Abstract
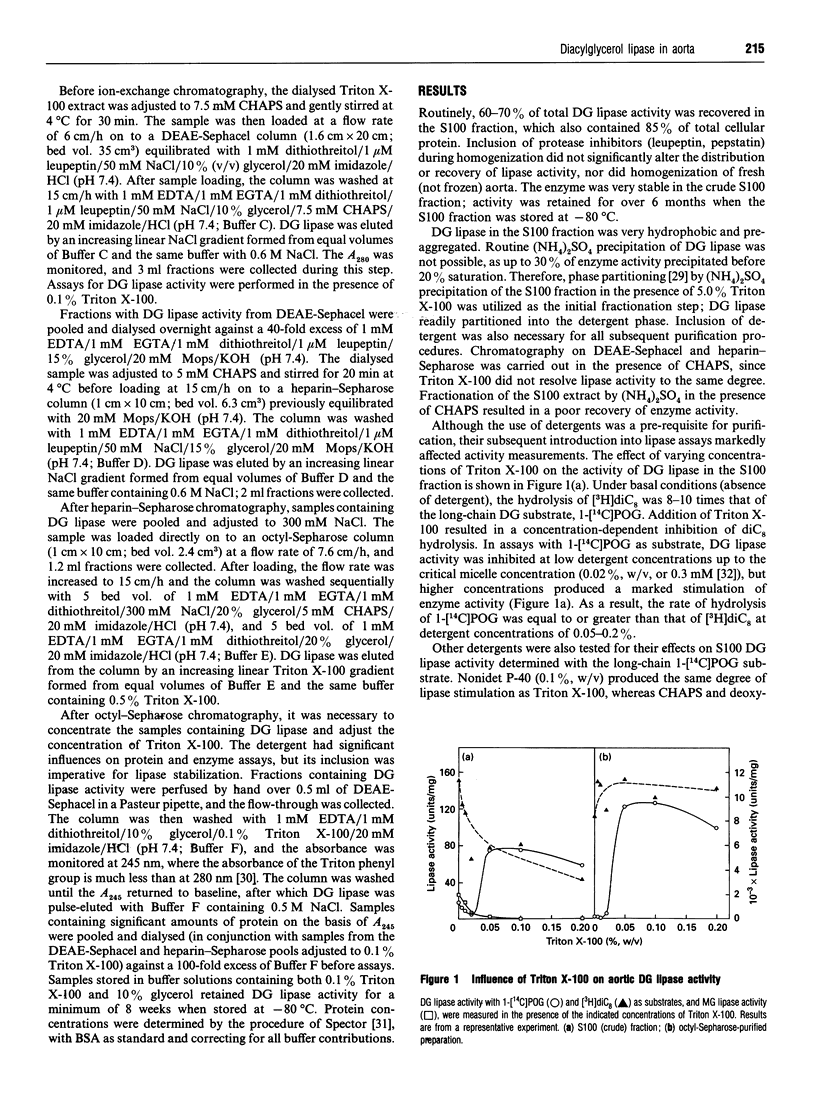
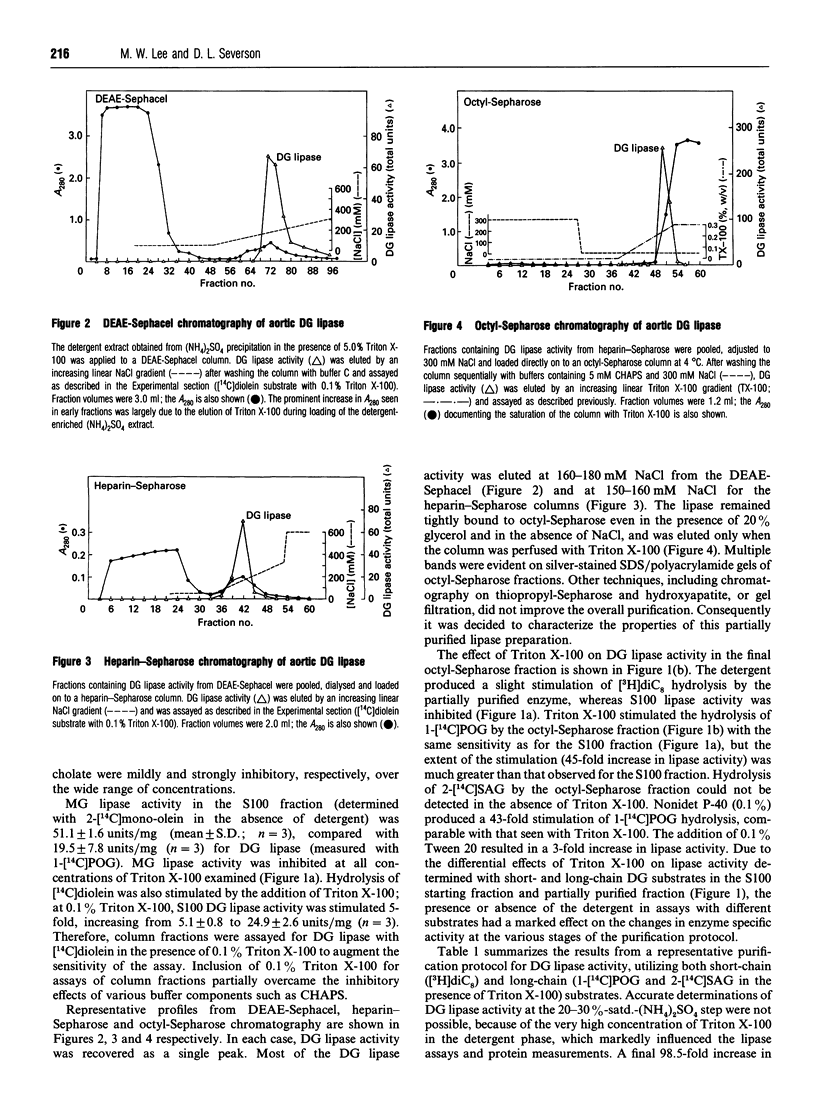
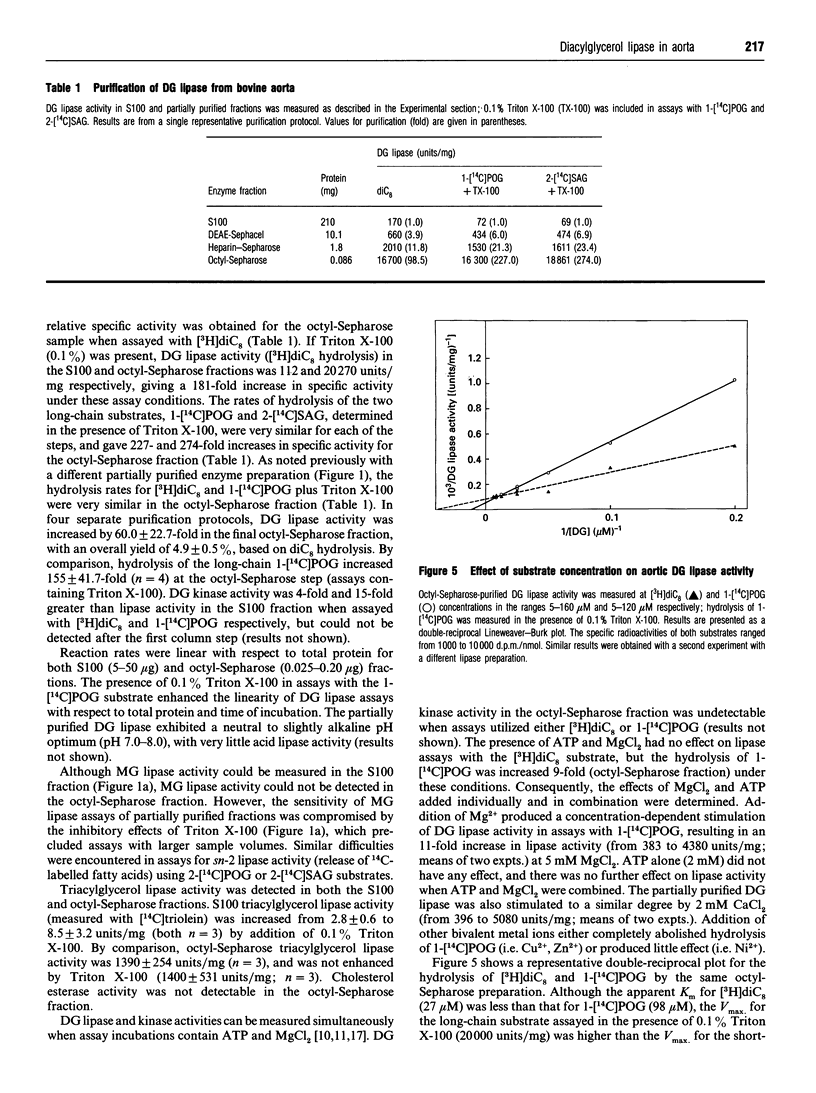
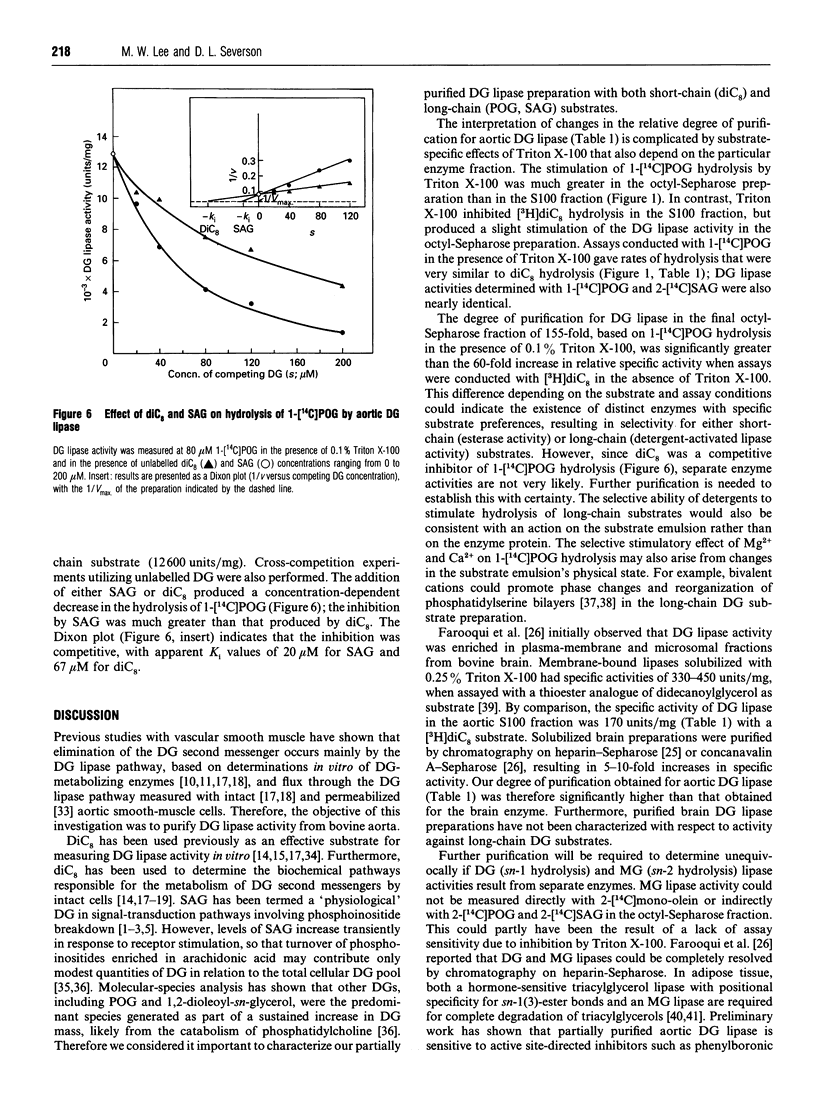
A diacylglycerol (DG) lipase has been purified from a soluble subcellular fraction of bovine aorta by (NH4)2SO4 precipitation in the presence of 5.0% (w/v) Triton X-100, followed by chromatography on DEAE-Sephacel, heparin-Sepharose and octyl-Sepharose in the presence of either CHAPS or Triton X-100 detergents. Under basal conditions, the hydrolysis of a short-chain [3H]dioctanoylglycerol ([3H]diC8) substrate was much greater than that of a long-chain 1-[1-14C]palmitoyl-2-oleoyl-sn-glycerol (1-[14C]POG) substrate. Lipase activity measured with 1-[14C]POG was markedly enhanced by Triton X-100. In the presence of 0.1% Triton X-100, specific enzyme activities in the octyl-Sepharose fraction determined with 1-[14C]POG or 1-stearoyl-2-[1-14C]-arachidonoyl-sn-glycerol as substrates were the same as that measured with [3H]diC8. MgCl2 (5mM) or CaCl2 (2 mM) also selectively stimulated lipase activity (up to 10-13-fold) measured with the long-chain (1-[14C]POG) substrate only. The increase in relative specific activity in the octyl-Sepharose fraction was 60-fold and 155-fold, based on hydrolysis of [3H]diC8 and 1-[14C]POG (+ Triton X-100), respectively. Unlabelled diC8 was a competitive inhibitor of 1-[14C]POG hydrolysis, suggesting that a single lipase hydrolyses both the short-chain and long-chain DG substrates; selective stimulatory effects of non-ionic detergents and bivalent cations on the hydrolysis of 1-[14C]POG may be due to effects on the physical properties of the substrate preparation. Monoacylglycerol lipase, DG kinase and cholesterol esterase activities could not be detected in the partially purified lipase preparation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. C., Gammon C. M., Ousley A. H., McCarthy K. D., Morell P. Bradykinin stimulates arachidonic acid release through the sequential actions of an sn-1 diacylglycerol lipase and a monoacylglycerol lipase. J Neurochem. 1992 Mar;58(3):1130–1139. doi: 10.1111/j.1471-4159.1992.tb09372.x. [DOI] [PubMed] [Google Scholar]
- Balsinde J., Diez E., Mollinedo F. Arachidonic acid release from diacylglycerol in human neutrophils. Translocation of diacylglycerol-deacylating enzyme activities from an intracellular pool to plasma membrane upon cell activation. J Biol Chem. 1991 Aug 25;266(24):15638–15643. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers. Metabolism of exogenous diacylglycerols by human platelets. J Biol Chem. 1986 Sep 25;261(27):12513–12519. [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Functions of diacylglycerol in glycerolipid metabolism, signal transduction and cellular transformation. Oncogene Res. 1988 Feb;2(3):205–218. [PubMed] [Google Scholar]
- Bishop W. R., Ganong B. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986 May 25;261(15):6993–7000. [PubMed] [Google Scholar]
- Chau L. Y., Tai H. H. Monoglyceride and diglyceride lipases from human platelet microsomes. Biochim Biophys Acta. 1988 Dec 16;963(3):436–444. doi: 10.1016/0005-2760(88)90312-8. [DOI] [PubMed] [Google Scholar]
- Chuang M., Dell K. R., Severson D. L. Protein kinase C does not regulate diacylglycerol metabolism in aortic smooth muscle cells. Mol Cell Biochem. 1990 Jul 17;96(1):69–77. doi: 10.1007/BF00228454. [DOI] [PubMed] [Google Scholar]
- Chuang M., Lee M. W., Zhao D., Severson D. L. Metabolism of a long-chain diacylglycerol by permeabilized A10 smooth muscle cells. Am J Physiol. 1993 Oct;265(4 Pt 1):C927–C933. doi: 10.1152/ajpcell.1993.265.4.C927. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. sn-1,2-Dioctanoylglycerol. A cell-permeable diacylglycerol that mimics phorbol diester action on the epidermal growth factor receptor and mitogenesis. J Biol Chem. 1985 Feb 10;260(3):1562–1566. [PubMed] [Google Scholar]
- Farooqui A. A., Taylor W. A., Horrocks L. A. Characterization and solubilization of membrane bound diacylglycerol lipases from bovine brain. Int J Biochem. 1986;18(11):991–997. doi: 10.1016/0020-711x(86)90244-2. [DOI] [PubMed] [Google Scholar]
- Farooqui A. A., Taylor W. A., Horrocks L. A. Separation of bovine brain mono- and diacylglycerol lipases by heparin sepharose affinity chromatography. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1241–1246. doi: 10.1016/0006-291x(84)91225-7. [DOI] [PubMed] [Google Scholar]
- Farooqui A. A., Taylor W. A., Pendley C. E., 2nd, Cox J. W., Horrocks L. A. Spectrophotometric determination of lipases, lysophospholipases, and phospholipases. J Lipid Res. 1984 Dec 15;25(13):1555–1562. [PubMed] [Google Scholar]
- Florin-Christensen J., Florin-Christensen M., Delfino J. M., Rasmussen H. New patterns of diacylglycerol metabolism in intact cells. Biochem J. 1993 Feb 1;289(Pt 3):783–788. doi: 10.1042/bj2890783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson G., Belfrage P. Positional specificity of hormone-sensitive lipase from rat adipose tissue. J Biol Chem. 1983 Dec 10;258(23):14253–14256. [PubMed] [Google Scholar]
- Fredrikson G., Tornqvist H., Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta. 1986 Apr 15;876(2):288–293. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- Hee-Cheong M., Fletcher T., Kryski S. K., Severson D. L. Diacylglycerol lipase and kinase activities in rat brain microvessels. Biochim Biophys Acta. 1985 Jan 9;833(1):59–68. doi: 10.1016/0005-2760(85)90253-x. [DOI] [PubMed] [Google Scholar]
- Hee-Cheong M., Severson D. L. Metabolism of dioctanoylglycerol by isolated cardiac myocytes. J Mol Cell Cardiol. 1989 Aug;21(8):829–837. doi: 10.1016/0022-2828(89)90722-0. [DOI] [PubMed] [Google Scholar]
- Hee-Cheong M., Severson D. L. Properties of monoacylglycerol lipase in rabbit aorta. Lipids. 1987 Dec;22(12):999–1004. doi: 10.1007/BF02536439. [DOI] [PubMed] [Google Scholar]
- Helenius A., McCaslin D. R., Fries E., Tanford C. Properties of detergents. Methods Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Cullis P. R. Effects of divalent cations and pH on phosphatidylserine model membranes: a 31P NMR study. Biochem Biophys Res Commun. 1980 Feb 12;92(3):846–852. doi: 10.1016/0006-291x(80)90780-9. [DOI] [PubMed] [Google Scholar]
- Ide H., Koyama S., Nakazawa Y. Diacylglycerol generated in the phospholipid vesicles by phospholipase C is effectively utilized by diacylglycerol lipase in rat liver cytosol. Biochim Biophys Acta. 1990 May 22;1044(2):179–186. doi: 10.1016/0005-2760(90)90301-d. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Kondoh H., Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983 Feb 10;258(3):1767–1774. [PubMed] [Google Scholar]
- Kato M., Takenawa T. Purification and characterization of membrane-bound and cytosolic forms of diacylglycerol kinase from rat brain. J Biol Chem. 1990 Jan 15;265(2):794–800. [PubMed] [Google Scholar]
- Kennerly D. A. Diacylglycerol metabolism in mast cells. Analysis of lipid metabolic pathways using molecular species analysis of intermediates. J Biol Chem. 1987 Dec 5;262(34):16305–16313. [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A., Baer E. Ionic influences on the phase transition of dipalmitoylphosphatidylserine. Biochemistry. 1976 Feb 24;15(4):885–891. doi: 10.1021/bi00649a025. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Sagawa N., Okita J. R., Bleasdale J. E., MacDonald P. C., Johnston J. M. Diacylglycerol metabolism and arachidonic acid release in human fetal membranes and decidua vera. J Biol Chem. 1981 Jul 25;256(14):7316–7321. [PubMed] [Google Scholar]
- Parish C. R., Classon B. J., Tsagaratos J., Walker I. D., Kirszbaum L., McKenzie I. F. Fractionation of detergent lysates of cells by ammonium sulphate-induced phase separation. Anal Biochem. 1986 Aug 1;156(2):495–502. doi: 10.1016/0003-2697(86)90284-8. [DOI] [PubMed] [Google Scholar]
- Pessin M. S., Raben D. M. Molecular species analysis of 1,2-diglycerides stimulated by alpha-thrombin in cultured fibroblasts. J Biol Chem. 1989 May 25;264(15):8729–8738. [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Sagawa N., Okazaki T., MacDonald P. C., Johnston J. M. Regulation of diacylglycerol metabolism and arachidonic acid release in human amnionic tissue. J Biol Chem. 1982 Jul 25;257(14):8158–8162. [PubMed] [Google Scholar]
- Schubert D., Boss K., Dorst H. J., Flossdorf J., Pappert G. The nature of the stable noncovalent dimers of band 3 protein from erythrocyte membranes in solutions of Triton X-100. FEBS Lett. 1983 Oct 31;163(1):81–84. doi: 10.1016/0014-5793(83)81168-5. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Fletcher T., Groves G., Hurley B., Sloan S. Hydrolysis of triolein, cholesterol oleate, and 4-methylumbelliferyl stearate by acid and neutral ester hydrolases (lipases) from pigeon adipose tissue: effect of cAMP-dependent protein kinase. Can J Biochem. 1981 Jun;59(6):418–429. doi: 10.1139/o81-058. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Hee-Cheong M. Diacylglycerol lipase and kinase activities in rabbit aorta and coronary microvessels. Biochem Cell Biol. 1986 Oct;64(10):976–983. doi: 10.1139/o86-130. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Hee-Cheong M. Diacylglycerol metabolism in isolated aortic smooth muscle cells. Am J Physiol. 1989 Jan;256(1 Pt 1):C11–C17. doi: 10.1152/ajpcell.1989.256.1.C11. [DOI] [PubMed] [Google Scholar]
- Shears S. B. Regulation of the metabolism of 1,2-diacylglycerols and inositol phosphates that respond to receptor activation. Pharmacol Ther. 1991;49(1-2):79–104. doi: 10.1016/0163-7258(91)90023-f. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Stam H., Broekhoven-Schokker S., Hülsmann W. C. Characterization of mono-, di- and triacylglycerol lipase activities in the isolated rat heart. Biochim Biophys Acta. 1986 Jan 3;875(1):76–86. doi: 10.1016/0005-2760(86)90013-5. [DOI] [PubMed] [Google Scholar]
- Yang S. G., Saifeddine M., Chuang M., Severson D. L., Hollenberg M. D. Diacylglycerol lipase and the contractile action of epidermal growth factor-urogastrone: evidence for distinct signal pathways in a single strip of gastric smooth muscle. Eur J Pharmacol. 1991 Jul 12;207(3):225–230. doi: 10.1016/0922-4106(91)90034-f. [DOI] [PubMed] [Google Scholar]


