Graphical abstract
Keywords: CD47, Phagocytosis, Immunotherapy, CD47-SIRPα
Highlights
-
•
CD47 has emerged as a target in cancer immunotherapy.
-
•
CD47 is highly expressed in cancers to prevent phagocytosis by phagocytes.
-
•
Targeting CD47 has been verified as a promising therapeutic strategy in cancer immunotherapy.
-
•
Antibodies and small molecules targeting CD47 exhibited both safety and efficacy in clinical trials.
-
•
Formidable challenges of targeting CD47 including anemia can be avoided by targeting the regulators of CD47 or its receptor SIRPα.
Abstract
Background
Immunotherapy has emerged as a novel strategy for cancer treatment following surgery, radiotherapy, and chemotherapy. Immune checkpoint blockade and Chimeric antigen receptor (CAR)-T cell therapies have been successful in clinical trials. Cancer cells evade immune surveillance by hijacking inhibitory pathways via overexpression of checkpoint genes. The Cluster of Differentiation 47 (CD47) has emerged as a crucial checkpoint for cancer immunotherapy by working as a “don’t eat me” signal and suppressing innate immune signaling. Furthermore, CD47 is highly expressed in many cancer types to protect cancer cells from phagocytosis via binding to SIRPα on phagocytes. Targeting CD47 by either interrupting the CD47-SIRPα axis or combing with other therapies has been demonstrated as an encouraging therapeutic strategy in cancer immunotherapy. Antibodies and small molecules that target CD47 have been explored in pre- and clinical trials. However, formidable challenges such as the anemia and palate aggregation cannot be avoided because of the wide presentation of CD47 on erythrocytes.
Aim of view
This review summarizes the current knowledge on the regulation and function of CD47, and provides a new perspective for immunotherapy targeting CD47. It also highlights the clinical progress of targeting CD47 and discusses challenges and potential strategies.
Key scientific concepts of review
This review provides a comprehensive understanding of targeting CD47 in cancer immunotherapy, it also augments the concept of combination immunotherapy strategies by employing both innate and adaptive immune responses.
Introduction
Cancer immunotherapy has become an innovative therapy owing to its incomparable advantages over traditional anti-tumor therapies. Remarkable advances have been made in recent years following the clinical success of immune checkpoint blockade and chimeric antigen receptor (CAR)-T cell therapies. Both checkpoint inhibitors and adoptive cell therapy manipulate the immune system to kill cancer cells [1], which usually escape immune surveillance by hijacking inhibitory pathways via overexpression of checkpoint genes [2]. Immune checkpoint inhibitors such as antibodies and small molecules of programmed cell death ligand 1(PD-1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), blocking the interaction between immune checkpoints and their ligands have achieved success [3]. However, there are still problems and side effects including but not limited to low response rate, high cost, and non-specific toxicity. Therefore, it is necessary to investigate these novel checkpoints.
Most previous immunotherapies were based on stimulating adaptive immunity, particularly T-cell function. The Cluster of Differentiation 47 (CD47)- Signal regulatory protein alpha (SIRPα) axis, was the first tumor phagocytosis checkpoint identified in the late 2000s. It is an immune checkpoint on myeloid-specific system and functions via the innate immune system. CD47, serving as a “don’t eat me” signal on tumor cells inhibits the phagocytosis of macrophages in the immunity system [4], [5], [6]. Several antibodies and CD47 inhibitors of CD47 have been explored, many of which are in clinical trials [7], [8]. Striking responses in clinical trials have been achieved for several types of hematologic malignancies and solid tumors by targeting CD47 [9], [10]. Furthermore, CD47-SIRPα signaling depends on the phagocytic function of macrophages, which are the most abundant infiltrating leukocytes in tumors. Therefore, targeting CD47 may be a turning point in cancer immunotherapy.
In this review article, we summarize the comprehensive understanding of CD47 regulation at the transcriptional, translational, and post-translational levels to explore its role in signal transduction. We also discuss its expression in immune cells and functions in innate and adaptive immune responses. Finally, we highlight the clinical progress of targeting CD47 and discuss the challenges and potential strategies for CD47 targeting in cancer immunotherapy. This comprehensive review aims to not only help understand the molecular mechanisms of CD47 function, but also aid in combination treatment methods, such as immunotherapy against the backbone of chemotherapy in the future.
Basic knowledge of CD47
CD47, a heavily N-glycosylated 47–52 kD integral membrane glycoprotein [11], is expressed in almost all normal cells including red blood cells (RBCs) and platelets [12]. Furthermore, CD47 is highly expressed in various tumor cells and is a biomarker of malignant tumors. In this section, we review the discovery of CD47 in retrospect and discuss its structure and binding proteins.
A brief history of CD47
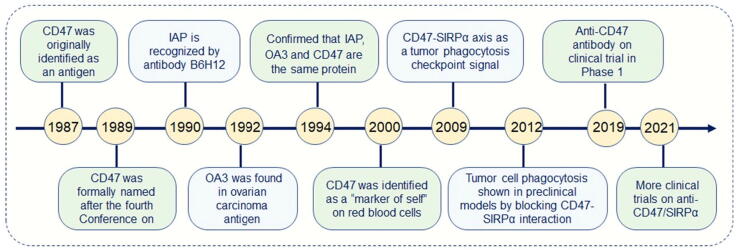
CD47 was originally identified in 1987 as a missing antigen by the antibody 1D8 in Rh-negative RBCs [13]. In 1990, a cell surface protein named integrin-associated protein (IAP) was purified using the monoclonal antibody B6H12 [12], which functions by inhibiting Arg-Gly-Asp binding to the leukocyte response integrin [14]. IAP plays a role in the cell adhesion in non-erythroid cells [15] and was found to be identical to the erythrocyte cell surface antigen CD47 soon after its cloning [16]. The term IAP is currently used less frequently, considering that CD47 was found to interact with more proteins than integrins [17]. Later, in 1992, an overexpressed ovarian carcinoma antigen (OA3) was found to be abundantly expressed in ovarian cancer but rarely expressed in normal tissues. OA3 was used as as an immune target. In 1994, Tanner et al. demonstrated that IAP, OA3, and CD47 are the same proteins by comparing their sequences [16].
In 2000, for the first time, CD47 was identified as a “marker of self” on murine RBCs to prevent the clearance of RBCs by splenic red pulp macrophages in the bloodstream by binding to SIRPα [18].
In 2009, the CD47-SIRPα axis was identified as a tumor phagocytosis checkpoint that conveys a “don't eat me” signal to macrophages and is regarded as an immune escape mechanism acquired by many tumor cells [19]. Subsequently, the unique role of CD47 in cancer immunotherapy has been highlighted. In 2012, the experimental proof of phagocytosis in cancer cells by phagocytes came to light through preclinical experiments. This significant finding was achieved by disrupting the CD47-SIRPα interaction in cancer cells [20]. In 2019, a first-in-human and first-in-class phase I trial of the anti-CD47 antibody Hu5F-G4 was conducted in patients with solid tumors [21]. In 2021, multiple anti-CD47/SIRPα antibodies were tested for clinical trials in different cancers [22].
In summary, research on CD47 has continued over the last 30 years, especially since its function as a phagocytosis checkpoint was discovered (Fig. 1). Although clinical trials targeting CD47 are under way, more effort is needed to further understand the detailed CD47 function to optimize immunotherapies targeting CD47 in the future.
Fig. 1.
The brief history of CD47.
The architecture of CD47
CD47comprises a heavily glycosylated N-terminal extracellular domain (ECD), a 5-TM spanning domain and a short C-terminal domain (CTD) [18], [23], [24], [25]. Its ECD consists of a V-set immunoglobulin superfamily (IgSF) domain, which functions as a cell surface marker of self via binding to SIRPα.
The CTD of CD47 is alternatively spliced, with variants ranging in length from 3 to 36 amino acids in the cytoplasmic tail and arise by alternative splicing of the intricate splice donor site in the 3′ UTR exon (Fig. 2).
Fig. 2.
CD47 ligand proteins a CD47 structure and its ligand proteins b SIRP family members c Binding of CD47 on the surface of HSCs and RBCs and SIRPα on macrophages prevents them from phagocytosis by macrophages d CD47 on tumor cells binds to SIRPα on macrophages to protect tumor cells from being phagocytized by macrophages, blocking CD47 by antibody or interrupting the CD47-SIRPα axis promotes phagocytosis and tumor clearance.
CD47 binding proteins
The multiple functions of CD47 may be attributed to its numerous binding partners. As a transmembrane protein, CD47 bridges the communication with molecules located extracellularly, on the membrane, and inside cells (Table 1). Moreover, CD47 has several ligand proteins including thrombospondins (TSPs), SIRPα and SIRPγ, etc. (Fig. 2a).
Table 1.
Comprehensive list of CD47 associated/interacted proteins and their major functions.
| CD47 interacting proteins | Biological functions | References | ||
|---|---|---|---|---|
| Extracellular | TSP-1 (C-terminal domain) | apoptosis, inhibition of angiogenesis, immunosuppressive, promoting ROS production, and inflammatory response | [37] | |
| SIRPα | inhibition of phagocytosis, adhesion and migration, dendritic cell function and T Cell activation. | [60] | ||
| SIRPγ | T-cell proliferation and T-cell trans-endothelial migration. | [61], [62] | ||
| Serpin A1 (C-terminal) | proliferation stimulation | [63] | ||
| COMP | inflammation and joint destruction in osteoarthritis | [64] | ||
| Membrane | Integrin | αvβ3 | human melanoma cells spreading (C32), binding to vitronectin, PMN activation, calcium regulation, and proinflammatory cytokine synthesis | [65] |
| αIIbβ3 | platelet activation | [66], [67], | ||
| α2β1 | chemotactic response of smooth muscle cells | [68] | ||
| α4β1 | T cell antigen receptor antagonist, anti-proliferative activities and T cell adhesion, and B-cell migration | [69] | ||
| α5β1 | chondrocyte mechano-transduction | [70] | ||
| α6β1 | microglial activation | [71] | ||
| αMβ2 | neutrophil transepithelial migration | [72] | ||
| VEGFR-2 | immunosuppressive activity of VEGF in T cells, inhibition of VEGFR2activation | [73], [74] | ||
| CD36 | inhibition of NO-stimulated phenotypic responses and cGMP signaling in vascular cells | [75], [76] | ||
| Fas/CD95 | activation of Fas (CD95) mediates apoptosis | [77] | ||
| Intracellular | PLIC-1 | inducing redistribution of vimentin and cell spreading, and inhibition of Gi signaling | [78], [79] | |
| BNIP3 | T Cell apoptosis | [80] | ||
| ERK | inhibiting smooth muscle cell migration | [81] | ||
| Syk | phosphorylation of Lyn, Syk and FAK | [66] | ||
| Gαs, Gαq and Gβγ | stimulating astrocytoma proliferation via Akt | [82] | ||
| RAC | neurite and filopodium formation | [33] | ||
| CDC42 | neurite and filopodium formation | [33], [83] | ||
| Src | dendritic development | [83] | ||
| PI3K | cell spreading | [84] | ||
| ENO1 | enhancing aerobic glycolysis | [85] | ||
| Drp1 | caspase-Independent Cell Death | [86] | ||
CD47 binds to Thrombospondin 1 (TSP-1)
Thrombospondin 1 (TSP-1) is the first reported ligand for CD47 [26]. TSP-1 is synthesized and secreted by many cell types including macrophages, monocytes, smooth muscle cells, endothelial cells, and tumor cells. TSP-1 interacts with CD47 via its RFYVVMWK sequence (commonly known as 4N1K) in the C-terminal CBD domain. TSP-1/CD47 signaling is crucial for cell apoptosis, proliferation and adhesion. It also inhibits angiogenesis, inflammatory reactions, and platelet activation and aggregation in various cell types [27], [28], [29], [30], [31], [32], [33].
The interaction of CD47 and TSP-1 disrupts angiogenesis to inhibit tumor growth
TSP-1 regulates angiogenesis by binding to CD47 [34]. The interaction between CD47 and TSP-1 inhibits the activation of vascular endothelial growth factor receptor-2 (VEGFR2) and thus disrupting angiogenesis in endothelial cells, and these anti-angiogenic effects of CD47 worsen with age in mammalians, indicating that CD47/TSP-1 signaling is a potential therapeutic target [35], [36]. It works similarly in T cells by inhibiting the downstream target signal of the VEGFR2-Nitric Oxide (NO) signal pathway to inhibit angiogenesis [34]. In addition, the combination of CD47 and TSP-1 promoted the early activation of platelets and spreading of platelets. Since tumor growth and metastasis require angiogenesis, CD47/TSP-1 signaling is considered a potent inhibitor in the context of tumor development and metastasis [37].
The interaction between CD47 and TSP-1 inhibits inflammatory responses
The CD47 to TSP-1 ligation is associated with the regulation of the inflammatory response. The CD47/TSP-1 immune complex temporarily accumulates at the inflammatory site and inhibits cell activation and cytokine secretion, thus affecting the intensity and duration of the inflammatory reaction [38]. TSP-1 deficiency decreased the inflammatory phenotype of macrophages and limited their phagocytic capacity [39].
The interaction between CD47 and TSP-1 augments the regeneration of stem cells
In normal stem cells, CD47 deletion or in combination with TSP-1 augments the regenerative ability of stem cells and up-regulates the expression of stem-cell -related transcription factors Sox2, c-Myc, KLF4 and Oct4 [40]. Therefore, CD47-targeted drugs used to inhibit cancer stem cells (CSCs) in immunotherapy can also be used to enhance the function of normal stem cells during regeneration.
CD47 binds to SIRPα
In 2000, SIRPαwas identified as a second CD47 ligand protein of CD47 in 2000. SIRPα is a type of inhibitory receptor, which belongs to the immunoglobulin superfamily.
The SIRP family contains five members: the inhibitory receptor SIRPα, activated receptor SIRPβ, non-signal receptor SIRPγ, SIRP δ and SIRPβ2 (Fig. 2b). However, SIRP δ and SIRPβ2 have rarely been studied. In addition, SIRPβ1 shows no significant binding to CD47 due to the subtle difference in the binding region compared with SIRPα through the analysis of the X-ray crystal structure [41]. They have the same homologous extracellular immunoglobulin (Ig)-like domains, but their transmembrane and intracytoplasmic domains are different [42], [43], [44], [45], [46]. The SIRP family is present in the immune cells of the central nervous system.
SIRPα is the most crucial and conservative member of the SIRP family, mainly present in monocytes, macrophages, dendritic cells, granulocytes, bone marrow precursors and neurons. Unlike CD47, SIRPα is barely detectable on RBCs, T or B lymphocytes [17], [24], [47]. SIRPα expressed on the surface of phagocytes is relatively stable and not affected by the degree of inflammation [48]. SIRPα is a transmembrane glycoprotein, composed of three Ig-like domains, one transmembrane domain, and four tyrosine phosphorylation sites with two immune receptor tyrosine-based inhibitor motif (ITIMs) in the cytoplasmic tail. The interaction between SIRPα and CD47 involves the NH2-terminal domain of SIRPαbinding to the single Lg-V domain of CD47.
CD47-SIRPα prevents hematopoietic stem cells and RBCs from phagocytosis
The inhibitory effect of CD47 on phagocytosis was initially observed in RBCs. CD47, present on the surface of hematopoietic stem cells (HSCs) and RBCs, interacts with SIRPα on macrophages, protecting them from being phagocytized by macrophages [49]. This protective mechanism has been tested and validated in various organisms, including humans [50], mice, pigs [51], etc. CD47 expression is decreased in hematopoietic progenitor cells in patients with myelofibrosis [52]. The hematopoietic function of HSCs in CD47 deficient mice was not significantly affected, but CD47-/- HSCs and RBCs disappeared in wild-type mice within four weeks [49], [53]. Young RBCs express high levels of CD47 to avoid phagocytosis; damaged or senescent RBCs are phagocytized by macrophages owing to the low expression of CD47. In addition, cytokines and inflammatory stimulation cause a transient up-regulation of CD47 in HSCs before and during migration. Thus, HSCs achieve partial immunity by regulating the expression of CD47. Therefore, degradation of CD47 or interruption of this axis disturbs homeostasis and causes blood cell clearance, thus leading to the pathogenesis of blood cell disorders. Taken together, the binding of CD47 on HSCs and RBCs to SIRPα on splenic macrophages prevented the phagocytosis of the former cell by the latter (Fig. 2c).
CD47-SIRPα prompts tumor immune evasion in cancer immunotherapy
The CD47-SIRPα axis plays a key role in tumor immune escape and innate immunotherapy [6]. The binding of CD47 on tumor cells and SIRPα on myeloid enhances the phosphorylation of SIRPα ITIM by Src family kinases SHP-1 and SHP-2 [54]. Recruitment of SHP-1 and SHP-2 inhibits the function of non-muscle myosin IIA, which regulates phagolysosomal biogenesis in macrophages and plays a critical role in limiting phagocytosis. CD47-SIRPα inhibits phagocytosis of myeloid cells via the above mechanism. Since CD47-SIRPα triggers a “don’t eat me” signal, cancer cells evade macrophage-mediated phagocytosis dependent on the expression of CD47 on their membrane (Fig. 2d). CD47 inhibits the phagocytic function of macrophages, activates cell–cell fusion, modulates neutrophil migration, and stimulates the immune response of T cells.
CD47-SIRPα interaction also functions in cancer stemness maintenance and immunoresistance of CSCs. CD47 helps CSCs escape macrophage phagocytosis and enhances the self-renewal and proliferative ability of some CSCs [55].
CD47-SIRPα regulates immunity via the Hedgehog/SMO/Gli1 pathway
The CD47-SIRPα axis activates the hedgehog/smoothened (SMO)/GLI family zinc finger 1 (Gli1) pathway in mesenchymal stem cells (MSC) -treated livers following ischemia/ reperfusion (IR) stress, which modulates cell differentiation, growth, and immune function. The interaction of CD47 on MSC and SIRPα on macrophages activates the Hedgehog/SMO/Gli1 pathway and Notch 1 signaling and inhibits NEK7/NLRP3 activity affecting the inflammatory responses [56]. CD47-SIRPα axis also regulates the interaction between macrophage GLI1 and Notch1 intracellular domain (NICD) in MSC-mediated immune regulation, indicating this pathway may be a potential therapeutic target in MSC-mediated immunotherapy of sterile inflammatory liver injury [56].
CD47-SIRPα trans-interaction disrupts the transduction of SIRPα
The interaction between CD47 and SIRPα constitutes a bidirectional cell–cell communication system that enhances the formation of filopodia and neurites. The trans-interaction of CD47 and SIRPα between Chinese hamster ovary (CHO) cells results in the engulfment of the ligand-receptor complex into either cell type [57]. This process eliminates the CD47-SIRPα complex on the cell surface and disrupts the signal transduction of SIRPα, thereby eliminating the positive regulation of filopodium or neurite formation in neurons. They play a significant role in the formation and maintenance of synapses. The interaction between CD47 and SIRPα induces the degradation of SIRPα by the proteasome-mediated pathway. Thus, the lysosome may modulate the degradation of the CD47-SIRPα complex after trans-endocytosis [57].
CD47 binds to SIRPγ
SIRPγ also has the ability to bind to CD47, but its binding affinity is approximately ten times lower than that of SIRPα (SIRPγ Kd ≈ 23 μM, SIRPα Kd ≈ 1.5 μM). SIRPγ is primarily expressed in the T cells [58]. The binding of SIRPγ to CD47 plays a critical role in the trans-endothelial migration of T cells, leukocyte migration and T cell proliferation. Blocking SIRPγ or CD47 reduces T-cell migration. Furthermore, theCD47-SIRPγ interaction enhances superantigen-mediated T-cell co-stimulation, and blocking their binding eliminates IFN-γ secretion in chronically activated T cells [58], [59].
CD47 regulation and function in cancer
Regulation of CD47
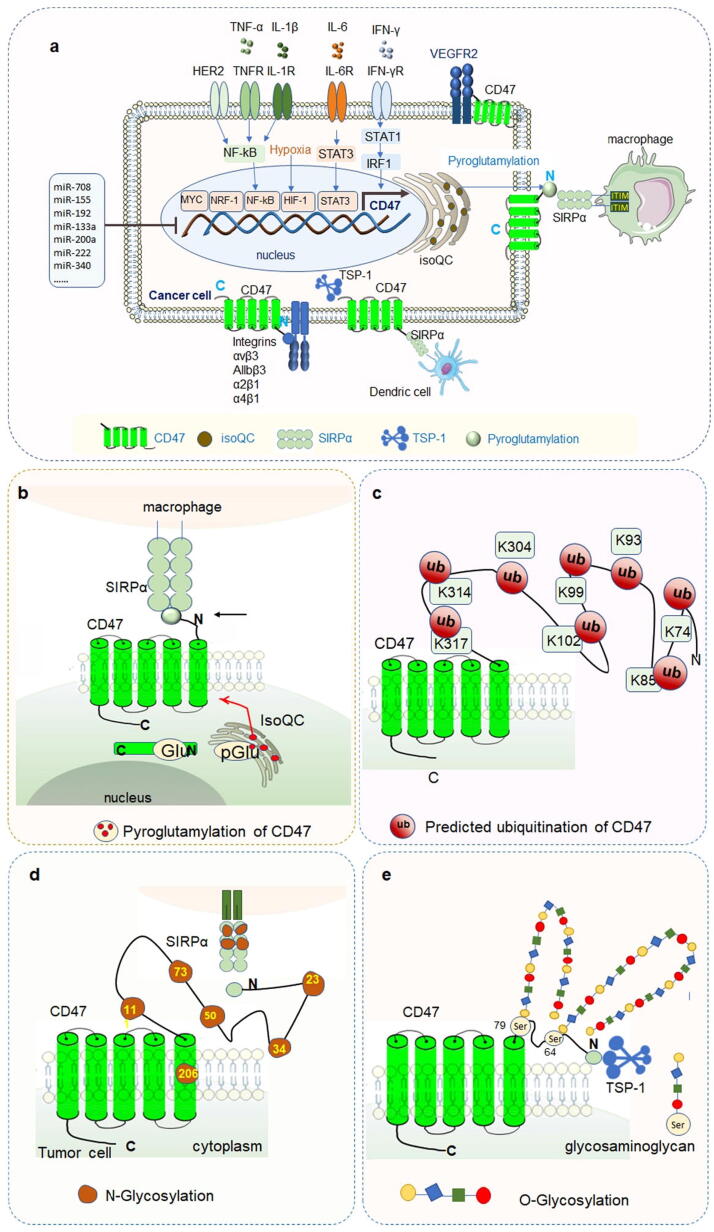
The CD47 expression is accurately regulated at the transcriptional, post-transcriptional and post-translational levels (Fig. 3a).
Fig. 3.
Regulation and modification of CD47 expression a Regulators of CD47 expression in cancer cells. TNF-α, IL-1β and HER2 can stimulate the expression of CD47 via activating NF-κB pathway. IL-6 increases the expression of CD47 by activating the STAT3 signaling pathway. IFN-γ up-regulates the expression of CD47 via the STAT1-IRF1 signaling pathway. HIF-1 directly binds to the CD47 promoter to promote the transcriptional expression of CD47 in a hypoxic environment. Myc also binds to the CD47 promoter directly to up-regulate CD47 expression. Pyroglutamylation of CD47 protein catalyzed by QPCTL is critical for the binding of CD47-SIRPα and then influences the immune response of macrophages. b Pyroglutamylation of CD47 c Predicted ubiquitination of CD47, ubiquitination sites listed here are the potential sites predicted in the phosphosite website. d N-glycosylation of CD47 e O-glycosylation of CD47.
Transcriptional regulation of CD47
Transcription factors regulate CD47 expression
Multiple studies have demonstrated that CD47 is regulated by several transcription factors, including Myc, nuclear factor kappa B (NF-κB), hypoxia-inducible factor-1 (HIF-1), and nuclear respiratory factor 1 (NRF-1), etc.
Myc potentiates CD47 expression
The oncogene Myc regulates CD47 transcription and partially contributes to tumor cell evasion from the immune surveillance [87], [88], [89]. Myc protein potentiates the expression of CD47 by binding to the CD47 promoters [87]. In cutaneous T-cell lymphoma (CTCL) tumor cells, ectopic expression of CD47 can be moderately induced by high c-Myc. In a Tet-off transgenic Myc-induced T-ALL mouse model elevated Myc levels trigger the upregulation of CD47 and PD-L1. Interestingly, this process also results in the loss of “don’t find me” and “don’t eat me” signals, which collectively sustains the tumor regression [87]. Myc knockout in tumor down-regulates CD47 mRNA and protein levels. Suppression of Myc by JQ1 reduces cell surface CD47 expression in hit lymphoma cells [90]. When the immune system is activated, the inactivation or interference of Myc expression can significantly enhance the ability to clear tumor cells.
HIF-1 enhances CD47 expression
The rapid proliferation of tumors reshapes the special microenvironment of tumor cells including the anaerobic microenvironment. This highly hypoxic environment leads to the genetic reprogramming of both cancer and immune cells via hypoxia-inducible factors [91], [92]. HIF-1 directly binds to the CD47 promoter to activate its transcription, thereby enhancing CD47 expression to prevent phagocytosis by macrophages and maintain the stemness of tumor stem cells [93]. The enhancement of CD47 by HIF-1 has been manifested in breast cancer [93], [94], colon cancer [95] and mouse cone photoreceptor cells 661 W [96].
NF-κB regulates CD47
NF-κB is a transcriptional factor involved in many diseases. CD47 expression is elevated in Sorafenib-resistant Hepatocellular carcinoma (HCC) cells and regulated by NF-κB through a specific response element in the CD47 promoter. NF-κB inhibitors suppress the radiation-induced CD47 in HER2-expressing breast cancer cells via the HER2-NF-κB pathway [97]. In turn, the CD47-SIRPα interaction results in the phosphorylation of the cytoplasmic ITIM of SIRPα, and finally leads to the inhibition of NF-κB signaling with the reduction of the release of NF-κB-dependent cytokines in macrophages.
NRF-1 enhances CD47
The transcription factor NRF-1 contains a unique putative basic leucine zipper (bZip) DNA-binding domain in its N-terminal [98]. The core promoter sequence of human CD47 has a consensus sequence for the NRF-1 DNA binding domain and can be recognized by NRF-1. Overexpression of NRF-1 significantly enhances CD47 promoter activity and acts as a key modulator of its binding to the CD47 promoter in human hepatoma and neuroblastoma cell lines [99].
Cytokines regulate CD47 expression
Besides transcription factors, CD47 expression is also regulated by a series of cytokines.
Tumor necrosis factor alpha (TNF-α) enhances CD47
TNF-α acts as a CD47 inducer in the breast cancer [100], non-small cell lung cancer (NSCLC) [101], HCC [97] etc. TNF-α promotes the binding of NF-κB to the CD47 promoter region [97]. CD47 is upregulated by the TNF-α/NF-κB signal pathway in NSCLC tumor models [101] and promotes tumor cell proliferation and invasion [102]. Treatment with 20 ng/ml TNF-α increased cell surface CD47 localization in HCCs by activating the NF-κB pathway [97]. CD47 upregulation in lung cancer is also mediated by the TNF-α/NF-κB1 signal pathway [101]. Infliximab, which inhibits the binding of TNF-α to a cellular receptor, decreases the expression of CD47 in breast cancer [100].
Interferon-γ (IFN-γ) potentiates CD47
Interferon-γ (IFN-γ) is the only member of the type II IFN and is restrictively expressed compared to IFN-α and IFN-β. It is a cytokine that is clinically used to treat cancers owing to its function in tumor immune surveillance. IFN-γ correlates with CD47 and it potentiates CD47 in many cancers [103], [104]. IFN-γ modulates CD47 by the IFN-γ/JAK/STAT1/IRF1 signaling pathway. On the other hand, CD47 deficiency increases the proportion of IFN-γ production in CD90+ natural killer (NK) cells [105].
Interleukin stimulates or upregulates CD47
Interleukin-6 (IL-6), derived from tumor-infiltrating macrophages, upregulates CD47 by activating the STAT3 pathway, and blocking CD47 or interrupting the IL-6/STAT3 axis restores macrophage-mediated phagocytosis [106]. In cervical cancer, Interleukin-1β (IL-1β) was found to activate CD47 by stimulating NF-κB pathway [107]. Interaction between CD47-SIRPα and IL-10 confined inflammation-induced macrophage phagocytosis of healthy self-cells [108]. Additionally, CD47 is regulated by many other interleukins, such as IL-4, IL-7, and IL-13 [107], [109]. However, the mechanisms by which these cytokines regulate CD47 expression remain unclear.
MicroRNAs (miRNAs) and long noncoding RNAs (lncRNAs) regulate CD47 expression
MiRNAs negatively regulate CD47
MiRNAs targeting CD47 or SIRPα have emerged as promising targets for cancer treatment [20]. Here, we have summarized the miRNAs that down-regulate CD47 and promote phagocytosis in different tumor types (Table 2).
Table 2.
MiRNAs regulate CD47 expression.
| miRNA name | Mechanism and function | Resource |
|---|---|---|
| miR-708 | miR-708 directly binds the 3′-UTR of CD47 and inhibits CD47 in T cells acute lymphoblastic leukemia. | [110] |
| miR-155 | miR-155 downregulates CD47 in myeloma cells and increases phagocytosis of cancer cells by macrophages and inhibits tumor growth in mice. | [111] |
| miR-192 | miR-192 binds the 3′-UTR of CD47 and suppresses CD47 at the post-transcriptional level in medulloblastoma. | [112] |
| miR-133a | miR-133a binds to the 3′-UTR of CD47 directly and suppresses its protein expression in esophageal squamous cell carcinoma and laryngeal carcinoma. | [113], [114] |
| miR-200a | miR-200a affects NPC cell growth and invasion by regulating CD47 in Nasopharyngeal carcinoma. | [115] |
| miR-222 | miR-222 directly decreases CD47 expression in human kidney carcinoma cells. | [116] |
| miR-340 | miR-340 down-regulates the expression of CD47 in pancreatic cancer cells. | [117] |
| miR-34a | miR-34a targets the 3′UTR of CD47/PD-L1. | [118], [119] |
| miR-326 | miR-326 is upregulated in multiple sclerosis (MS) and inhibits CD47 by targeting 3′UTR CD47. | [120] |
| miR-141 | miR-141 has a negative regulatory effect on CD47 and cullin 3 by binding to the 3′UTR of CD47 and CUL3. | [121] |
| miR-378a | miR-378a regulates macrophage phagocytosis and differentiation by blocking the CD47-SIRPα pathway in atherosclerosis. | [122] |
| miR-149 | LncRNA MIAT sponges miR-149-5p to suppress phagocytosis via upregulating CD47 in advanced atherosclerosis. | [123] |
| miR-423-5p | In 92.1 UM cell lines, transfection with miR-423-5p down-regulates CD47 expression. | [124], [125] |
| miR-17/20a/106a | Inhibition of miR-1720a/106a in mouse peritoneal macrophage upregulates SIRPα at the post-transcriptional level and reduction of phagocytosis. | [126] |
| miR-7 | miR-7 downregulates EGFR mRNA by binding to its 3′ UTR, and the expression of EGFR promotes CD47 expression. | [127] |
| Let-7i-5p | Targeting TSP-1 in HCC, TSP-1 competes with SIRPα to bind to CD47 to prevent anti-apoptotic and angiogenesis signal transduction between macrophage cells and HCCs. | [128], [129] |
LncRNAs regulate CD47
LncRNAs are non-protein-coding RNAs longer than 200 nucleotides that function in the epigenetic regulation [130]. It has been reported that lncRNA MIAT sponges miR-149-5p to suppress phagocytosis by upregulating CD47 expression [123]. The lncRNA metastasis-associated lung adenocarcinoma transcription 1 (MALAT1) is related to cancer cell growth and metastasis. In addition, MALAT1 regulated Myc and CD47 expression in breast cancer cell lines [131].
The above regulatory mechanisms are of great significance for understanding of the transcriptional regulation of CD47. This finding has significance for the specific inhibition of CD47 expression in clinical applications.
Post-translational modifications (PTMs) of CD47
PTMs play critical roles in normal cellular processes including protein expression and degradation, cell differentiation, and signal transduction and regulation [132]. These modifications, including acetylation, methylation, ubiquitination, glycosylation, and phosphorylation, affect both physiological processes and pathogenesis [133], [134]. Here we summarize the reported PTM of CD47 and its functions in cancers.
Pyroglutamylation of CD47
Pyroglutamylation is a type of protein PTM discovered in the late 1880s. It refers to the cyclization of N-terminal glutamine or glutamic acid residues to form pyroglutamate residues in the protein, which is catalyzed by the enzyme glutaminyl cyclase (QC) and its isoform isoQC. The QC protein is encoded by the QPCT gene, while the isoQC is encoded by the QPCTL gene. Both are ubiquitously present but vary in different tissues. Notably, isoQC is primarily located in the Golgi apparatus inside the cell.
Pyroglutamylation is a biological process that occurs spontaneously and slowly in cells, serving to protect the N-terminus against exopeptidase degradation and is a necessary process for the maturation of many proteins. It also prompts regulatory peptides to adopt a proper conformation as well as helps stabilize protein expression by binding to their receptors. The structures of the CD47-SIRPα axis have shown the pyro-glutamate at the N-terminus of CD47 based on its hydrogen bonding [41]. By screening a novel regulator of CD47, it was found that CD47 is a potential substrate of isoQC. The N-terminus of CD47 was glutamine-cyclized, and the pyroglutamylation of CD47 was catalyzed by isoQC [135], [136], [137], which has also been identified in many cancer cells [135]. Pyroglutamylated CD47 is specifically recognized by SIRPα binding and contributed to the binding affinity of CD47 and SIRPα binding [41], [138]. In the co-culture of macrophages and tumor cells, isoQC was found to prevent tumor cells from phagocytosis by macrophages. Therefore, isoQC is an enzymatic modifier of the CD47-SIRPα pathway and may be a potential target for cancer immunotherapy [136], [139].
Glycosylation of CD47
The molecular weight of glycosylated CD47 is more than 250kD. There are six potential N-glycosylation sites in CD47: N23, N34, N50, N73, N111, and N206 [140]. Five of these are located in the extracellular IgV domain of CD47. Putative N-linked glycosylation sites can be disrupted by an asparagine-to-glutamine substitution at one or more desired locations [141]. In erythrocytes, three of these sites are modified by N-glycosylation. In addition, glycosaminoglycan modification of CD47 was found in Jurkat cells [142]. Further studies have shown that glycosaminoglycan modification mainly occurs at Ser-64 and Ser-79 of the CD47 IgV domain, and modification of Ser-64 is necessary for the interaction between CD47 and TSP-1. Furthermore, Ser-64 of CD47 is essential for TSP-1 in inhibiting of T cell activation [142].
Predicted ubiquitination of CD47
Protein ubiquitination modifies a protein substrate and determines of its function. The results of proteomic discovery mass spectrometry on the phosphosite website showed that K74, K85, K93, K99, K102, K304, K314, and K317 of CD47 were ubiquitinated; however, the functions of these ubiquitinated sites have not been reported in the literature (https://www.phosphosite.org).
In summary, pyroglutamylation and glycosylation of CD47 have been extensively studied in the last few years. Other PTMs such as ubiquitination and phosphorylation have seldomly been reported in the literature. Post-translational modifications of CD47 deserve further investigation because of their important roles in CD47 function and tumor immunotherapy.
Cellular function of CD47
CD47 plays versatile roles in a series of physiological and pathological processes such as proliferation [33], [40], [127], apoptosis, adhesion and migration, immune regulation, and homeostasis via signaling through SIRPα [44], [143], TSP-1 and integrins [12].
CD47 enhances or suppresses cell proliferation
In terms of proliferation, CD47 plays both enhancing and suppressing roles depending on the cell type. For example, the activation of CD47 induces the proliferation of human U87 and U373 astrocytoma cells rather than normal astrocytes via the Akt pathway [33]. InU87 cell, the blockade of CD47 attenuated cancer cell proliferation. In non-transformed and malignant cells, CD47 signaling regulates self-renewal. The treatment of breast cancer stem cells MDA-MB-231 with the CD47 antibody B6H12 decreases cell proliferation and asymmetric cell division [127]. Increased CD47 expression in glioblastoma (GBM) cells is demonstrated to promote proliferation and invasion via the PI3K/ATK pathway [33], [144], [145], [146]. However, in primary murine endothelial cells, it is shown that the deficiency of CD47 permits sustained proliferation, upregulates asymmetric division, and promotes the expression of stem cell transcription factors, including Sox2, c-Myc, and Klf4. Therefore, the inhibition of CD47 enables self-renewal and reprogramming in endothelial cells [40].
CD47 promotes or limits autophagy
The role of CD47 in autophagy regulation varies depending on cell type.
Targeting CD47 has been shown to suppress tumor growth by triggering or increasing autophagy in some cases. For instance, in NSCLC cells, targeting the CD47-SIPRα pathway induces autophagic flux, leading to the formation and degradation of the autophagosomes in lysosomes [147]. Similarly, in breast cancers, the protective effects of CD47 blockade are mediated by the upregulation of the autophagic flux [148].
However, in other cancers, targeting CD47 suppresses or attenuates autophagy. For example, VEGF and CD47 dual-targeting therapy suppresses autophagy and increases tumor cell apoptosis, angiogenesis inhibition, and macrophage infiltration in GBM, thus augmenting anti-glioblastoma effects and prolonging survival [149], [150], [151].
CD47 enhances glycolysis
Glycolysis suppresses immune cell activity, even though it is a hallmark of cancer cells [152]. Glioblastoma stem cells (GSCs) rewire the glycolysis-dominate metabolism to fatty acid oxidation (FAO)-dominated pathway for better energy consumption and defense of the resistant GSCs from macrophage-mediated phagocytosis. FAO inhibition demonstrated that FAO-derived acetyl-CoA promoted CD47 expression. Therefore, this glycolysis-to-FAO reprogramming is related to CD47 anti-phagocytosis in GSCs [153].
CD47 potentiates the aerobic glycolysis and ERK activity by stabilizing CD47 binding protein ENO1 in colorectal cancer cells (CRCs) and promotes the progression and metastasis of CRCs [85]. Activation of CD47 contributes to the activation of PI3K/PDK1/AKT/mTOR oncogenic signalling [127], [154].
Function of CD47 in the immune system
CD47 is a versatile cell-surface molecule that is widely present in immune cells including dendritic cells (DCs), macrophages, monocytes and T cells. CD47, as a self-recognition marker, is critical for both innate and adaptive immune responses. It plays an integral role in immune responses by delivering a “don't eat me” signal to inhibit phagocytosis [155]. Previous studies have verified that CD47 co-stimulates T cells, promotes leukocyte migration, and suppresses macrophage-clearing functions [156]. Loss of CD47 activates DCs, NK cells, and T cells, suggesting a critical role of CD47 in the regulation of immune cells [105]. CD47 has been treated as a checkpoint in both the innate and adaptive immune systems. CD47 blockade or targeting its binding protein SIRPα enhances both innate and adaptive antitumor immune responses [157], [89], [158]. Therefore, the expression of CD47 correlates with both innate and adaptive immune systems.
CD47 in innate immunity
The innate immune system is a natural immune defense system that was gradually formed by organisms during evolution and is mainly composed of tissue barriers, innate immune cells, and innate immune molecules. CD47 is closely associated with cells in the innate immunity system, including macrophages, DCs, NKs, etc. Conversely, the expression of CD47 modulates the function of innate immune cells.
CD47 and macrophages
Macrophages are found in all tissues of the human body [159], maintaining homeostasis and resisting pathogen invasion [160], [161], [162], [163]. They also regulate the tumor microenvironment (TME) via phagocytosis.
CD47 is overexpressed in many types of cancers and directly binds to SIRPα directly on macrophages. The CD47-SIRPα delivers a “don't eat me” signal protecting cancer cells from immune clearance [164] (Fig. 2d). CD47 has been regarded as a novel effective macrophage immune checkpoint for cancer immunotherapy [155], [165]. CD47 binding protein TSP-1, a major component of platelet alpha granules, is also expressed on macrophages [38]. The interaction between CD47 and TSP-1 participates in the migration of monocytes through cerebral vascular endothelial cells, contributing to the nervous system inflammation and the occurrence and development of diseases.
Monocytes are thought to be a source of tissue macrophages and are the primary mononuclear phagocyte [166]. CD47 induces rapid monocyte apoptosis via TSP-1. Freshly isolated monocytes undergo a rapid, caspase-independent cell death. Therefore, CD47 may mediate rapid apoptosis-like cell death in human monocytes.
CD47 and natural killer cells
NK cells resist pathogen invasion and malignant cell transformation. They are the first line of defense against malignant cell transformations. NK cells specifically express suppressor receptors, including leukocyte immunoglobulin-like receptor families such as PD-1, CTLA-4, CD94 and activated receptors including NKG2D and natural cytotoxic receptors [167].
CD47 regulates the proliferation, activation and recruitment of NK cells [168]. NK cells express high levels of CD47 and cell-surface proteins that regulate NK cells’ homeostasis and their responses to viral infection in mice [168]. High CD47 expression augmented NK cell recruitment into the TME (Fig. 4d). The NK cell response and immune function are significantly impaired in CD47-deficient mice. NK precursor depletion has been observed in bone marrow in CD47-/- mice and consistent with the antiphagocytic role of CD47 [168]. In contrast, CD47 deficiency results in the accumulation of mature and immature NK cells in spleen [168] and increases the proportion of IFN-γ producing CD90+ NK cells. In CD47 deficient mice, NK cells play a key role in enhancing disease [105]. In terms of proliferation, the CD47 ligand protein TSP-1 inhibited CD69 expression and NK cell proliferation in vitro in CD47-/- NK cells; and treatment of NK cells with a CD47 inhibitor blocking TSP-1 binding lead to the abrogation of its inhibitory function on NK cell proliferation [169] (Fig. 4e).
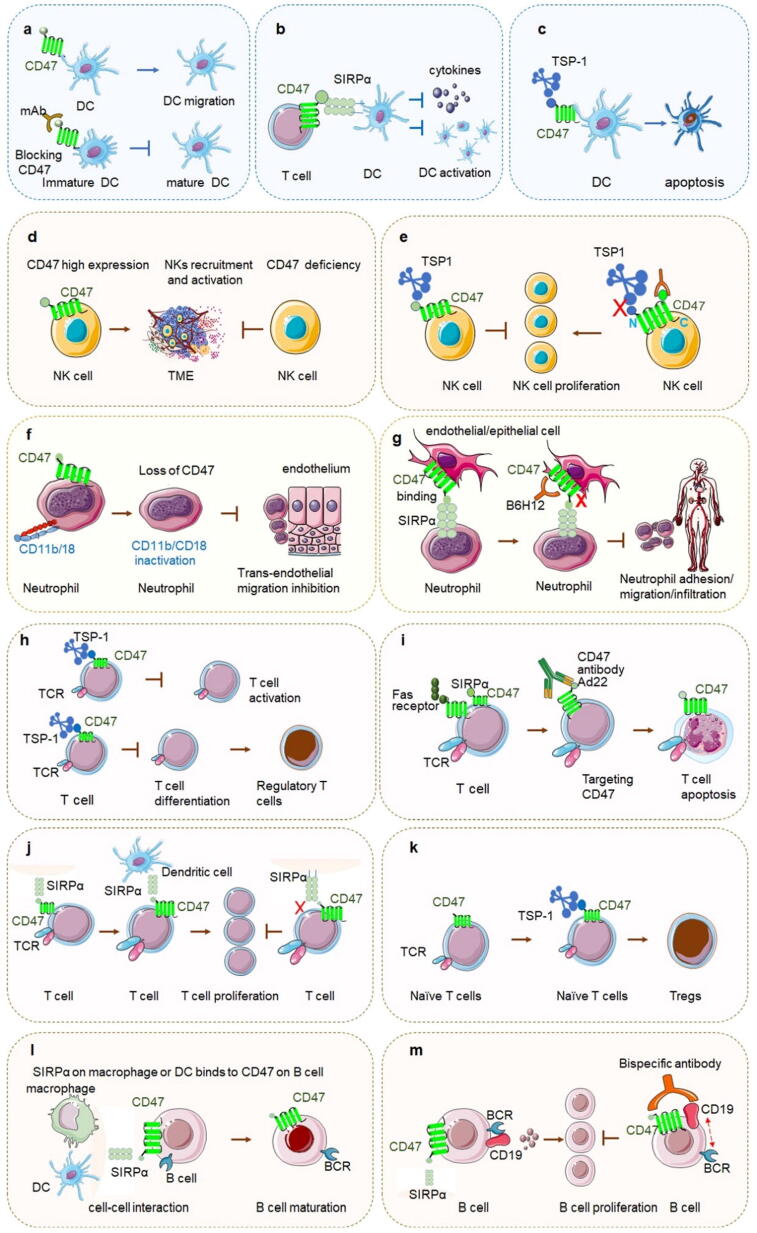
Fig. 4.
CD47 in innate immunity and adaptive immunity a. CD47 on DC regulates its migration and CD47 inhibits the transformation of immature DC to mature DC. b. CD47 on T cells binds with SIRPα on dendritic cells to inhibit the activation of dendritic cells, secretion of cytokines, and negatively regulate T cell expression. c. The interaction of TSP-1 and CD47 induces DC to apoptosis. d. CD47 high expression on NK cells promotes NK recruitment and activation in the TME, and CD47 deficiency on NK cells inhibits above activities. e. The CD47 ligand protein TSP-1 inhibits NK cell proliferation by binding CD47. CD47 regulates NK cell recruitment and activation. Targeting CD47 by antibody miap301 blocks the binding between TSP-1 and CD47 and enhances NK cell’s proliferation. f. CD47 modulates dysregulated neutrophil transmigration across epithelial surfaces. CD47 associates with leukocyte-specific integrin CD11b/CD18 in the plasma membrane of neutrophils, and the loss of CD47 results in impaired CD11b/CD18 activation.g. CD47 plays an essential role in the migration of neutrophils to the injured site. CD47 on the epithelial cells binding with SIPRα on the neutrophils regulates the adhesion function of neutrophils and helps them infiltrate into the tissue space through vascular endothelium. Disrupting CD47 or its binding signal pathway reduces neutrophils’ migration and infiltration.h. TSP-1 on T cell inhibits its activation, and the interaction of TSP-1 and CD47 on T cell promotes naïve T cells differentiation to regulatory T cells.i. Targeting CD47 by antibodies such as Ad22 induces T cell apoptosis promptly, and this process can be completed without TNFRI/p55, TNFRII/p75 or Fas signal pathway. j. By binding CD47, SIRPα enhances T cell proliferation and activates T cell. This may probably be due to the adhesion between T cell and APC cell together with TCR signal transduction. Inhibition of SIRPα and CD47 mAb reduces the proliferation of T lymphocytes.k. Blocking the interaction of CD47 on naïve T cells with TSP-1 prompts the generation of Tregs.l. The interaction between CD47 on B cell and SIRPα on macrophage and DC brings cell–cell interaction which is important to B cell maturation.m. CD19 and BCR co-transduce B cell signal to promote B cell activation and proliferation. The bispecific antibody targeting both CD19 and CD47 inhibits CD19 cluster to move to BCR and inhibits B cell proliferation finally.
CD47 and dendritic cells
DCs, as the main antigen-presenting cells, express both CD47 and SIRPα. As a self-marker of DC, CD47 not only modulates the migration, maturity, activation, and apoptosis of DCs, but also initiates immune responses.
CD47 regulates the activation, quantity, maturity, migration and apoptosis of DCs
CD47-SIRPα inhibits DC activation (Fig. 4a). CD47 on T cells interacting with SIRPα on DCs negatively regulates both DC and T cells. SIRPα on DCs inhibits phagocytosis. Inhibition of the CD47-SIRPα axis activates DCs to engulf tumor cells and presents tumor cell-related antigens to CD8+ T cells, thus exerting the specific killing effect of CD8+ T cells. Furthermore, CD47-SIRPα also affects DC quantity as there are a reduced number of DCs in DC-specific SIRPα knockout mice and fibroblastic reticular cells in the spleen [170].
CD47 regulates the maturity process of DCs (Fig. 4a). It inhibits the transformation of immature dendritic cells (iDCs) to mature cells [171]. Under resting conditions, iDCs are present in most tissues. Once exposed to microorganisms, they undergo maturational changes involving costimulatory molecules upregulation and inflammatory cytokines, thus becoming efficient stimulators of naive T cells. Ligation of CD47 to the surface of iDCs by a monoclonal antibody (mAb) counteracts the maturation of iDCs in both phenotype and function. However, it did not affect their phagocytic ability of the apoptotic cells [172].
CD47 regulates DC migration (Fig. 4a). CD47 selectively regulates DCs but not T or B cell migration in lymphatic organs by penetrating endothelial barrier in vivo. CD47-SIRPα interactions are necessary for skin DC migration under inflammatory conditions. When CD47 binds to TSP-1 and SIRPα, it inhibits not only the synthesis of cytokines stimulated by micro-organisms (Fig. 4b) but also the expression of the co-stimulatory proteins CD40, CD54, CD86 and CD80 on the surface of DCs, thus negatively regulating the phenotype and function of immature DCs. The inhibition of CD47 facilitates DC maturation and enhance antigen presentation by DCs, and eventually propagates the antitumor immunity against immunogenic cell death [173].
CD47 induced the rapid apoptosis of DC through TSP-1(Fig. 4c). The characterization is the same as that for the induction of apoptosis of monocytes. Monocyte-derived DCs undergo rapid and caspase-independent cell death. Therefore, CD47 mediates apoptosis-like cell death of human DCs [174].
CD47 participates in the initiation of DCs’ immune responses
CD47 plays a critical role in initiating an immune response in DCs. Although macrophages absorb tumor DNA more effectively, the increased DNA sensing by blocking CD47-SIRPα occurs preferentially on DCs rather than on macrophages (inhibition of cytoplasmic DNA sensing is a method for the immune escape of tumor cells). CD47 blocks the activation of the NADPH oxidase NOX2 in DCs, thus suppressing phagosome acidification and reducing the degradation of tumor mitochondrial DNA (mtDNA) in DCs. Consequently, tumor mtDNA is recognized by cyclic GMP-AMP synthase (cGAS) in the cytoplasm of DCs, thus promoting the production of type I interferons and anti-tumor adaptive immunity [175].
CD47 and neutrophil cells
Neutrophils are the most abundant leukocytes in the blood. Neutrophil migration and aggregation are important features of the inflammatory response. As the first-line cells of inflammatory response, neutrophils play an important role in infectious and aseptic inflammation [176], [177].
Trans-endothelial migration of neutrophils is an important process in inflammation. CD47 on neutrophils modulates their trans-epithelial migration and adhesion, and dysregulates neutrophil transmigration across epithelial surfaces. CD47 associates with leukocyte-specific integrin CD11b/CD18 in the plasma membrane of polymorphonuclear neutrophils (PMN), and the loss of CD47 leads to the inactivation of CD11b/CD18 [178] (Fig. 4f). Furthermore, the interaction between SIRPα on neutrophils or monocytes and CD47 on epithelial/endothelial cells plays an important role in cell migration across endothelium and the epithelial cells, particularly during blood cell exudation. Anti-CD47 antibody F(ab) 2 fragments of B6H12 strongly inhibits neutrophil trans-endothelial migration [179] (Fig. 4g).
In a mouse model of cerebral ischemia–reperfusion, the migration and adhesion of neutrophils to microvascular endothelial cells led to the infiltration of neutrophils into ischemic brain tissue. In contrast, the infiltration of monocytes and neutrophils into the brain tissue of CD47-deficient mice was significantly reduced. A possible reason for this is that CD47 regulates the adhesion function of neutrophils and helps them infiltrate the tissue space through the vascular endothelium, thus damaging local tissues through direct contact and the release of various cytokines. Simultaneously, inflammatory cells are collected by chemokines and continuously infiltrate the tissue space, resulting in the expansion of inflammation. In addition, in CD47 knockout mice, after intraperitoneal injection of Escherichia coli, the neutrophil mobilization is delayed. The deletion of CD47 can also eliminate the pathological reaction in the acute stage of colitis mediated by innate immunity. The mechanism may be that it damages the migration function of neutrophils and the expression of cytokine mRNA. Furthermore, CD47 also regulates human PMN chemotaxis as SIRPα regulates neutrophil transmigration in vitro.
CD47 in adaptive immunity
CD47 and T cells
CD47 signaling plays important roles in various processes including the activation, proliferation and differentiation of T cells to induce their death.
CD47 regulates the activation, differentiation, proliferation and apoptosis of T cells
CD47 in T cell activation functions as a costimulatory factor. The main role of CD47 in T cell activation is to augment the TCR signal transduction efficiency by inducing T cells to diffuse on the surface of antigen-presenting cells (APC). The synergistic effect of CD47 and TCR signal transduction may be due to their function in stimulating adhesion to the surface of expression ligand or APC rather than by initiating a new signaling cascade. The binding of CD47 on T cells and SIRPα on DCs can induce T lymphocyte activation by DCs. The most important feature of the CD47 costimulatory pathway is that the mutated antigen peptide (APL)/TCR molecule pair inhibits the activation of T lymphocytes. On the other hand, TSP-1 binding to CD47 inhibits T cell activation (Fig. 4h) [142].
The interaction of CD47 on T cells and SIRPα on DCs can promote the proliferation of T cells, whereas the inhibition of SIRPα and CD47 can reduce the proliferation of T lymphocytes (Fig. 4i). However, SIRPα antibodies such as SIRP-1 and SIRP-2 induce signal-agent phagocytosis of tumor cells while preserving T cells [180]. Blocking SIRPα overcomes T-cell exclusion and counteracts the cancer immunotherapy resistance [181].
CD47 regulates T-cell differentiation by regulating T-cells and APCs. The CD47-specific TSP-1 peptide suppresses the differentiation of Th17 cells and potentiates the generation of regulatory T (Treg) cells [182], [183] (Fig. 4h). Treatment with anti-CD47 or anti-TSP-1 antibodies promotes naïve T cells (CD4+CD25-) to differentiate into Tregs [184]. CD47/TSP-1 also inhibits T lymphocyte differentiation and development into Th1-type cells. CD47-null T cells exhibit elevated levels of Th1 differentiation compared to CD47-expressing cells [185].
CD47/TSP-1 interaction or blocking CD47 induces T cell apoptosis. Treatment with the anti-CD47 mAb Ad22 rapidly induced the apoptosis of T cells, independent of the ligation of Fas and TNF receptors (Fig. 4j). Apoptosis mediated by the Fas and TNF signaling pathways causes DNA fragmentation, whereas the CD47 pathway does not necessarily cause DNA fragmentation. Normal T cells need to be pre-activated to respond to CD47-induced apoptosis. However, the specific physiological role of CD47 in scavenging activated T cells via a new apoptotic pathway remains unclear. A recent study indicated that the expression of CD47 is critical for CD47-chimeric antigen receptor (CAR) T cells, CD47-deficient T cells are sensitive to macrophage-mediated phagocytosis [186].
CD47 regulates T cell immunity
CD47 regulates T-cell function by regulating other important genes including its binding genes TSP-1, and Fas (CD95), as well as other regulator genes in T cells.
CD47 has been treated as an inhibitory signaling receptor for TSP-1 on T cells which can limit T-cell receptor signaling and kill irradiated target cells [187]. CD47-TSP-1 binding inhibits TCR signal transduction and induces active T cells anergy. TSP-1 forms a costimulatory pathway with CD47 and CD36, which reduces the activation threshold of T lymphocytes and activates the proliferation of reactive T cells, leading to the occurrence of autoimmune diseases.
Fas is a member of the tumor necrosis factor receptor (TNFR) superfamily and is involved in the apoptotic signalling [188]. Fas was demonstrated as a lateral binding partner of CD47 in T cells [28]. The activation of Fas receptors on T cells by antibodies or Fas ligand binding induces cell death only in CD47 expressed cells [77]. The binding of CD47 to Fas was weak under normal conditions, but Fas activation increased the lateral interaction, as evaluated by co-immunoprecipitation and co-localization researches. This interaction is essential for Fas downstream signal transduction of Fas, including caspase-7 and PARP cleavage [189].
TCGA data indicate a positive correlation between CD47 and T-cell regulators including CTLA4 and its counter receptors CD86 and CD80. Inhibition of CD47 on T cells or in combination with anti-CTLA4 enhances antigen-dependent elimination of irradiated melanoma cells [190]. CD47 also negatively regulates the expression of vascular endothelial growth factor (VEGF)and VEGF2 in T cells, thus affecting anti-angiogenesis.
c. Therapeutic effect of CD47 blockade requires T cell function
The importance of CD47 in T cell function is clear as mentioned above. Conversely, blocking CD47 function requires T cells. Blocking SIRPα binding to CD47 has been demonstrated to augment the phagocytosis of syngeneic tumors in immune-deficient mice, but it is generally not sufficient, therefore, better immune stimuli are needed to induce CD8+ T cell-mediated tumor killing. The therapeutic effect of CD47 blocking is dependent on the cross-initiation of T-cell responses by dendritic cells rather than macrophages. In T-cell-deficient mice, the CD47 antibody has no therapeutic effect. In addition, some studies have shown that CD47 blocking anti-tumor effect requires the expression of a stimulator of interferon genes (STING) in the innate immunity [191].
CD47 and regulatory T cells
Treg cells are originally termed “suppressor cells” and play a key modulating role in suppressing autoimmunity and maintaining immune homeostasis [192]. CD47 on Tregs modulates Treg cell generation, proliferation, and differentiation and contributes to Tregs’ neuroprotection via interacting with its receptors SIRPα or TSP-1.
CD47 promotes Tregs generation
CD47 promotes Treg generation via binding to TSP-1 [182], [183]. In the absence of CD25+ cells, the TSP-1/CD47 interaction in primary naïve cells promoted Treg cell generation (Fig. 4k). When TSP-1 is released during an inflammatory response, it promotes the generation of peripheral Tregs that maintain the inflammatory process and prevent further collateral damage from antigens [184]. In melanoma, TSP-1 generates Tregs from naïve T cells through interaction with CD47. Patients with advanced melanoma exhibit higher level of TSP-1 and a higher proportion of peripheral Tregs that evade the immune system. In the presence of a CD47 antibody or 4N1K peptide (CD47 binding sequence derived from TSP-1), CD47 ligation on naïve T cells (CD4+CD25-) increased the expression of FoxP3, which is considered a hallmark of Tregs, thereby differentiating naïve T cells into Tregs [184].
CD47 contributes to Tregs proliferation
CD47 regulates the proliferation and expansion of Foxp3+Treg cells in vivo, and the continuous expression of CD47 functions in CD103+Treg homeostasis. CD47 ligation of human cord blood mononuclear cells induce naive T cell anergy and enhances the generation of peripheral CD103−Foxp3+ Tregs [184], [193].
High CD47 expression levels on the surface of peripheral Treg cells were observed in patients with atopic dermatitis (AD) compared to healthy controls. Higher CD47 expression in peripheral Tregs may prolong the life of these cells and lead to a larger population of Tregs in AD [194]. In another study, the addition of TSP-1 induced an increment significantly via TSP-1/CD47 interaction in the percentage of Tregs (CD25+FoxP3+) in psoriasis patients [195].
In CD47-deficient mice, CD103+Foxp3+ (CD44highCD62Llow) Tregs rapidly increased as compared to age-matched control littermates, while quiescent CD103−Foxp3+ (CD44lowCD62Lhigh) Tregs remained stable [196].
CD47 contributes to immune tolerance
CD47 on Tregs contributes to the induction of immune tolerance in donor-specific transfusion (DST) in skin-heart transplantation mice, and an elevated quantity of Treg cells contributes to immune tolerance induced by CD47+/+ DST [197]. Dual targeting CD47 and CTLA-4 on Treg cells delivers an “eat me signal” and increases Treg depletion and antitumor efficacy in solid tumors [198].
Tregs maintain the immune balance and facilitate self-tolerance in the central immune nervous system. Treg cells play a critical role in neuroprotection via suppressing microglial activation in Parkinson’s disease. They protect dopaminergic neurons against 1-methyl-4-phenylpyridinium (MPP+) neurotoxicity through the interaction of CD47 on Tregs and SIRPα on Primary ventral mesencephalic neurons [199]. Disrupting of CD47-SIPRα signaling compromises Treg cell neuroprotection.
CD47 and B cells
B cells, derived from hematopoietic stem cells (HSC) in the bone marrow, are present at different densities and locations in the tumor microenvironment (TME) of different cancer types. Tumor-infiltrating B cells facilitates T cell-mediated antitumor immunity [200].
CD47 is expressed in B cells and limits antibody-mediated phagocytosis. The depletion of mature B cells improves clinical outcomes in various autoimmune diseases. B cell depletion via anti-CD19 treatment in vivo was more efficient in CD47-/- mice than in wild-type mice [201].
CD47-SIRPα interaction is involved in the adhesion and recruitment of B lymphocytes through endothelial cells. The interaction between CD47 on B cells and SIRPα on macrophages or DCs also plays a role in the cell–cell contact between B lymphocytes and macrophages or DCs, which is essential for the differentiation of B cells. In addition, CD47 on B lymphocytes showed a distinct affinity for SIRPα during B cell maturation, suggesting the function of CD47-SIPRα in B cell maturation (Fig. 4l).
CD47 inhibits the growth of B cells. CD47 expression is induced in Epstein-Barr virus (EBV)-transformed B cells by EBV infection and CD47 ligation suppresses the growth of EBV-transformed B cells, and induces cell cycle arrest at G1 phase.
CD47 regulates the motility of human B-lymphocytes through CDC42. In addition, a bispecific antibody targeting CD47 and CD19 abrogates B-cell receptor/CD19 interaction, resulting in the termination of B-cell proliferation (Fig. 4m). CD19 is a specific receptor of B cells. Monoclonal antibodies targeting CD19 can inhibit the proliferation of B cells, whereas those targeting CD47 alone do not. Interestingly, when bispecific antibodies were used to target both CD19 and CD47, the inhibitory effect was stronger than that of CD19 monoclonal antibodies. The main reason is that targeting CD19 and CD47 prevents the migration of the CD19 cluster to B Cell Receptor (BCR) cluster, while the use of CD19 mAb has no effect on the migration of CD19 cluster [202].
In conclusion, CD47 signaling plays a critical role in immune cells’ biological processes such as activation, proliferation and apoptosis. Conversely, blocking CD47 or combining it with other drugs requires both innate and adaptive immune responses. For example, combination treatment with the CD47 antibody and blinatumomab can enhance efficacy through the combination of both innate and adaptive immune responses by inducing phagocytosis and T cell cytotoxicity in human non-Hodgkin lymphoma cell-engrafted mice.
Role of CD47 in cancer and tumor microenvironment
CD47 is highly expressed in many cancers, associated with patient survival and has the potential to predict immune responses to immunotherapy. Targeting of CD47 cooperates with all other elements of the TME. Here, we summarize the role of CD47 in various cancers and its relationship with the TME.
Role of CD47 in cancer
CD47 overexpression has been observed in many cancers and is correlated with a poor prognosis and short survival time. This inspired the study of CD47 as a therapeutic target in cancer. CD47 contributes to tumor initiation and metastasis. Blocking CD47 expression suppresses the stem cell characteristics of hepatocellular tumor-initiating cells. Next, we reviewed the roles of CD47 in hematopoietic tumors and solid tumors, while investigating the regulatory function of CD47 in other diseases.
CD47 in hematological neoplasms
Increasing evidence indicates that CD47 is overexpressed in hematological cancers and its binding with SIRPα on monocytes inhibits the phagocytosis of cancer cells and helps tumor evasion of immune surveillance [203]. CD47 overexpression is closely associated with poor clinical outcomes in various hematological neoplasms [155]. It has been demonstrated that the combination of antibodies targeting CD47, and other hematological surface markers exerts synergistic effects [179].
CD47 in lymphoma
CD47 in B-cell lymphomas
CD47 is widely present in B-cell lymphomas, including diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL) [204], [205]. CD47 is overexpressed in DLBCL and can be downregulated by berberine via suppressing c-Myc. Phagocytosis was potentiated when CD47 expression was suppressed in DLBCL cells. Co-expression of CD47 and SIRPα is a prognostic factor for DLBCL [206]. Berberine-induced CD47 inhibition potentiated phagocytosis by macrophages, thus clearing DLBCL cells in vivo and in vitro [207]. In Burkitt’s lymphoma (BL), tumor cells dissemination relies on CD47 expression, which can be inhibited by blocking the CD47-SIRPα pathway. Disrupting the binding between CD47 and SIRPα by the monoclonal antibody KWAR23 inhibits tumor growth in a BL mouse modal [208]. CD47 engagement by CD47 mAb promotes rapid apoptosis of CLL tumor cells independent of caspase pathways, such as decreased mitochondrial transmembrane potential, phosphatidylserine translocation and DNA fragmentation [209].
CD47 in T-cell lymphoma (TCL)
TCL is an uncommon NHL form of non-Hodgkin lymphoma with an aggressive clinical course[210]. CD47 is variably expressed in human TCL cells. Targeting the CD47-SIRPα interaction enhanced phagocytosis and improved survival time in a patient-derived xenograft model. Furthermore, CD47 potentiated TCL metastasis by enhancing the AKAP13-mediated RhoA activation [211]. Patients holding a higher CD47 expression have a worse life span than those patients with lower CD47 expression [109], [212], [213], [214]. It has been demonstrated that major histocompatibility complex class I (MHC-I) in TCL suppresses the phagocytosis of tumor cells. Targeting CD47 in combination with an antibody targeting MHC class I(W6/32) exerted a synergistic effect on TCL [215].
CD47 in non-Hodgkin lymphoma
Malignant lymphomas are classified as Hodgkin's disease and NHL [216]. NHL is much less predictive than Hodgkin’s [217]. Chemotherapy is the traditional treatment for NHL is chemotherapy and rituximab has emerged in the era of the NHL immunotherapy [218]. CD47 expression is increased in NHL cell lines, and the high CD47 expression indicates a worse clinical prognosis. Therefore, CD47 has been demonstrated to be a prognostic factor and therapeutic target for NHL [204].
CD47 in myeloid hematoma
CD47 is also expressed ectopically in myeloid malignancies, resulting in the escape of tumors from phagocytosis by macrophages [219]. Pre-clinical data have indicated that CD47 blockade has potent anti-cancer activity in multiple hematologic cancers such as acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) [219], [220].
CD47 in acute myeloid leukemia (AML) and chronic myeloid leukemia (CML)
The expression of CD47 is increased in AML/CML leukemia stem cells (LSC) and increased CD47 expression has been identified as a marker of LSC in AML [221]. CD47 is closely associated with FLT3-ITD mutations, which lead to the shortest lifespan with normal cytogenetics.
CD47 in multiple myeloma (MM)
It has been found that 80 % of patients with MM express CD47 and elevated TSP-1 or TSP-2, indicating that CD47 and TSP-1 or TSP-2 may have a potential role in the pathophysiology of MM, probably through the interaction between MM plasma cells and the microenvironment [222]. In the bone marrow, MM cells express CD47 remarkably higher than other cell types. It can be treated as a potential therapeutic target and blocking CD47 leads to macrophage activation and MM cell elimination [223]. High CD47 expression in patients with MM is associated with p53 deficiency and increased β-2 macroglobulin level [111].
CD47 in solid tumors
CD47 in lung cancer
CD47 is highly expressed in both Small-cell lung cancer (SCLC) and NSCLC [224]. CD47 overexpression correlates with decreased neutrophil apoptosis and phagocytosis in NSCLC [225], [226]. CD47 is considered a therapeutic target and an independent prognostic factor for the recurrence of SCLC and NSCLC [50], [227], [228]. The disruption of the CD47-SIRPα signal pathway markedly inhibits SCLC tumor growth and targeting CD47 by antibody or siRNA has become a promising immunotherapeutic method for NSCLC. In EGFR-mutant NSCLCs, the augmented CD47expression is related to off-target resistance to gefitinib, a tyrosine kinase inhibitor (TKI) [229]. Inhibition of EGFR significantly reduced CD47 expression on the surface of pro-apoptotic cells in EGFR-mutant mutations. Inhibition of CD47 enhances the elimination of EGFR-TKI-resistant cells by phagocytes [230].
CD47 in glioblastoma (GBM)
CD47 is highly expressed in GBM cells. GBM cells with high levels of CD47 exhibit stem cell characteristics and poor clinical outcomes [231]. CD47 knockout (KO) significantly increases phagocytosis of tumor cells by macrophages in GBM, increases tumor-associated extracellular matrix protein tenascin C (TNC) in xenografts and promotes phagocytosis [232].
Currently, standard therapies for GBM include irradiation and temozolomide (TMZ) chemotherapy [233]. A recent study indicated that irradiation or TMZ significantly augmented anti-CD47-mediated phagocytosis of GBM cells in vitro and in vivo, which may support the future of targeting in combination with irradiation or chemotherapy to augment GBM treatment efficacy [5], [234]. Dual targeting of CD47 and PD-L1 nanoparticles enhanced the phagocytic ability of macrophages in GBM [235]. Moreover, blocking the CD47-SRIPα axis and knocking down soluble LRIG2 synergistically suppress GBM growth in a mouse model [236]. In addition, inhibition of TSP-1 and CD47 interactions with an antagonist peptide attenuated GBM cell invasion [237].
Mechanistically, the therapeutic regulation of phagocytosis in GBM activates both the innate and adaptive anti-tumor immunity [238]. Using orthotopically xenografted mouse models, Hutter et al. found that microglias are effector cells for cancer cell elimination by phagocytosis in response to an anti-CD47 blockade in GBM when phagocytizing macrophages are absent [239]. Blocking CD47 suppresses microglia-dependent phagocytosis and monocyte transition into macrophages [240].
In addition to the CD47-SIRPα and TSP-1/CD47 pathways, other CD47-related pathways may contribute to GBM therapy. The co-enhancement of mitochondrial FAO enzymes and CD47 is dominant in patient with recurrent GBM with poor prognoses. The FAO-CD47 pathway may be a target for increasing GBM control by clearing the radioresistant phagocytosis-proofing cancer cells during GBM radioimmunotherapy [153].
CD47 in breast cancer
Breast cancer is the most common cancer in women, and its incidence and mortality rates have increased significantly in recent years [241]. Breast cancer is divided into the following subtypes according to its molecule characteristics: Luminal A, Luminal B, HER 2+, Triple-negative breast cancer (TNBC), and basal-like type [242]. Patients with BC with high CD47 levels have poor survival rates [243]. High CD47 expression may be a key factor in CSC resistance to chemotherapy [244]. Metformin treatment of breast CSCs leads to a significant increase in miR-708 expression, then inhibits the expression of CD47 and ultimately exerts a positive effect on breast cancer chemotherapy [245]. CD47 is enhanced in HER2-expressing cells preferentially, and the interaction between CD47 and HER2 can be shown by the different CD47 expression levels in HER2+ versus HER2− breast cancer cells.
In TNBC, intra-tumoral co-injection of the STING ligand cGAMP and CD47 antibody inhibits tumor growth by promoting macrophage phagocytosis of cancer cells and inducing a systemic anti-tumor immune response, which relies on STING and type I IFN signalling [243]. Taken together, all these results suggest that a combination of blocking CD47 with other drugs or molecules could be a potentially effective therapeutic strategy for the treatment of breast cancer.
CD47 in ovarian cancer
CD47 plays a crucial role in endometriosis-associated ovarian cancer (EAOC). High CD47 expression was positively and significantly associated with ovarian cancer grade and poor prognosis. CD47 inhibits phagocytosis and enhances cell proliferation and migration in EAOC, and decreased CD47 levels can reverse these negative effect [246]. Furthermore, low CD47 expression in ovarian cancer stem-like cells protected by surrounding bulk tumor cells indicate immune vulnerability [247]. Therefore, CD47 may be a useful surface marker and a potential therapeutic target in ovarian cancer [248].
CD47 and hepatocellular carcinoma (HCC)
CD47 is over-expressed in conventional HCC, but not in the fibrolamellar subtype of HCC (FL-HCC) [249]. CD47 is closely associated with pathological features and poor clinical outcomes in patients with HCC [250]. Macrophages induce the upregulation of CD47 in HCC via IL-6 and are associated with poor prognosis [106].
Macrophage-mediated phagocytosis of HCC cells is upregulated after blocking CD47. Targeting CD47 has a potential immunotherapeutic efficacy against human HCC. 4-methylumbelliferone attenuates the expression of CD47 in hepatic cancer stem cells and provides a robust anti-tumor T cell response induced by Interleukin-12 [251]. A bispecific antibody targeting GPC3 and CD47 exerts potentiated anti-tumor efficacy against dual antigen-expressing HCC [252]. Mechanistically, the METTL3/IGF2BP1/CD47 pathway may be a potential therapeutic target because it functions in sublethal heat treatment induced mesenchymal transition in HCC [253].
Blocking the TSP-1/CD47 pathway also suppresses HCC growth. In a spontaneous mouse HCC model, treatment with the HDAC6 plasmid AS-let-7i-5p and recombinant TSP-1 inhibited neoplastic and antiphagocytic behavior of HCC by interacting with CD47 in the TME [128].
CD47 and cholangiocarcinoma (CCA)
CD47 is more highly expressed in cholangiocarcinoma (CCA) than in HCC. Interruption of the CD47-SIRPα axis augments macrophage phagocytosis in all macrophage subtypes and consequently inhibits CCA growth and metastasis [254].
CD47 and tumor microenvironment
The TME contains a variety of tumor-infiltrating cells that interact with tumor cells through a complex network. The cells include tumor cells, endothelial cells immune cells, fibroblasts, soluble signals and extracellular matrix (ECM) [255], [256]. The TME is crucial for tumor progression and metastasis [257], [258]. Single-cell sequencing data suggest that the expression of CD47 varies significantly among different cell types in the TME, with the highest expression in tumor cells [259], which is required for establishing tumor metastasis [155].
CD47 on tumor cells facilitates their immune escape in TME
Mouse tumor models and patient samples have indicated that macrophages accumulate significantly in the TME. Experimental data have shown that CD47 directly inhibits the phagocytosis of tumor cells by macrophages. Targeting CD47 exerts tumor-suppressive effects by enhancing macrophage phagocytosis of tumor cells. Treatment of mice with clodronate reversed this effect, partly supporting the hypothesis that CD47 enhances tumor cell immune escape by antagonizing macrophage phagocytosis [19].
Tumor-associated macrophages (TAMs) are immune infiltration macrophages abundant in most solid tumors. The phagocytic function of TAM has been verified as a crucial factor contributing to tumor progression control and correlates with the TME [187]. Blocking CD47 or SIRPα with antagonistic antibodies upregulates the phagocytic activity of TAM and restrains tumor growth. This approach has demonstrated promising results in glioblastoma [260], melanoma, lymphoma [208], breast [261], colorectal cancer [262] and other tumor types. In breast cancer, increased expression of CD47 is closely related to the infiltration of Tregs and TAMs into the TME. Tregs and TAMs help create an inhibitory immune microenvironment that promote tumor progression [112], [263].
Phagocytosis of tumor cells causes the secretion of cytokines and chemokines that recruit other immune cells to the TME to promote adaptive immune responses. For instance, treating with anti-CD47 antibodies of colon cancer models promotes the proliferation of antigen-specific CD8+ T cells and macrophage phagocytosis, thereby reducing the number of Treg cells, indicating that anti-CD47 treatment promotes adaptive immune responses.
Furthermore, CD47 is closely related to T-cell exhaustion markers such as PD-1 and CTLA-4. These results suggest that T cells lose their ability to fight tumors in the TME when CD47 is highly expressed. In summary, CD47 may help tumor cells achieve immune escape by affecting T cell exhaustion, immune cell infiltration and changing the TME [259].
CD47/TSP-1 interaction affects TME
TSP-1 is a component of particles secreted by immune cells that modulates metabolism in the TME and induces resistance to cancer treatments [264]. The function of TSP-1 in TME has been previously reviewed [264]. CD47 is a receptor of TSP-1. TSP-1 and CD47 expression level vary depending on the TME. Under hypoxia, the expression and interaction of TSP-1 and CD47 were upregulated to enhance tumor growth.
Strategies and clinical progress of targeting CD47 in cancer immunotherapy
Because CD47 is highly expressed in various tumors and blocking CD47-SIRPα interactions promotes the phagocytosis of cancer cells by macrophages and other myeloid cells [23], CD47 has been treated as a novel potent macrophage immune checkpoint in cancer immunotherapy. Correspondingly, research and clinical trials targeting CD47 and its related pathways have attracted increasing attention from scientists, companies, and all others.
CD47-SIRPα as an immune checkpoint blockade in cancer immunotherapy
CD47-SIRPα as an immune checkpoint has been reviewed [47], [265], [266], [267]. CD47 is highly expressed in various cancer cells and functions as a key antiphagocytic molecule that renders tumor cells resistant to the host immune surveillance [266], [268], [269]. In CSCs, high expression of CD47 is predicted to protect CSCs from SIRPα-dependent macrophage clearance [270]. Blocking CD47 with antibodies promotes macrophage phagocytosis of tumor cells and reduces tumor size [19], [20], [271]. CD47-blocking therapies stimulate macrophage cytokine secretion and thus stimulating the patient’s immune system to attack and eliminate cancer cells. The CD47-SIRPα pathway is a phagocytosis checkpoint in macrophages and other innate immune cells, and CD47 has been demonstrated as an interesting therapeutic target due to its anti-phagocytosis function on tumor cells.
Targeting CD47-SIRPα not only destroys the binding of CD47 and SIRPα and enhances the phagocytic ability of macrophages to tumor cells [19], [204], but also kills cancer cells via the NK cell-mediated ADCC (antibody-dependent cell-mediated cytotoxicity) effect [272], even directly inducing tumor cell apoptosis. Targeting CD47 also promotes dendritic cells to phagocytose tumor cells, thus leading to tumor antigen presenting to T cells and the activation of an adaptive immune response.
Development of CD47 antibodies and their clinical application
In the 1980 s, CD47 was first identified as a tumor antigen in human ovarian cancer, and was found to be highly expressed in hematopoietic tumors and other solid tumors such as ovarian, breast, and colon tumor, etc. CD47 transduces inhibitory signals via SIRPα on macrophages and other myeloid cells. High CD47 expression is positively correlated with poor clinical prognosis and adverse molecular features in multiple cancer types.
Progression of CD47 antibody development
Many antibodies targeting CD47 have been developed in clinical trials. It has been shown that anti-CD47 is effective against many tumor types. Targeting CD47 with an anti-CD47 antibody alone or in combination with another molecule or antibody augments therapeutic efficacy. The CD47 antibody was initially used in malignant brain tumors including GBM in children [260]. The CD47 antibody Hu5F9 exhibited robust effects in children with brain tumors. Hu5F9-G4 is the 1st humanized antibody targeting CD47, and can be used to treat a variety of central nervous system malignant tumors. Furthermore, Hu5F9-G4 inhibited tumor growth in SCLC cells in vivo.
SIRPαD1-Fc, a novel fusion antibody targeting CD47, enhances the autophagy of NSCLC cells by inhibiting Akt/mTOR signaling and improving ROS quantity. Inhibition of autophagy enhances macrophage-mediated phagocytosis and cytotoxicity against SIRPαD1-Fc-treated NSCLC [147], [273]. The combined approach of targeting autophagy and CD47 with SIRPαD1–Fc demonstrated significant suppression of NSCLC xenografts growth, leading to increased apoptosis and macrophage recruitment in vivo in NCI-H1975 cells. Therefore, autophagy plays an important role in CD47 targeting NSCLC therapy [147]. In addition, a glutamine-rich carrier that efficiently delivers anti-CD47 siRNA suppresses cancer cell growth in lung cancer [274].
Further studies showed that the CD47 antibody B6H12 treatment of breast cancer cell lines MDA-MB-231 and T47D effectively reduced the proliferation efficiency and rate of the asymmetric division [127]. After treatment with the CD47 monoclonal antibody B16H12, the expression levels of EGFR and KLF4 in breast CSCs were decreased remarkably. Theoretically, this type of synergistic effect could produce a good therapeutic effect on tumors [127]. In addition, in a mice-engrafted MM model, anti-CD47 mAb B6H12 inhibited myeloma cell growth and led to obvious tumor suppression and eradication. B6H12.2 and BRIC126 preferentially potentiated the phagocytosis of leukemia stem cells.
Progression of combined therapy targeting CD47
Targeting CD47-SIRPα has been demonstrated to exert therapeutic effects in many tumor models and combining it with other treatments may result in better efficacy. Common combination therapies include chemotherapy and radiotherapy [275], recruitment of macrophages, treatment with other therapeutic antibodies, and inhibition of tumor metastasis. The improved efficacy of the combination therapy may be attributed to the following mechanisms:
The additive effect of antibodies targeting CD47 and other antigens
Combination of CD47 targeting and FCR activation antibodies
The CD47 antibody functions with other tumor-targeted antibodies to amplify the therapeutic effect through different mechanisms. The main mechanism of the CD47 antibody is to potentiate the phagocytic ability of macrophages by destroying the binding effect of CD47-SIRPα, which is independent of the FC regulation of the antibody. Therefore, the ability to kill tumor cells can be enhanced by binding to another antibody to stimulate FC-regulated functions, such as ADCC or FCR-regulated phagocytosis. Similarly, the SIRPα antibody and trastuzumab can augment the curative effect on breast cancer by enhancing the ADCC effect. These findings suggest that antibodies targeting CD47-SIRPα and some FCR-activated antibodies exert better therapeutic effects in various tumor models.
Combination of CD47 targeting and other tumor-specific antigen targeting therapy
Dual antigen targeting binds to a single molecule in the form of a bispecific antibody, which not only blocks CD47, but also binds to tumor-specific antigens. Considering that CD47 is expressed on both normal and tumor cells, this bispecific antibody reduces potential off-target toxicity and achieves specific clearance of tumor cells.
In hematological neoplasms, dual blockade of hematological surface markers and CD47 have been demonstrated to have synergistic effects. Specifically-designed antibodies particularly those targeting neoplastic cells, can also prevent the adverse effects, including anemia or thrombocytopenia as CD47 is relatively highly expressed on RBCs and platelets [276]. NI-1701, which specifically and effectively targets both CD19 and CD47, showed a more robust anti-tumor effect than either antibody alone in mice. NI-1701 was much more effective than rituximab monotherapy; however, the combination of rituximab and NI-1701 showed a synergistic effect in NHL. Another example comes from the combination of sodium stibogluconate (SSG) with blocking the CD47-SIRPα axis, which effectively counterbalances the resistance of anti-CD20-opsonized B-cell lymphoma cells to neutrophil killing [277]. The combination of Hu5F9-G4 (Magrolimab) and Azathioprine (AZA) has exhibited a safe and curative effect in clinical trials of AML [21], [278]. Magrolimab plus AZA has shown efficacy in AML patients with TP53 mutations [279], [280].
Previous studies have shown that targeting both PD-L1 and CD47 in melanoma, lung cancer, colon carcinoma and syngeneic tumor models boosts anti-tumor effects [4], [104], [281], [282], [283]. RRx-001 is a small molecule inhibitor of Myc that negatively regulates CD47 expression on SCLC cells. Treatment of patients with SCLC with RRx-001 attenuates CD47 and PD-L1 expression and significantly correlates with clinical benefit [284]. RRX001 (Abdnaz) developed by EpicentRx Inc., is in phase I/II clinical trials for various cancers.
Another bispecific anti-human CD70/KWAR23 antibody exhibited a synergistic effect in renal carcinoma cell lines compared to any single targeted drug [208]. Bispecific κλ bodies, which targets tumor-associated antigen and selective CD47 (CD47-neutralizing κλ bodies), selectively target cancer cells and bind with healthy cells weakly, partially overcoming tolerability and “antigen sink” issues arising from wide expression of CD47 in normal tissue [285].
In NSCLC, the combination of targeting of both CD68 and CD47 exhibits excellent results in predicting the NSCLC patients’ survival [286]. In breast cancer, dual inhibition of HER2 and CD47 clear cancer cells with radio-resistance [287]. The combination of CD47 blockade and Trastuzumab (an antibody against HER2) dramatically improves the survival of patients with HER2+ breast cancer [288]. Furthermore, in GBM, the combination of using CD24 and CD47 antibodies offers an additive effect compared with either treatment alone [289].
Combination of inhibition of CD47 and enhancement of macrophage
The CD47 antibody combined with other reagents enhances macrophage function.. For example, Hu5F9-G4 combined with the anti-CD20 antibody rituximab potentiates macrophages to recognize and attack cancer cells in NHL [290], [291]. Therefore, tumor cells are removed by an increased number of macrophages combined with anti-CD47 antibodies, and macrophage stimulating cytokines, including M−CSF and GM-CSF. These cytokines exert an anti-tumor effect in cancers when combined with antibodies. In addition, the combination of hydrogel loaded with the functionalized PAMAM/shRNA complex downregulates CD47 and increases macrophage infiltration and prolongs the survival of GBM mice [292]. The combination of targeting CD24 and CD47 offers an additive effect against GBM compared with either treatment alone [289].
Combination of CD47 antibody and chemoradiotherapy
The combination of chemoradiotherapy and CD47 antibodies clears tumor cells through two different mechanisms. Chemotherapy increases the number of effector macrophages before the injection of the CD47 antibody because chemotherapy triggers an inflammatory response that allows macrophages to be recruited to tumor cells. Chemotherapy also induces a phagocytic signal and further enhances phagocytosis induced by the CD47 antibody. Radiotherapy and chemotherapy enhance the pro-phagocytic signals on tumor cells, such as calreticulin (acting as an “eat me” signal). For example, in GBM, RRx-001 targeting CD47 increased the uptake and accumulation of TMZ in an orthotopic GBM model, thus improving the outcome of the cancer therapy [293]. Finally, CD47 was also used as an adjuvant in conventional therapy to inhibit tumor metastasis. CD47 promotes tumor metastasis, and CD47 antibody inhibits the metastasis of NHL and some solid tumors to peripheral blood and other locations. Therefore, the injection of the CD47 antibody to prevent tumor metastasis plus surgical resection is an ideal treatment.
The clinical application progress of targeting CD47 in cancer immunotherapy
Targeting the CD47-SIRPα pathway effectively inhibits the development of multiple tumors. Many companies have developed monoclonal antibody drugs targeting this pathway. So far, a few clinical trials have been conducted to access the feasibility of blocking the interaction of CD47-SIRPα drugs. At present, the main clinical trials are conducted on leukemia, lymphoma and some solid tumors [9]. Some clinical trials are in the phase I stage, the main purpose of which is to determine the maximum tolerable dose of the drug and the dose toxicity of the treatment regimen, whereas others are in phase II which aims to test the safety and efficacy of drugs. In addition, some clinical trials have evaluated the clinical effects of blocking anti-phagocytic signals alone or in combination with other drugs and obtained exciting results.
These drugs block the interaction of CD47-SIRPα in a variety of ways, including anti-CD47 or anti-SIRPα monoclonal antibodies targeting binding sites, using recombinant proteins such as the extracellular domain of CD47 or SIRPα, to compete with endogenous proteins. Drugs targeting the regulation of CD47 transcription or transport pathways to inhibit the expression of CD47 in tumor cells [27]. Here, we summarize the large body of preclinical and clinical drugs designed to target CD47-SIRPα interactions (Table 3 and Table 4).
Table 3.
CD47-targeting drugs in the clinical trial and investigational new drug (IND) stage (Please refer the separate excel).
| Company Name (sponsor) | Drug name | main component | Target | Disease | Clinical phase | Combination drug | single-drug therapy or combination therapy | National Clinical Traial Number (NCT NO.) |
|---|---|---|---|---|---|---|---|---|
| (a) CD47-targeting drugs in clinical trial (registered in US) and IND stage | ||||||||
| Akeso | AK117 | Monoclonal Antibody | CD47 | MDS | Phase 1/2 | Azacitidine | combination therapy | NCT04900350 |
| AML | Phase 1/2 | Azacitidine | combination therapy | NCT04980885 | ||||
| Advanced Malignancies | Phase 1/2 | AK112/Chemotherapy | combination therapy | NCT05214482 | ||||
| Advanced Malignancies | Phase 1/2 | AK112/Carboplatin/Cisplatin/5-Fluorouracil | combination therapy | NCT05229497 | ||||
| Advanced Malignancies | Phase 1/2 | AK104/Capecitabine tablets/Oxaliplatin/Cisplatin/Paclitaxel/Irinotecan/Docetaxel/5-FU | combination therapy | NCT05235542 | ||||
| Neoplasms Malignant | Phase 1 | single-drug therapy |
NCT04728334/ NCT04349969 |
|||||
| ALX Oncology | ALX148/evorpacept | Fusion protein | CD47 | Gastric Cancer | Phase 2/3 | Trastuzumab/Ramucirumab/Paclitaxel | combination therapy | NCT05002127 |
| NHL | Phase 1/2 | Lenalidomide/Biological: Rituximab | combination therapy | NCT05025800 | ||||
| MDS | Phase 1/2 | Azacitidine | combination therapy | NCT04417517 | ||||
| AML | Phase 1/2 | Venetoclax/Drug: Azacitidine | combination therapy | NCT04755244 | ||||
| Head and Neck Cancer | Phase 2 | Pembrolizumab/Cisplatin/Carboplatin; 5FU | combination therapy | NCT04675333 | ||||
| Head and Neck Cancer | Phase 2 | Pembrolizumab | combination therapy | NCT04675294 | ||||
| MSS Metastatic Colorectal Cancer | Phase 2 | Cetuximab/Drug: Pembrolizumab | combination therapy | NCT05167409 | ||||
| Solid Tumor/NHL | Phase 1 | Pembrolizumab/Trastuzumab/Rituximab/Ramucirumab + Paclitaxel/5-FU + Cisplatin | combination therapy | NCT03013218 | ||||
| Arch Oncology | AO-176 | Monoclonal Antibody | CD47 | Solid Tumor | Phase 1/2 | Paclitaxel/Pembrolizumab | combination therapy | NCT03834948 |
| Multiple Myeloma | Phase 1/2 | Dex/Dex + Bort | combination therapy | NCT04445701 | ||||
| Bio-Thera Solutions | BAT7104 | bispecific antibody | CD47/PD-L1 | Solid Tumor | Phase 1 | single-drug therapy | NCT05200013 | |
| Chia Tai Tianqing | TQB2928 | Monoclonal Antibody | CD47 | Advanced Malignancies | Phase 1 | single-drug therapy | NCT05192512 | |
| Elpiscience | ES004 | Monoclonal antibody | SIRPα | Malignant tumor | IND | / | ||
| EpicentRx | RRx-001 | Small molecular | CD47/SIRPα axis | Small Cell Lung Cancer | Phase 3 | Cisplatin/carboplatin plus etoposide | combination therapy | NCT03699956 |
| Colorectal Neoplasms | Phase 2 | Regorafenib/Irinotecan | combination therapy | NCT02096354 | ||||
| Solid Tumor | Phase 2 | Cisplatin/Cisplatin/Etoposide/Carboplatin/Irinotecan/Vinorelbine/Doxil/Gemcitabine/Taxane/Paclitaxel/ Nab-Paclitaxel/Pemetrexed | combination therapy | NCT02489903 | ||||
| Oral Mucositis | Phase 2 | Cisplatin | combination therapy | NCT03515538 | ||||
| Cholangiocarcinoma | Phase 2 | Gemcitabine and cisplatin | combination therapy | NCT02452970; erminated (Resensitization or clinical benefit was not observed) | ||||
| Brain Metastases | Phase 1 | WBRT | combination therapy | NCT02215512 | ||||
| Solid Tumor/Lymphoma | Phase 1 | single-drug therapy | NCT02096341 | |||||
| Progressive Malignant Solid and Central Nervous System Tumors (PIRATE) | Phase 1 | Temozolomide/ Irinotecan | combination therapy | NCT04525014 | ||||
| Metastatic or Advanced Cancer | Phase 1 | Irinotecan | combination therapy | NCT02801097 | ||||
| Solid Tumor/Lymphoma | Phase 1 | Nivolumab | combination therapy | NCT02518958 | ||||
| Glioblastoma and Anaplastic Gliomas | Phase 1 | Temozolomide/TMZ | combination therapy | NCT02871843 | ||||
| Solid Tumor/Lymphoma | Phase 1 | single-drug therapy | NCT01359982 | |||||
| Conjupro | CPO107 | bispecific antibody | SIRPα/CD20 | CD20 Positive NHL | Phase 1/2 | single-drug therapy | NCT04853329 | |
| GeneScience | Gentulizumab | Monoclonal Antibody | CD47 | Solid Tumor/NHL | Phase 1 | single-drug therapy | NCT05221385 | |
| CD47 | AML/MDS | Phase 1 | single-drug therapy | NCT05263271 | ||||
| Gilead Sciences | Magrolimab | Monoclonal Antibody | CD47 | HL | Phase 2 | Drug: Pembrolizumab/Procedure: PET/CT | combination therapy | NCT04788043 |
| MDS/AML | Phase 1/2 | Drug: Sabatolimab/Drug: Azacitidine | combination therapy | NCT05367401 | ||||
| Solid Tumor | Phase 1 | single-drug therapy | NCT02216409 | |||||
| Hematological Malignancies | Phase 1 | Drug: Azacitidine | combination therapy | NCT03248479 | ||||
| Lymphoma | Phase 1 | Drug: Obinutuzumab/Drug: Venetoclax | combination therapy | NCT04599634 | ||||
| Hengrui Pharmaceuticals | SHR-1603 | Monoclonal Antibody | CD47 | Nasopharyngeal Carcinoma | Phase1 | single and combined withGemcitabine/Cisplatin/Albumin Paclitaxel | single-drug therapy and combination therapy | NCT04282070 |
| Solid Tumor | Phase 1 | single-drug therapy | NCT03710265 | |||||
| I-MAB | TJC4 | monoclonal antibody | CD47 | AML/MDS | Phase1 | Lemzoparlimab/Azacitidine/Venetoclax | combination therapy | NCT04912063 |
| Multiple Myeloma | Phase1 | Lemzoparlimab/Dexamethasone/Carfilzomib/Pomalidomide/Daratumumab | combination therapy | NCT04895410 | ||||
| TJ-011133 (Lemzoparlimab) | Solid Tumor | Phase 1/2 | toripalimab | combination therapy | NCT05148533 | |||
| AML/MDS | Phase 1/2 | single-drug therapy | NCT04202003 | |||||
| MDS | Phase 1 | Azacitidine/Venetoclax | combination therapy | NCT04912063 | ||||
| Multiple Myeloma | Phase 1 | Single or combined with examethasone/Carfilzomib/Pomalidomide/Daratumumab | single-drug therapy and combination therapy | NCT04895410 | ||||
| Solid Tumor/Lymphoma | Phase 1 | Pembrolizumab/Rituximab | combination therapy | NCT03934814 | ||||
| MDS | Phase 1 | Azacitidine/Venetoclax | combination therapy | NCT04912063 | ||||
| ImmuneOncia | IMC-002 | Monoclonal Antibody | CD47 | Advanced Malignancies | Phase 1 | single-drug therapy | NCT05276310/NCT04306224 | |
| ImmuneOnco Biopharma | IMM-01 | Fusion protein | CD47 | AML/MDS | Phase 1/2 | Azacitidine | combination therapy | NCT05140811 |
| IMM-2505 | bispecific antibody | PD-L1/CD47 | Advanced Malignancies | IND | single-drug therapy | IND | ||
| IMM2902 | bispecific antibody | CD47/SIRPα | HER2-expressing Advanced Solid Tumor | Phase 1 | single-drug therapy | NCT05076591 | ||
| IMM0306 | bispecific antibody | CD47/CD20 | B-NHL | Phase 1 | single-drug therapy | NCT04746131 | ||
| Innovent | SG2501 | bispecific antibody | CD38/CD47 | Hematological Malignancy | Phase 1 | single-drug therapy | NCT05293912 | |
| IBI397 | Monoclonal Antibody | SIRPα | IND | IND | ||||
| IBI188 | Monoclonal Antibody | CD47 | Advanced Malignancies | Phase 1 | single-drug therapy | NCT03763149/NCT03717103 | ||
| IBI322 | bispecific antibody | CD47/PDL1 | Advanced Malignancies | Phase 1 | single-drug therapy | NCT04338659/NCT04328831 | ||
| Solid Tumor | Phase 1 | single-drug therapy | NCT04912466 | |||||
| Hematological Malignancy | Phase 1 | single-drug therapy | NCT04795128 | |||||
| Myeloid Tumor | Phase 1 | HMA | combination therapy | NCT05148442 | ||||
| Company Name (sponsor) | Drug name | main component | Target | Disease | Clinical phase | Combination drug | single-drug therapy or combination therapy |
National Clinical Traial Number (NCT NO.) |
| JMT BIO | JMT601 | Fusion protein | CD20/CD47 | NHL | Phase 1/2 | single-drug therapy | NCT04853329 | |
| Advanced Malignancies | Phase 1 | single-drug therapy |
NCT03722186; Suspended (Business Decision) |
|||||
| KAHR Medical | DSP107 | bispecific antibody | CD47/41BB | NSCLC | Phase1/2 | Atezolizumab | single-drug therapy and combination therapy | NCT04440735 |
| Hematological Malignancies | Phase1 | Azacitidine/Venetoclax | single-drug therapy and combination therapy | NCT04937166 | ||||
| Lunan Pharmacy | Monoclonal antibody | CD47 | Malignant tumor | IND | single-drug therapy | / | ||
| MABWELL | 6MW3211 | Bispecific antibody | CD47/PD-L1 | Advanced Malignant Neoplasm | Phase1/2 | single-drug therapy | NCT05048160 | |
| MAB WORKS | MIL-95 | Monoclonal antibody | CD47 | Advanced Malignancies | Phase 1 | single-drug therapy | NCT04651348 | |
| OSE Immunotherapeutics | OSE-172 | Monoclonal antibody | SIRPα | Solid Tumor | Phase1 | BI 754091 | single-drug therapy and combination therapy | NCT03990233 |
| Advanced Cancer | Phase 1 | ezabenlimab/[89Zr]Zr- BI 765063 | combination therapy | NCT05068102 | ||||
| HNSCC | Phase1 | Ezabenlimab/BI 836880/Cetuximab/Investigatoŕs Choice Chemotherapy | combination therapy | NCT05249426 | ||||
| Solid Tumor | Phase1 | BI 754091 | single-drug therapy and combination therapy | NCT04653142 | ||||
| Pfizer | PF-07257876 | bispecific antibody | CD47/PDL1 | Solid Tumor | Phase 1 | single-drug therapy | NCT04881045 | |
| Seagen | SGN-CD47M | Antibody–Drug Conjugates | CD47 | Solid Tumor | Phase 1 | single-drug therapy | NCT03957096 | |
| Shattuck Labs | SL-172154 | bispecific antibody | SIRPα/CD40L | SCC | Phase 1 | single-drug therapy | NCT04502888 | |
| Sorrento Therapeutics | STI-6643 | Monoclonal Antibody | CD47 | Solid Tumor | Phase 1 | single-drug therapy | NCT04900519 | |
| SUNHO(China) | IBC0966 | bispecific antibody | CD47/PDL1 | Advanced Malignancies | Phase 1/2 | single-drug therapy | NCT04980690 | |
| Surface Oncology | SRF231628,629 | Monoclonal Antibody | CD47 | Solid Tumor/Hematological Malignancy | Phase1 | single-drug therapy | NCT03512340 | |
| SUMGEN | SG12473 | bispecific antibody | CD47/PD-1 | Malignant tumor | Phase 1 | single-drug therapy | CTR20211029 | |
| SG2501 | bispecific antibody | CD47/CD38 | Hematological Malignancy | Phase 1 | single-drug therapy | NCT05293912 | ||
| SG404 | Fusion protein | CD47 | Malignant tumor | Phase 1 | single-drug therapy | CTR20202489 | ||
| TG Therapeutics | TG-1801 | bispecific antibody | CD47/CD19 | Hematological Malignancy | Phase 1 | Biological: Ublituximab | combination therapy | NCT04806035 |
| Trillium | TTI-622 | Fusion protein | CD47 | Solid Tumor | Phase 1/2 | Pegylated Liposomal Doxorubicin | combination therapy | NCT05261490 |
| Leiomyosarcoma | Phase 1/2 | Doxorubicin | combination therapy | NCT04996004 | ||||
| Multiple Myeloma | Phase 1 | Daratumumab Hyaluronidase-fihj | combination therapy | NCT05139225 | ||||
| Hematological Malignancy | Phase 1 | Azacitidine/Venetoclax/Carfilzomib/Dexamethasone/anti-CD20 targeting agent | single-drug therapy and combination therapy | NCT03530683 | ||||
| Solid Tumor | Phase 1 | Monotherapy/Drug: PD-1/PD-L1 Inhibitor/pegylated interferon-α2a/Other:T-Vec/Other: radiation | combination therapy | NCT02890368 | ||||
| Advanced Malignancies | Phase 1 | Drug: Rituximab/Drug: Nivolumab | combination therapy | NCT02663518 | ||||
| Trillium Therapeutics | TT1-621 | Fusion protein | CD47 | Leiomyosarcoma | Phase 1/2 | Doxorubicin | combination therapy | NCT04996004 |
| Solid Tumor/Hematological Malignancy | Phase1 | Rituximab/Drug: Nivolumab | single-drug therapy and combination therapy | NCT02663518 | ||||
| Solid Tumor | Phase1 | PD-1/PD-L1 Inhibitor/pegylated interferon-α2a/T-Vec/radiation | single-drug therapy and combination therapy | NCT02890368 | ||||
| Waterstone | HX009 | bispecific antibody | CD47/PD1 | Lymphoma | Phase 2 | single-drug therapy | NCT05189093 | |
| Solid Tumor | Phase 1/2 | single-drug therapy | NCT04886271/NCT04097769 | |||||
| ZAI LAB | ZL-1201 | Monoclonal Antibody | CD47 | Advanced Cancer | Phase 1 | single-drug therapy | NCT04257617 | |
| Celgene Corporation | Anti-SIRPα | CC-95251 | SIRPα | Advanced Solid and Hematologic Cancers | Phase1 | Cetuximab, Rituximab | Alone and in Combination with Cetuximab or Rituximab |
NCT03783403 |
| Celgene | CC-90002 | Monoclonal Antibody | CD47 | AML/MDS | Phase1 | single-drug therapy | NCT02641002 | |
| Insilico Medicine | ISM004-1057D | targeting pyroglutamylation of CD47 | CD47(isoQC) | Solid Tumor/Hematological Malignancy | IND | IND | ||
| Nantes University Hospital | Monoclonal Antibody | SIRPα | HCC | Phase 1 | single-drug therapy |
NCT02868255 completed in Sep.2021 |
||
| Gilead Sciences(Bought Forty Seven in 2020) | Magrolimab (Hu5F9 G4) | Monoclonal Antibody | CD47 | Myelodysplastic Syndromes | Phase 3 | Azacitidine/Placebo | combination therapy | NCT04313881 |
| AML | Phase 3 | Venetoclax/Azacitidine | combination therapy | NCT05079230 | ||||
| AML | Phase 3 | Azacitidine | combination therapy | NCT04778397 | ||||
| Hodgkin Lymphoma | Phase 2 | Pembrolizumab | combination therapy | NCT04788043 | ||||
| Solid Tumors | Phase 2 | Docetaxel | combination therapy | NCT04827576 | ||||
| Metastatic Colorectal Cancer | Phase 2 | Bevacizumab/Irinotecan/Fluorouracil/Leucovorin | combination therapy | NCT05330429 | ||||
| Triple-Negative Breast Cancer | Phase 2 | Nab-Paclitaxel/Paclitaxel/Sacituzumab govitecan | combination therapy | NCT04958785 | ||||
| Multiple Myeloma | Phase 2 | Daratumumab/Pomalidomide/Dexamethasone/Bortezomib/Carfilzomib | combination therapy | NCT04892446 | ||||
| Head and Neck Squamous Cell Carcinoma | Phase 2 | pembrolizumab/5-FU/platinum/docetaxel | combination therapy | NCT04854499 | ||||
| Myeloid Malignancies | Phase 2 | venetoclax/azacitidine /mitoxantrone/etoposide/cytarabine/CC-486 | combination therapy | NCT04778410 | ||||
| Solid Tumor | Phase 1/2 | Cetuximab | combination therapy |
NCT02953782 finished in Mar.2021 |
||||
| Non Hodgkin Lymphoma | Phase 1/2 | rituximab/gemcitabine/oxaliplatin | combination therapy | NCT02953509 | ||||
| AML | Phase 1/2 | Azacitidine/Venetoclax | combination therapy | NCT04435691 | ||||
| Urothelial Carcinoma | Phase 1/2 | Atezolizumab | combination therapy | NCT03869190 | ||||
| MDS/AML | Phase 1/2 | Sabatolimab/Azacitidine | combination therapy | NCT05367401 | ||||
| T-Cell Lymphoma | Phase 1/2 | mogamulizumab | combination therapy | NCT04541017;Suspended (Other - Amendment Request) | ||||
| B-cell Malignancies | Phase 1 | Obinutuzumab/Venetoclax | combination therapy | NCT04599634 | ||||
| Hematological Malignancies | Phase 1 | Azacitidine | combination therapy | NCT03248479 | ||||
| AML | Phase 1 | Atezolizumab | combination therapy | NCT03922477 | ||||
| Ovarian Cancer | Phase 1 | Avelumab | combination therapy |
NCT03558139 Completed in Dec.2020 |
||||
| Non-Hodgkin's Lymphoma | Phase 1 | Acalabrutinib/AZD6738/Rituximab/AZD5153 | combination therapy | NCT03527147 | ||||
| Neuroblastoma/Osteosarcoma | Phase 1 | Dinutuximab/ | combination therapy | NCT04751383 | ||||
| MDS/AML | Phase 1 | single-drug therapy | NCT02678338 | |||||
| Solid Tumor | Phase 1 | single-drug therapy | NCT02216409 | |||||
| Brain Tumors | Phase 1 | single-drug therapy | NCT05169944 | |||||
| Shandong New Time Pharmaceutical | F527 | Monoclonal Antibody | CD47 | Lymphoma | Phase 1 | single-drug therapy | NCT05293028 | |
| (b) CD47-targeting drugs in clinical trial (registered in China) and IND stage | ||||||||
| 3D Medicines/ImmuneOncia Therapeutics | 3D-197/IMC-002 | Monoclonal Antibody | CD47 | Solid Tumor/Lymphoma | Phase 1 | single-drug therapy | CTR20220544 | |
| Akeso | AK117 | Monoclonal Antibody | CD47 | Advanced Malignancies | Phase 1/2 | AK112 | combination therapy | CTR20220121 |
| Advanced Malignancies | Phase 1/2 | AK112 | combination therapy | CTR20212989 | ||||
| Advanced Malignancies | Phase 1/2 | AK104 | combination therapy | CTR20220284 | ||||
| BioRay | BR105 | Monoclonal Antibody | SIRPα | Advanced Malignancies | Phase 1 | single-drug therapy | CTR20220467 | |
| Bio-Thera Solutions | BAT7104 | bispecific antibody | CD47/PDL1 | Advanced Malignancies | Phase 1 | single-drug therapy | CTR20220098 | |
| Chia Tai Tianqing | TQB2928 | Monoclonal Antibody | CD47 | Advanced Malignancies | Phase 1 | single-drug therapy | CTR20213324 | |
| GeneScience | gentulizumab | Monoclonal Antibody | CD47 | Hematological Malignancy | Phase 1 | single-drug therapy | CTR20210066 | |
| Hengrui Pharmaceutical | SHR-1603 | Monoclonal Antibody | CD47 | Advanced Malignancies | Phase 1 | not declared publically | CTR20181964,stopped | |
| ImmuneOnco Biopharma | IMM01 | Fusion protein | CD47 | Lymphoma | Phase 1 | not declared publically | CTR20191531 | |
| HL/B-NHL/AML/MDS/MM | Phase 2 | single-drug therapy | CTR20212227 | |||||
| AML/MDS | Phase 1 | Azacitidine | combination therapy | CTR20212519 | ||||
| Solid Tumor | Phase 1/2 | BGB-A317 | combination therapy | CTR20220791 | ||||
| IMM0306 | bispecific antibody | CD47/CD20 | NHL | Phase 1 | not declared publically | CTR20192612 | ||
| IMM2902 | bispecific antibody | CD47/SIRPα | Solid Tumor | Phase 1 | single-drug therapy | CTR20212375 | ||
| Innovent | IBI188/Letaplimab | Monoclonal Antibody | CD47 | Advanced Malignancies | Phase 1 | single-drug therapy | CTR20210761 | |
| Advanced Malignancies | Phase 1 | not declared publically | CTR20182140 | |||||
| AML | Phase 1/2 | Azacitidine/Decitabine | single-drug therapy and combination therapy | CTR20200938 | ||||
| MDS | Phase 1/3 | Azacitidine | combination therapy | CTR20201039 | ||||
| IBI322 | bispecific antibody | CD47/PDL1 | Advanced Malignancies | Phase 1 | Bevacizumab/Docetaxel | single-drug therapy and combination therapy | CTR20200175 | |
| Solid Tumor | Phase 1 | Sintilimab/Bevacizumab | single-drug therapy and combination therapy | CTR20211251 | ||||
| Hematological Malignancy | Phase 1 | single-drug therapy | CTR20210385 | |||||
| Myeloid Malignancies | Phase 1 | single-drug therapy | CTR20213120 | |||||
| IBI397 | Advanced Malignancies | Phase 1 | single-drug therapy | CTR20220193 | ||||
| ZL-1201 | Monoclonal Antibody | CD47 | Solid Tumor or Hematological Malignancy | Phase 1 | single-drug therapy | CTR20210973 | ||
| JMT BIO | JMT601 | Fusion protein | CD20/CD47 | NHL | Phase 1 | single-drug therapy | CTR20211365 | |
| Mabwell | 6MW3211 | bispecific antibody | CD47/PDL1 | Advanced Malignancies | Phase 1/2 | single-drug therapy | CTR20211936 | |
| SUMGEN | SG12473 | bispecific antibody | CD47/PDL1 | Advanced Malignancies | Phase 1 | single-drug therapy | CTR20211029 | |
| SG404 | Fusion protein | CD47 | Advanced Malignancies | Phase 1 | single-drug therapy | CTR20202489 | ||
| SUNHO(China) | IBC0966 | bispecific antibody | CD47/PDL1 | Advanced Malignancies | Phase 1/2 | single-drug therapy | CTR20211609 | |
| Waterstone | HX009 | bispecific antibody | Solid Tumor | Phase 2 | single-drug therapy | CTR20211292 | ||
| CD47/PD1 | Solid Tumor | Phase 1 | not declared publically | CTR20192299 | ||||
| Lymphoma | Phase 1/2 | single-drug therapy | CTR20213391 | |||||
| Shandong New Time Pharmaceutical | F527 | Monoclonal Antibody | CD47 | Lymphoma | Phase 1 | single-drug therapy | CTR20220738 | |
| MAB WORKS | MIL95 | Monoclonal Antibody | CD47 | Solid Tumor or Hematological Malignancy | Phase 1 | single-drug therapy | CTR20201108 | |
Table 4.
CD47 agonists in pre-clinical stage.
| Name | Mechanism | Target | Disease |
|---|---|---|---|
| B16H12.2 | CD47 antibody, it enhances phagocytosis of tumor cells | CD47 | leukemia |
| AMMS4-G4 [295] | CD47 antibody, potentiates macrophage infiltration and modestly augments the anti-tumor activity of opsonizing antibody and antiangiogenic therapy | CD47 | leukemia |
| ZF1 [297] | ZF1 specifically binds CD47 and induces phagocytosis of leukemic cancer cells | CD47 | leukemia |
| ADU-1805 [296] | Binding to SIRPα and blocking the interaction of SIRPα with CD47 | SIRPα | lymphadenoma |
| Luteolin | Binding to isoQC, a CD47 pyroglutamylation enzyme, and disrupts CD47 binding to SIRPα | CD47(isoQC) | multiple myeloma |
| KWAR23 [208] | Human SIRPα mAB, and their binding disrupts SIRPα’ binding to CD47. | SIRPα | Burkitt’s lymphoma |
| 4N1K/4N1 | It may bind to receptors independent from CD47. It is analogous to the C-terminal part of TSP-1. | CD47 | AML |
| PKHB1 | A CD47 agonist peptide derived from TSP-1, it induces a calcium-dependent and caspase-independent cell death in T-ALL cell lines. | CD47 | T-ALL |
A couple of transactions witnessed the prosperity of CD47 targeting drug development and market prospects. For example, Gilead Science bought Forty-Seven (a pioneering company focused on the development of an inhibitor of the CD47-SIRPα pathway) for USD4.9 billion in March. 2020. SciClone Pharmaceuticals obtained an exclusive license for RRX-001 in China from Epicent Rx at a cost of approximately USD120 million, and RX-001 is in Phase III for SCLC. The Chinese company ImmunoOnco received USD25 million from Eli Lilly & Company to conduct research and clinical trials on IMM01, IMM0306 and IMM2510.
The CD47 targeting strategy has mainly been studied in leukemia and hematologic malignancies and has exhibited better results in hematologic cancers, mainly for the following reasons. First, the immune infiltration of immune cells is much better in hematological cancers than in solid tumors, and targeting CD47 responds to the combined function of both innate ‘and adaptive immune cells. Second, various antibodies blocking the CD47-SIRPα axis exert better effects in hematological cancers. However, the new antibody BC31M4 selectively targets solid tumors, works in a pH-dependent manner, and exhibits anti-tumor efficacy in mouse models [294].
Safety assessment and future perspectives
Safety assessment of CD47 blockade in cancer immunotherapy
Targeting CD47 is a hot topic in cancer immunotherapy and has attracted the attention of many pharmaceutical companies. Many preclinical and clinical studies have demonstrated the safety and efficacy of targeting CD47 in hematological cancers [155]. However, CD47 is widely expressed in the hematopoietic system, especially in RBCs and pallets. This widespread expression raises concerns regarding the safety of targeted therapy, as it may pose potential risks [297]. Therefore, balancing the safety and efficacy of targeting CD47 is currently a formidable challenge [267], [295].
The biological mechanism of CD47 targeting is complex, however, the theoretical basis of CD47 as a tumor immunotherapy target is relatively clear [33]. Antibodies such as Hu47F9-G4, alone or in combination, may kill normal hematopoietic cells accidentally [276]. Low-dose or low-erythrocyte agglutination reactions in vitro and long-term treatment have been attempted [290], [298], however, anemia and other adverse reactions still occur in actual clinical trials, therefore, erythrocyte agglutination is not the main cause of anemia [299]. Several strategies have been discussed to alleviate this adverse effect.
Potential challenges in clinical trials of CD47 blockade in cancer immunotherapy
The CD47-SIRPα blockade is not enough to trigger phagocytosis. In addition, it requires the assistance of “eat me” signals including calreticulin. However, blocking CD47-SIRPαin tumor cells, aging RBCs and platelets can directly lead to phagocytosis. In other words, erythrocytopenia and thrombocytopenia are the biggest challenges in the development of CD47 target drugs. On May.24, 2017, Arch Oncology conducted its 1st clinical trial and the 1st enrolled patient died on the 2nd day of CD47 targeting drugs due to uncontrolled erythrocyte agglutination. And in October.2018, Celgene stopped patient recruitment in its clinical trial for CC-90002 due to its poor efficacy and safety. Killing tumor cells while avoiding accidental injury to RBCs has become a thorny problem.
Another obstacle limiting the translation of CD47-targeting therapy into the clinic is the limited efficacy of CD47 targeting in solid tumors because of the insufficient phagocytes and the reduced access of antibodies into the solid tumors [300]. In human AML and xenotransplantation models, CD47 antibodies cannot completely remove tumor cells, and these cells cannot be phagocytized in vivo, which may be due to the lack of enough macrophages in the bone marrow.
Suggested strategies to conquer the side effects of CD47 targeting therapies
Designing CD47 antibody with weak binding to RBCs
Because of the high expression of CD47 on RBCs, they can easily to bind to CD47 monoclonal antibodies [301]. In developing a new generation CD47 antibody, researchers tend to develop an antibody with anti-tumor activity but without hematological toxicity. The molecular structure of CD47 on tumor cells may be different from that on RBCs, and thus they bind to different proteins. TJC4, a new antibody targeting CD47, screened and designed by I-MAB based on the above concept [302], is currently undergoing its phase I/II clinical trials.
Designing CD47 antibody-dependent on the distinctions between RBCs and tumor cells
Physical morphology analysis revealed that tumor cells were generally spherical, while RBCs were generally thin in the center and thick in the periphery, with a round cake shape and a concave center on both sides. Therefore, there are significant differences in membrane fluidity and topological conformation between those two cell types. It is suggested that CD47 antibodies should be designed based on the above points. AO-176 (a product of Arch Oncology) was designed according to the above difference [303]. This mAb can selectively bind to tumor cells rather than RBCs, which has good application prospect. RRx-001 has been demonstrated as a down regulator of the c-Myc and CD47- SIRPα checkpoint axis. Furthermore, it does not result in it won’t bring anemia or palate aggregation, and the use of such checkpoint inhibitors may limit hematologic toxicity [304], [305], [306].
Targeting SIRPα instead of CD47
ADU-1805 is a humanized IgG2 mAB binding to SIRPα and possibly the best antibody targeting SIRPα. It is different from CD47 targeted drug in terms of safety since it does not cause phagocytosis of RBCs and platelets. Therefore, ADU-1805 has potential clinical development [296]. Besides ADU1805, other targeted SIRPα recombinant fusion antibodies ALX148 and TTI-621 and high-affinity monomeric SIRPα have lower affinities for normal blood cells [271], [307], [308]. The novel recombinant SIRPα-Fc fusion protein IMM01 has no RBC binding ability, it targets the CD47-SIRPα by blocking this axis and meanwhile activating the “eat me” signal. Furthermore, it also converts the “cold tumor” into “hot tumor” to potentiate the immune cells’ infiltration [309]. The preclinical antibody BYON4228 targeting the pan-allelic SIRPα can enhance the destruction of antibody-opsonized cancer cells, the clinical trials are expected to start soon [310].
Targeting isoQC-mediated CD47 pyroglutamylation in CD47 and SIRPα interaction
Interaction between CD47 and SIRPα is regulated by several factors, and one of the key regulators is IsoQC, which acts as the key enzyme responsible for the pyroglutamylation of CD47. This pyroglutamylation process plays a significant role in determining the binding affinity of CD47 to SIRPα, thereby influencing their interaction [311]. Targeting isoQC prevents anemia because it is not expressed in mature RBCs.
The function of isoQC and targeting isoQC
A recent study targeting isoQC found that intracellular isoQC deficiency restricted chemokine function and reshaped myeloid infiltration to enhance tumor immunity [312]. IsoQC also regulates inflammatory signatures, monocyte abundance and macrophage in the tumor microenvironment, providing support for targeting QPCTL to promote tumor-specific immunity [313], [314], [315]. The knockout of isoQC disrupts monocyte homeostasis and suppresses tumor growth, indicating that isoQC is a promising target for cancer therapy [312].
Targeting isoQC can significantly reduce the binding ability of CD47 to SIRPα and thus regulate the tumor immunity [135], [136], [137]. Treatment with isoQC inhibitors resulted in a significant reduction in the pyroglutamylation of CD47 without affecting its CD47 surface levels. IsoQC inhibitors enhance the phagocytic ability of macrophages to tumor cells in vitro and in vivo. Tumor cells with isoQC deficiencies were easier to be removed [135], [139]. Therefore, isoQC inhibition induces myeloid checkpoint blockade [316]. IsoQC is present in the Golgi complex, because of the lack of the Golgi apparatus in erythrocytes. IsoQC is not expressed on red blood cells. IsoQC inhibitors may not affect mature CD47 on RBC surface [317]. Therefore, isoQC inhibitors may help avoid the common defects of CD47 monoclonal antibody-based drugs.
QC and isoQC inhibitors development and clinical application
As catalytic enzymes, isoQC and QC have similar binding sites and substrate selectivity, and the inhibitors suitable for QC may also be suitable for isoQC. Various QC inhibitors have been designed for this purpose. SEN177 is an isoQC inhibitor that has been verified to disturb the interaction between CD47 and SIRPα, it attenuates SIRPα-Fc staining and reduces the binding efficiency between CD47 and SIRPα [138]. PQ912 is a first-in-class QC antagonist currently used in clinical development. It was the first QCs inhibitor approved for Alzheimer's disease (AD) clinical trials and in the phase IIb stage. In addition to these chemical molecules, some natural compounds including flavonoid derivatives, marine products and natural phenolic compounds, also have demonstrated inhibitory effects on QC [318], [319]. Luteolin, a natural flavonoid screened in our lab, can significantly inhibit the activity of isoQC and ultimately help macrophages to phagocytize tumor cells in vitro [320]. No QC/isoQC inhibitors have been approved in clinical trials for treatment of tumors using immunotherapy. The latest news comes from Insilico Medicine is that they nominated ISM004-1057D, a compound targeting isoQC in cancer immunotherapy. This compound is in the investigational new drug (IND) stage waiting for clinical trial approval. The clinical trials later will be conducted in cooperation with Fosun Pharma.
Besides all above-mentioned suggestions, the combination strategy discussed in part 5.2.2 would be one of the best options and it is currently used in clinical trials.
Taken together, although various CD47-targeting reagents are currently in different phases of clinical trials, and have demonstrated efficacy in combination with other therapies, dosing and safety issues such as acute anemia and thrombocytopenia need to be fully considered as adverse events [296].
Conclusions and further perspectives
Immune checkpoint inhibitors, including those targeting CTLA-4 and PD-1/PD-L1, have achieved unexpected success in clinical applications, and cancer immunotherapy has entered a new phase of cancer treatment. However, low overall response rate and immune tolerance are challenging issues. Clinical trials have shown that targeting CTLA-4 or PD-1 increases the number of autoimmune lymphocytes, which in turn increases the risk of autoimmune side effects, including vitiligo, hepatitis, pneumonia, and colitis. In contrast, targeting CD47 is less toxic, because cancer cells are completely phagocyted by macrophages and no garbage is left inside the cell in the TME. In addition, targeting CD47 and SIRPα also augments the effect of the antibody-dependent cellular phagocytosis (ADCP) of targeted antibodies and thus it has another unique advance. Furthermore, the combination of CAR-T therapy and CD47 blocking has exhibited better results that any monotherapy [321]. Interestingly, anti-CD47 antibodies can putatively target quiescent cancer stem cells with high CD47 expression. However, considering the high expression of CD47 in the circulatory system, hemato-toxicity can’t be avoided, and various strategies have been developed to mitigate this toxicity when designing antibodies or other inhibitors.
Recently, more phagocytosis checkpoints have emerged including CD24-Siglec 10, STC1, MHC-1-LILRB1, CD22, GD2, and PD-1-PD-L1 was also identified as a phagocytosis checkpoint [2].
In summary, CD47 has emerged as a phagocytosis checkpoint in cancer immunotherapy. Targeting the phagocytosis checkpoint introduces a new era of immunotherapy, but also confronts new challenges. In of the coming years, scientists may likely find answers to several pending questions in the field of CD47-SIRPα research: (1) When will the first CD47-SIRPα targeting drug be available on the market? (2) What are the clinical trial outcomes for targeting QPCTL? (3) Are there more effective strategies than the current methods to mitigate existing side effects? These questions reflect the ongoing efforts and advancements in CD47-SIRPα research, and the answers will undoubtedly provide valuable insights into the potential clinical applications and therapeutic benefits of these targeting approaches. As research progresses, we can expect exciting developments in this field, potentially leading to improved treatments and patient outcomes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 31830053, 31920103007, 8207112072, 82122056), the National Key Research and Development Program of China (2020YFA0803201), and the Science Technology Commission of Shanghai Municipality (No. 20S11900700).
Author information
These authors contributed equally: Yu’e Liu, Linjun Weng, Yanjin Wang.
Author contributions
L.F, P.W designed the project, reviewed and revised the manuscript. Y.L., L.W., Y.W. summarized the literature and wrote part of the manuscript. Y.L. draw figures of the article. J.Z., P.Z polished the language of the manuscript. Q.W, Y.S and J.Z gave suggestions for revision. All authors contributed to the article and approved the submitted version.
Biographies

Prof. Lan Fang is a tenure-track professor in Tongji University, China. She has been awarded as “The Excellent Young Scholar” by National Natural Science Foundation of China (NSFC), and she is the principle investigator of General Funds supported by NSFC. She is mainly engaged in protein post-translational modification to regulate the molecular mechanisms of key proteins and signaling pathways in tumor occurrence and metastasis. As the first/Co-first and Corresponding/Co-corresponding author, she has published articles in Cancer Cell (2018,2023), Molecular Cell (2014), Cell Death and Differentiation (2020), Cell Discovery (2016) etc.

Prof. Ping Wang is a tenure-track professor in Tongji University. He is the Director of Tongji Cancer Center and the Vice Dean of School of Medicine in Tongji University. He has been awarded as the “Cheung Kong Scholars Program” distinguished professor (2019), The Forth “Ten Thousand Talent Program” (2019), “The Distinguished Young Scholar of China” (2016), supported by the National Natural Science Foundation of China. He is mainly studying the tumor microenvironment. He has published more than 50 articles in this field including Cancer Cell, Molecular Cell, Nature Communications etc. His H index is 44 and i10 index is 73 in Google Scholar now.

Prof. Yufeng Shi is a tenure-track professor in Tongji University. He has been awarded as the “The High-level Talent from Overseas” in Shanghai. He has finished his post-doc training from Cornell University and the Memorial Sloan Kettering Cancer Center in USA. He mainly studies the cancer metabolism. As first/Co-first/corresponding and co-corresponding author, he has published articles in Nature (2019), Cell (2005), Nature Immunology (2007), Cell Research (2014), Advanced Science (2022), etc.

Dr. Yu’e Liu obtained her doctorate degree from the Grenoble Ecole De Management (France) in 2019, and now she is studying for her second decorate degree in basic medicine in Tongji University. She had worked in Prof. Ping Wang lab as a research fellow and now she is doing her Ph.D. research under the supervision of Prof. Yufeng Shi. As first/co-first author, she has published papers in Advanced Science (2022), Signal Transduction and Targeted Therapy (2020,2023), International Journal of Biological Sciences (2023), etc. Her research interest is tumor microenvironment and cancer metabolism.

Dr. Linjun Weng is the Associate Researcher in Tongji University affiliated Tenth Hospital. He obtained his Ph.D. from Tongji University under the supervision of Prof. Ping Wang. He is an expert in the CRISPR screening and he screened the regulator of CD47 including QPCTL, this work was published in Cell Research (2019).

Dr. Yanjin Wang is a post-doctoral fellow in Tongji University affiliated East Hospital now. He obtained his Ph.D. from Tongji University under the supervision of Prof. Ping Wang. He is mainly studying the tumor immunity, he has studied PD-L1 cytosol and nuclear location, his work has been published in Signal Transduction and Targeted Therapy (2020,2023).
Contributor Information
Yufeng Shi, Email: yshi@tongji.edu.cn.
Ping Wang, Email: wangp@tongji.edu.cn.
Lan Fang, Email: lanfang@tongji.edu.cn.
References
- 1.Kennedy L.B., Salama A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y., Wang Y., Yang Y., et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. 2023;8(1):104. doi: 10.1038/s41392-023-01365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He J., Xiong X., Yang H., et al. Defined tumor antigen-specific T cells potentiate personalized TCR-T cell therapy and prediction of immunotherapy response. Cell Res. 2022;32(6):530–542. doi: 10.1038/s41422-022-00627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lian S., Xie R., Ye Y., et al. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBioMedicine. 2019;42:281–295. doi: 10.1016/j.ebiom.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu J., Xiao Q., Dong M., et al. Glioblastoma immunotherapy targeting the innate immune checkpoint CD47-SIRPalpha Axis. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.593219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z., Li Y., Gao J., et al. The role of CD47-SIRPalpha immune checkpoint in tumor immune evasion and innate immunotherapy. Life Sci. 2021;273 doi: 10.1016/j.lfs.2021.119150. [DOI] [PubMed] [Google Scholar]
- 7.Yu W.B., Ye Z.H., Chen X., Shi J.J., Lu J.J. The development of small-molecule inhibitors targeting CD47. Drug Discov Today. 2021;26(2):561–568. doi: 10.1016/j.drudis.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., Yang Z., Yang Y. Potential role of CD47-directed bispecific antibodies in cancer immunotherapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.686031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittet M.J., Michielin O., Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol. 2022;19(6):402–421. doi: 10.1038/s41571-022-00620-6. [DOI] [PubMed] [Google Scholar]
- 11.Matlung H.L., Szilagyi K., Barclay N.A., van den Berg T.K. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276(1):145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 12.Brown E., Hooper L., Ho T., Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111(6 Pt 1):2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller Y.E., Daniels G.L., Jones C., Palmer D.K. Identification of a cell-surface antigen produced by a gene on human chromosome 3 (cen-q22) and not expressed by Rhnull cells. Am J Hum Genet. 1987;41(6):1061–1070. [PMC free article] [PubMed] [Google Scholar]
- 14.Gresham H.D., Goodwin J.L., Allen P.M., Anderson D.C., Brown E.J. A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J Cell Biol. 1989;108(5):1935–1943. doi: 10.1083/jcb.108.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oldenborg P.-A. CD47: a cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol. 2013;2013 doi: 10.1155/2013/614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindberg F.P., Lublin D.M., Telen M.J., et al. Rh-related antigen CD47 is the signal-transducer integrin-associated protein. J Biol Chem. 1994;269(3):1567–1570. [PubMed] [Google Scholar]
- 17.Brown E.J., Frazier W.A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11(3):130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 18.Oldenborg P.A., Zheleznyak A., Fang Y.F., et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):pp. 2051-+. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 19.Majeti R., Chao M.P., Alizadeh A.A., et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willingham S.B., Volkmer J.P., Gentles A.J., et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. PNAS. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikic B.I., Lakhani N., Patnaik A., et al. First-in-human, first-in-class phase i trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol. 2019;37(12):946–953. doi: 10.1200/JCO.18.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansell S.M., Maris M.B., Lesokhin A.M., et al. Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2021;27(8):2190–2199. doi: 10.1158/1078-0432.CCR-20-3706. [DOI] [PubMed] [Google Scholar]
- 23.Matlung H.L., Szilagyi K., Barclay N.A., van den Berg T.K. The CD47-SIRP alpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276(1):145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 24.Reinhold M.I., Lindberg F.P., Plas D., et al. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J Cell Sci. 1995;108(Pt 11):3419–3425. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- 25.Lindberg F.P., Gresham H.D., Schwarz E., Brown E.J. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993;123(2):485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isenberg J.S., Roberts D.D. The role of CD47 in pathogenesis and treatment of renal ischemia reperfusion injury. Pediatr Nephrol. 2019;34(12):2479–2494. doi: 10.1007/s00467-018-4123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorahy D.J., Thorne R.F., Fecondo J.V., Burns G.F. Stimulation of platelet activation and aggregation by a carboxyl-terminal peptide from thrombospondin binding to the integrin-associated protein receptor. J Biol Chem. 1997;272(2):1323–1330. doi: 10.1074/jbc.272.2.1323. [DOI] [PubMed] [Google Scholar]
- 28.Manna P.P., Frazier W.A. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol. 2003;170(7):3544–3553. doi: 10.4049/jimmunol.170.7.3544. [DOI] [PubMed] [Google Scholar]
- 29.Rath G.M., Schneider C., Dedieu S., et al. Thrombospondin-1 C-terminal-derived peptide protects thyroid cells from ceramide-induced apoptosis through the adenylyl cyclase pathway. Int J Biochem Cell Biol. 2006;38(12):2219–2228. doi: 10.1016/j.biocel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Lamy L., Foussat A., Brown E., et al. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178(9):5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 31.Isenberg J.S., Hyodo F., Matsumoto K., et al. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109(5):1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing C., Lee S., Kim W.J., et al. Neurovascular effects of CD47 signaling: promotion of cell death, inflammation, and suppression of angiogenesis in brain endothelial cells in vitro. J Neurosci Res. 2009;87(11):2571–2577. doi: 10.1002/jnr.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sick E., Boukhari A., Deramaudt T., et al. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia. 2011;59(2):308–319. doi: 10.1002/glia.21102. [DOI] [PubMed] [Google Scholar]
- 34.Isenberg J.S., Ridnour L.A., Dimitry J., et al. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281(36):26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 35.Kaur S., Martin-Manso G., Pendrak M.L., et al. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285(50):38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghimire K., Li Y., Chiba T., et al. CD47 promotes age-associated deterioration in angiogenesis, blood flow and glucose homeostasis. Cells. 2020;9(7):pp. doi: 10.3390/cells9071695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jian Y.K., Zhu H.Y., Wu X.L., Li B. Thrombospondin 1 triggers osteosarcoma cell metastasis and tumor angiogenesis. Oncol Res. 2019;27(2):211–218. doi: 10.3727/096504018X15208993118389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y., Qi X., Tong X., Wang S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol Immunol. 2013;10(6):506–512. doi: 10.1038/cmi.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Dee Z., Pidcock K., Gutierrez L.S. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011 doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaur S., Soto-Pantoja D.R., Stein E.V., et al. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep. 2013;3:1673. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatherley D., Graham S.C., Turner J., et al. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell. 2008;31(2):266–277. doi: 10.1016/j.molcel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Barclay A.N., Brown M.H. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6(6):457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 43.Jiang P.H., Lagenaur C.F., Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274(2):559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 44.Vernon-Wilson E.F., Kee W.J., Willis A.C., et al. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur J Immunol. 2000;30(8):2130–2137. doi: 10.1002/1521-4141(2000)30:8<2130::AID-IMMU2130>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Seiffert M., Cant C., Chen Z.J., et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94(11):3633–3643. [PubMed] [Google Scholar]
- 46.Seiffert M., Brossart P., Cant C., et al. Signal-regulatory protein alpha (SIRP alpha) but not SIRP beta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(-) hematopoietic cells. Blood. 2001;97(9):2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- 47.Logtenberg M.E.W., Scheeren F.A., Schumacher T.N. The CD47-SIRPalpha immune checkpoint. Immunity. 2020;52(5):742–752. doi: 10.1016/j.immuni.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams S., van der Laan L.J.W., Vernon-Wilson E., et al. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol. 1998;161(4):1853–1859. [PubMed] [Google Scholar]
- 49.Oldenborg P.A., Zheleznyak A., Fang Y.F., et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 50.Weiskopf K., Jahchan N.S., Schnorr P.J., et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest. 2016;126(7):2610–2620. doi: 10.1172/JCI81603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boettcher A.N., Cunnick J.E., Powell E.J., et al. Porcine signal regulatory protein alpha binds to human CD47 to inhibit phagocytosis: Implications for human hematopoietic stem cell transplantation into severe combined immunodeficient pigs. Xenotransplantation. 2019;26(2):e12466. doi: 10.1111/xen.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nonino A., Nascimento J.M., Mascarenhas C.C., et al. CD47 expression is decreased in hematopoietic progenitor cells in patients with myelofibrosis. Braz J Med Biol Res. 2019;52(1):pp. doi: 10.1590/1414-431X20187784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng M., Chen J.Y., Weissman-Tsukamoto R., et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. PNAS. 2015;112(7):2145–2150. doi: 10.1073/pnas.1424907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timms J.F., Swanson K.D., Marie-Cardine A., et al. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr Biol. 1999;9(16):927–930. doi: 10.1016/s0960-9822(99)80401-1. [DOI] [PubMed] [Google Scholar]
- 55.Chhabra A., Ring A.M., Weiskopf K., et al. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci Transl Med. 2016;8(351):pp. doi: 10.1126/scitranslmed.aae0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheng M., Lin Y., Xu D., et al. CD47-Mediated hedgehog/SMO/GLI1 signaling promotes mesenchymal stem cell immunomodulation in mouse liver inflammation. Hepatology. 2021;74(3):1560–1577. doi: 10.1002/hep.31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kusakari S., Ohnishi H., Jin F.J., et al. Trans-endocytosis of CD47 and SHPS-1 and its role in regulation of the CD47-SHPS-1 system. J Cell Sci. 2008;121(Pt 8):1213–1223. doi: 10.1242/jcs.025015. [DOI] [PubMed] [Google Scholar]
- 58.Piccio L., Vermi W., Boles K.S., et al. Adhesion of human T cells to antigen-presenting cells through SIRP beta 2-CD47 interaction costimulates T-cell proliferation. Blood. 2005;105(6):2421–2427. doi: 10.1182/blood-2004-07-2823. [DOI] [PubMed] [Google Scholar]
- 59.Dehmani S., Nerriere-Daguin V., Neel M., et al. SIRPgamma-CD47 interaction positively regulates the activation of human T cells in situation of chronic stimulation. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.732530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagnerud S., Manna P.P., Cella M., et al. Deficit of CD47 results in a defect of marginal zone dendritic cells, blunted immune response to particulate antigen and impairment of skin dendritic cell migration. J Immunol. 2006;176(10):5772–5778. doi: 10.4049/jimmunol.176.10.5772. [DOI] [PubMed] [Google Scholar]
- 61.Piccio L., Vermi W., Boles K.S., et al. Adhesion of human T cells to antigen-presenting cells through SIRPβ2-CD47 interaction costimulates T-cell proliferation. 2005;105(6):2421–2427. doi: 10.1182/blood-2004-07-2823. [DOI] [PubMed] [Google Scholar]
- 62.Stefanidakis M., Newton G., Lee W.Y., Parkos C.A., Luscinskas F.W.J.B. The Journal of the American Society of Hematology, Endothelial CD47 interaction with SIRPγ is required for human T-cell transendothelial migration under shear flow conditions in vitro. 2008;112(4):1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Congote L.F., Temmel N.J.F.l. The C-terminal 26-residue peptide of serpin A1 stimulates proliferation of breast and liver cancer cells: role of protein kinase C and CD47. 2004;576(3):343–347. doi: 10.1016/j.febslet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 64.Wang Q., Onuma K., Liu C., et al. Dysregulated integrin alphaVbeta3 and CD47 signaling promotes joint inflammation, cartilage breakdown, and progression of osteoarthritis. JCI Insight. 2019;4(18):pp. doi: 10.1172/jci.insight.128616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saumet A., Slimane M.B., Lanotte M., Lawler J., Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood. 2005;106(2):658–667. doi: 10.1182/blood-2004-09-3585. [DOI] [PubMed] [Google Scholar]
- 66.Chung J., Gao A.-G., Frazier W.A. Thrombspondin acts via integrin associated protein to activate the platelet integrin aIIbb3. J Biol Chem. 1997;272(23):14740–14746. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- 67.Fujimoto T.-T., Katsutani S., Shimomura T., Fujimura K. Thrombospondin bound integrin-associated protein (CD47) physically and functionally modifies integrin aIIbb3 by its extracellular domain. J Biol Chem. 2003;278(29):26655–26665. doi: 10.1074/jbc.M302194200. [DOI] [PubMed] [Google Scholar]
- 68.Wang X.-Q., Frazier W.A. The thrombospondin receptor CD47 (IAP) modulates and associates with a2b1 integrin in vascular smooth muscle cells. Mol Biol Cell. 1998;9(4):865–874. doi: 10.1091/mbc.9.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z., Calzada M.J., Sipes J.M., et al. Interactions of thrombospondins with alpha4beta1 integrin and CD47 differentially modulate T cell behavior. J Cell Biol. 2002;157(3):509–519. doi: 10.1083/jcb.200109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.M. Orazizadeh, H.S. Lee, B. Groenendijk, et al., CD47 associates with alpha 5 integrin and regulates responses of human articular chondrocytes to mechanical stimulation in an in vitro model 2008; 10 (1): 1–11. [DOI] [PMC free article] [PubMed]
- 71.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GEJJoN. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation 2003; 23 (7): 2665-2674. [DOI] [PMC free article] [PubMed]
- 72.V. Azcutia M. Kelm A.-C. Luissint et al. Neutrophil expressed CD47 regulates CD11b/CD18-dependent neutrophil transepithelial migration in the intestine in vivo 14 2 2021 331 341 [DOI] [PMC free article] [PubMed]
- 73.Kaur S, Chang T, Singh SP, et al. CD47 signaling regulates the immunosuppressive activity of VEGF in T cells 2014; 193 (8): 3914-3924. [DOI] [PMC free article] [PubMed]
- 74.S. Kaur, G. Martin-Manso, M.L. Pendrak, et al., Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47 2010; 285 (50): 38923-38932. [DOI] [PMC free article] [PubMed]
- 75.J.S. Isenberg, L.A. Ridnour, J. Dimitry, et al., CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1 2006; 281 (36): 26069–26080. [DOI] [PubMed]
- 76.T.W. Miller, J.S. Isenberg, H.B. Shih, Y. Wang, D.D.J.P.o. Roberts, Amyloid-β inhibits No-cGMP signaling in a CD36-and CD47-dependent manner 2010; 5(12): e15686 [DOI] [PMC free article] [PubMed]
- 77.Manna PP, Dimitry J, Oldenborg P-A, Frazier WAJJoBC. CD47 augments Fas/CD95-mediated apoptosis 2005; 280 (33): 29637–29644. [DOI] [PubMed]
- 78.Wu A-L, Wang J, Zheleznyak A, Brown EJJMc. Ubiquitin-related proteins regulate interaction of vimentin intermediate filaments with the plasma membrane 1999; 4(4): 619–625. [DOI] [PubMed]
- 79.N'Diaye E-N, Brown EJJTJocb. The ubiquitin-related protein PLIC-1 regulates heterotrimeric G protein function through association with Gβγ 2003; 163(5): 1157–1165. [DOI] [PMC free article] [PubMed]
- 80.Lamy L, Ticchioni M, Rouquette-Jazdanian AK, et al. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis 2003; 278(26): 23915–23921. [DOI] [PubMed]
- 81.Wang X-Q, Lindberg FP, Frazier WAJTJocb. Integrin-associated protein stimulates α2β1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases 1999; 147(2): 389–400. [DOI] [PMC free article] [PubMed]
- 82.Sick E., Boukhari A., Deramaudt T., et al. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia. 2011;59(2):308–319. doi: 10.1002/glia.21102. [DOI] [PubMed] [Google Scholar]
- 83.Murata T., Ohnishi H., Okazawa H., et al. CD47 promotes neuronal development through Src- and FRG/Vav2-mediated activation of Rac and Cdc42. J Neurosci. 2006;26(48):12397–12407. doi: 10.1523/JNEUROSCI.3981-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao A-G, Lindberg FP, Dimitry JM, Brown EJ, Frazier WAJTJocb. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein 1996; 135 (2): 533–44. [DOI] [PMC free article] [PubMed]
- 85.Hu T., Liu H., Liang Z., et al. Tumor-intrinsic CD47 signal regulates glycolysis and promotes colorectal cancer cell growth and metastasis. Theranostics. 2020;10(9):4056–4072. doi: 10.7150/thno.40860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bras M., Yuste V.J., Roué G., et al. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. 2007;27(20):7073. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Casey S.C., Tong L., Li Y., et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Casey S.C., Baylot V., Felsher D.W. The MYC oncogene is a global regulator of the immune response. Blood. 2018;131(18):2007–2015. doi: 10.1182/blood-2017-11-742577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia X., Yan B., Tian X., et al. CD47/SIRPalpha pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci. 2021;17(13):3281–3287. doi: 10.7150/ijbs.60782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li W., Gupta S.K., Han W., et al. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J Hematol Oncol. 2019;12(1):73. doi: 10.1186/s13045-019-0761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bertout J.A., Patel S.A., Simon M.C. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martins F., Oliveira R., Cavadas B., et al. Hypoxia and macrophages act in concert towards a beneficial outcome in colon cancer. Cancers (Basel) 2020;12(4):pp. doi: 10.3390/cancers12040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang H., Lu H., Xiang L., et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. PNAS. 2015;112(45):E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samanta D., Park Y., Ni X., et al. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. PNAS. 2018;115(6):E1239–E1248. doi: 10.1073/pnas.1718197115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monti E., Marras E., Prini P., Gariboldi M.B. Luteolin impairs hypoxia adaptation and progression in human breast and colon cancer cells. Eur J Pharmacol. 2020;881 doi: 10.1016/j.ejphar.2020.173210. [DOI] [PubMed] [Google Scholar]
- 96.Liu J., Tang M., Harkin K., et al. Single-cell RNA sequencing study of retinal immune regulators identified CD47 and CD59a expression in photoreceptors-Implications in subretinal immune regulation. J Neurosci Res. 2020;98(7):1498–1513. doi: 10.1002/jnr.24618. [DOI] [PubMed] [Google Scholar]
- 97.Lo J., Lau E.Y., Ching R.H., et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62(2):534–545. doi: 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- 98.Efiok B.J., Chiorini J.A., Safer B. A key transcription factor for eukaryotic initiation factor-2 alpha is strongly homologous to developmental transcription factors and may link metabolic genes to cellular growth and development. J Biol Chem. 1994;269(29):18921–18930. [PubMed] [Google Scholar]
- 99.Chang W.T., Huang A.M. Alpha-Pal/NRF-1 regulates the promoter of the human integrin-associated protein/CD47 gene. J Biol Chem. 2004;279(15):14542–14550. doi: 10.1074/jbc.M309825200. [DOI] [PubMed] [Google Scholar]
- 100.Betancur P.A., Abraham B.J., Yiu Y.Y., et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun. 2017;8:14802. doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X., Wang Y., Fan J., et al. Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J Immunother Cancer. 2019;7(1):346. doi: 10.1186/s40425-019-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao H., Wang J., Kong X., et al. CD47 promotes tumor invasion and metastasis in non-small cell lung cancer. Sci Rep. 2016;6:29719. doi: 10.1038/srep29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye Z.H., Jiang X.M., Huang M.Y., et al. Regulation of CD47 expression by interferon-gamma in cancer cells. Transl Oncol. 2021;14(9) doi: 10.1016/j.tranon.2021.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sockolosky J.T., Dougan M., Ingram J.R., et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. PNAS. 2016;113(19):E2646–E2654. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Engelbertsen D., Autio A., Verwilligen R.A.F., et al. Increased lymphocyte activation and atherosclerosis in CD47-deficient mice. Sci Rep. 2019;9(1):10608. doi: 10.1038/s41598-019-46942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen J., Zheng D.X., Yu X.J., et al. Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology. 2019;8(11):e1652540. doi: 10.1080/2162402X.2019.1652540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu F., Dai M., Xu Q., et al. SRSF10-mediated IL1RAP alternative splicing regulates cervical cancer oncogenesis via mIL1RAP-NF-kappaB-CD47 axis. Oncogene. 2018;37(18):2394–2409. doi: 10.1038/s41388-017-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bian Z., Shi L., Guo Y.L., et al. Cd47-Sirpalpha interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy self-cells. PNAS. 2016;113(37):E5434–E5443. doi: 10.1073/pnas.1521069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnson L.D.S., Banerjee S., Kruglov O., et al. Targeting CD47 in Sezary syndrome with SIRPalphaFc. Blood Adv. 2019;3(7):1145–1153. doi: 10.1182/bloodadvances.2018030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang W., Wang W.T., Fang K., et al. MIR-708 promotes phagocytosis to eradicate T-ALL cells by targeting CD47. Mol Cancer. 2018;17(1):12. doi: 10.1186/s12943-018-0768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rastgoo N., Wu J., Liu A., et al. Targeting CD47/TNFAIP8 by miR-155 overcomes drug resistance and inhibits tumor growth through induction of phagocytosis and apoptosis in multiple myeloma. Haematologica. 2020;105(12):2813–2823. doi: 10.3324/haematol.2019.227579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang S.Y., Choi S.A., Lee J.Y., et al. miR-192 suppresses leptomeningeal dissemination of medulloblastoma by modulating cell proliferation and anchoring through the regulation of DHFR, integrins, and CD47. Oncotarget. 2015;6(41):43712–43730. doi: 10.18632/oncotarget.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki S., Yokobori T., Tanaka N., et al. CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol Rep. 2012;28(2):465–472. doi: 10.3892/or.2012.1831. [DOI] [PubMed] [Google Scholar]
- 114.Li H., Wang Y., Li Y.Z. MicroRNA-133a suppresses the proliferation, migration, and invasion of laryngeal carcinoma cells by targeting CD47. Tumour Biol. 2016:pp. doi: 10.1007/s13277-016-5451-x. [DOI] [PubMed] [Google Scholar]
- 115.Zhao Y., Yu X., Tang H., et al. MicroRNA-200a promotes phagocytosis of macrophages and suppresses cell proliferation, migration, and invasion in nasopharyngeal carcinoma by targeting CD47. Biomed Res Int. 2020;2020:3723781. doi: 10.1155/2020/3723781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi L., Wang X., Hu B., Wang D., Ren Z. miR-222 enhances radiosensitivity of cancer cells by inhibiting the expression of CD47. Int J Clin Exp Path. 2019;12(11):4204–4213. [PMC free article] [PubMed] [Google Scholar]
- 117.Xi Q., Zhang J., Yang G., et al. Restoration of miR-340 controls pancreatic cancer cell CD47 expression to promote macrophage phagocytosis and enhance antitumor immunity. J Immunother Cancer. 2020;8(1):pp. doi: 10.1136/jitc-2019-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beizavi Z., Gheibihayat S.M., Moghadasian H., et al. The regulation of CD47-SIRPalpha signaling axis by microRNAs in combination with conventional cytotoxic drugs together with the help of nano-delivery: a choice for therapy? Mol Biol Rep. 2021;48(7):5707–5722. doi: 10.1007/s11033-021-06547-y. [DOI] [PubMed] [Google Scholar]
- 119.Xu S., Wang X., Yang Y., Li Y., Wu S. LSD1 silencing contributes to enhanced efficacy of anti-CD47/PD-L1 immunotherapy in cervical cancer. Cell Death Dis. 2021;12(4):282. doi: 10.1038/s41419-021-03556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Junker A., Krumbholz M., Eisele S., et al. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009;132(Pt 12):3342–3352. doi: 10.1093/brain/awp300. [DOI] [PubMed] [Google Scholar]
- 121.Tang W., Qin J., Tang J., et al. Aberrant reduction of MiR-141 increased CD47/CUL3 in Hirschsprung's disease. Cell Physiol Biochem. 2013;32(6):1655–1667. doi: 10.1159/000356601. [DOI] [PubMed] [Google Scholar]
- 122.Chen W.N., Li X.M., Wang J.Y., et al. miR-378a modulates macrophage phagocytosis and differentiation through targeting CD47-SIRP alpha axis in atherosclerosis. Scand J Immunol. 2019;90(1):pp. doi: 10.1111/sji.12766. [DOI] [PubMed] [Google Scholar]
- 123.Ye Z.M., Yang S., Xia Y.P., et al. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. 2019;10(2):138. doi: 10.1038/s41419-019-1409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Petralia M.C., Mazzon E., Fagone P., et al. Characterization of the pathophysiological Role of CD47 in Uveal Melanoma. Molecules. 2019;24(13):pp. doi: 10.3390/molecules24132450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Michaels A.D., Newhook T.E., Adair S.J., et al. CD47 blockade as an adjuvant immunotherapy for resectable pancreatic cancer. Clin Cancer Res. 2018;24(6):1415–1425. doi: 10.1158/1078-0432.CCR-17-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu D.H., Pan C.Y., Li L.M., et al. MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein alpha. J Allergy Clin Immun. 2013;132(2):pp. 426-+. doi: 10.1016/j.jaci.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaur S., Elkahloun A.G., Singh S.P., et al. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget. 2016;7(9):10133–10152. doi: 10.18632/oncotarget.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang H.D., Kim H.S., Kim S.Y., et al. HDAC6 suppresses Let-7i-5p to elicit TSP1/CD47-mediated anti-tumorigenesis and phagocytosis of hepatocellular carcinoma. Hepatology. 2019;70(4):1262–1279. doi: 10.1002/hep.30657. [DOI] [PubMed] [Google Scholar]
- 129.Gao Q., Chen K., Gao L., Zheng Y., Yang Y.-G. Thrombospondin-1 signaling through CD47 inhibits cell cycle progression and induces senescence in endothelial cells. Cell Death Dis. 2016;7(9):e2368–e. doi: 10.1038/cddis.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kopp F., Mendell J.T. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xiping Z., Bo C., Shifeng Y., et al. Roles of MALAT1 in development and migration of triple negative and Her-2 positive breast cancer. Oncotarget. 2018;9(2):2255–2267. doi: 10.18632/oncotarget.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chiang C., Gack M.U. Post-translational control of intracellular pathogen sensing pathways. Trends Immunol. 2017;38(1):39–52. doi: 10.1016/j.it.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deribe Y.L., Pawson T., Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010;17(6):666–672. doi: 10.1038/nsmb.1842. [DOI] [PubMed] [Google Scholar]
- 134.Lin H., Begley T. Protein posttranslational modifications: chemistry, biology, and applications. Mol Biosyst. 2011;7(1):14–15. doi: 10.1039/c0mb90037k. [DOI] [PubMed] [Google Scholar]
- 135.Wu Z.Q., Weng L.J., Zhang T.B., et al. Identification of Glutaminyl Cyclase isoenzyme isoQC as a regulator of SIRP alpha-CD47 axis. Cell Res. 2019;29(6):502–505. doi: 10.1038/s41422-019-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Logtenberg M.E.W. Glutaminyl cyclase is an enzymatic modifier of the CD47-SIRP alpha axis and target for immunotherapy. Cancer Immunol Res. 2019;7(2):pp. doi: 10.1038/s41591-019-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mair B., Aldridge P.M., Atwal R.S., et al. High-throughput genome-wide phenotypic screening via immunomagnetic cell sorting. Nat Biomed Eng. 2019;3(10):796–805. doi: 10.1038/s41551-019-0454-8. [DOI] [PubMed] [Google Scholar]
- 138.Logtenberg M.E.W., Jansen J.H.M., Raaben M., et al. Glutaminyl cyclase is an enzymatic modifier of the CD47-SIRP alpha axis and a target for cancer immunotherapy. Nat Med. 2019;25(4):pp. 612-+. doi: 10.1038/s41591-019-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Logtenberg M.E.W., Jansen J.H.M., Raaben M., et al. Glutaminyl cyclase is an enzymatic modifier of the CD47-SIRP alpha axis and a target for cancer immunotherapy. Nat Med. 2019;25(4):pp. 612-+. doi: 10.1038/s41591-019-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi R., Chai Y., Duan X., et al. The identification of a CD47-blocking “hotspot” and design of a CD47/PD-L1 dual-specific antibody with limited hemagglutination. Signal Transduct Target Ther. 2020;5(1):16. doi: 10.1038/s41392-020-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parthasarathy R., Subramanian S., Boder E.T., Discher D.E. Post-translational regulation of expression and conformation of an immunoglobulin domain in yeast surface display. Biotechnol Bioeng. 2006;93(1):159–168. doi: 10.1002/bit.20684. [DOI] [PubMed] [Google Scholar]
- 142.Kaur S., Kuznetsova S.A., Pendrak M.L., et al. Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by thrombospondin-1. J Biol Chem. 2011;286(17):14991–15002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matozaki T., Murata Y., Okazawa H., Ohnish H. Functions and molecular mechanisms of the CD47-SIRP alpha signalling pathway. Trends Cell Biol. 2009;19(2):72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 144.Boukhari A., Alhosin M., Bronner C., et al. CD47 activation-induced UHRF1 over-expression is associated with silencing of tumor suppressor gene p16(INK4A) in glioblastoma cells. Anticancer Res. 2015;35(1):149–157. [PubMed] [Google Scholar]
- 145.Lombardo S.D., Bramanti A., Ciurleo R., et al. Profiling of inhibitory immune checkpoints in glioblastoma: Potential pathogenetic players. Oncol Lett. 2020;20(6):332. doi: 10.3892/ol.2020.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu X.J., Wu X., Wang Y.M., et al. CD47 promotes human glioblastoma invasion through activation of the PI3K/Akt pathway. Oncol Res. 2019;27(4):415–422. doi: 10.3727/096504018X15155538502359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang X., Fan J., Wang S., et al. Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol Res. 2017;5(5):363–375. doi: 10.1158/2326-6066.CIR-16-0398. [DOI] [PubMed] [Google Scholar]
- 148.Feliz-Mosquea Y.R., Christensen A.A., Wilson A.S., et al. Combination of anthracyclines and anti-CD47 therapy inhibit invasive breast cancer growth while preventing cardiac toxicity by regulation of autophagy. Breast Cancer Res Treat. 2018;172(1):69–82. doi: 10.1007/s10549-018-4884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.El-Rashid M., Ghimire K., Sanganeria B., Lu B., Rogers N.M. CD47 limits autophagy to promote acute kidney injury. FASEB J. 2019;33(11):12735–12749. doi: 10.1096/fj.201900120RR. [DOI] [PubMed] [Google Scholar]
- 150.Zhang X., Wang S., Nan Y., et al. Inhibition of autophagy potentiated the anti-tumor effects of VEGF and CD47 bispecific therapy in glioblastoma. Appl Microbiol Biotechnol. 2018;102(15):6503–6513. doi: 10.1007/s00253-018-9069-3. [DOI] [PubMed] [Google Scholar]
- 151.Zhang X., Chen W., Fan J., et al. Disrupting CD47-SIRPalpha axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis. 2018;39(5):689–699. doi: 10.1093/carcin/bgy041. [DOI] [PubMed] [Google Scholar]
- 152.Allen R., Ivtchenko E., Thuamsang B., Sangsuwan R., Lewis J.S. Polymer-loaded hydrogels serve as depots for lactate and mimic “cold” tumor microenvironments. Biomater Sci-Uk. 2020;8(21):6056–6068. doi: 10.1039/d0bm01196g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang N., Xie B., Xiao W., et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun. 2022;13(1):1511. doi: 10.1038/s41467-022-29137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Y., Chang Y., He X., et al. CD47 enhances cell viability and migration ability but inhibits apoptosis in endometrial carcinoma cells via the PI3K/Akt/mTOR signaling pathway. Front Oncol. 2020;10:1525. doi: 10.3389/fonc.2020.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eladl E., Tremblay-LeMay R., Rastgoo N., et al. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13(1):96. doi: 10.1186/s13045-020-00930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Blazar B.R., Lindberg F.P., Ingulli E., et al. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med. 2001;194(4):541–549. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu L., Wang S., Li J., Li B. CD47/SIRPalpha blocking enhances CD19/CD3-bispecific T cell engager antibody-mediated lysis of B cell malignancies. Biochem Biophys Res Commun. 2019;509(3):739–745. doi: 10.1016/j.bbrc.2018.12.175. [DOI] [PubMed] [Google Scholar]
- 158.Kuo T.C., Chen A., Harrabi O., et al. Targeting the myeloid checkpoint receptor SIRPalpha potentiates innate and adaptive immune responses to promote anti-tumor activity. J Hematol Oncol. 2020;13(1):160. doi: 10.1186/s13045-020-00989-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schlundt C., Fischer H., Bucher C.H., et al. The multifaceted roles of macrophages in bone regeneration: a story of polarization, activation and time. Acta Biomater. 2021;133:46–57. doi: 10.1016/j.actbio.2021.04.052. [DOI] [PubMed] [Google Scholar]
- 160.Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Murray P.J., Allen J.E., Biswas S.K., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Appelqvist H., Waster P., Kagedal K., Ollinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5(4):214–226. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]
- 163.Lavine K.J., Pinto A.R., Epelman S., et al. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (Part 4) J Am Coll Cardiol. 2018;72(18):2213–2230. doi: 10.1016/j.jacc.2018.08.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang C.Y., Ye Z.H., Huang M.Y., Lu J.J. Regulation of CD47 expression in cancer cells. Transl Oncol. 2020;13(12) doi: 10.1016/j.tranon.2020.100862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Feng R., Zhao H., Xu J., Shen C. CD47: the next checkpoint target for cancer immunotherapy. Crit Rev Oncol Hematol. 2020;152 doi: 10.1016/j.critrevonc.2020.103014. [DOI] [PubMed] [Google Scholar]
- 166.Jakubzick C.V., Randolph G.J., Henson P.M. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17(6):349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 167.Kim N., Kim H.S. Targeting checkpoint receptors and molecules for therapeutic modulation of natural killer cells. Front Immunol. 2018;9:2041. doi: 10.3389/fimmu.2018.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nath P.R., Gangaplara A., Pal-Nath D., et al. CD47 expression in natural killer cells regulates homeostasis and modulates immune response to lymphocytic choriomeningitis virus. Front Immunol. 2018;9:2985. doi: 10.3389/fimmu.2018.02985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nath P.R., Pal-Nath D., Mandal A., et al. Natural killer cell recruitment and activation are regulated by CD47 expression in the tumor microenvironment. Cancer Immunol Res. 2019;7(9):1547–1561. doi: 10.1158/2326-6066.CIR-18-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saito Y., Respatika D., Komori S., et al. SIRPalpha(+) dendritic cells regulate homeostasis of fibroblastic reticular cells via TNF receptor ligands in the adult spleen. Proc Natl Acad Sci USA. 2017;114(47):E10151–E10160. doi: 10.1073/pnas.1711345114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Latour S., Tanaka H., Demeure C., et al. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167(5):2547–2554. doi: 10.4049/jimmunol.167.5.2547. [DOI] [PubMed] [Google Scholar]
- 172.Demeure C.E., Tanaka H., Mateo V., et al. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000;164(4):2193–2199. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- 173.Zhou F., Feng B., Yu H., et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31(14):e1805888. doi: 10.1002/adma.201805888. [DOI] [PubMed] [Google Scholar]
- 174.Johansson U., Higginbottom K., Londei M. CD47 ligation induces a rapid caspase-independent apoptosis-like cell death in human monocytes and dendritic cells. Scand J Immunol. 2004;59(1):40–49. doi: 10.1111/j.0300-9475.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 175.Xu M.M., Pu Y., Han D.L., et al. Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein alpha signaling. Immunity. 2017;47(2):pp. 363-+. doi: 10.1016/j.immuni.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Giese M.A., Hind L.E., Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159–2167. doi: 10.1182/blood-2018-11-844548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu W., Wang P., Petri B., et al. Integrin-induced PIP5K1C kinase polarization regulates neutrophil polarization, directionality, and in vivo infiltration. Immunity. 2010;33(3):340–350. doi: 10.1016/j.immuni.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Azcutia V., Kelm M., Luissint A.C., et al. Neutrophil expressed CD47 regulates CD11b/CD18-dependent neutrophil transepithelial migration in the intestine in vivo. Mucosal Immunol. 2021;14(2):331–341. doi: 10.1038/s41385-020-0316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hayat S.M.G., Bianconi V., Pirro M., et al. CD47: role in the immune system and application to cancer therapy. Cell Oncol (Dordr) 2020;43(1):19–30. doi: 10.1007/s13402-019-00469-5. [DOI] [PubMed] [Google Scholar]
- 180.Andrejeva G., Capoccia B.J., Hiebsch R.R., et al. Novel SIRP alpha antibodies that induce single-agent phagocytosis of tumor cells while preserving T cells. J Immunol. 2021;206(4):712–721. doi: 10.4049/jimmunol.2001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gauttier V., Pengam S., Durand J., et al. Selective SIRPalpha blockade reverses tumor T cell exclusion and overcomes cancer immunotherapy resistance. J Clin Invest. 2020;130(11):6109–6123. doi: 10.1172/JCI135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grimbert P., Bouguermouh S., Baba N., et al. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25− T cells in response to inflammation. 2006;177(6):3534–3541. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- 183.Rodríguez-Jiménez P., Chicharro P., Llamas-Velasco M., et al. Thrombospondin-1/CD47 Interaction Regulates Th17 and Treg Differentiation in Psoriasis. 2019;10:1268. doi: 10.3389/fimmu.2019.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grimbert P., Bouguermouh S., Baba N., et al. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol. 2006;177(6):3534–3541. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- 185.Bouguermouh S., Van V.Q., Martel J., et al. CD47 expression on T cell is a self-control negative regulator of type 1 immune response. J Immunol. 2008;180(12):8073–8082. doi: 10.4049/jimmunol.180.12.8073. [DOI] [PubMed] [Google Scholar]
- 186.Beckett A.N., Chockley P., Pruett-Miller S.M., et al. CD47 expression is critical for CAR T-cell survival in vivo. J Immunother Cancer. 2023;11(3):pp. doi: 10.1136/jitc-2022-005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lecoultre M., Dutoit V., Walker P.R. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. J Immunother Cancer. 2020;8(2):pp. doi: 10.1136/jitc-2020-001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guegan J.P., Legembre P. Nonapoptotic functions of Fas/CD95 in the immune response. FEBS J. 2018;285(5):809–827. doi: 10.1111/febs.14292. [DOI] [PubMed] [Google Scholar]
- 189.Soto-Pantoja D.R., Kaur S., Roberts D.D. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol. 2015;50(3):212–230. doi: 10.3109/10409238.2015.1014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schwartz A.L., Nath P.R., Allgauer M., et al. Antisense targeting of CD47 enhances human cytotoxic T-cell activity and increases survival of mice bearing B16 melanoma when combined with anti-CTLA4 and tumor irradiation. Cancer Immunol Immunother. 2019;68(11):1805–1817. doi: 10.1007/s00262-019-02397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu X., Pu Y., Cron K., et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21(10):1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ohue Y., Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–2089. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Avice M.N., Rubio M., Sergerie M., Delespesse G., Sarfati M. Role of CD47 in the induction of human naive T cell anergy. J Immunol. 2001;167(5):2459–2468. doi: 10.4049/jimmunol.167.5.2459. [DOI] [PubMed] [Google Scholar]
- 194.Lee N., Shin J.U., Jin S., et al. Upregulation of CD47 in regulatory T cells in atopic dermatitis. Yonsei Med J. 2016;57(6):1435–1445. doi: 10.3349/ymj.2016.57.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rodriguez-Jimenez P., Chicharro P., Llamas-Velasco M., et al. Thrombospondin-1/CD47 interaction regulates Th17 and treg differentiation in psoriasis. Front Immunol. 2019;10:1268. doi: 10.3389/fimmu.2019.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Van V.Q., Darwiche J., Raymond M., et al. Cutting edge: CD47 controls the in vivo proliferation and homeostasis of peripheral CD4+ CD25+ Foxp3+ regulatory T cells that express CD103. J Immunol. 2008;181(8):5204–5208. doi: 10.4049/jimmunol.181.8.5204. [DOI] [PubMed] [Google Scholar]
- 197.Hu Y., Zhou H., Gao B., Wang G., Wang Y. Role of regulatory T cells in CD47/donor-specific transfusion-induced immune tolerance in skin-heart transplantation mice. Transpl Infect Dis. 2019;21(1):e13012. doi: 10.1111/tid.13012. [DOI] [PubMed] [Google Scholar]
- 198.Zhang A., Ren Z., Tseng K.F., et al. Dual targeting of CTLA-4 and CD47 on Treg cells promotes immunity against solid tumors. Sci Transl Med. 2021;13(605):pp. doi: 10.1126/scitranslmed.abg8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang Y., Liu Z., Cao B.B., Qiu Y.H., Peng Y.P. Treg cells protect dopaminergic neurons against MPP+ neurotoxicity via CD47-SIRPA interaction. Cell Physiol Biochem. 2017;41(3):1240–1254. doi: 10.1159/000464388. [DOI] [PubMed] [Google Scholar]
- 200.Engelhard V., Conejo-Garcia J.R., Ahmed R., et al. B cells and cancer. Cancer Cell. 2021;39(10):1293–1296. doi: 10.1016/j.ccell.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 201.Gallagher S., Turman S., Lekstrom K., et al. CD47 limits antibody dependent phagocytosis against non-malignant B cells. Mol Immunol. 2017;85:57–65. doi: 10.1016/j.molimm.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 202.Hatterer E., Barba L., Noraz N., et al. Co-engaging CD47 and CD19 with a bispecific antibody abrogates B-cell receptor/CD19 association leading to impaired B-cell proliferation. MAbs. 2019;11(2):322–334. doi: 10.1080/19420862.2018.1558698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Murata Y., Saito Y., Kotani T., Matozaki T. CD47-signal regulatory protein alpha signaling system and its application to cancer immunotherapy. Cancer Sci. 2018;109(8):2349–2357. doi: 10.1111/cas.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chao M.P., Alizadeh A.A., Tang C., et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang W., Fan Y., Li M., et al. Therapy strategy of CD47 in diffuse large B-cell lymphoma (DLBCL) Dis Markers. 2021;2021:4894022. doi: 10.1155/2021/4894022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 206.Kazama R., Miyoshi H., Takeuchi M., et al. Combination of CD47 and signal-regulatory protein-alpha constituting the “don't eat me signal” is a prognostic factor in diffuse large B-cell lymphoma. Cancer Sci. 2020;111(7):2608–2619. doi: 10.1111/cas.14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ren S., Cai Y., Hu S., et al. Berberine exerts anti-tumor activity in diffuse large B-cell lymphoma by modulating c-myc/CD47 axis. Biochem Pharmacol. 2021;188 doi: 10.1016/j.bcp.2021.114576. [DOI] [PubMed] [Google Scholar]
- 208.Ring N.G., Herndler-Brandstetter D., Weiskopf K., et al. Anti-SIRPalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. 2017;114(49):E10578–E10585. doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mateo V., Brown E.J., Biron G., et al. Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood. 2002;100(8):2882–2890. doi: 10.1182/blood-2001-12-0217. [DOI] [PubMed] [Google Scholar]
- 210.Bennani N.N., Ansell S.M. Tumor Microenvironment in T-Cell Lymphomas. Cancer Treat Res. 2019;176:69–82. doi: 10.1007/978-3-319-99716-2_3. [DOI] [PubMed] [Google Scholar]
- 211.Kitai Y., Ishiura M., Saitoh K., et al. CD47 promotes T-cell lymphoma metastasis by up-regulating AKAP13-mediated RhoA activation. Int Immunol. 2021;33(5):273–280. doi: 10.1093/intimm/dxab002. [DOI] [PubMed] [Google Scholar]
- 212.Yang K., Xu J., Liu Q., Li J., Xi Y. Expression and significance of CD47, PD1 and PDL1 in T-cell acute lymphoblastic lymphoma/leukemia. Pathol Res Pract. 2019;215(2):265–271. doi: 10.1016/j.prp.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 213.Folkes A.S., Feng M., Zain J.M., et al. Targeting CD47 as a cancer therapeutic strategy: the cutaneous T-cell lymphoma experience. Curr Opin Oncol. 2018;30(5):332–337. doi: 10.1097/CCO.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yanagida E., Miyoshi H., Takeuchi M., et al. Clinicopathological analysis of immunohistochemical expression of CD47 and SIRPalpha in adult T-cell leukemia/lymphoma. Hematol Oncol. 2020;38(5):680–688. doi: 10.1002/hon.2768. [DOI] [PubMed] [Google Scholar]
- 215.Jain S., Van Scoyk A., Morgan E.A., et al. Targeted inhibition of CD47-SIRPalpha requires Fc-FcgammaR interactions to maximize activity in T-cell lymphomas. Blood. 2019;134(17):1430–1440. doi: 10.1182/blood.2019001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Singh R., Shaik S., Negi B.S., et al. Non-Hodgkin's lymphoma: a review. J Family Med Prim Care. 2020;9(4):1834–1840. doi: 10.4103/jfmpc.jfmpc_1037_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bowzyk Al-Naeeb A., Ajithkumar T., Behan S., Hodson D.J. Non-Hodgkin lymphoma. BMJ. 2018;362 doi: 10.1136/bmj.k3204. [DOI] [PubMed] [Google Scholar]
- 218.Herrera A.F. Noncellular immune therapies for non-hodgkin lymphoma. Hematol Oncol Clin N Am. 2019;33(4):707–725. doi: 10.1016/j.hoc.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 219.Chao M.P., Takimoto C.H., Feng D.D., et al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol. 2019;9:1380. doi: 10.3389/fonc.2019.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu Y., Bewersdorf J.P., Stahl M., Zeidan A.M. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: the dawn of a new era? Blood Rev. 2019;34:67–83. doi: 10.1016/j.blre.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 221.Herrmann H., Sadovnik I., Eisenwort G., et al. Delineation of target expression profiles in CD34+/CD38- and CD34+/CD38+ stem and progenitor cells in AML and CML. Blood Adv. 2020;4(20):5118–5132. doi: 10.1182/bloodadvances.2020001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rendtlew Danielsen J.M., Knudsen L.M., Dahl I.M., Lodahl M., Rasmussen T. Dysregulation of CD47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br J Haematol. 2007;138(6):756–760. doi: 10.1111/j.1365-2141.2007.06729.x. [DOI] [PubMed] [Google Scholar]
- 223.Sun J., Muz B., Alhallak K., et al. Targeting CD47 as a novel immunotherapy for multiple myeloma. Cancers (Basel) 2020;12(2):pp. doi: 10.3390/cancers12020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Orozco-Morales M., Aviles-Salas A., Hernandez-Pedro N., et al. Clinicopathological and prognostic significance of CD47 expression in lung neuroendocrine tumors. J Immunol Res. 2021;2021:6632249. doi: 10.1155/2021/6632249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Barrera L., Montes-Servin E., Hernandez-Martinez J.M., et al. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Brit J Cancer. 2017;117(3):385–397. doi: 10.1038/bjc.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Catalan R., Orozco-Morales M., Hernandez-Pedro N.Y., et al. CD47-SIRP alpha Axis as a Biomarker and Therapeutic Target in Cancer: Current Perspectives and Future Challenges in Nonsmall Cell Lung Cancer. J Immunol Res. 2020 (2020),:pp. doi: 10.1155/2020/9435030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Calles A., Aguado G., Sandoval C., Alvarez R. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol. 2019;21(8):961–976. doi: 10.1007/s12094-018-02011-9. [DOI] [PubMed] [Google Scholar]
- 228.Xu Y., Li J., Tong B., et al. Positive tumour CD47 expression is an independent prognostic factor for recurrence in resected non-small cell lung cancer. Esmo Open. 2020;5(4):pp. doi: 10.1136/esmoopen-2020-000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nigro A., Ricciardi L., Salvato I., et al. Enhanced expression of CD47 is associated with off-target resistance to tyrosine kinase inhibitor gefitinib in NSCLC. Front Immunol. 2020;10:pp. doi: 10.3389/fimmu.2019.03135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Arrieta O., Aviles-Salas A., Orozco-Morales M., et al. Association between CD47 expression, clinical characteristics and prognosis in patients with advanced non-small cell lung cancer. Cancer Med-Us. 2020;9(7):2390–2402. doi: 10.1002/cam4.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li F., Lv B.K., Liu Y., et al. Blocking the CD47-SIRP alpha axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology. 2018;7(2):pp. doi: 10.1080/2162402X.2017.1391973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ma D., Liu S., Lal B., et al. Extracellular matrix protein tenascin c increases phagocytosis mediated by CD47 loss of function in glioblastoma. Cancer Res. 2019;79(10):2697–2708. doi: 10.1158/0008-5472.CAN-18-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bakas S., Akbari H., Pisapia J., et al. In vivo detection of EGFRvIII in glioblastoma via perfusion magnetic resonance imaging signature consistent with deep peritumoral infiltration: the phi-index. Clin Cancer Res. 2017;23(16):4724–4734. doi: 10.1158/1078-0432.CCR-16-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gholamin S., Youssef O.A., Rafat M., et al. Irradiation or temozolomide chemotherapy enhances anti-CD47 treatment of glioblastoma. Innate Immun. 2020;26(2):130–137. doi: 10.1177/1753425919876690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang P., Rashidi A., Zhao J., et al. STING agonist-loaded, CD47/PD-L1-targeting nanoparticles potentiate antitumor immunity and radiotherapy for glioblastoma. Nat Commun. 2023;14(1):1610. doi: 10.1038/s41467-023-37328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hu J., Dong F., He Y., et al. LRIG2 promotes glioblastoma progression by modulating innate antitumor immunity through macrophage infiltration and polarization. J Immunother Cancer. 2022;10(9):pp. doi: 10.1136/jitc-2021-004452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Daubon T., Leon C., Clarke K., et al. Deciphering the complex role of thrombospondin-1 in glioblastoma development. Nat Commun. 2019;10(1):1146. doi: 10.1038/s41467-019-08480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.von Roemeling C.A., Wang Y.F., Qie Y.Q., et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat Commun. 2020;11(1):pp. doi: 10.1038/s41467-020-15129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hutter G., Theruvath J., Graef C.M., et al. Microglia are effector cells of CD47-SIRPalpha antiphagocytic axis disruption against glioblastoma. PNAS. 2019;116(3):997–1006. doi: 10.1073/pnas.1721434116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang H., Newton G., Wu L., et al. CD47 antibody blockade suppresses microglia-dependent phagocytosis and monocyte transition to macrophages, impairing recovery in EAE, JCI. Insight. 2021;6(21):pp. doi: 10.1172/jci.insight.148719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Anastasiadi Z., Lianos G.D., Ignatiadou E., Harissis H.V., Mitsis M. Breast cancer in young women: an overview. Updates Surg. 2017;69(3):313–317. doi: 10.1007/s13304-017-0424-1. [DOI] [PubMed] [Google Scholar]
- 242.Roulot A., Hequet D., Guinebretiere J.M., et al. Tumoral heterogeneity of breast cancer. Ann Biol Clin (Paris) 2016;74(6):653–660. doi: 10.1684/abc.2016.1192. [DOI] [PubMed] [Google Scholar]
- 243.Kosaka A., Ishibashi K., Nagato T., et al. CD47 blockade enhances the efficacy of intratumoral STING-targeting therapy by activating phagocytes. J Exp Med. 2021;218(11):pp. doi: 10.1084/jem.20200792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lv C., Li F., Li X., et al. MiR-31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists. Nat Commun. 2017;8(1):1036. doi: 10.1038/s41467-017-01059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tan W., Tang H., Jiang X., et al. Metformin mediates induction of miR-708 to inhibit self-renewal and chemoresistance of breast cancer stem cells through targeting CD47. J Cell Mol Med. 2019;23(9):5994–6004. doi: 10.1111/jcmm.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shu R., Evtimov V.J., Hammett M.V., et al. Engineered CAR-T cells targeting TAG-72 and CD47 in ovarian cancer. Mol Ther Oncolytics. 2021;20:325–341. doi: 10.1016/j.omto.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chang C.L., Wu C.C., Hsu Y.T., Hsu Y.C. Immune vulnerability of ovarian cancer stem-like cells due to low CD47 expression is protected by surrounding bulk tumor cells. Oncoimmunology. 2020;9(1):pp. doi: 10.1080/2162402X.2020.1803530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang C.L., Lin M.J., Hsu C.Y., et al. CD47 promotes cell growth and motility in epithelial ovarian cancer. Biomed Pharmacother. 2019;119 doi: 10.1016/j.biopha.2019.109105. [DOI] [PubMed] [Google Scholar]
- 249.Cooney T., Wei M.C., Rangaswami A., et al. CD47 is not over-expressed in fibrolamellar hepatocellular carcinoma. Ann Clin Lab Sci. 2017;47(4):395–402. [PubMed] [Google Scholar]
- 250.Kim H., Bang S., Jee S., Paik S.S., Jang K. Clinicopathological significance of CD47 expression in hepatocellular carcinoma. J Clin Pathol. 2021;74(2):111–115. doi: 10.1136/jclinpath-2020-206611. [DOI] [PubMed] [Google Scholar]
- 251.Rodriguez M.M., Fiore E., Bayo J., et al. 4Mu decreases CD47 expression on hepatic cancer stem cells and primes a potent antitumor T cell response induced by interleukin-12. Mol Ther. 2018;26(12):2738–2750. doi: 10.1016/j.ymthe.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Du K., Li Y., Liu J., et al. A bispecific antibody targeting GPC3 and CD47 induced enhanced antitumor efficacy against dual antigen-expressing HCC. Mol Ther. 2021;29(4):1572–1584. doi: 10.1016/j.ymthe.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fan Z., Gao Y., Zhang W., et al. METTL3/IGF2BP1/CD47 contributes to the sublethal heat treatment induced mesenchymal transition in HCC. Biochem Biophys Res Commun. 2021;546:169–177. doi: 10.1016/j.bbrc.2021.01.085. [DOI] [PubMed] [Google Scholar]
- 254.Vaeteewoottacharn K., Kariya R., Pothipan P., et al. Attenuation of CD47-SIRPalpha signal in cholangiocarcinoma potentiates tumor-associated macrophage-mediated phagocytosis and suppresses intrahepatic metastasis. Transl Oncol. 2019;12(2):217–225. doi: 10.1016/j.tranon.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Novikova M.V., Khromova N.V., Kopnin P.B. Components of the hepatocellular carcinoma microenvironment and their role in tumor progression. Biochemistry (Mosc) 2017;82(8):861–873. doi: 10.1134/S0006297917080016. [DOI] [PubMed] [Google Scholar]
- 256.Chen S.M.Y., Krinsky A.L., Woolaver R.A., et al. Tumor immune microenvironment in head and neck cancers. Mol Carcinog. 2020;59(7):766–774. doi: 10.1002/mc.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 258.Wu K., Lin K., Li X., et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. 2020;11:1731. doi: 10.3389/fimmu.2020.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yu L., Ding Y., Wan T., et al. Significance of CD47 and its association with tumor immune microenvironment heterogeneity in ovarian cancer. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.768115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gholamin S., Mitra S.S., Feroze A.H., et al. Disrupting the CD47-SIRP alpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med. 2017;9(381):pp. doi: 10.1126/scitranslmed.aaf2968. [DOI] [PubMed] [Google Scholar]
- 261.Iribarren K., Buque A., Mondragon L., et al. Anticancer effects of anti-CD47 immunotherapy in vivo. Oncoimmunology. 2019;8(3):1550619. doi: 10.1080/2162402X.2018.1550619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Abe T., Tanaka Y., Piao J., et al. Signal regulatory protein alpha blockade potentiates tumoricidal effects of macrophages on gastroenterological neoplastic cells in syngeneic immunocompetent mice. Ann Gastroenterol Surg. 2018;2(6):451–462. doi: 10.1002/ags3.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Saleh R., Elkord E. FoxP3(+) T regulatory cells in cancer: prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–185. doi: 10.1016/j.canlet.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 264.Kaur S., Bronson S.M., Pal-Nath D., et al. Functions of thrombospondin-1 in the tumor microenvironment. Int J Mol Sci. 2021;22(9):pp. doi: 10.3390/ijms22094570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Veillette A., Chen J. SIRPalpha-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018;39(3):173–184. doi: 10.1016/j.it.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 266.Vonderheide R.H. CD47 blockade as another immune checkpoint therapy for cancer. Nat Med. 2015;21(10):1122–1123. doi: 10.1038/nm.3965. [DOI] [PubMed] [Google Scholar]
- 267.Chen Y.C., Shi W., Shi J.J., Lu J.J. Progress of CD47 immune checkpoint blockade agents in anticancer therapy: a hematotoxic perspective. J Cancer Res Clin Oncol. 2021:pp. doi: 10.1007/s00432-021-03815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kojima Y., Volkmer J.P., McKenna K., et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536(7614):86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Piccione E.C., Juarez S., Tseng S., et al. SIRPalpha-antibody fusion proteins selectively bind and eliminate dual antigen-expressing tumor cells. Clin Cancer Res. 2016;22(20):5109–5119. doi: 10.1158/1078-0432.CCR-15-2503. [DOI] [PubMed] [Google Scholar]
- 270.Jaiswal S., Jamieson C.H.M., Pang W.W., et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Weiskopf K., Ring A.M., Ho C.C.M., et al. Engineered SIRP alpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341(6141):88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kim M.J., Lee J.C., Lee J.J., et al. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumour Biol. 2008;29(1):28–34. doi: 10.1159/000132568. [DOI] [PubMed] [Google Scholar]
- 273.Kaufman H.L. Rational combination immunotherapy: understand the biology. Cancer Immunol Res. 2017;5(5):355–356. doi: 10.1158/2326-6066.CIR-17-0128. [DOI] [PubMed] [Google Scholar]
- 274.Wu J., Li Z., Yang Z., et al. A glutamine-rich carrier efficiently delivers anti-CD47 siRNA driven by a “Glutamine Trap” to inhibit lung cancer cell growth. Mol Pharm. 2018;15(8):3032–3045. doi: 10.1021/acs.molpharmaceut.8b00076. [DOI] [PubMed] [Google Scholar]
- 275.Nishiga Y., Drainas A.P., Baron M., et al. Radiotherapy in combination with CD47 blockade elicits a macrophage-mediated abscopal effect. Nat Cancer. 2022;3(11):1351–1366. doi: 10.1038/s43018-022-00456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Buatois V., Johnson Z., Salgado-Pires S., et al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol Cancer Ther. 2018;17(8):1739–1751. doi: 10.1158/1535-7163.MCT-17-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.van Rees D.J., Brinkhaus M., Klein B., et al. Sodium stibogluconate and CD47-SIRPalpha blockade overcome resistance of anti-CD20-opsonized B cells to neutrophil killing. Blood Adv. 2022;6(7):2156–2166. doi: 10.1182/bloodadvances.2021005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sallman D.A., Asch A.S., Al Malki M.M., et al. The first-in-class anti-CD47 antibody magrolimab (5F9) in combination with azacitidine is effective in MDS and AML patients: ongoing phase 1b results. Blood. 2019;134 [Google Scholar]
- 279.Saxena K., Konopleva M. An expert overview of emerging therapies for acute myeloid leukemia: novel small molecules targeting apoptosis, p53, transcriptional regulation and metabolism. Expert Opin Invest Drugs. 2020;29(9):973–988. doi: 10.1080/13543784.2020.1804856. [DOI] [PubMed] [Google Scholar]
- 280.Swoboda D.M., Sallman D.A. The promise of macrophage directed checkpoint inhibitors in myeloid malignancies. Best Pract Res Clin Haematol. 2020;33(4) doi: 10.1016/j.beha.2020.101221. [DOI] [PubMed] [Google Scholar]
- 281.Liu B.N., Guo H.Z., Xu J., et al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs. 2018;10(2):315–324. doi: 10.1080/19420862.2017.1409319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chen S.H., Dominik P.K., Stanfield J., et al. Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity. J Immunother Cancer. 2021;9(10):pp. doi: 10.1136/jitc-2021-003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yang Z., Peng Y., Guo W., et al. PD-L1 and CD47 co-expression predicts survival and enlightens future dual-targeting immunotherapy in non-small cell lung cancer. Thorac Cancer. 2021;12(11):1743–1751. doi: 10.1111/1759-7714.13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tomita Y., Oronsky B., Abrouk N., et al. In small cell lung cancer patients treated with RRx-001, a downregulator of CD47, decreased expression of PD-L1 on circulating tumor cells significantly correlates with clinical benefit. Transl Lung Cancer Res. 2021;10(1):274–278. doi: 10.21037/tlcr-20-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Dheilly E., Moine V., Broyer L., et al. Selective blockade of the ubiquitous checkpoint receptor CD47 is enabled by dual-targeting bispecific antibodies. Mol Ther. 2017;25(2):523–533. doi: 10.1016/j.ymthe.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fu F., Zhang Y., Gao Z., et al. Combination of CD47 and CD68 expression predicts survival in eastern-Asian patients with non-small cell lung cancer. J Cancer Res Clin Oncol. 2021;147(3):739–747. doi: 10.1007/s00432-020-03477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Candas-Green D., Xie B., Huang J., et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat Commun. 2020;11(1):4591. doi: 10.1038/s41467-020-18245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Upton R., Banuelos A., Feng D., et al. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. PNAS. 2021;118(29):pp. doi: 10.1073/pnas.2026849118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wu H., Liu J., Wang Z., Yuan W., Chen L. Prospects of antibodies targeting CD47 or CD24 in the treatment of glioblastoma. CNS Neurosci Ther. 2021;27(10):1105–1117. doi: 10.1111/cns.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Advani R., Flinn I., Popplewell L., et al. CD47 blockade by Hu5F9-G4 and rituximab in non-hodgkin's lymphoma. N Engl J Med. 2018;379(18):1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen Y.P., Kim H.J., Wu H., et al. SIRPalpha expression delineates subsets of intratumoral monocyte/macrophages with different functional and prognostic impact in follicular lymphoma. Blood Cancer J. 2019;9(10):84. doi: 10.1038/s41408-019-0246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Song J., Zhang H., Wang D., et al. Hydrogel loading functionalized PAMAM/shRNA complex for postsurgical glioblastoma treatment. J Control Release. 2021;338:583–592. doi: 10.1016/j.jconrel.2021.08.052. [DOI] [PubMed] [Google Scholar]
- 293.Oronsky B., Reid T., Cabrales P. Vascular priming with RRx-001 to increase the uptake and accumulation of temozolomide and irinotecan in orthotopically implanted gliomas. J Drug Target. 2021:1–6. doi: 10.1080/1061186X.2021.1904248. [DOI] [PubMed] [Google Scholar]
- 294.Li Y., Liu J., Chen W., et al. A pH-dependent anti-CD47 antibody that selectively targets solid tumors and improves therapeutic efficacy and safety. J Hematol Oncol. 2023;16(1):2. doi: 10.1186/s13045-023-01399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yu X.Y., Qiu W.Y., Long F., et al. A novel fully human anti-CD47 antibody as a potential therapy for human neoplasms with good safety. Biochimie. 2018;151:54–66. doi: 10.1016/j.biochi.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 296.Voets E., Parade M., Lutje Hulsik D., et al. Functional characterization of the selective pan-allele anti-SIRPalpha antibody ADU-1805 that blocks the SIRPalpha-CD47 innate immune checkpoint. J Immunother Cancer. 2019;7(1):340. doi: 10.1186/s40425-019-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Pietsch E.C., Dong J., Cardoso R., et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. 2017;7(2):e536. doi: 10.1038/bcj.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Han X., Sterling H., Chen Y., et al. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J Biol Chem. 2000;275(48):37984–37992. doi: 10.1074/jbc.M002334200. [DOI] [PubMed] [Google Scholar]
- 299.Brierley C.K., Staves J., Roberts C., et al. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion. 2019;59(7):2248–2254. doi: 10.1111/trf.15397. [DOI] [PubMed] [Google Scholar]
- 300.Chen J., Zhong M.C., Guo H.J., et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544(7651):pp. 493-+. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Velliquette R.W., Aeschlimann J., Kirkegaard J., et al. Monoclonal anti-CD47 interference in red cell and platelet testing. Transfusion. 2019;59(2):730–737. doi: 10.1111/trf.15033. [DOI] [PubMed] [Google Scholar]
- 302.Li M, Yu H, Qi F, et al. Anti-CD47 immunotherapy in combination with BCL-2 inhibitor to enhance anti-tumor activity in B-cell lymphoma, Hematol Oncol; 2022. [DOI] [PubMed]
- 303.Puro R.J., Bouchlaka M.N., Hiebsch R.R., et al. Development of AO-176, a Next-Generation Humanized Anti-CD47 Antibody with Novel Anticancer Properties and Negligible Red Blood Cell Binding. Mol Cancer Ther. 2020;19(3):835–846. doi: 10.1158/1535-7163.MCT-19-1079. [DOI] [PubMed] [Google Scholar]
- 304.Oronsky B., Cabrales P., Caroen S., et al. RRx-001, a downregulator of the CD47- SIRPα checkpoint pathway, does not cause anemia or thrombocytopenia. Exp Opin Drug Metab Toxicol. 2021;17(4):355–357. doi: 10.1080/17425255.2021.1876025. [DOI] [PubMed] [Google Scholar]
- 305.Oronsky B., Carter C.A., Caroen S., et al. RRx-001, a first-in-class small molecule inhibitor of MYC and a downregulator of CD47, is an “erythrophagoimmunotherapeutic”. Oncoimmunology. 2020;9(1):1746172. doi: 10.1080/2162402X.2020.1746172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Jurgensen K.J., Skinner W.K.J., Oronsky B., et al. RRx-001 radioprotection: enhancement of survival and hematopoietic recovery in gamma-irradiated mice. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.676396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Petrova P.S., Viller N.N., Wong M., et al. TTI-621 (SIRPalphaFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23(4):1068–1079. doi: 10.1158/1078-0432.CCR-16-1700. [DOI] [PubMed] [Google Scholar]
- 308.Kauder S.E., Kuo T.C., Chen A., et al. ALX148 is a high affinity sirp alpha fusion protein that blocks CD47, enhances the activity of anti-cancer antibodies and checkpoint inhibitors, and has a favorable safety profile in preclinical models. Blood. 2017;130:pp. [Google Scholar]
- 309.Yu J., Li S., Chen D., et al. SIRPalpha-Fc fusion protein IMM01 exhibits dual anti-tumor activities by targeting CD47/SIRPalpha signal pathway via blocking the “don't eat me” signal and activating the “eat me” signal. J Hematol Oncol. 2022;15(1):167. doi: 10.1186/s13045-022-01385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.van Helden M.J., Zwarthoff S.A., Arends R.J., et al. BYON4228 is a pan-allelic antagonistic SIRPalpha antibody that potentiates destruction of antibody-opsonized tumor cells and lacks binding to SIRPgamma on T cells. J Immunother Cancer. 2023;11(4):pp. doi: 10.1136/jitc-2022-006567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mateo V., Lagneaux L., Bron D., et al. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med. 1999;5(11):1277–1284. doi: 10.1038/15233. [DOI] [PubMed] [Google Scholar]
- 312.Barreira da Silva R., Leitao R.M., Pechuan-Jorge X., et al. Loss of the intracellular enzyme QPCTL limits chemokine function and reshapes myeloid infiltration to augment tumor immunity. Nat Immunol. 2022;23(4):568–580. doi: 10.1038/s41590-022-01153-x. [DOI] [PubMed] [Google Scholar]
- 313.Bresser K., Logtenberg M.E.W., Toebes M., et al. QPCTL regulates macrophage and monocyte abundance and inflammatory signatures in the tumor microenvironment. Oncoimmunology. 2022;11(1):2049486. doi: 10.1080/2162402X.2022.2049486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Cynis H., Hoffmann T., Friedrich D., et al. The isoenzyme of glutaminyl cyclase is an important regulator of monocyte infiltration under inflammatory conditions. EMBO Mol Med. 2011;3(9):545–558. doi: 10.1002/emmm.201100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Liu Y., Lu S., Sun Y., et al. Deciphering the role of QPCTL in glioma progression and cancer immunotherapy. Front Immunol. 2023;14:1166377. doi: 10.3389/fimmu.2023.1166377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Inhibition of QPCTL Induces Myeloid Immune Checkpoint Blockade, Cancer Discov 2019; 9 (5): OF8.
- 317.Liu Y.E., Shi Y., Wang P. Functions of glutaminyl cyclase and its isoform in diseases. Vis Cancer Med. 2023;4:1. [Google Scholar]
- 318.Coimbra J.R., Sobral P.J., Santos A.E., Moreira P.I., Salvador J.A. An overview of glutaminyl cyclase inhibitors for Alzheimer's disease. Future Med Chem. 2019;11(24):3179–3194. doi: 10.4155/fmc-2019-0163. [DOI] [PubMed] [Google Scholar]
- 319.Li M., Dong Y., Yu X., et al. Inhibitory effect of flavonoids on human glutaminyl cyclase. Bioorg Med Chem. 2016;24(10):2280–2286. doi: 10.1016/j.bmc.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 320.Li Z., Gu X., Rao D., et al. Luteolin promotes macrophage-mediated phagocytosis by inhibiting CD47 pyroglutamation. Transl Oncol. 2021;14(8) doi: 10.1016/j.tranon.2021.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Dacek M.M., Kurtz K.G., Wallisch P., et al. Potentiating antibody-dependent killing of cancers with CAR T cells secreting CD47-SIRPalpha checkpoint blocker. Blood. 2023;141(16):2003–2015. doi: 10.1182/blood.2022016101. [DOI] [PMC free article] [PubMed] [Google Scholar]