates undergoing different clinical phases worldwide by applying an adequate search method and conducted a brief cheminformatic analysis. To our knowledge, this is the most systematic summarization of flavonoid-based drugs and clinical candidates to date. It was found that nineteen flavonoid-based drugs have been approved, and 36 candidates are undergoing or suspended in different clinical phases. Of these candidates, natural flavonoids account for 47.3 %. Thus, flavonoid-based drugs remain the best options for finding novel agents or active templates in drug development. In particular, flavonoid glycosides account for 36.8 % of the present marketed drugs. How is it possible for these glycoside drugs to be potentially developed into pharmaceuticals, even though they do not adhere to Lipinski's rule of five? It is conceivable that the impact of glycosylation on the bioactivity of flavonoids in vitro may diverge from that observed in vivo. Specifically, when administered orally, flavonoid glycosides exhibit comparable or even heightened bioactivity compared to their aglycone counterparts. Moreover, flavonoid glycosides exhibit elevated plasma concentrations and a prolonged mean residence time relative to aglycones [101]. Additionally, developing flavonoids for treating cardiovascular diseases seems to have a higher probability of success than developing them as antitumor drugs. The present review might narrow the screening scope and reduce the cost of drug research and development. Notably, we have tried our best to include all the information, but the retrieval method used in this article may also have its own limitations. If you find any inaccuracies in the relevant information, please do not hesitate to contact us.
CRediT authorship contribution statement
Kuo Xu: Conceptualization, Writing – original draft, Funding acquisition. Xia Ren: Visualization, Writing – review & editing. Jintao Wang: Investigation, Resources. Qin Zhang: Investigation, Resources. Xian-Jun Fu: Conceptualization, Supervision, Funding acquisition. Pei-Cheng Zhang: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This project was funded byShandong Provincial Natural Science Foundation (No. ZR2022YQ71 and ZR2022LZY026), NATCM's Project of High-level Construction of Key TCM Disciplines (Marine Traditional Chinese Medicine; No. zyyzdxk-2023124), Key R&D Program of Shandong Province, China (No. 2021CXGC010510), Shandong Province Key Discipline Construction Project of Traditional Chinese Medicine (Marine Chinese Medicinals), Shandong Province Traditional Chinese Medicine High-level Talent Cultivation Project, and Youth Innovation Team of Shandong University of Traditional Chinese Medicine.
Biographies

Xu Kuo is an Associate Professor of Medicinal Chemistry at Shandong University of Traditional Chinese Medicine. He obtained his degree from the Institute of Materia Medica of Peking Union Medical College in 2017 and his research primarily focuses on the active compounds found in coastal and marine plants. His selection for the National Postdoctoral Program for Innovative Talents ( Grant No. BX201700247) serves as recognition of his significant contributions to the field. To date, he has successfully led over 10 research projects and has published 35 papers as the first author or corresponding author, with 16 of these papers appearing in journals classified under the Q1 partition of the Journal Citation Reports. Additionally, he has been granted 8 patents and has authored 3 monographs.

Dr. Ren Xia, a highly accomplished academic with a Ph.D. and extensive experience as a master tutor, primarily dedicates her research efforts to the exploration of the development and utilization of Marine traditional Chinese medicine. Her research interests encompass the investigation of pharmacodynamic material basis, quality evaluation, and the development of innovative drugs derived from Marine traditional Chinese medicine. Dr. Xia has successfully led and concluded numerous scientific research projects, including the prestigious National Natural Science Foundation Youth Fund and Shandong Natural Science Foundation Fund. She has authored a total of 15 papers, with 10 of them being included in the SCI index and seven published in esteemed journals. Furthermore, one of their papers has been recognized as a highly cited paper, ranking within the top 1% according to Essential Science Indicators (ESI).

Jintao Wang is a seasoned professional in the pharmaceutical industry, having commenced his career in pharmaceutical research and development in 2010. During this period, he successfully obtained approval for clinical trials of two Class 3 generic drugs and one Class 2.2 new drug for production. In 2018, he transitioned to Chongqing Kangzhou Big data Group Co., Ltd. (YAOZH.com), where he has been actively involved in consulting activities within the pharmaceutical sector. Notably, he has led a proficient consulting team, overseeing the completion of over 60 medical consulting projects annually. In recent years, his focus has been on data-driven pharmaceutical consulting services, enabling him to deliver specialized and professional consulting support to pharmaceutical companies.

Qin Zhang, born in Chongqing, China in 1991, earned his bachelor's degree from Chongqing University of Arts and Sciences in 2014. In 2019, he joined Chongqing Kangzhou Big data Group Co., Ltd (YAOZH.com), where he specializes in the research and development of medical big data, demonstrating expertise in data value mining and application transformation.

Xianjun Fu, Ph.D., professor, and doctoral supervisor, currently serves as the deputy dean of Qingdao College of Traditional Chinese Medicine at Shandong University of Traditional Chinese Medicine. He is also a member of the international scientific organization Vebleo Fellow and serves as a guest editor for Frontiers in Pharmacology. Throughout his career, he has dedicated his research efforts to the exploration of modern drug development within the framework of traditional Chinese medicine theory. He has effectively overseen over 10 scientific research projects at the national, provincial, and ministerial levels. Moreover, he has authored and published more than 160 papers, including 2 highly cited papers ranking in the top 1% in the ESI database. Additionally, he has secured over 30 domestic and international patent authorizations, authored 8 monographs, and received the second Prize of the National Science and Technology Progress Award.

Pei-Cheng Zhang, Ph.D., is a distinguished professor of Medicinal Chemistry at Peking Union Medical College and Chinese Academy of Medical Sciences. He earned his doctoral degree in Medicinal Chemistry from Shenyang Pharmaceutical University in 1999. Since then, he has dedicated his research efforts to the comprehensive investigation of flavonoids and other natural products. His significant contributions in this field have been published in reputable scientific journals such as Angewandte Chemie-International Edition, Organic Letters, Acta Pharmaceutica Sinica B, Organic Chemistry Frontiers, Journal of Natural Products, Bioorganic Chemistry, Organic & Biomolecular Chemistry, Chinese Chemical Letters, etc. Additionally, Professor Dr. Pei-Cheng Zhang held the esteemed position of Editor in Chief for the publication “Flavonoid Chemistry” (Chemical Industry Press, the People's Republic of China).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.11.007.
Contributor Information
Xianjun Fu, Email: fuxianjun@sdutcm.edu.cn.
Pei-Cheng Zhang, Email: pczhang@imm.ac.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.IUPAC. Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. 10.1351/goldbook.
- 2.Engelkemeir D.W., Geissman T.A., Crowell W.R., Friess S.L. Flavanones and related compounds. IV. the reduction of some naturally-occurring flavones at the dropping mercury electrode. J Am Chem Soc. 1947;69:155–159. doi: 10.1021/ja01193a042. [DOI] [PubMed] [Google Scholar]
- 3.Geissman T.A., Mehlquist G.A. Inheritance in the carnation, Dianthus caryophyllus. IV. the chemistry of flower color variation, I. Genetics. 1947;32:410–433. doi: 10.1093/genetics/32.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinreiner G.E. Theories of the biogenesis of flavonoid compounds. Bot Rev. 1952;18:77–164. doi: 10.1007/BF02960588. [DOI] [Google Scholar]
- 5.Uivarosi V., Munteanu A.C., Nițulescu G.M. An overview of synthetic and semisynthetic flavonoid derivatives and analogues: perspectives in drug discovery. Stud Nat Prod Chem. 2019;60:29–84. doi: 10.1016/B978-0-444-64181-6.00002-4. [DOI] [Google Scholar]
- 6.Brodowska K. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur J Biol Res. 2017;7:108–123. doi: 10.5281/zenodo.545778. [DOI] [Google Scholar]
- 7.Shen N., Wang T., Gan Q., Liu S., Wang L., Jin B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383 doi: 10.1016/j.foodchem.2022.132531. [DOI] [PubMed] [Google Scholar]
- 8.Veitch N.C., Grayer R.J. Flavonoids and their glycosides, including anthocyanins. Nat Prod Rep. 2011;28:1626–1695. doi: 10.1039/B718040N. [DOI] [PubMed] [Google Scholar]
- 9.Gupta M., Mishra A. Bioactive Flavonoids: A comparative overview of the biogenetic and chemical synthesis approach. Mini Rev Med Chem. 2023;23 doi: 10.2174/1389557523666230214101821. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J., Liu W. A tale of two databases: the use of Web of Science and Scopus in academic papers. Scientometrics. 2020;123:321–335. doi: 10.1007/s11192-020-03387-8. [DOI] [Google Scholar]
- 11.Xue J.C., Yuan S., Meng H., Hou X.T., Li J., Zhang H.M., et al. The role and mechanism of flavonoid herbal natural products in ulcerative colitis. Biomed Pharmacother. 2023;158 doi: 10.1016/j.biopha.2022.114086. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z., Nian M., Qiao H., Yang X., Wu S., Zheng X. Review of bioactivity and structure-activity relationship on baicalein (5,6,7-trihydroxyflavone) and wogonin (5,7-dihydroxy-8-methoxyflavone) derivatives: Structural modifications inspired from flavonoids in Scutellaria baicalensis. Eur J Med Chem. 2022;243 doi: 10.1016/j.ejmech.2022.114733. [DOI] [PubMed] [Google Scholar]
- 13.Quintal Martínez J.P., Segura Campos M.R. Flavonoids as a therapeutical option for the treatment of thrombotic complications associated with COVID-19. Phytother Res. 2022;8 doi: 10.1002/ptr.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rufino A.T., Costa V.M., Carvalho F., Fernandes E. Flavonoids as antiobesity agents: A review. Med Res Rev. 2021;41:556–585. doi: 10.1002/med.21740. [DOI] [PubMed] [Google Scholar]
- 15.Ganai S.A., Sheikh F.A., Baba Z.A., Mir M.A., Mantoo M.A., Yatoo M.A. Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother Res. 2021;35:3509–3532. doi: 10.1002/ptr.7044. [DOI] [PubMed] [Google Scholar]
- 16.Yan H.W., Zhu H., Yuan X., Yang Y.N., Feng Z.M., Jiang J.S., et al. Eight new biflavonoids with lavandulyl units from the roots of Sophora flavescens and their inhibitory effect on PTP1B. Bioorg Chem. 2019;86:679–685. doi: 10.1016/j.bioorg.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 17.Gao W., Jiang J.S., Chen Z., Yang Y.N., Feng Z.M., Zhang X., et al. Stereospecific acyloin ring contraction controlled by glucose and concise total synthesis of saffloneoside. Org Chem Front. 2019;6:1858. doi: 10.1039/C9QO00279K. [DOI] [Google Scholar]
- 18.Zhang F., Yang Y.N., Song X.Y., Shao S.Y., Feng Z.M., Jiang J.S., et al. Forsythoneosides A-D, Neuroprotective phenethanoid and flavone glycoside heterodimers from the fruits of Forsythia suspensa. J Nat Prod. 2015;78:2390–2397. doi: 10.1021/acs.jnatprod.5b00372. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y.N., Zhu H., Chen Z., Liu F., An Y.W., Feng Z.M., et al. NMR spectroscopic method for the assignment of 3,5-dioxygenated aromatic rings in natural products. J Nat Prod. 2015;78:705–711. doi: 10.1021/np5008679. [DOI] [PubMed] [Google Scholar]
- 20.He J., Yang Y.N., Jiang J.S., Feng Z.M., Zhang P.C. Saffloflavonesides A and B, two rearranged derivatives of flavonoid C-glycosides with a furan-tetrahydrofuran ring from Carthamus tinctorius. Org Lett. 2014;16:5714–5717. doi: 10.1021/ol502789x. [DOI] [PubMed] [Google Scholar]
- 21.Li F., Zhan Z., Liu F., Yang Y., Li L., Feng Z., et al. Polyflavanostilbene A, a new flavanol-fused stilbene glycoside from Polygonum cuspidatum. Org Lett. 2013;15:674–677. doi: 10.1021/ol3035033. [DOI] [PubMed] [Google Scholar]
- 22.Feng Z.M., He J., Jiang J.S., Chen Z., Yang Y.N., Zhang P.C. NMR solution structure study of the representative component Hydroxysafflor Yellow A and other quinochalcone C-glycosides from Carthamus tinctorius. J Nat Prod. 2013;76:270–274. doi: 10.1021/np300814k. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J.S., He J., Feng Z.M., Zhang P.C. Two new quinochalcones from the florets of Carthamus tinctorius. Org Lett. 2010;12:1196–1199. doi: 10.1021/ol902971w. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P., Feng Z., Wang Y. Flavonoids, including an unusual flavonoid-Mg2+ salt, from roots of Cudrania cochinchinensis. Phytochemistry. 2005;66:2759–2765. doi: 10.1016/j.phytochem.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P.C., Wang S., Wu Y., Chen R.Y., Yu D.Q. Five new diprenylated flavonols from the leaves of Broussonetia kazinoki. J Nat Prod. 2001;64:1206–1209. doi: 10.1021/np010283o. [DOI] [PubMed] [Google Scholar]
- 26.López-López E., Naveja J.J., Medina-Franco J.L. DataWarrior: an evaluation of the open-source drug discovery tool. Expert Opin Drug Discov. 2019;14:335–341. doi: 10.1080/17460441.2019.1581170. [DOI] [PubMed] [Google Scholar]
- 27.Sander T., Freyss J., von Korff M., Rufener C. DataWarrior: an open-source program for chemistry aware data visualization and analysis. J Chem Inf Model. 2015;55:460–473. doi: 10.1021/ci500588j. [DOI] [PubMed] [Google Scholar]
- 28.Su M., Guo C., Liu M., Liang X., Yang B. Therapeutic targets of vitamin C on liver injury and associated biological mechanisms: A study of network pharmacology. Int Immunopharmacol. 2019;66:383–387. doi: 10.1016/j.intimp.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 29.Li X., Yu J., Zhang Z., Ren J., Peluffo A.E., Zhang W., et al. Network bioinformatics analysis provides insight into drug repurposing for COVID-19. Med Drug Discov. 2021;10 doi: 10.1016/j.medidd.2021.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S.N., Lian Y.J., Qiu S.L., Liu X.Y., Yang Y.W., Shang J.J. Compatibility rules of Chinese patent medicines with the effect of “Simultaneous Treatment of Brain and Heart Diseases”: Based on data mining. World Chinese Medicine. 2022;17:1928–1933. [Google Scholar]
- 31.Stone S., Newman D.J., Colletti S.L., Tan D.S. Cheminformatic analysis of natural product-based drugs and chemical probes. Nat Prod Rep. 2022;39:20–32. doi: 10.1039/D1NP00039J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stratton C.F., Newman D.J., Tan D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorg Med Chem Lett. 2015;25:4802–4807. doi: 10.1016/j.bmcl.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer R.A., Wurst J.M., Tan D.S. Expanding the range of ‘druggable’ targets with natural product-based libraries: an academic perspective. Curr Opin Chem Biol. 2010;14:308–314. doi: 10.1016/j.cbpa.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wenderski T.A., Stratton C.F., Bauer R.A., Kopp F., Tan D.S. Principal component analysis as a tool for library design: a case study investigating natural products, brand-name drugs, natural product-like libraries, and drug-like libraries. Methods Mol Biol. 2015;1263:225–242. doi: 10.1007/978-1-4939-2269-7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X., Guo J.Y., Li Q.Y., Wei X.J., Li J.Y., Wan G.H., et al. Study on mechanism of Valerianae Jatamansi Rhizoma et Radix against post-traumatic stress disorder based on molecular docking and network pharmacology. China Journal of Chinese Materia Medica. 2021;46(2380–2391) doi: 10.19540/j.cnki.cjcmm.20201229.401. [DOI] [PubMed] [Google Scholar]
- 36.Ryoo S.B., Oh H.K., Yu S.A., Moon S.H., Choe E.K., Oh T.Y., et al. The effects of Eupatilin (Stillen (R)) on motility of human lower gastrointestinal tracts. Korean J Physiol Pharmacol. 2014;18:383–390. doi: 10.4196/kjpp.2014.18.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Qin'e W.u., Wenxi Z.S., Zhao Jun Y.u., Hao L.D., Fei L., Renfu Z. Phase II clinical study of domestic Diosmin tablets. Jiangsu Med J. 2006;32(379–381) doi: 10.19460/j.cnki.0253-3685.2006.04.034. [DOI] [Google Scholar]
- 38.De Haen P. Current trends in the introduction of new drugs. Clin Pharmacol Ther. 1974;16:413–423. doi: 10.1002/cpt1974163part1413. [DOI] [PubMed] [Google Scholar]
- 39.Steinhilber D., Hofmann B. Recent advances in the search for novel 5-lipoxygenase inhibitors. Basic Clin Paharmacol Toxicol. 2014;114:70–77. doi: 10.1111/bcpt.12114. [DOI] [PubMed] [Google Scholar]
- 40.Wu L.L., Zhang Z.J., Xu F.G., Tian Y., Chen Y. Bioequivalence study of baicalin capsules in healthy volunteers. Chin J New Drugs Clin Rem. 2005;24:687–690. [Google Scholar]
- 41.Villegas I., Alarcón de la Lastra C., Orjales A., La Casa C. A new flavonoid derivative, dosmalfate, attenuates the development of dextran sulphate sodium-induced colitis in mice. Int Immunopharmacol. 2003;3:1731–1741. doi: 10.1016/j.intimp.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Gong B. The appraisal meeting of breviscapine, a new drug for treating paralysis, was held in Kunming. Chin Tradit Herb Drug. 1980;11:480. [Google Scholar]
- 43.Sarfati R., Brux J. Vascular fragility of the endometrium under estrogen-progestin treatment. Treatment by disodium flavodate. J Gynecol Obstet Biol Reprod. 1973;2:87–94. [PubMed] [Google Scholar]
- 44.Glacet-Bernard A., Coscas G., Chabanel A., Zourdani A., Lelong F., Samama M.M. A randomized, double-masked study on the treatment of retinal vein occlusion with troxerutin. Am J Ophthalmol. 1994;118:421–429. doi: 10.1016/S0002-9394(14)75791-5. [DOI] [PubMed] [Google Scholar]
- 45.Peng Y., Zhang W.J., Lian Z.L., Jin R., Li J.S., Meng K. The effects of Icaritin on the expression of estrogen receptor ER-α36 in endometrial cancer cell line. Chin Med Biotechnol. 2012;7:106–109. doi: 10.3969/cmba.j.issn.1673-713X.2012.02.004. [DOI] [Google Scholar]
- 46.Colding A., Krogsgaard A., Norregaard S. Dimefline, a new respiratory analeptic. pneumotachographic studies on patients with respiratory insufficiency. Acta Anaesthesiol Scand. 1963;7:97–106. doi: 10.1111/j.1399-6576.1963.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 47.Ruffmann R. A review of flavoxate hydrochloride in the treatment of urge incontinence. J Int Med Res. 1988;16:317–330. doi: 10.1177/030006058801600501. [DOI] [PubMed] [Google Scholar]
- 48.Mas-Capdevila A., Teichenne J., Domenech-Coca C., Caimari A., Del Bas J.M., Escoté X., et al. Effect of hesperidin on cardiovascular disease risk factors: the role of intestinal microbiota on hesperidin bioavailability. Nutrients. 2020;12:1488. doi: 10.3390/nu12051488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polachi N., Bai G., Li T., Chu Y., Wang X., Li S., et al. Modulatory effects of silibinin in various cell signaling pathways against liver disorders and cancer - A comprehensive review. Eur J Med Chem. 2016;123:577–595. doi: 10.1016/j.ejmech.2016.07.070. [DOI] [PubMed] [Google Scholar]
- 50.Polyak S.J., Oberlies N.H., Pécheur E.I., Dahari H., Ferenci P., Pawlotsky J.M. Silymarin for HCV infection. Antivir Ther. 2013;18:141–147. doi: 10.3851/IMP2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhillon S. Keam SJ. Umbralisib: first approval. Drugs. 2021;81:857–866. doi: 10.1007/s40265-021-01504-2. [DOI] [PubMed] [Google Scholar]
- 52.Lang X.C., Liu S.M. Puerarin injection, a new drug for the treatment of cardiovascular diseases, recently passed the technical identification in Weifang. Qilu Pharmaceutical Affairs. 1985;3:50. [Google Scholar]
- 53.Tsuda M., Kitazaki T., Ito T., Fujita T. The effect of ipriflavone (TC-80) on bone resorption in tissue culture. J Bone Miner Res. 1986;1:207–211. doi: 10.1002/jbmr.5650010207. [DOI] [PubMed] [Google Scholar]
- 54.He P., Jin Y., Ma L., Meng X.J., Zheng J., Sun L.L., et al. Ipriflavone-a new drug for osteoporosis. Chinese New Drugs Journal. 1999;8:670–672. [Google Scholar]
- 55.Liu P., Zhao Y.X., Zhang Y., Wang S.J., Jing X.W., Luo W.M., et al. Clinical observation of daidzein intervention on serum inflammatory factors in senile patients with coronary. Chinese Journal of Integrated Traditional and Western Medicine. 2006;26:42–45. [PubMed] [Google Scholar]
- 56.Levy R.M., Saikovsky R., Shmidt E., Khokhlov A., Burnett B.P. Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: a short-term randomized, double-blind pilot study. Nutr Res. 2009;29:298–304. doi: 10.1016/j.nutres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Higuchi K., Watanabe T., Tanigawa T., Tominaga K., Fujiwara Y., Arakawa T. Sofalcone, a gastroprotective drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori: a randomized controlled comparative trial with cimetidine, an H2-receptor antagonist. J Gastroen Hepatol. 2010;25:155–160. doi: 10.1111/j.1440-1746.2010.06232.x. [DOI] [PubMed] [Google Scholar]
- 58.Shan H., Du Y., Bai H., Chen J., He X., Wang Q., et al. Progress in the development of baicalein and its clinical pharmacology study. Chin J Clin Pharm Therap. 2020;25(701–8) doi: 10.12092/j.issn.1009-2501.2020.06.013. [DOI] [Google Scholar]
- 59.Li T., Weng T., Wang J., Wei Z., Zhao L., Li Z. A practical and efficient approach to the preparation of bioactive natural product Wogonin. Org Process Res Dev. 2017;21:171–176. doi: 10.1021/acs.oprd.6b00249. [DOI] [Google Scholar]
- 60.Lin T.S., Andritsos L.A., Jones J.A., Fischer B., Heerema N.A., Blum K.A., et al. Activity of the cyclin-dependent kinase (CDK) inhibitor flavopiridol in relapsed, genetically high risk chronic lymphocytic leukemia (CLL) J Clin Oncol. 2008;26:7007. doi: 10.1200/jco.2008.26.15_suppl.7007. [DOI] [Google Scholar]
- 61.Zeidner J.F., Karp J.E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk Res. 2015;39:1312–1318. doi: 10.1016/j.leukres.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 62.George B., Richards D.A., Edenfield W.J., Warner S.L., Mouritsen L., Bishop R., et al. A phase I, first-in-human, open-label, dose-escalation, safety, pharmacokinetic, and pharmacodynamic study of oral TP-1287 administered daily to patients with advanced solid tumors. J Clin Oncol. 2020;38:3611. doi: 10.1200/JCO.2020.38.15_suppl.3611. [DOI] [Google Scholar]
- 63.Diab A., Martin A., Simpson L., Algazi A.P., Chawla P., Kim D.W., et al. Phase I trial of the CDK 4/6 inhibitor, P1446A–05 (voruciclib) in combination with the BRAF inhibitor (BRAFi), vemurafenib in advanced. BRAF-mutant melanoma J Clin Oncol. 2015;33:9076. doi: 10.1200/jco.2015.33.15_suppl.9076. [DOI] [Google Scholar]
- 64.Butler M.S., Robertson A.A., Cooper M.A. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep. 2014;31:1612–1661. doi: 10.1039/C4NP00064A. [DOI] [PubMed] [Google Scholar]
- 65.Wei L., Dai Y., Zhou Y., He Z., Yao J., Zhao L., et al. Oroxylin A activates PKM1/HNF4 alpha to induce hepatoma differentiation and block cancer progression. Cell Death Dis. 2017;8:e2944. doi: 10.1038/cddis.2017.335. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Sakai E., Shimada-Sugawara M., Yamaguchi Y., Sakamoto H., Fumimoto R., Fukuma Y., et al. Fisetin inhibits osteoclastogenesis through prevention of RANKL-induced ROS production by Nrf2-mediated up-regulation of phase II antioxidant enzymes. J Pharmacol Sci. 2013;121:288–298. doi: 10.1254/jphs.12243FP. [DOI] [PubMed] [Google Scholar]
- 67.Han M.K., Barreto T.A., Martinez F.J., Comstock A.T., Sajjan U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2020;7:e000392. doi: 10.1136/bmjresp-2018-000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zwicker J.I., Schlechter B.L., Stopa J.D., Liebman H.A., Aggarwal A., Puligandla M., et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight. 2019;4:e125851. doi: 10.1172/jci.insight.125851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Q.Y., Tong Y.F., Chen F., Qi Y., Li W., Wu S. Identification and synthesis of impurities in pinocembrin-a new drug for the treatment of ischemic stroke. Chinese J Chem. 2012;30:1315–1319. doi: 10.1002/cjoc.201200201. [DOI] [Google Scholar]
- 70.Hampton T. Grapefruit compound battles hepatitis C. J Am Med Assoc. 2008;299:1532. doi: 10.1001/jama.299.13.1532. [DOI] [PubMed] [Google Scholar]
- 71.Nahmias Y., Goldwasser J., Casali M., van Poll D., Wakita T., Chung R.T., et al. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47:1437–1445. doi: 10.1002/hep.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo Y.L., Zhang C.C., Li P.B., Nie Y.C., Wu H., Shen J.G., et al. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int Immunopharmacol. 2012;13:301–307. doi: 10.1016/j.intimp.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 73.Iyer S.P., Huen A., Ferreri A.J.M., Haverkos B.M., Carlo-Stella C. Pooled safety analysis and efficacy of Tenalisib (RP6530), a PI3Kδ/γ inhibitor, in patients with relapsed/refractory lymphoid malignancies. Blood. 2018;132:2925. doi: 10.1182/blood-2018-99-112757. [DOI] [Google Scholar]
- 74.Nadadur R., Umar S., Matori H., Iorga A., Mai D., Amjedi M., et al. Genistein therapy reverses lung inflammation and fibrosis during severe pulmonary hypertension through estrogen receptor beta. Biophys J. 2012;102:140a. doi: 10.1016/j.bpj.2011.11.772. [DOI] [Google Scholar]
- 75.Qureshi M.Y., Patterson M.C., Clark V., Johnson J.N., Moutvic M.A., Driscoll S.W., et al. Safety and efficacy of (+)-epicatechin in subjects with Friedreich's ataxia: A phase II, open-label, prospective study. J Inherit Metab Dis. 2021;44:502–514. doi: 10.1002/jimd.12285. [DOI] [PubMed] [Google Scholar]
- 76.Shiha G., Soliman R., Elbasiony M., Darwish N.H.E., Mousa S.A. Addition of epigallocatechin gallate 400 mg to Sofosbuvir 400 mg + Daclatisvir 60 mg with or without ribavirin in treatment of patients with chronic hepatitis c improves the safety profile: a pilot study. Sci Rep. 2019;9:13593. doi: 10.1038/s41598-019-49973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scanio M.J.C., Searle X.B., Liu B., Koenig J.R., Altenbach R., Gfesser G.A., et al. Discovery of ABBV/GLPG-3221, a potent corrector of CFTR for the treatment of cystic fibrosis. ACS Med Chem Lett. 2019;10:1543–1548. doi: 10.1021/acsmedchemlett.9b00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bell S.C., Barry P.J., De Boeck K., Drevinek P., Elborn J.S., Plant B.J., et al. CFTR activity is enhanced by the novel corrector GLPG2222, given with and without ivacaftor in two randomized trials. J Cyst Fibros. 2019;18:700–707. doi: 10.1016/j.jcf.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 79.Sareddy G.R., Li X., Liu J., Viswanadhapalli S., Garcia L., Gruslova A., et al. Selective estrogen receptor β agonist LY500307 as a novel therapeutic agent for glioblastoma. Sci Rep. 2016;6:24185. doi: 10.1038/srep24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L., et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 81.Schattenberg J.M., Pares A., Kowdley K.V., Heneghan M.A., Caldwell S., Pratt D., et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol. 2021;74:1344–1354. doi: 10.1016/j.jhep.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 82.Plowman J., Narayanan V.L., Dykes D., Szarvasi E., Briet P., Yoder O.C., et al. Flavone acetic acid: a novel agent with preclinical antitumor activity against colon adenocarcinoma 38 in mice. Cancer Treat Rep. 1986;70:631–635. [PubMed] [Google Scholar]
- 83.Cassaday R.D., Goy A., Advani S., Chawla P., Nachankar R., Gandhi M., et al. A phase II, single-arm, open-label, multicenter study to evaluate the efficacy and safety of P276–00, a cyclin-dependent kinase inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2015;15:392–397. doi: 10.1016/j.clml.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joshi K.S., Rathos M.J., Joshi R.D., Sivakumar M., Mascarenhas M., Kamble S., et al. In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276–00. Mol Cancer Ther. 2007;6:918–925. doi: 10.1158/1535-7163.MCT-06-0613. [DOI] [PubMed] [Google Scholar]
- 85.Alessi D.R., Cuenda A., Cohen P., Dudley D.T., Saltiel A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 86.Goetz M.P., Reid J.M., Qi Y., Chen A., McGovern R.M., Scanlon M.J.K.D., et al. A phase I study of once-weekly aminoflavone prodrug (AFP464) in solid tumor patients. J Clin Oncol. 2011;29:2546. doi: 10.1200/jco.2011.29.15_suppl.2546. [DOI] [Google Scholar]
- 87.Brüning A., Mylonas I. New emerging drugs targeting the genomic integrity and replication machinery in ovarian cancer. Arch Gynecol Obstet. 2011;283:1087–1096. doi: 10.1007/s00404-010-1757-x. [DOI] [PubMed] [Google Scholar]
- 88.Blosser J.C., McCreedy S., Parker R.B., Kinsolving C.R., Watkins B.E., Wu E.S. Flavodilol: a new antihypertensive agent that preferentially depletes peripheral biogenic amines. J Cardiovasc Pharmacol. 1989;14:142–156. [PubMed] [Google Scholar]
- 89.Wu E.S., Cole T.E., Davidson T.A., Dailey M.A., Doring K.G., Fedorchuk M., et al. 2. Synthesis and structure-activity relationship of flavodilol and its analogues, a novel class of antihypertensive agents with catecholamine depleting properties. J Med Chem. 1989;32:183–192. doi: 10.1021/jm00121a034. [DOI] [PubMed] [Google Scholar]
- 90.Hien T.V., Huong N.B., Hung P.M., Duc N.B. Radioprotective effects of vitexina for breast cancer patients undergoing radiotherapy with cobalt-60. Integr Cancer Ther. 2002;1:38–134. doi: 10.1177/153473540200100103. [DOI] [PubMed] [Google Scholar]
- 91.Testa R., Guarneri L., Taddei C., Poggesi E., Angelico P., Sartani A., et al. Functional antagonistic activity of Rec 15/2739, a novel alpha-1 antagonist selective for the lower urinary tract, on noradrenaline-induced contraction of human prostate and mesenteric artery. J Pharmacol Exp Ther. 1996;277:1237–1246. [PubMed] [Google Scholar]
- 92.Testa R., Guarneri L., Bernasconi P., Angelico P., Ibba M., Poggesi E., et al. Effects of terflavoxate on stimulated contractions of urinary bladder in vitro. Arzneimittelforsch. 1993;43:122–128. [PubMed] [Google Scholar]
- 93.Karbon E.W., Abreu M.E., Erickson R.H., Kaiser C., Natalie K.J.Jr., Clissold D.B., et al. NPC 16377, a potent and selective sigma-ligand. I. Receptor binding, neurochemical and neuroendocrine profile. J Pharmacol Exp Ther. 1993;265:866–875. [PubMed] [Google Scholar]
- 94.Ursini F., Maiorino M., Morazzoni P., Roveri A., Pifferi G. A novel antioxidant flavonoid (IdB 1031) affecting molecular mechanisms of cellular activation. Free Radic Biol Med. 1994;16:547–553. doi: 10.1016/0891-5849(94)90054-X. [DOI] [PubMed] [Google Scholar]
- 95.Mersereau J.E., Levy N., Staub R.E., Baggett S., Zogovic T., Chow S., et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008;283:49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakayama O., Yagi M., Tanaka M., Kiyoto S., Uchida I., Hashimoto M., Okuhara M., Kohsaka M. WS-7528, a new isoflavanone with estrogen activity isolated from Streptomyces sp. No. 7528. Taxonomy, fermentation, isolation, physico-chemical properties and biological activities. J Antibiot (Tokyo) 1990;43:1394–1402. doi: 10.7164/antibiotics.43.1394. [DOI] [PubMed] [Google Scholar]
- 97.Giacomelli S., Gallo D., Apollonio P., Ferlini C., Distefano M., Morazzoni P., et al. Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin. Life Sci. 2002;70:1447–1459. doi: 10.1016/s0024-3205(01)01511-9. [DOI] [PubMed] [Google Scholar]
- 98.Yuan C.J., Wang J., Chen S., Luo S., Zhou T.Y., Xu X.M. HPLC determination of the content and the related substances of tectorigenin sodium sulfonite and its preparation Taike Jining injection. Nat Prod Res Dev. 2010;22(104–6) doi: 10.16333/j.1001-6880.2010.01.013. [DOI] [Google Scholar]
- 99.Miyake A., Yamamoto H., Kubota E., Hamaguchi K., Kouda A., Honda K., et al. Suppression of inflammatory responses to 12-O-tetradecanoyl-phorbol-13-acetate and carrageenin by YM-26734, a selective inhibitor of extracellular group II phospholipase A2. Br J Pharmacol. 1993;110:447–453. doi: 10.1111/j.1476-5381.1993.tb13831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ito S., Demachi M., Toriumi Y., Adachi T., Itai S., Hrayama F., et al. Improvement of dissolution characteristics of a new chalcone derivative, SU-740: comparison between size reduction, solid dispersion and inclusion complexation. Chem Pharm Bull. 1995;43:2221–2225. doi: 10.1248/cpb.43.2221. [DOI] [Google Scholar]
- 101.Xiao J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit Rev Food Sci Nutr. 2017;57:1874–1905. doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.