Graphical abstract
Keywords: Norharmane, Quaternized dimer, Anti-MRSA, Membrane, Metabolomics, DNA
Highlights
-
•
Design and synthesis of quaternization or dimerization norharmane analogues are described.
-
•
Their anti-MRSA activities are evaluated, and SARs are summarized.
-
•
Compound 5a exhibits potent bactericidal activity in vitro and in vivo with low toxicity.
-
•
Compound 5a could disrupt bacterial biofilm and damage the membrane.
-
•
Metabolomics analysis is used to systematically investigate the mechanisms.
Abstract
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA)-caused infections greatly threaten public health. The discovery of natural-product-based anti-MRSA agents for treating infectious diseases has become one of the current research focuses.
Objectives
This study aims to identify promising anti-MRSA agents with a clear mechanism based on natural norharmane modified by quaternization or dimerization.
Methods
A total of 32 norharmane analogues were prepared and characterized. Their antibacterial activities and resistance development propensity were tested by the broth double-dilution method. Cell counting kit-8 and hemolysis experiments were used to assess their biosafety. The plasma stability, bactericidal mode, and biofilm disruption effects were examined by colony counting and crystal violet staining assays. Fluorescence microscopy, metabolomic analysis, docking simulation and spectra titration revealed its anti-MRSA mechanisms. The mouse skin infection model was used to investigate the in vivo efficacy.
Results
Compound 5a was selected as a potential anti-MRSA agent, which exhibited potent anti-MRSA activity in vitro and in vivo, low cytotoxicity and hemolysis under an effective dose. Moreover, compound 5a showed good stability in 50% plasma, a low tendency of resistance development and capabilities to disrupt bacterial biofilms. The mechanism studies revealed that compound 5a could inhibit the biosynthesis of bacteria cell walls, damage the membrane, disturb energy metabolism and amino acid metabolism pathways, and interfere with protein synthesis and nucleic acid function.
Conclusions
These results suggested that compound 5a is a promising candidate for combating MRSA infections, providing valuable information for further exploiting a new generation of therapeutic antibiotics.
Introduction
Infectious diseases caused by drug-resistant bacteria in hospitals and communities are widespread and growing threats to global human health [1]. It is predicted that drug-resistant bacterial infections could result in 10 million deaths globally each year and has also been listed as one of the top ten public threats [2]. Methicillin-resistant Staphylococcus aureus (MRSA) is a principal cause of osteomyelitis, pneumonia, endocarditis, skin infections and bacteremia, and has spread worldwide since firstly reported in 1961 [3]. Moreover, MRSA can infect nearly anybody's site with nearly any item in contact with the skin [4]. In the past decades, numerous antibiotics have been successfully used to treat MRSA infections in the clinic, including macrolides, quinolones, tetracycline, glycopeptide, and cephalosporins [5]. However, the abuse of antibiotics results in a dramatic increase in drug resistance, and the species of FDA-approved new antibiotics are limited because of low earnings for pharmaceutical companies. Thus, the clinically effective treatments of MRSA-related diseases have met enormous challenges [6]. Furthermore, similar molecular skeletons for most traditional antibiotics can cause cross-resistance [7]. Hence, the development of new molecular entities to effectively address MRSA infection issues is urgently needed.
Natural products and derivatives with diverse structures play a vital role in the discovery of antibiotics [8]. Approximately 50% of FDA-approved antibacterial drugs from 1981 to 2019 are derived from natural products [2]. The seed of Peganum harmala L., a traditional Chinese medicine, was used for wound therapy in the Middle East and North Africa due to their antibacterial activity [9], [10]. Modern bioassay-guided fractionation indicates that 2–6% of pharmacological alkaloids are their basis of antibacterial activity [10]. Most alkaloids are harmane, harmine, harmaline, and harmalol, which contain the same skeleton norharmane, also known as β-carboline. Interestingly, norharmane hydrochloride exhibited attractive antibacterial activity against S. aureus with minimum inhibitory concentrations (MIC) of 16 μg/mL (Fig. 1) [11]. Therefore, norharmane can be used as an anti-MRSA lead skeleton.
Fig. 1.
Progress of norharmane derivatives as anti-MRSA agents and the design of present work.
As is known, quaternization or dimerization design strategies can enhance antibacterial activity [12], [13]. After quaternization at N-2 position for compound A (MIC = 21 μg/mL), its analogue compound B showed better activity against MRSA (MIC = 4 μg/mL) (Fig. 1) [14]. Our previous work also provided a quaternized anti-MRSA molecule C (MIC = 4 μg/mL) based on norharmane [15]. Moreover, the structure–activity relationships (SARs) study indicated that introducing the methoxy group at the C-6 position could promote anti-MRSA activity. The tactics of dimerization, demonstrated as a valid method to improve the antibacterial activity previously [16], were also feasible for norharmane confirmed by the activity change of compounds D and E [17]. Hence, this study aims to design a series of novel quaternization or dimerization norharmane analogues with a methoxy group at the C-6 position to discover natural product-based anti-MRSA agents.
Multi-target drugs can synergistically regulate multiple targets in the drug-resistant bacterial system to avoid the development of drug resistance [18]. Notably, 52% (14/27) of the FDA-approved antibiotics between 2000 and 2020 are multi-target drugs [18]. Quaternary ammonium agents were widely used as bactericides due to their biofilm-eradicating activity and membrane-targeting mechanism [12], [13]. In addition, the norharmane can act on multiple targets such as DNA, DNA gyrase and so on [15]. Therefore, the targeted compounds we designed could act on MRSA by a multi-target mechanism. In this work, we evaluated the cytotoxicity, hemolysis, plasma stability, drug resistance development, killing kinetics and in vivo antibacterial effect of the promising compound. More importantly, the anti-MRSA mechanism was clarified by disruption of biofilms, cell membrane damage, intracellular metabolic analysis and DNA interaction assays.
Materials and methods
Reagents, materials, and instruments
Deuterium generation reagents, including deuterated chloroform (CDCl3), deuterated dimethyl sulfoxide (DMSO‑d6) and deuterated methanol (CD3OD), were provided by J&K Scientific LTD. (Beijing, China). Other commercial reagents were directly used without further purification. Nuclear magnetic resonance (NMR) analysis was conducted on a Bruker AV 600 spectrometer (Bruker Biospin, Rheinstetten, Germany). Mass spectra data were acquired using a Waters ACQUITY™ UPLC system coupled with a SYNAPT G2-Si QTOF high-definition mass spectrometer (Waters, Manchester, UK). Fluorescence microscopy analysis was performed on LECIA DM4 B microscope (Leica, Wetzlar, Germany). Gemini EM microplate reader (Molecular Devices, CA, USA) was used for optical density measurements. The molecular docking study was performed by Sybyl-X 2.0 software (Tripos Inc., NJ, USA).
Synthesis of compounds 2–10
The detailed synthesis method and data characterization can be found in Supporting Information.
In vitro antibacterial assay
The MIC values were measured by the broth double-dilution method, as reported previously [16]. S. aureus strains CGMCC 1.12409, ATCC 43300, ATCC 6538 and E. coli ATCC 8739 were purchased from China General Microbiological Culture Collection Center. The clinical-isolated MRSA strains (M−15, M−16, M−17, M−18, and M−23) were kindly provided by Dr. Qin Shang-Shang from Zhengzhou University [5]. Vancomycin and ampicillin sodium were selected as positive drugs. The compounds (2–10) solutions were initially prepared with dimethyl sulfoxide (DMSO). Then, they were diluted with Mueller–Hinton Broth (MHB) media. The required final concentrations were 0.25–128 μM and 0.25–128 μg/mL (DMSO < 0.5%). A concentration of logarithmic-growth-phase bacterial suspension was adjusted to 1 × 106 CFU/mL by turbidimetry. After being cultured for 18 h, the concentration of visible inhibition on bacteria growth was defined as the MIC value for each compound.
In vitro cytotoxicity assay
The cytotoxicity tests against human normal prostate stromal cells (WPMY-1), human normal trophoblastic cells (HTR-8/SVneo) and human prostate cancer cells (DU-145, PC-3) were performed by cell counting kit-8 (CCK-8) assay, as reported previously [5]. Briefly, the cell inoculation concentration was 5 × 103 cells/well. After being cultured, the medium was discarded and replaced with a fresh medium containing compound in various concentrations (2–512 μg/mL). After incubation for 24 h, CCK-8 was used to monitor the cell viability. Absorbance values at 450 nm were obtained by a Microplate Reader.
Hemolytic assay
The hemolysis assay was conducted as described previously [19]. Fresh red blood cells (RBCs) were collected from rabbit blood. RBCs suspension (5% v/v, 150 μL) was added to compound 5a (50 μL) solutions with indicated concentrations in a 96-well plate. After incubation (37 °C, 1 h) and centrifugation (3500 rpm, 5 min), 100 μL of supernat was transferred to a new plate. The absorbance values at 540 nm were obtained. Triton X-100 (1%) and PBS (1 × ) were used as positive and negative controls, respectively. Hemolytic activity was calculated as the following formula: .
Plasma stability assay
The bacterial suspension of MRSA CGMCC 1.12409 was adjusted to 1 × 106 CFU/mL. Compound 5a was dissolved in 50 µL of 50% fresh plasma. After being preincubated at 37 °C for 0, 3 and 6 h, respectively, the bacterial solution (150 µL) was added to the 96-well plate, which was cultured for 18–24 h. The test methods of MIC and minimum bactericidal concentrations (MBC) values were described as reported previously [20].
Drug resistance assay
As described previously [7], the bacterial suspension of MRSA CGMCC 1.12409 was cultured with compound 5a or norfloxacin at a concentration of sub-MIC (0.5 × MIC) at 37 °C for 10 h, which was further used to determine the new MIC value. The new MIC value was used for the next process, and the experiments were repeated for 10 passages.
Growth and time-kill kinetics assay
The efficacy of compound 5a on the growth of MRSA CGMCC 1.12409 was analyzed as previously described [21]. Briefly, the logarithmic-growth-phase MRSA CGMCC 1.12409 was inoculated into fresh MHB media (1 × 106 CFU/mL), and then compound 5a was added with final concentrations of 0, 0.5, 1, 2, and 4 × MIC, respectively. Vancomycin (1 and 4 × MIC) was the positive control. Each sample was incubated at 37 °C, and the OD600nm value was recorded at appointed times (0, 0.5, 1, 2, 4, 6, 8, 12, and 24 h).
Time-kill kinetics of compound 5a was also carried out as reported previously [7]. Briefly, the logarithmic-growth-phase MRSA CGMCC 1.12409 (1 × 106 CFU/mL) was prepared and incubated with compound 5a (0, 1, 2, 4, and 8 × MIC) and vancomycin (1 and 4 × MIC) at 37 °C for 0, 0.5, 1, 2, 4, 6, 8 and 12 h, respectively. The bacteria and drug mixture were diluted with saline solution and spread on the Mueller–Hinton agar (MHA) plates. After incubation and colony counting, the values (Log10 CFU/mL) were obtained.
Determination of MBIC and MBEC
As described previously [20], the 200 μL of logarithmic-growth-phase MRSA CGMCC 1.12409 suspension (5 × 107 CFU/mL) was seeded to the 96-well plates. The mature MRSA biofilm model was constructed after incubation at 37 °C for 24 h. Then, the medium and planktonic bacteria were discarded, and MRSA biofilms were washed with 1 × PBS. Subsequently, fresh MHB medium (200 μL) with serial dilutions of compound 5a (1–128 μg/mL) was added to the wells and incubated at 37 °C for 24 h. The lowest concentration with no turbidity (visible bacterial growth) was determined as the minimum biofilm inhibitory concentration (MBIC). Subsequently, the medium was discarded and washed with 1 × PBS. The fresh medium (200 μL) was put in to let viable MRSA biofilms for recovery. After incubation at 37 °C for 24 h, planktonic bacteria spread into the medium, leading to turbidity. The lowest concentration with no turbidity was determined as the minimum biofilm eradication concentration (MBEC) because of eradicated biofilms.
Determination of biofilm-sustaining bacteria
Compound 5a with different concentrations was added to the mature MRSA biofilm prepared by the above method. After incubation at 37 °C for 24 h, dilution and colony counting, the values (Log10 CFU/mL) were obtained accordingly (n = 3).
Effect on biofilm biomass
As reported previously [20], [12], compound 5a with different concentrations was added to the mature MRSA biofilm prepared by the above method. MHB broth without compound as the positive control. MHB broth without compound and prepared bacterial biofilm as the blank control. The medium and planktonic bacteria were removed, and the biofilms were fixed by 200 μL of methanol. The crystal violet (0.1%, 200 μL) was pipetted into the wells and stained for 20 min. After washing with 1 × PBS, MRSA biofilm was added with 200 μL of 33% acetic acid to remove the redundant dyestuff. The OD570 values were obtained to calculate the residue of MRSA biofilm.
Exopolysaccharide (EPS) reduction assay
As reported previously [12], 1 mL of MRSA suspension (1 × 106 CFU/mL) was incubated in 6-well microtiter plates with or without compound 5a (2–64 μg/mL) at 37 °C for 24 h. Then, the planktonic cells were discarded, and biofilms were washed with 0.9% NaCl solution. After harvesting the biofilms by adding 0.5 mL of 0.9% NaCl solution into the wells followed by scrapping the adhered biofilm with a sterile scraper, 5% phenol (0.5 mL) and H2SO4 (2.5 mL) were added to the extracted mixture, which was incubated for 1 h in the dark. The OD490nm values were measured. The percentage reduction of EPS content in biofilm was calculated as the following formula:.
SYTOX green assay
As reported previously [7], the logarithmic-growth-phase MRSA CGMCC 1.12409 suspension (150 μL, 1 × 108 CFU/mL) with SYTOX green solution (50 μL, 1 μM) was cultured for 30 min under dark. The fluorescence intensity of the mixture was monitored under an emission/excitation wavelength of 523/504 nm. After the signal was stable, it was measured for 6 min with 30-second intervals. Then compound 5a with final concentrations of 0, 4 × and 8 × MIC was added. The fluorescence intensity values were measured every 30 s for the next 1 h.
4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) and propidium iodide (PI) staining assay
The logarithmic-growth-phase MRSA CGMCC 1.12409 suspension (1 × 108 CFU/mL) treated by compound 5a (0 and 4 × MIC) was cultured at 37 °C (250 rpm) for 2 h. After rinse and resuspension for the MRSA samples, DAPI (20 μL, 10 μg/mL) and PI (20 μL, 20 μg/mL) were mixed into them, which were incubated at 37 °C for 30 min. Subsequently, the MRSA cells were made into smears and observed under a LECIA DM4 B microscope.
Metabolomics analysis
Sample preparation for the metabolomics study was conducted as reported previously [23], [24], [25]. The logarithmic-growth-phase MRSA CGMCC 1.12409 suspension was adjusted to an appointed concentration (OD600 = 0.4) and then diluted 100-fold with MHB media. Compound 5a (0.5 × MIC, 1 μg/mL) was put into the MRSA suspension (20 mL) in 125 mL flasks. An equal amount of sterile water containing DMSO was used as the control group. Ten independent biological replicates were prepared. After incubation (37 °C, 250 rpm) for 12 h, the OD600 value was regulated to 0.4. Then, the 15 mL of suspension was moved to a centrifuge tube. After centrifugation (4 °C, 3500 rpm, 6 min) and rinse with 0.9% NaCl solution twice, MRSA cells were snap-frozen and stored at −80 °C until metabolomic analysis. After thawing on ice, 4 mL of methanol was added. After resuspending, the suspension underwent three freeze–thaw cycles (10 min) in liquid nitrogen and ice. After ultrasonic extraction for 5 min in ice water, 3 mL of supernatant was obtained by centrifugation (4 °C, 8000 rpm, 10 min). To optimize the LC-MS conditions and validate the LC-MS method, a quality control sample (QCs) was obtained by mixing equal aliquots of each sample (200 μL). The QC samples were repeatedly injected once every four experimental samples. The bacterial metabolome was profiled using an ACQUITY UPLC coupled to a SYNAPT G2-Si QTOF high-definition MS mass spectrometer (Waters, Manchester, UK). Chromatographic separation was performed on an ACQUITY HSS T3 column (100 mm × 2.1 mm i.d., 1.8 μm) at 50 °C. The mobile phases were water (phase A) and acetonitrile (phase B), which were all added 0.1% (v/v) formic acid. The gradient proceeded at the flow rate of 0.4 mL/min as follows: isocratic 5% B (0–1 min); linear gradient from 5% to 95% B (1–16 min); isocratic 95% B (16–20 min); linear gradient from 95% to 5% B (20–22 min); isocratic 5% B (22–26 min). The key MS operating parameters were as follows: capillary voltage, 3.0/−2.5 kV; sample cone voltage, 40 V; source temperature, 140 °C; desolvation temperature, 500 °C; desolvation gas, 800 L/h; cone gas, 10 L/h; mass range, 100–1200 Da; MSE scan mode for data acquisition. A solution of leucine − enkephalin (400 pg/mL) was used for lock mass correction. The raw data acquired by LC-MS were processed using Progenesis QI software (Nonlinear Dynamics, Newcastle, UK). A matrix comprising accurate mass, retention time, normalized intensity, and P-values was obtained. After pretreatment by the “80% rule”, the resultant data were processed by orthogonal partial least squares discriminant analysis (OPLS–DA), which was validated through a permutation analysis (200 times). The putative metabolites were tentatively assigned by databases searching, including METLIN (http://metlin.scripps.edu/xcms/) and the Human Metabolome Database (HMDB, http://www.hmdb.ca). The metabolomics pathway was analyzed by MetaboAnalyst 5.0 (https://www.metaboanalyst.ca) based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic pathway database (https://www.kegg.jp/kegg/) [25].
Molecular docking studies
Molecular docking simulation was performed by the Surflex-Dock program of the Sybyl-X 2.0 package. The complex structures of DNA gyrase (PDB ID: 2XCS) [26] and DNA (PDB: 454D) [27] were obtained from Protein Data Bank (https://www.rcsb.org/). All hydrogen atoms were added to the crystal structures. Moreover, the AMBER7 FF99 method was used to add a charge. The chemical structure of compound 5a was prepared in the package. Tripos force field and Gasteiger–Hückel methods were applied to optimize the energy. The interactions were displayed by Discover Studio 2019 clients.
UV absorption and fluorescence spectra assay
As reported previously [28], the UV and fluorescence spectra of compound 5a (20 μM) titrated with calf thymus DNA (0–12 μM) were recorded at 37 °C by UV-2450 (Shimadzu) and F-4600 (Hitachi) spectrophotometers, respectively. Tris-HCl buffer solution (pH = 7.4) was used to prepare the stock solution.
Ethics statement
Animal experiments were approved by the Animal Care and Use Committee of Weifang Medical University (license no. 2020SDL082) and performed according to the ethical policies.
In vivo antibacterial assay
As reported previously [5], healthy female KM mice (body mass ∼25 g) purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd. were used to construct a mouse skin infection model. The mice were randomly divided into six groups (n = 6): a blank group (no injection of MRSA and compound), a control group (only injection of MRSA), a positive control group (vancomycin, 3.3 mg/kg) and three 5a-treated groups (3.3, 6.6 and 13.2 mg/kg). After being treated with cyclophosphamide, depilated and reared for 24 h, the mice were subcutaneously injected with MRSA solution (50 μL, 1 × 107 CFU/mL), which caused the visible abscesses. Then 50 μL of different concentrations of 5a, vancomycin and 0.9% NaCl solution were injected into the site of infection, which were continued for four days. Finally, the mice were euthanized after 24 h administration of the last dose, and the infected skin was aseptically separated. The infected skin (0.06 g) was placed into 2 mL of sterile 0.9% NaCl solution and homogenized, which were further diluted and plated onto agar plates. In addition, hematoxylin and eosin (H&E) staining was used to investigate their pathological changes under a light microscope at 100× magnification.
Statistical analysis
Three biological replicates were performed unless stated. Data were expressed as mean ± standard deviation (SD). The significance of difference was analyzed by Student’s t-test or one-way analysis of variance (ANOVA) with GraphPad Prism 8.0 software.
Results and discussion
Chemistry
The synthesis of these molecular entities was shown in Schemes S1 and S2. Briefly, to realize a synthetic pathway for preparing quaternization or dimerization norharmane analogues, we felt that the commercially available 5-methoxytryptamine 1 would be a useful precursor (Scheme S1). Compound 2 was obtained with a yield of 98% by the Pictet–Spengler cyclization reaction using polyformaldehyde in acetic acid and methanol. After treatment with potassium permanganate in anhydrous dimethylformamide, the scaffold 3 was smoothly prepared (35% yield). Subsequently, some norharmane dimers 4 were synthesized using sodium hydride as the base, followed by the addition of corresponding dibromoalkane (62–87% yield). Quaternization of compound 4 by treatment with iodomethane using acetonitrile as solvent at 50 °C proceeded successfully, affording compound 5 with a yield of 33–48%. Then quaternized norharmane monomers 6 (54–71% yield) and dimers 7 (42–67% yield) modified at N-2 position were synthesized with corresponding dibromoalkane by the same method, which provides sufficient theoretical support for further SARs study. Methylation product 8 modified at N-9 position was prepared in the presence of sodium hydride with 92% yield. Next, compounds 9 (43–77% yield) and 10 (34–52% yield) were synthesized through a quaternization reaction.
A total of 32 synthesized norharmane analogues were fully characterized through 1H NMR, 13C NMR, and HRMS (Supplementary Fig. S1). All the spectral data are in accordance with the proposed chemical structures. Taking compound 5a as an example, the signals of the methyl and methoxy groups were detected around δ = 3.98 ppm and δ = 4.66 ppm in 1H NMR spectra, respectively. The signals of benzene and pyridine rings presented to the aromatic ring region in the NMR spectra. Besides, the signal of [M−2I]2+ appeared at 268.1574 Da in the HRMS of compound 5a with a relative error of 0.37 ppm, which conformed to the theoretical value of 268.1573 Da.
Antibacterial activity and SARs
The MIC values were measured to examine the in vitro antibacterial activity of norharmane analogues against both Gram-positive and Gram-negative bacterial strains, including S. aureus ATCC 6538, ATCC 43300, CGMCC 1.12409, and E. coli ATCC 8739. Generally, the activity of antibacterial drugs is expressed by mass concentration. To study the SARs more accurately, the molar concentration was used to test the MIC values in this section. In this work, good activity was defined when the MIC ≤ 2 μM. The potential activity was defined when the 2 < MIC ≤ 4 μM. The moderate activity was defined when the 4 < MIC ≤ 32 μM. The poor activity was defined when the MIC > 32 μM. As displayed in Table 1, the parent compound 3 displayed poor activity with MIC > 128 μM. Moreover, all compounds were not sensitive toward E. coli. For S. aureus ATCC 6538, compounds 5a and 7a exhibited good activity (MIC = 2 μM) compared with ampicillin sodium (MIC = 2 μM) and vancomycin (MIC = 1 μM). Eleven compounds, 5c, 5d, 6a, 7b, 9a, 9b, 9c, 9d, 9e, 10a and 10b, showed potential or moderate activity with MICs ranging from 4 to 32 μM. For MRSA ATCC 43300 and CGMCC 1.12409, antibacterial activity was weakened. Ampicillin sodium displayed moderate (MIC = 32 μM) and poor activity (MIC > 128 μM) against MRSA ATCC 43300 and CGMCC 1.12409, respectively. Interestingly, compound 5a maintained its activity with a MIC of 4 μM against MRSA ATCC 43300 and CGMCC 1.12409, which is close to the activity of vancomycin (MIC = 1 μM). Compounds 6a, 6b and 9a showed moderate activity with MICs ranging from 8 to 32 μM. Moreover, compound 5a showed activity against E. coli ATCC 8739, whereas vancomycin is not sensitive. Collectively, compound 5a displayed the most potent antibacterial activity across the synthesized harmane analogues.
Table 1.
In vitro antibacterial activities of the synthesized compounds (MIC in μM).
| Compd. | Gram-positive |
Gram-negative |
Compd. | Gram-positive |
Gram-negative |
||||
|---|---|---|---|---|---|---|---|---|---|
|
S. aureus ATCC 6538 |
MRSA ATCC 43,300 |
MRSA CGMCC 1.12409 |
E. coli ATCC8739 |
S. aureus ATCC 6538 |
MRSA ATCC 43,300 |
MRSA CGMCC 1.12409 |
E. coli ATCC8739 |
||
| 2 | >128 | >128 | >128 | >128 | 6e | 64 | 128 | 128 | >128 |
| 3 | >128 | >128 | >128 | >128 | 7a | 2 | 128 | 64 | 128 |
| 4a | >128 | >128 | >128 | >128 | 7b | 8 | 128 | 64 | 128 |
| 4b | >128 | >128 | >128 | >128 | 7c | 128 | >128 | >128 | >128 |
| 4c | >128 | >128 | >128 | >128 | 7d | 128 | >128 | >128 | >128 |
| 4d | >128 | >128 | >128 | >128 | 7e | >128 | >128 | >128 | >128 |
| 4e | >128 | >128 | >128 | >128 | 8 | >128 | >128 | >128 | >128 |
| 4f | >128 | >128 | >128 | >128 | 9a | 8 | 32 | 8 | 128 |
| 5a | 2 | 4 | 4 | 64 | 9b | 8 | 64 | 128 | >128 |
| 5c | 8 | 64 | 32 | >128 | 9c | 32 | >128 | >128 | >128 |
| 5d | 16 | 128 | 64 | >128 | 9d | 32 | 128 | >128 | >128 |
| 5e | 128 | >128 | >128 | >128 | 9e | 16 | >128 | >128 | >128 |
| 6a | 32 | 32 | 32 | 128 | 10a | 4 | 128 | 64 | 128 |
| 6b | 64 | 32 | 32 | 128 | 10b | 32 | >128 | 128 | >128 |
| 6c | 64 | 64 | 128 | >128 | 10c | >128 | >128 | >128 | >128 |
| 6d | 64 | 128 | 128 | >128 | 10d | >128 | >128 | >128 | >128 |
| Ampa | 2 | 32 | >128 | 8 | Vanb | 1 | 1 | 1 | >128 |
Amp: ampicillin sodium.
Van: vancomycin.
The SARs against S. aureus were carefully summarized based on the antibacterial activity, as shown in Fig. S2. For norharmane monomers modified at N-2 position: (a) Quaternization modification is favorable, such as compounds 3 and 9a; (b) Overall, the longer chain gives more potent anti-MRSA activity. For example, compound 6a exhibited better activity (MIC = 32 μM) than compounds 6b–6e, with MICs ranging from 32 to 128 μM; (c) The monomer is better against MRSA, such as compounds 6a and 7a; (d) Methyl substitution at N-9 position is beneficial against S. aureus ATCC 6538 such as compounds 6 and 9, whereas miscellaneous for MRSA. For norharmane dimers modified at N-2 position: (a) Quaternization modification is beneficial, such as compounds 3 and 7a; (b) The long-chain is advantageous, such as compounds 10a (MIC = 4 μM) and 10b (MIC = 32 μM) against S. aureus ATCC 6538; (c) Methyl substitution at N-9 position is undesirable such as compounds 7a (MIC = 2 μM) and 10a (MIC = 4 μM) against S. aureus ATCC 6538. For norharmane dimers modified at N-9 position: (a) Quaternization modification is beneficial, such as compounds 4a and 5a; (b) The long-chain is favorable such as compounds 5a (MIC = 4 μM) and 5e (MIC > 128 μM) against MRSA CGMCC 1.12409. In general, quaternized dimers based on norharmane could effectively improve antibacterial activity. Specifically, dimerization at N-9 position with N-2 quaternization is better than directly quaternized dimers at N-2 position.
Hence, compound 5a was selected for in-depth biological research. Subsequently, the superior compound 5a was further evaluated for its anti-MRSA activity containing MRSA standard strain (ATCC 43300), community-acquired strain (CGMCC 1.12409), and 5 clinical-isolated strains under mass concentration (Table 2). Compound 5a exhibited remarkable antibacterial effects against all tested MRSA strains with MIC of 2–4 μg/mL, close to vancomycin's activity (MIC = 1 μg/mL), the most potent anti-MRSA drug.
Table 2.
Antibacterial activity values of compound 5a against MRSA (MIC in μg/mL).
| Compd. | Standard strain |
Community-acquired strain |
Clinical-isolated strains |
||||
|---|---|---|---|---|---|---|---|
| ATCC 43,300 | CGMCC 1.12409 | M-15 | M-16 | M-17 | M-18 | M-23 | |
| 5a | 4 | 2 | 4 | 4 | 2 | 4 | 2 |
| Ampa | 16 | >64 | >64 | >64 | >64 | >64 | >64 |
| Vanb | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Amp: ampicillin sodium.
Van: vancomycin.
Cytotoxicity and hemolysis evaluation
As depicted in Fig. S3A, the viability of WPMY-1, HTR-8/SVneo, DU-145 and PC-3 cells exceeded 90% under an effective anti-MRSA dose (2 μg/mL), indicating low in vitro cytotoxicity. Moreover, the viability of all tested cells still exceeded 70% at 16 μg/mL (8 × MIC) of compound 5a. Interestingly, compound 5a can promote DU-145 cell proliferation at low concentrations (2–8 μg/mL) and maintain 76.83% viability even at 64 μg/mL (32 × MIC), which are comparable to vancomycin (80.68%) and norfloxacin (71.51%) as shown in Fig. S3B. Overall, these results are still very inspiring when considering the low MIC value of compound 5a against MRSA compared with the previous literature [7].
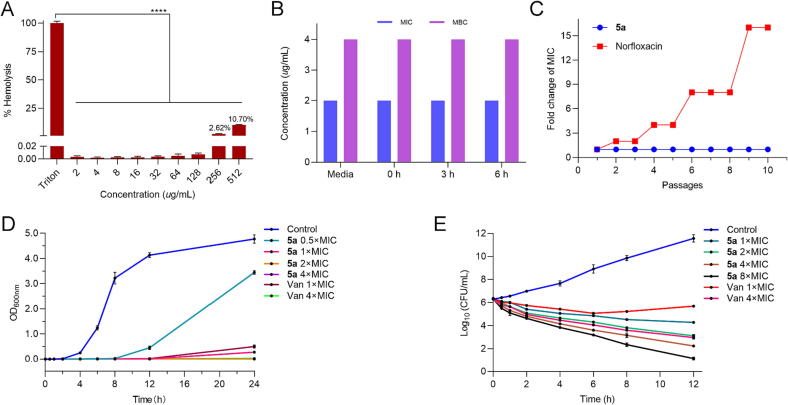
To further study the potential applications of compound 5a, we measured the hemolysis rate of rabbit RBCs [19], [20]. As illustrated in Fig. 2A, apparent hemolysis for the RBCs was not observed when concentrations of compound 5a were lower than 128 μg/mL. Moreover, only 10.7% hemolysis was presented at 512 μg/mL (256 × MIC). The results indicated that compound 5a has low hemolytic activity in mammals compared with the previous report [20].
Fig. 2.
(A) Hemolysis rate of compound 5a to red blood cells, ****P < 0.0001 vs Triton. (B) Anti-MRSA efficacy of compound 5a preincubated in 50% plasma for different periods. (C) Bacterial resistance study of compound 5a. (D) Growth kinetics of compound 5a and vancomycin. (E) Time-kill kinetics of compound 5a and vancomycin. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Plasma stability evaluation
Some proteins in the plasma might interact with antibiotics, decreasing their actual concentration and reducing antibacterial efficacy [12], [20]. Thus, it is essential to evaluate the plasma stability of anti-MRSA candidates. MRSA CGMCC 1.12409 was confirmed as the test strain because of its sensitivity against compound 5a and commercial availability. After preincubation in 50% plasma for different periods (0, 3 and 6 h), the MIC and MBC values of compound 5a against MRSA did not increase (Fig. 2B), which indicated that the activity of compound 5a was stable under the influence of 50% plasma.
Resistance development evaluation
The low tendency of developing bacterial resistance has become an essential pursuit for the discovery of new anti-MRSA drugs [7], [13]. Therefore, a laboratory simulation study was implemented with norfloxacin as the reference drug, which can quickly induce bacterial resistance. As displayed in Fig. 2C, the MIC of compound 5a was kept constant after 10 passages, while a 16-fold enhancement for norfloxacin was observed. The result indicated that compound 5a was challenging to develop resistance to MRSA CGMCC 1.12409, which might be attributed to its potential multi-target mechanism of action.
Growth and time-kill kinetics evaluation
The growth and time-killing kinetic curves were used to explore the bactericidal action of compound 5a against MRSA CGMCC 1.12409 [7], [21]. As depicted in Fig. 2D, evident growth retardation was observed after treatment with compound 5a (0.5 ×, 1 ×, 2 × and 4 × MIC). Specifically, 0.5 × MIC (1 μg/mL) of compound 5a could effectively control the growth of bacteria in the beginning 8 h.
MRSA CGMCC 1.12409 was treated with different concentrations of compound 5a for colony counting at different time points. As depicted in Fig. 2E, the gradual decrease of cell population upon the treatment with compound 5a was observed, indicating the killing and lysis of MRSA in a dose-dependent manner. Compound 5a could obtain 3.11 log-unit reduction at 8 × MIC in 6 h, 3.14 log-unit reduction at 4 × MIC in 8 h, and 3.24 log-unit reduction at 2 × MIC in 12 h, suggesting that compound 5a had a bactericidal action [5]. In addition, the bactericidal efficiency of compound 5a was more potent than vancomycin at the same multiple MIC changes and times [13].
Bacteria biofilm disruption evaluation
Bacterial biofilms, the main factor for persistent bacterial infection, could protect bacterial cells from outside influences. Therefore, it is necessary to evaluate the activity of disrupting biofilm for compound 5a. MBIC and MBEC values were used to evaluate the anti-biofilm efficiency of compound 5a [20]. Generally, biofilm assays mainly included three phases: (i) Phase 1, initial biofilm establishment, (ii) Phase 2, biofilm treatment with tested compound, and (iii) Phase 3, recovery of viable biofilms. The MBIC value was determined in Phase 2, and the MBEC value was obtained in Phase 3 [20]. The turbidity could not be observed based on the established MRSA biofilm model at concentrations of 32 μg/mL (MBIC) and 64 μg/mL (MBEC), respectively. Then, compound 5a on MRSA cell viability in biofilm was assessed. As shown in Fig. 3A, the living cells in the MRSA biofilms reduced from an initial amount of 8.01 to 5.05 log-unit at the concentration of 32 μg/mL (P < 0.0001). Moreover, approximately 4 log-unit reduction at 64 μg/mL could be observed (P < 0.0001). Additionally, the biofilm-disrupting effect of compound 5a was further assessed by the crystal violet staining method. As displayed in Fig. 3B, the amount of MRSA biofilm decreased notably at a high concentration of compound 5a. Approximately 86% biofilm biomass was disrupted by compound 5a at 32 μg/mL (P < 0.0001). These data indicated that compound 5a could disrupt the established biofilm.
Fig. 3.
(A) The cell viability in MRSA biofilm treated with compound 5a, *P < 0.05, **P < 0.01, ****P < 0.0001 vs Control. (B) The biofilm biomass of MRSA treated with compound 5a, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs Control. (C) Effect of compound 5a on EPS reduction in MRSA biofilm, *P < 0.05, **P < 0.01, ****P < 0.0001. (D) Cytoplasmic membrane permeabilization of compound 5a against MRSA by SYTOX green assay. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As a main participator in the biofilm matrix, EPS is essential in nutrient uptake and resistance to antibacterial drug diffusion [12]. Therefore, the ability of compound 5a to reduce EPS content in MRSA biofilm was investigated. As depicted in Fig. 3C, compound 5a could effectively reduce the contents of EPS in a dose-dependent manner. Compared to the control, 72.82% and 83.50% of EPS contents were decreased at 32 and 64 μg/mL (P < 0.0001), respectively, which indicated that compound 5a could loosen biofilm architecture by reducing EPS content to block bacterial adhesion.
Membrane damage study
As reported, one of the main mechanisms of action of quaternary ammonium agents is supposed to be the bacterial cell membrane. So the membrane damage assays were conducted to investigate this mode of action [5], [12], [13]. SYTOX green, unable to penetrate intact bacterial membranes, could bind highly to nucleic acids and generate a strong fluorescence when the membrane is incomplete [7]. As shown in Fig. 3D, the fluorescence intensity was enhanced in the mixture of MRSA CGMCC 1.12409 after treatment with compound 5a in a concentration-dependent manner. This result was further confirmed by a live/dead staining assay with DAPI and PI as monitors [5]. DAPI is used to stain cells disregarding viability by binding to the double-stranded DNA and emitting blue fluorescence. However, PI could cross the damaged bacterial cell membrane and emit red fluorescence. As illustrated in Fig. S4, only blue fluorescence was observed in the control group, indicating intact bacterial cell membranes in the absence of compound 5a. By comparison, both blue and red fluorescence were observed after treatment with compound 5a. These data suggested that compound 5a could damage the integrity of the bacterial cell membrane of MRSA.
Global analysis of the metabolomics response
To further explore the anti-MRSA mechanisms of compound 5a, the intracellular metabolomics study was carried out by UPLC-QTof-MS. The QC sample, a representative “mean” sample containing all metabolites, was used to validate the LC-MS method. As shown in Fig. S5, 80.3% and 85.9% of the variables had RSD<20% for positive and negative ion modes, respectively, illustrating that UPLC-QTof-MS method with excellent stability and repeatability could be used for metabolomics analysis [25]. After the data pretreatment, 14,467 and 9,380 ions were extracted from the chromatograms in positive and negative modes, respectively. Specifically, 6,227 and 4,629 ion peaks were obtained with P-value < 0.05, respectively. To reveal the effects of compound 5a on intracellular metabolic changes of MRSA, OPLS–DA score plots were constructed [28]. As depicted in Fig. S6A and S6D, the control and treated groups were unambiguously clustered according to their entire metabolome acquired by both positive (R2Y = 0.996, Q2 = 0.912) and negative (R2Y = 0.992, Q2 = 0.932) ions (Table S1). Furthermore, cross-validations were carried out to evaluate the robustness of the model by permutation test (n = 200, Fig. S6B and S6E) [29]. The Q2 values of −0.404 and −0.892 confirmed the stability of the models. These data indicated that the constructed models were reliable, with good fitness and prediction, and the treatment with compound 5a greatly perturbed the intracellular metabolic profile of MRSA. To identify the differential metabolites mainly contributing to the group separation, the Venn diagrams were subsequently constructed. As shown in Fig. S6C and S6F, 793 and 98 differential variables with P < 0.05, VIP > 1 and abs(Log2FC) > 1 were highlighted in the positive and negative ion modes, respectively. Among them, 54 down-regulated and 21 up-regulated differential metabolites were putatively identified according to their molecular weight and MS/MS spectra (Fig. S7 and Table S2).
The KEGG pathway enrichment analysis was conducted based on the potential differential metabolites. As shown in Fig. 4A and Table S3, 36 biochemical pathways were altered by compound 5a in MRSA. The pathways mainly comprised amino acid metabolism (10), carbohydrate metabolism (6), lipid metabolism (2), metabolism of cofactors and vitamins (9), and nucleic acid metabolism (2), as depicted in Fig. S8.
Fig. 4.
(A) The ingenuity pathway analysis of putatively identified metabolites. (B) Significantly impacted lipids in MRSA after treatment with compound 5a. (C) Carbohydrate metabolism. (D) Pantothenate and CoA biosynthesis. (E) Amino acid metabolism, aminoacyl-tRNA biosynthesis, folate biosynthesis, and one carbon pool by folate. (F) Purine metabolism. Green means down-regulated, and red means up-regulated. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Lipids play a crucial role in the formation and maintenance of bacteria cell structure [24]. Teichoic acid and peptidoglycan are important components of the cell wall of MRSA. As shown in Fig. 4B, CDP-glycerol, a fundamental precursor for teichoic acid and lipid biosynthesis [30], was reduced dramatically with Log2FC of −2.97. Amino-sugar and sugar-nucleotide metabolism, essential pools feeding key precursors into the peptidoglycan metabolism [30], was also perturbed, as depicted in Fig. 5A. The results indicated that the biosynthesis of bacteria cell wall might be influenced because of the potential perturbations of teichoic acid and peptidoglycan.
Fig. 5.
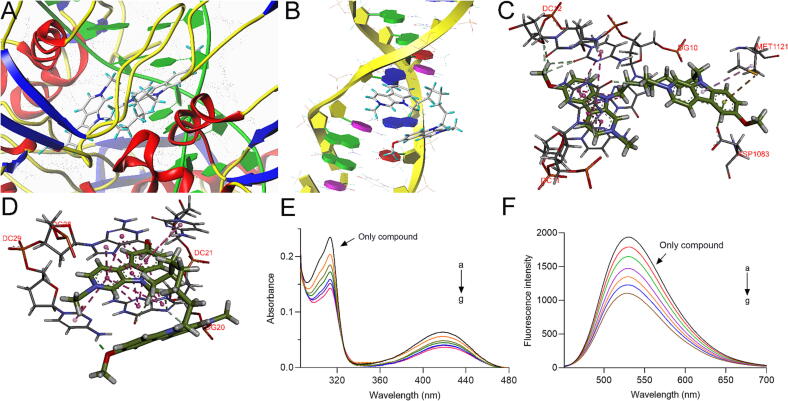
(A) Docking simulation of compound 5a with DNA gyrase. (B) Docking simulation of compound 5a with DNA. (C) The interactions of compound 5a in the active cavity of DNA gyrase. (D) The interactions between compound 5a and DNA. (E) The UV absorption spectra of compound 5a (20 μM) titrated with ctDNA (0–12 μM). (F) The fluorescence spectra of compound 5a (20 μM) titrated with ctDNA (0–12 μM).
Phosphatidylethanolamine (PE) was essential for membrane integrity and fluidity [24]. As shown in Fig. 4B, apparent perturbations could be observed with 14 down-regulated and 4 up-regulated PE, indicating membrane damage. In addition, CoA and its derivatives are crucial for enzymes of phospholipids synthesis and for reducing cell apoptosis and destruction [31]. The levels of three intermediates in pantothenate and CoA biosynthesis were notably reduced after treatment with compound 5a, including D-pantothenoyl-L-cysteine, dephospho-CoA, and pantetheine (Fig. 4D), which indicated the possibly blocked bacteria membrane repair.
As shown in Fig. 4C and Table S3, six carbohydrate pathways closely related to the energy supply were enriched, including starch and sucrose metabolism, pyruvate metabolism, TCA cycle, glycolysis and gluconeogenesis, galactose metabolism, amino sugar and nucleotide sugar metabolism. Specifically, most metabolites such as trehalose, sucrose, 2-hydroxyethyl-ThPP, and N-acetyl-D-mannosamine 6-phosphate are down-regulated, which have probably resulted from the direct damage on the energy metabolism system, indirect effects because of membrane damage, or activation of the defence system when encountering unfavorable environments [22], [24]. Whatever the reason, several changes caused by energy metabolism disorders were spotted in this research.
Amino acids, the blocks for proteins and signaling molecules, are vital for bacteria growth [28]. Previous studies have demonstrated that starvation could protect bacteria cells, resulting in amino acid restriction due to amino acids consuming as a substitute for carbon sources when treated with stress [32]. As shown in Fig. 4E and Table S3, ten amino acid metabolism pathways were mapped to the KEGG database. Notably, arginine metabolism has been used as a new strategy to develop novel bactericides [33]. Arginine biosynthesis and metabolism were influenced after MRSA treated with compound 5a in this study. The aminoacyl-tRNA biosynthesis pathway, closely related to amino acid metabolism, was also disturbed, which plays an essential role in protein synthesis, cell proliferation, and information transduction [34].
Folic acid metabolism is necessary to ensure bacteria cells' proper operation, such as the accurate synthesis of proteins or nucleic acids [35]. Sulfonamides, the oldest antibiotics, could block bacterial biosynthesis of folic acid and influence the synthesis of pyrimidine and purine, the raw materials of nucleic acids [36]. After treatment with compound 5a, two pathways of one carbon pool by folate and folate biosynthesis were disturbed, as depicted in Fig. 4E, indicating the probable bacteriostatic mechanism. Nucleotides are essential for energy transfer, and the biosynthesis of nucleic acids and lipids. Specifically, the balance of purine and pyrimidine is an essential factor for the physiological activities of bacteria, such as DNA synthesis and metabolism. In addition, it was reported that purine could carry out critical repair and survival functions in acid-stressed E. coli [24], [37]. In this study, the disturbance of purine metabolism (Fig. 4F) and pyrimidine metabolism (Table S3) were detected with main down-regulated metabolites of guanosine, guanosine diphosphate, cytidine, dCMP, xanthosine, and up-regulated metabolites of thymidine, deoxyguanosine, dGMP. These data indicated that nucleic acid function would be interfered with by compound 5a.
Interaction with DNA-related targets
DNA gyrase and DNA are potent targets for antibacterial agents' discovery and development due to the difference between bacteria and mammals [26], [27]. In particular, DNA gyrase, absent in mammals, is essential for bacterial DNA replication [19]. Bacterial DNA is located in nucleoids without the protection of a nuclear envelope and is more vulnerable than its eukaryotic counterpart [38]. Considering the membrane damage ability of compound 5a, it is essential to explore whether compound 5a could interact with DNA-related targets when reaching the inner structure of bacteria cells. Specifically, numerous studies have also shown that this kind of skeleton could bind with DNA gyrase and DNA [39].
A docking study was carried out to explore the binding mode and possible sites between compound 5a and DNA gyrase as well as DNA. Interestingly, the docking simulations provided good total scores of 14.2113 and 9.3603 for DNA gyrase and DNA, respectively, which are superior or equivalent to their ligands in complex (9.3774 and 9.9265), indicating the potential interactions. Binding sites analysis displayed that part of norharmane fragment of compound 5a bound to the DNA area of DNA gyrase mainly by π–π stacked interactions with DNA base (DG10, DC11, DC12), and some hydrophobic interactions formed between other fragments and amino acid residues (Fig. 5A and 5C). For DNA, the norharmane skeleton of compound 5a could intercalate into DNA bases by π–π stacked interactions. Moreover, it could bind to the DNA groove by hydrogen bonds (Fig. 5B and D). Interestingly, the oxygen atom of the methoxy group is adjacent to the DC29 residue, forming a strong hydrogen bond with a length of 1.89 Å.
Subsequently, their interactions were further investigated by UV absorption and fluorescence spectra [28], [40]. As shown in Fig. 5E, the intensity of the characteristic absorption wavelength (313 nm and 414 nm) of compound 5a decreased gradually with the proportional additions of ctDNA. Similarly, the intensity of the maximum peak at 530 nm for compound 5a was also reduced in a dose-dependent manner (Fig. 5F). The results above suggested that compound 5a could interact with DNA.
In vivo antibacterial efficacy
Inspired by the potential in vitro activity and good biosecurity under effective doses of compound 5a, the in vivo antibacterial efficacy was further evaluated by a mouse skin infection model [5], [41]. As shown in Fig. 6A, low-dose (3.3 mg/Kg), medium-dose (6.6 mg/Kg) and high-dose (13.2 mg/Kg) of compound 5a were administered after the mice infected by 1 × 107 CFU/mL dose of MRSA, referring to the previous literature [41]. Vancomycin (3.3 mg/kg) was used as the positive control. As shown in Fig. 6B, an obvious abscess on the dorsal skin was observed in the control group. After treatment by vancomycin (3.3 mg/kg) and the indicated dose of compound 5a, visible scabs were formed at the infected sites. Furthermore, the bacterial load in the infected skin tissues was determined to evaluate the in vivo therapeutic efficacy of compound 5a. As displayed in Fig. 6C, the number of clones of MRSA was markedly reduced after treatment with vancomycin (3.3 mg/kg) and three 5a-treated groups (3.3/6.6/13.2 mg/kg). As depicted in Fig. 6D, compared with the control group, 0.62 and 1.50 log-unit reductions of bacterial load at abscess were observed after treatment with 3.3 mg/kg dose of vancomycin (P < 0.01) and compound 5a (P < 0.0001), respectively. Moreover, the anti-MRSA activity of compound 5a was significantly improved with increasing doses. Notably, the bacterial load at the abscess was decreased by approximately 3.34 log units after being treated with a 13.2 mg/kg dose of compound 5a. Interestingly, both three 5a-treated groups exhibited more potential than vancomycin. The skin tissues were further studied by H&E histological examination. As shown in Fig. 6E, obvious inflammatory cell infiltration was observed after infection with MRSA. By contrast, the inflammatory cell infiltration was markedly reduced after treatment with vancomycin and compound 5a. Moreover, the high-dose group tended to be normal. The above results suggested that compound 5a was efficient in vivo.
Fig. 6.
(A) The experimental protocol for the skin infection model. (B) Images of MRSA infection sites with various treatments 4 days. (C) Images of MRSA colonies cultured from the homogenate of infected skin after 1000-fold dilution. (D) In vivo efficacy of compound 5a against MRSA. The data are presented as the mean ± SD from six independent experiments, **P < 0.01, ****P < 0.0001 vs Control. (E) Pathological assay of infected skin that received different treatments based on H&E staining. The scale bar is 100 μm.
Conclusion
Natural norharmane analogues derived by quaternization or dimerization were prepared as new anti-MRSA agents. The SARs were summarized, which supplied essential guidance for the discovery of anti-MRSA agents based on this skeleton. Notably, a promising compound 5a exerted potent anti-MRSA activity in vitro and in vivo, low cytotoxicity and poor hemolysis under an effective dose. Additionally, it exhibited good plasma stability, low tendency of resistance development and capabilities of disrupting bacterial biofilms at 32 μg/mL. As shown in Fig. 7, mechanism studies revealed compound 5a could damage the membrane integrity. The metabolomics study indicated that compound 5a could interfere with the biosynthesis of the bacteria cell wall, damage the membrane and block its repair, disturb energy metabolism and amino acid metabolism pathways, intervene in protein synthesis, and influence nucleic acid synthesis and function. In addition, docking simulation and spectra titration showed that compound 5a could intercalate into DNA gyrase or DNA to form a complex, thus exerting anti-MRSA activity. These results provided valuable information for further exploiting quaternized norharmane dimers as antibacterial agents to treat life-threatening MRSA infections.
Fig. 7.
Schematic representation of anti-MRSA mechanism of compound 5a.
CRediT authorship contribution statement
Jiang-Kun Dai: Conceptualization, Methodology, Validation, Writing – original draft, Funding acquisition. Wen-Jia Dan: Methodology, Validation. Yi-Dan Cao: Methodology, Validation. Ji-Xiang Gao: Methodology, Validation. Jun-Ru Wang: Supervision. Jian-Bo Wan: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Shandong Province (2023HWYQ-096, ZR2020QH346), the National Natural Science Foundation of China (82003595), the Macao Young Scholars Program (AM2019032) and the Science and Technology Development Fund, Macau SAR (File no. 0074/2021/AFJ).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.11.005.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Hu Y., Qu R., He T., Tang X., Chen W., Li L., et al. Lysine stapling screening provides stable and low toxic cationic antimicrobial peptides combating multidrug-resistant bacteria in vitro and in vivo. J Med Chem. 2022;65:579–591. doi: 10.1021/acs.jmedchem.1c01754. [DOI] [PubMed] [Google Scholar]
- 2.Porras G., Chassagne F., Lyles J.T., Marquez L., Dettweiler M., Salam A.M., et al. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem Rev. 2021;121:3495–3560. doi: 10.1021/acs.chemrev.0c00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner N.A., Sharma-Kuinkel B.K., Maskarinec S.A., Eichenberger E.M., Shah P.P., Carugati M., et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203–218. doi: 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan P.G., Mork R.L., Thompson R.M. Environmental methicillin-resistant Staphylococcus aureus contamination, persistent colonization, and subsequent skin and soft tissue infection. Infect Control Hosp Epidemiol. 2020;174:552–562. doi: 10.1001/jamapediatrics.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng W., Xu T., Cui L., Xue Z., Liu J., Yang R., et al. Discovery of amphiphilic xanthohumol derivatives as membrane-targeting antimicrobials against methicillin-resistant Staphylococcus aureus. J Med Chem. 2023;66:962–975. doi: 10.1021/acs.jmedchem.2c01793. [DOI] [PubMed] [Google Scholar]
- 6.Durand G.A., Raoult D., Dubourg G. Antibiotic discovery: history, methods and perspectives. Int J Antimicrob Agents. 2019;53:371–382. doi: 10.1016/j.ijantimicag.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Lin S., Liu J., Li H., Liu Y., Chen Y., Luo Y., et al. Development of highly potent carbazole amphiphiles as membrane-targeting antimicrobials for treating gram-positive bacterial infections. J Med Chem. 2020;63:9284–9299. doi: 10.1021/acs.jmedchem.0c00433. [DOI] [PubMed] [Google Scholar]
- 8.Dai J., Dan W., Li N., Wang J. Computer-aided drug discovery: novel 3,9-disubstituted eudistomin U derivatives as potent antibacterial agents. Eur J Med Chem. 2018;157:333–338. doi: 10.1016/j.ejmech.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Dai J., Dan W., Schneider U., Wang J. β-Carboline alkaloid monomers and dimers: occurrence, structural diversity, and biological activities. Eur J Med Chem. 2018;157:622–656. doi: 10.1016/j.ejmech.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Nenaah G. Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia. 2010;81:779–782. doi: 10.1016/j.fitote.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Volk R.B., Furkert F.H. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol Res. 2006;161:180–186. doi: 10.1016/j.micres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Sun H., Huang S., Jeyakkumar P., Cai G., Fang B., Zhou C. Natural berberine-derived azolyl ethanols as new structural antibacterial agents against drug-resistant Escherichia coli. J Med Chem. 2022;65:436–459. doi: 10.1021/acs.jmedchem.1c01592. [DOI] [PubMed] [Google Scholar]
- 13.Yang R., Hou E., Cheng W., Yan X., Zhang T., Li S., et al. Membrane-targeting neolignan-antimicrobial peptide mimic conjugates to combat methicillin-resistant Staphylococcus aureus (MRSA) infections. J Med Chem. 2022;65:16879–16892. doi: 10.1021/acs.jmedchem.2c01674. [DOI] [PubMed] [Google Scholar]
- 14.Meurer L.C., Guthikonda R.N., Huber J.L., DiNinno F. The synthesis and antibacterial activity of 2-carbolinyl-carbapenems: potent anti-MRSA/MRCNS agents. Bioorg Med Chem Lett. 1995;5:767–772. [Google Scholar]
- 15.Dai J., Dan W., Ren S., Shang C., Wang J. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur J Med Chem. 2018;160:23–36. doi: 10.1016/j.ejmech.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Wang M., Gao R., Zheng M., Sang P., Li C., Zhang E., et al. Development of bis-cyclic imidazolidine-4-one derivatives as potent antibacterial agents. J Med Chem. 2020;63:15591–15602. doi: 10.1021/acs.jmedchem.0c00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatwichien J., Basu S., Murphy M.E., Hamann M.T., Winkler J.D. Design, synthesis and biological evaluation of β-carboline dimers based on the structure of neokauluamine. Tetrahedron Lett. 2015;56:3515–3517. doi: 10.1016/j.tetlet.2015.01.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wetzel C., Lonneman M., Wu C. Polypharmacological drug actions of recently FDA approved antibiotics. Eur J Med Chem. 2021;209 doi: 10.1016/j.ejmech.2020.112931. [DOI] [PubMed] [Google Scholar]
- 19.Chen J., Battini N., Ansari M.F., Zhou C. Membrane active 7-thiazoxime quinolones as novel DNA binding agents to decrease the genes expression and exert potent anti-methicillin-resistant Staphylococcus aureus activity. Eur J Med Chem. 2021;217 doi: 10.1016/j.ejmech.2021.113340. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y., Yang R., Chen F., Yan T., Wen T., Li F., et al. Triphenyl-sesquineolignan analogues derived from Illicium simonsii Maxim exhibit potent antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) by disrupting bacterial membranes. Bioorg Chem. 2021;110 doi: 10.1016/j.bioorg.2021.104824. [DOI] [PubMed] [Google Scholar]
- 21.Zeng P., Yi L., Cheng Q., Liu J., Chen S., Chan K., et al. An ornithine-rich dodecapeptide with improved proteolytic stability selectively kills gram-negative food-borne pathogens and its action mode on Escherichia coli O157:H7. Int J Food Microbiol. 2021;352 doi: 10.1016/j.ijfoodmicro.2021.109281. [DOI] [PubMed] [Google Scholar]
- 22.Liu M., Feng M., Yang K., Cao Y., Zhang J., Xu J., et al. Transcriptomic and metabolomic analyses reveal antibacterial mechanism of astringent persimmon tannin against methicillin-resistant Staphylococcus aureus isolated from pork. Food Chem. 2020;309 doi: 10.1016/j.foodchem.2019.125692. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J., Han M., Han M., Zhu Y., Lin Y., Wang Y., et al. Comparative metabolomics reveals key pathways associated with the synergistic activity of polymyxin B and rifampicin combination against multidrug-resistant Acinetobacter baumannii. Biochem Pharmacol. 2021;184 doi: 10.1016/j.bcp.2020.114400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He R., Chen W., Chen H., Zhong Q., Zhang H., Zhang M., et al. Antibacterial mechanism of linalool against L. monocytogenes, a metabolomic study. Food Control. 2022;132 [Google Scholar]
- 25.Wang Y., Zhou S., Wang M., Liu S., Hu Y., He C., et al. UHPLC/Q-TOFMS-based metabolomics for the characterization of cold and hot properties of Chinese materia medica. J Ethnopharmacol. 2016;179:234–242. doi: 10.1016/j.jep.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 26.Bax B.D., Chan P.F., Eggleston D.S., Fosberry A., Gentry D.R., Gorrec F., et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466:935–940. doi: 10.1038/nature09197. [DOI] [PubMed] [Google Scholar]
- 27.Cai D.H., Zhang C.L., Liu Q.Y., He L., Liu Y.J., Xiong Y.H., et al. Synthesis, DNA binding, antibacterial and anticancer properties of two novel water-soluble copper(II) complexes containing gluconate. Eur J Med Chem. 2021;213 doi: 10.1016/j.ejmech.2021.113182. [DOI] [PubMed] [Google Scholar]
- 28.Dan W., Gao J., Zhang J., Cao Y., Liu J., Sun Y., et al. Antibacterial activity and mechanism of action of canthin-6-one against Staphylococcus aureus and its application on beef preservation. Food Control. 2023;147 [Google Scholar]
- 29.Cao Y.Y., Zhu Z.Y., Chen X.F., Yao X.W., Zhao L.Y., Wang H., et al. Effect of amphotericin B on the metabolic profiles of Candida albicans. J Proteome Res. 2013;12:2921–2932. doi: 10.1021/pr4002178. [DOI] [PubMed] [Google Scholar]
- 30.Hussein M., Karas J.A., Schneider-Futschik E.K., Chen F., Swarbrick J., Paulin O.K.A., et al. The killing mechanism of teixobactin against methicillin-resistant Staphylococcus aureus: an untargeted metabolomics study. mSystems. 2020;5:e00077–e00120. doi: 10.1128/mSystems.00077-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y.S., Tao J.Z. Metabolomics and pathway analyses to characterize metabolic alterations in pregnant dairy cows on D 17 and D 45 after AI. Sci Rep. 2018;8:5973. doi: 10.1038/s41598-018-23983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horinouchi T., Tamaoka K., Furusawa C., Ono N., Suzuki S., Hirasawa T., et al. Transcriptome analysis of parallel-evolved Escherichia coli strains under ethanol stress. BMC Genomics. 2010;11:579. doi: 10.1186/1471-2164-11-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong L., Teng J.L.L., Botelho M.G., Lo R.C., Lau S.K.P., Woo P.C.Y. Arginine metabolism in bacterial pathogenesis and cancer therapy. Int J Mol Sci. 2016;17:363. doi: 10.3390/ijms17030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouz G., Zitko J. Inhibitors of aminoacyl-tRNA synthetases as antimycobacterial compounds: an up-to-date review. Bioorg Chem. 2021;110 doi: 10.1016/j.bioorg.2021.104806. [DOI] [PubMed] [Google Scholar]
- 35.Fernández-Villa D., Aguilar M.R., Rojo L. Folic acid antagonists: antimicrobial and immunomodulating mechanisms and applications. Int J Mol Sci. 2019;20:4996. doi: 10.3390/ijms20204996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baran A., Kwiatkowska A., Potocki L. Antibiotics and bacterial resistance-a short story of an endless arms race. Int J Mol Sci. 2023;24:5777. doi: 10.3390/ijms24065777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shayanfar S., Broumand A., Pillai S.D. Acid stress induces differential accumulation of metabolites in Escherichia coli O26:H11. J Appl Microbiol. 2018;125:1911–1919. doi: 10.1111/jam.14081. [DOI] [PubMed] [Google Scholar]
- 38.Bai S.L., Wang J.X., Yang K.L., Zhou C.L., Xu Y.F., Song J.F., et al. A polymeric approach toward resistance-resistant antimicrobial agent with dual-selective mechanisms of action. Sci Adv. 2021;7:eabc9917. doi: 10.1126/sciadv.abc9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai J., Dan W., Li N., Wang R., Zhang Y., Li N., et al. Synthesis and antibacterial activity of C2 or C5 modified and D ring rejiggered canthin-6-one analogues. Food Chem. 2018;253:211–220. doi: 10.1016/j.foodchem.2018.01.166. [DOI] [PubMed] [Google Scholar]
- 40.Hussain I., Fatima S., Siddiqui S., Ahmed S., Tabish M. Exploring the binding mechanism of β-resorcylic acid with calf thymus DNA: insights from multi-spectroscopic, thermodynamic and bioinformatics approaches. Spectrochim Acta A. 2021;260 doi: 10.1016/j.saa.2021.119952. [DOI] [PubMed] [Google Scholar]
- 41.Chu W., Yang Y., Qin S., Cai J., Bai M., Kong H., et al. Low-toxicity amphiphilic molecules linked by an aromatic nucleus show broad-spectrum antibacterial activity and low drug resistance. Chem Commun. 2019;55:4307–4310. doi: 10.1039/c9cc00857h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.