Abstract
Myocardial injury and cardiac dysfunction following traumatic brain injury (TBI) have been reported in observational studies, but there is no robust estimate of their incidences. We conducted a systematic review and meta-analysis to estimate the pooled incidence of myocardial injury and cardiac dysfunction among adult TBI patients. A literature search was conducted using MEDLINE and EMBASE databases from inception to November 2022. Observational studies were included if they reported at least one of abnormal ECG findings, elevated cardiac troponin level, or echocardiographic evaluation of systolic function or left ventricular wall motion in adult TBI patients. Myocardial injury was defined as elevated cardiac troponin level according to the original studies and cardiac dysfunction was defined as presence of left ventricular ejection fraction < 50% or regional wall motion abnormalities assessed by echocardiography. The meta-analysis of the pooled incidence of myocardial injury and cardiac dysfunction was performed using random-effects models. The pooled estimated incidence of myocardial injury following TBI (17 studies, 3,773 participants) was 33% (95% CI 27% - 39%, I2, 93%) and the pooled estimated incidence of cardiac dysfunction following TBI (9 studies, 557 participants) was 16.% (95% CI 9% - 25.%, I2 = 84%). Although there was significant heterogeneity between studies and potential over-estimation of the incidence of myocardial injury and cardiac dysfunction, our findings suggest that myocardial injury occurs in approximately one-third of adults following TBI and cardiac dysfunction occurs in approximately one-sixth of TBI patients.
Keywords: Traumatic brain injury, cardiac dysfunction, troponin, echocardiography, systematic review
INTRODUCTION
Traumatic brain injury (TBI) poses a significant burden of morbidity and mortality globally1. Although cranial/neuronal injury is the primary insult, patients with TBI can also develop multi-organ dysfunction2–4. Patients with severe TBI often develop hypotension following injury, which can reduce cerebral perfusion, contribute to secondary brain injury, and increase mortality risk.
Electrocardiographic (ECG) changes following non-traumatic brain injury, including subarachnoid hemorrhage and stroke, have been described for decades5–7. More recently, awareness of myocardial injury and cardiac dysfunction following TBI has increased due to evaluation with biomarkers of myocardial injury and echocardiography. Additionally, observational studies suggest that brain-heart interactions may play a pivotal role in the pathophysiology of hemodynamic instability following TBI8,9, though a causal relationship has not been clearly established.
While observational studies have contributed to the understanding of TBI-related cardiac abnormalities, estimates of the incidence of myocardial injury and cardiac dysfunction following TBI are limited due to studies with small sample sizes and heterogeneous definitions. To address this, we conducted a systematic review and meta-analysis to estimate the pooled incidence of myocardial injury and cardiac dysfunction among adult patients with TBI.
METHODS
This review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards (PRISMA) 2020 statement 10,11 and used the methodological guidance for systematic review of prevalence and incidence by a working group of the Joanna Briggs Institute12. The protocol was pre-registered in the International Prospective Register of Systematic Reviews (CRD42020179315). A literature search was conducted to identify studies that examined cardiac abnormalities in adult patients with TBI. We performed an electronic search using MEDLINE and EMBASE databases from inception to November 2022, as well as article reference lists. The search strategy included the use of text words and Medical Subject Headings (MeSH) related to “traumatic brain injury”, “echocardiography”, “troponin”, “myocardial injury”, or “cardiac function”. Full search terms for both databases are listed in Supplemental Digital Content 1. The inclusion was not limited by sample size or language used. No ethical approvals were sought for this systematic review and meta-analysis of previously published data.
Selection criteria
Observational study designs, including case-control, cross-sectional, and cohort studies, were eligible for this systematic review. We included studies in which the population included patients who were diagnosed with TBI, regardless of gender, injury severity, or presence of associated injuries. Studies published only in abstract form, case reports, editorials, opinion articles, and letters were excluded. We also excluded studies reporting cardiac dysfunction in the pediatric TBI patients (age < 16 years), and studies in patients with brain death. The included studies must report at least one of the following indicators for myocardial injury or cardiac dysfunction: 1) abnormal ECG findings, including arrhythmias, QT prolongation, and ST-T changes, 2) elevated cardiac troponin level, or 3) echocardiographic evaluation of systolic function or left ventricular wall motions at any time point within the same admission following TBI.
Two independent investigators (NC and TK) ran the electronic search according to the protocols. After removal of any duplicates, the titles and abstracts of the studies were screened and independently evaluated for eligibility (NC and TK). Any differences in the determination of the eligibility of a study were resolved by a third investigator (VK). To avoid double counting, only one set of results was selected when data from the same cohort/database was utilized in multiple publications.
Data extraction and outcomes
Two investigators (NC and TK) independently extracted data from each study using a structured information collection form in an excel spreadsheet. Data extracted from the eligible studies included the title of the study, first author last name, published journal, year of publication, year (s) the study was conducted, the country where the study was conducted, the type of the study, study population (case and control), the method used to recruit/identify myocardial injury and cardiac dysfunction, the definition of cardiac dysfunction used by echocardiographic evaluation, types and cut-off point for troponin elevation reported in each study, the number of TBIs, and demographic/clinical data of TBI with/without myocardial injury and cardiac dysfunction (gender, age, polytrauma, injury severity). Cardiac indices of interest were: 1) left ventricular ejection fraction (LVEF), 2) fractional shortening (FS), 3) fractional area change (FAC), 4) presence of regional wall motion abnormalities (RWMA), 5) echocardiographic strain abnormalities, and 6) troponin level. There were no restrictions on the types of troponin assays used. It is challenging to compare the cut-off value of different troponin assays; therefore, we defined troponin elevation according to that used in the original studies. Due to variations in the definitions of cardiac dysfunction, we also extracted definitions of cardiac dysfunction as reported in individual studies.
The primary outcomes were the incidence of myocardial injury and cardiac dysfunction in adult patients with TBI. Myocardial injury was defined as elevated cardiac troponin level compared to reference values in the original manuscript and cardiac dysfunction was defined as the presence of either impaired left ventricular systolic function assessed using echocardiography and as defined using criteria according to the original studies, or the presence of RWMA assessed using echocardiography. We did not include patients with abnormal ECG patterns in the meta-analysis because ECG changes are less specific to myocardial injury or cardiac dysfunction than elevated troponin or abnormal echocardiographic findings.
Risk of bias assessment
Two reviewers (NC and TK) independently assessed the risk of bias of the individual studies using The Joanna Briggs Institute Critical Appraisal Checklist for studies reporting prevalence data12. The appraisal checklist consisted of nine questions which could be divided into three categories: participants (questions 1,2,4, and 9), outcome measurement (questions 6 and 7), and statistical analysis (question 3, 5, and 8). Question 5 was excluded as it was redeemed irrelevant to the included studies. Therefore, a total of eight questions were assessed by the two reviewers. Any disagreement was resolved by the third investigator (VK). Since conventional funnel plots are inaccurate for assessing potential publication bias in meta-analyses of proportion studies13, we used the Doi plot and Luis Furuya-Kanamori asymmetry index (LFK index)14 to examine the publication bias. The Doi plot represents normal quantile against effect size to improve the visual precision for detecting asymmetry. The LFK index is used to quantify asymmetry of the overall study effects. A LFK index of within ± 1, within ± 1 to ± 2 interval, and beyond ± 2 interval indicate no asymmetry, minor asymmetry, and major asymmetry, respectively.
Statistical analysis
The pooled incidence of myocardial injury (elevated troponin levels) and cardiac dysfunction (LVEF < 50% or presence of RWMA) following TBI across studies was calculated as the proportion and confidence intervals (CIs)15. The meta-analysis of both the pooled incidence of myocardial injury and the pooled incidence of cardiac dysfunction was performed using random-effects models, assuming that all studies did not yield the same results because they were observational studies that included different populations. The pooled incidence of subsets according to types of TBI (isolated versus non-isolated, mild versus moderate-severe) was also explored. For secondary analysis of the outcomes of myocardial injury/cardiac dysfunction, the pooled effect estimates of mortality were calculated by combining the effect estimates of each eligible study using generic inverse variance method and presented as odds ratio (OR) with 95% CIs. We used a random-effects model due to the heterogeneity of the studies.
The magnitude of heterogeneity between studies was evaluated with the Q test and I2 statistics16. A range of I2 0 – 25%, 26 – 50%, 51 – 75%, and > 75% represent insignificant, low, moderate, and high heterogeneity, respectively17. We also calculated the 95% prediction intervals for the pooled incidence and the pooled ORs in order to provide an estimate of the expected effects if future studies might be conducted 18,19. Sensitivity analyses were performed to test the robustness of the overall estimates and to explore the potential causes of heterogeneity based on included the population, timing of evaluation, study design, and risk of bias. We used MetaXL v 5.3 (EpiGear International, Sunrise Beach, Queensland, Australia) in Microsoft Excel for Windows (https://www.epigear.com/index_files/metaxl.html) to perform the analyses of incidence, the Doi plot, and LFK index. Review Manager (RevMan [Computer program], Version 5.4, The Cochrane Collaboration, 2020) was used for the meta-analysis of mortality.
RESULTS
The search identified 22,435 articles of which 3,080 duplicates were removed. Following exclusion of a further 19,210 articles based on title, 145 abstracts were accessed for eligibility from which 64 articles were identified for full-text review. Four studies in the pediatric population, eight studies in patients with brain death, and three that did not report the number of patients with TBI were excluded. We also excluded eight studies because the primary outcome was not reported, and twelve studies which reported only ECG abnormalities or creatine kinase. Additionally, six studies that used overlapping datasets were also excluded. One study20 reported used FS < 25% as a definition for cardiac dysfunction, and was excluded. Finally, 22 eligible studies were included in the meta-analysis21–42 (Figure 1).
Figure 1. Flowchart of study selection.

Summary of the studies reporting myocardial injury and cardiac dysfunction following TBI
A total of 22 studies reporting troponin elevation or abnormal echocardiography involving 4,066 participants with TBI from 12 countries were included in the meta-analysis21–42. The characteristics of the studies and reported incidence of myocardial injury and cardiac dysfunction are shown in Table 1. The methods used to evaluate myocardial injury and cardiac dysfunction following TBI and the timing of evaluation varied among studies. Eleven studies reported only elevated troponin 21–23,26–28,30,32,34,37,38, six reported abnormal echocardiographic findings20,24,25,36,40,41, while six studies evaluated both troponin and echocardiography after TBI29,31,33,35,39,42. The troponin assays used and cut-off points also varied among the studies that reported troponin elevation. Fourteen studies used troponin I21–23,26,27,29–31,33–35,37–39 (threshold ranged between 0.056 ng/mL and 0.4 ng/mL), while three studies used troponin T28,32,42, two of which28,32 used high sensitivity troponin T with threshold of > 99th percentile. For timing of troponin evaluation, one study29 did not mention when serum troponin samples were obtained. Serum troponin data were available on admission in seven studies21,22,26,30,33,34,38, and within 24 h of admission in eight studies23,26–28,31,32,37,38. Serial serum troponin was obtained in only six studies21,26,30,31,38,39, of which three studies followed troponin levels until day 3 26,30,31 and one study until day 439. Moreover, one study followed troponin levels until day 5 and reported that serum troponin reached a peak on day 3 and reduced thereafter21.
Table 1.
Summary of the studies reporting incidence of troponin elevation and abnormal echocardiography in patients with traumatic brain injury
| Author/Year | Country | Study design | Time of evaluation | Definitions of myocardial injury/ cardiac dysfunction | Total TBI (n) | Prevalence n (%) |
|---|---|---|---|---|---|---|
| Troponin studies (11 studies) | ||||||
| Baffoun et al. 201120 | Tunisia | Prospective | On admission, D3, D5 | Elevated Troponin I | 35 | 12 (34%) |
| Bender et al. 202021 | Germany | Retrospective | On admission | Troponin I > 0.05 mcg/L | 288 | 59 (20%) |
| Cai et al. 201622 | USA | Retrospective | Within 24 hours | Troponin I ≥ 0.06 ng/mL | 580 | 179 (31%) |
| Dixit et al. 200025 | USA | Prospective | On admission, D1, D2, D3 | Troponin I ≥ 0.4 mcg/L | 34 | 6 (18%) |
| Dou et al. 202226 | China | Retrospective | Within 24 hours | Troponin I ≥ 0.04 ng/mL | 67 | 30 (45%) |
| El-Menyar et al. 201927 | Qatar | Retrospective | Within 24 hours | High-sensitivity troponin T > 14 ng/L | 1471 | 647 (44%) |
| Hamdi et al. 201229 | Egypt | Prospective | On admission, D3 | Troponin I > 0.2 mcg/L | 32 | 14 (44%) |
| Krishnamoorthy et al. 202232 | USA | Prospective multicenter | Within 4 h | High sensitivity troponin > 99th percentile | 133 | 26 (20%) |
| Lippi et al. 201634 | Italy | Retrospective | On admission | Troponin I > 99th percentile (> 40 ng/L) | 145 | 99 (68%) (>10 ng/L) 31 (21%) (> 40 ng/L) |
| Rimaz et al. 201937 | Iran | Prospective | Within 24 h | Troponin I ≥ 0.06 ng/mL | 166 | 87 (52%) |
| Salim et al. 200838 | USA | Retrospective | On admission, 24 to 48 hours | Troponin I > 0.3 ng/mL | 420 | 125 (29%) (on admission) 173 (41%) (during hospitalization) |
| Echocardiographic studies (6 studies) | ||||||
| Chaikitisilpa et al. 201923 | USA | Prospective | Within 24 hours | TTE (LVEF < 50%) | 15 | 2 (13%) |
| Cuisinier et al. 201624 | France | Prospective | Within 24 hours | TTE (LVEF < 50%) | 20 | 0 (0%) |
| Krishnamoorthy et al. 201731* | USA | Prospective | D1, D2–4, D7–9 | TTE (FS < 25%) | 64 | 7 (11%) |
| Stewart et al. 200741 | USA | Prospective | Within 24 h | TTE (LVEF < 40%) or diastolic dysfunction | 28 | 15 (54%) |
| Praveen et al. 202136 | India | Prospective | Pre- and postoperatively within 48 h | TTE (LVEF < 50% or RWMA) | 60 | 8 (13%) |
| Srinivasaiah et al. 202240 | India | Prospective | Within 48 hours (pre & postoperative D1) | TTE (LVEF < 50% or RWMA) | 110 | 13 (12%) |
| Studies using both troponin and echocardiography (6 studies) | ||||||
| Gibbons et al. 201928 | USA | Retrospective analysis of prospective cohort | Not mentioned | Troponin I ≥ 0.04 ng/mL, | 114 | 60 (53%) |
| TTE (LVEF < 50% or RWMA) | 72 | 23 (32%) | ||||
| Hasanin et al. 201630* | Egypt | Prospective | D1, 3 | Troponin I > 0.056 ng/mL | 50 | 27 (54%) |
| Within 12 h, D3, D5, D7 | TTE (LVEF < 55% or RWMA) | 50 | 14 (28%) | |||
| Lee et al. 201633 | Korea | Prospective | On admission (Troponin) Within 1 week (TTE) | Abnormal TTE (reduced LVEF or RWMA), ECG changes, and elevated troponin I > 0.4 ng/mL | 64 | 4 (6%) |
| Prathep et al. 201435 | USA | Retrospective | Within 14 days | Troponin I > 0.4 ng/mL | 107 | 24 (22%) |
| TTE (LVEF < 50% or RWMA) | 139 | 31(22%) | ||||
| Serri et al. 201639 | Canada | Prospective | Daily the first 4 days | Troponin I > 0.1 mcg/L | 43 | 15 (35%) |
| Within 4 days | TTE (LVEF < 50% or RWMA) | 49 | 4 (8%) | |||
| Venkata et al. 201842* | USA | Prospective | Anytime | Troponin T ≥ 0.04 ng/mL | 24 | 6 (25%) |
| Within 48 h | TTE (LVEF < 55% or RWMA) | 46 | 6 (13%) | |||
3 studies were excluded from the meta-analysis of the incidence of cardiac dysfunction due to the definitions used for cardiac dysfunction
FS, fractional shortening; LVEF, left ventricular ejection fraction; RWMA, regional wall motion abnormality; TBI, traumatic brain injury; TTE, transthoracic echocardiogram
In the 12 studies that included echocardiographic results20,24,25,29,31,33,35,36,39–42, impaired left ventricular (LV) systolic function was reported in all studies. Low LVEF was used to identify patients with cardiac dysfunction in 11 studies; LVEF < 50% in seven studies24,25,29,35,36,39,40, < 55% in two studies31,42, < 40% in one study41, and not defined in one study33. Fractional shortening < 25% was used in one study20. We excluded studies using LVEF < 55% and FS < 25% as criteria for cardiac dysfunction from the final analysis to remain consistency of the definition. Therefore, a total of 9 studies examining cardiac dysfunction were included in the final meta-analysis. The presence of RWMA was examined in seven studies29,33,35,36,39,40,42; however, most did not report any details about RWMA. Only five studies20,25,36,39,41 explored echocardiographic LV diastolic function abnormality but did not provide the definition for diastolic dysfunction; therefore, we could not perform meta-analysis of the incidence of diastolic dysfunction. For timing of echocardiographic evaluation, echocardiography was performed within 24–48 h in seven studies20,24,25,31,40–42. Two studies20,31 provided data on serial echocardiography throughout the first week, but only one20 reported echocardiography trajectory change in which cardiac dysfunction occurred within the first 24 h and then recovered.
The characteristics of the included patients also varied between studies (Supplemental digital content 2: Table showing patient characteristics). Sixteen studies included only patients with TBI, three studies included acute trauma patients27,34,41, and three included patients with other acute neurologic events21,26,33. Among the included studies, 13 (2,161 participants, 53%)21–25,31,32,34–38,40 reported myocardial injury/cardiac dysfunction in patients with isolated TBI. The severity of TBI was reported in 15 studies (2,219 participants)20–25,29,31,32,35–40,42, and most included patients with moderate-severe TBI (1,877 participants, 46%).
Quality of evidence
The quality of the 22 studies was assessed using The Joanna Briggs Institute Critical Appraisal Checklist for studies reporting prevalence data12. The risk of bias assessment (yes, no, unclear or not applicable) is shown in supplemental digital content 3 and details of how each of the domains were scored are explained in supplement digital content 4. Overall, the risk of bias was considered moderate, but all of the included studies had limitations in at least one of the three domains (participants, outcome measurement, and statistical analysis). Inappropriate sample frame and insufficient sample size were the main risks of bias in most of the studies. Only two studies 39,42 included our target population (adult patients with moderate-severe TBI with and without polytrauma), while 12 studies21–25,32,34–38,40 included only patients with isolated TBI. Concerning outcome measurement, studies described how serum troponin samples were obtained and reported cut-off values, except for one study21. Details of the echocardiogram performed, and timing of the evaluation were clearly described in most studies, though two29,35 did not mention the timing of the evaluation. Though most studies performed appropriate statistical analyses, the sample size was considered adequate in only 10 studies22,23,28,29,32,34,35,37,38,40.
Meta-analysis of the incidence of myocardial injury following TBI
The pooled incidence of myocardial injury (elevated troponin) after TBI (reported in 17 studies 21–23,26–35,37–39,42, 3,773 participants) was 33% (95% CI 27% - 39%, 95% prediction interval 21 % - 48%, I2 = 93%) (Figure 2). For subgroup analysis, 1,924 patients with isolated TBI were identified from 9 studies21–23,31,32,34,35,37,38. The pooled incidence of myocardial injury among patients with isolated TBI was 32% (95% CI 24% - 40%, 95% prediction interval 19% - 49%, I2 = 92%). The pooled incidence of myocardial injury among patients with moderate-severe TBI (10 studies21–23,29,32,35,37–39,42, 1,601 participants) was 37% (95%CI 30% - 44%, 95% prediction interval 25% - 51%, I2 = 85%).
Figure 2. Forest plots of the 17 studies reporting the incidence of myocardial injury (elevated troponin) following traumatic brain injury.
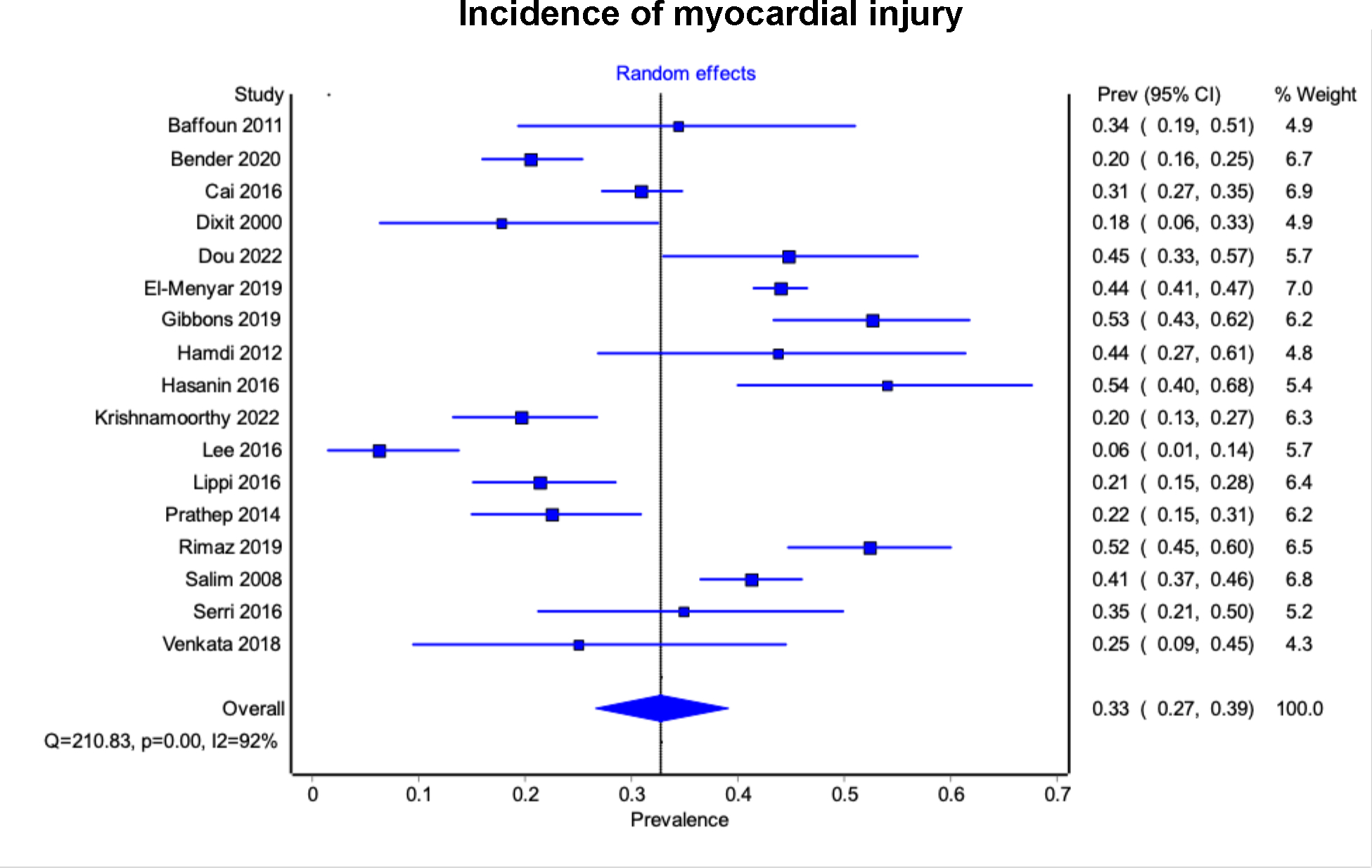
The diamond represents the pooled incidence from each of the included studies (squares) and 95% confidence interval.
Meta-analysis of the incidence of cardiac dysfunction following TBI
The pooled incidence of cardiac dysfunction (LVEF < 50% or RWMA) after TBI (reported in 9 studies 24,25,29,33,35,36,39–41, 557 participants) was 16% (95% CI 9% - 25%, 95% prediction interval 7% - 34%, I2 = 84%) (Figure 3). 344 patients with isolated TBI were identified from 5 studies24,25,35,36,40. The pooled incidence of cardiac dysfunction in patients with isolated TBI was 13% (95% CI 6% - 21%; 95% prediction interval 5% - 31%, I2 = 69%). The pooled incidence of cardiac dysfunction in patients with moderate-severe TBI (7 studies24,25,29,35,36,39,40, 375 participants) was 15% (95%CI 8% - 23%, 95% prediction interval 7% - 31%, I2 = 74%).
Figure 3. Forest plot of the 9 studies reporting incidence of cardiac dysfunction (left ventricular ejection fraction < 50% or regional wall motion abnormality from echocardiography) after traumatic brain injury.
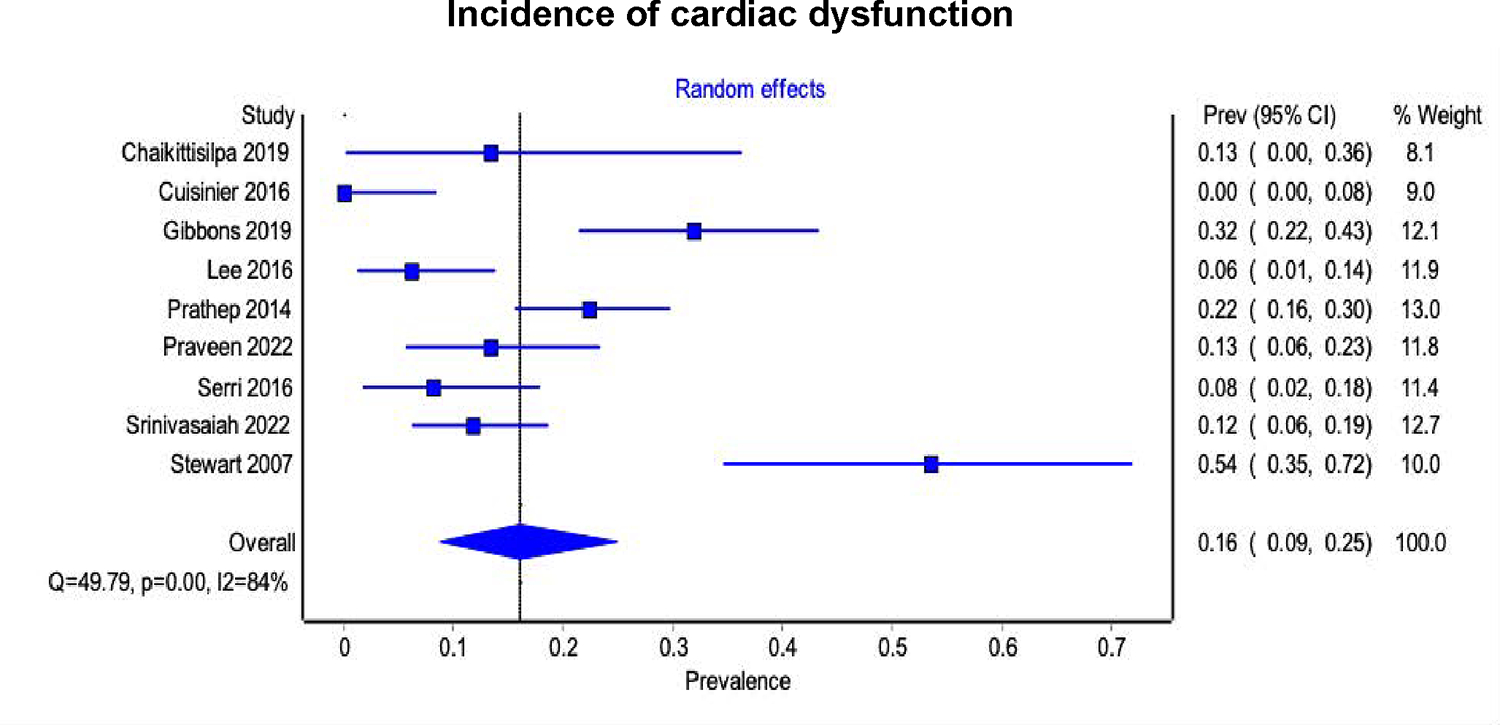
The diamond represents the pooled incidence from each of the included studies (squares) and 95% confidence interval.
Mortality among TBI patients with myocardial injury and cardiac dysfunction
In-hospital mortality among patients with and without myocardial injury after TBI was reported in 6 studies21–23,28,37,38, including 1,803 participants. A meta-analysis performed to determine the association between myocardial injury and mortality after TBI found that myocardial injury was associated with a higher risk of in-hospital mortality (OR 3.98, 95% CI 2.70 – 5.86, 95% prediction interval 1.19 – 13.31, p < 0.0001, I2 = 70%) (Figure 4A).
Figure 4. Forest plot of the included studies reporting pooled estimated odds ratios for mortality in patients with myocardial injury and cardiac dysfunction after traumatic brain injury.

A. Myocardial injuryB. Cardiac dysfunctionThe diamond represents the pooled incidence from each of the included studies (squares) and 95% confidence interval.
Among the included studies reporting cardiac dysfunction from echocardiographic evaluation, 6 studies24,25,35,36,39,40 (314 participants) also reported in-hospital mortality. In one study, none of the patients in the study died; therefore, this study was not included in the meta-analysis36. Compared with patients without cardiac dysfunction, the pooled OR for mortality in patients with cardiac dysfunction was 4.65 (95% CI 0.98 – 21.99, 95% prediction interval 0.05 – 390.59, p = 0.05, I2 = 44%) (Figure 4B).
Sensitivity analysis
Due to the significant heterogeneity, we performed a sensitivity analysis to test for data consistency between studies (Supplemental digital content 5: Table showing sensitivity analysis). Myocardial injury was observed more frequently when troponin samples were obtained several times during admission. The pooled incidence of myocardial injury when troponin levels were obtained on admission was 25% (95%CI 15% - 36%, I2 = 92%). Studies in which a single troponin sample was obtained tended to have lower incidence than those in which serial troponin samples were obtained (30% and 38%, respectively).
The incidence of cardiac dysfunction was similar when comparing studies evaluating single versus serial echocardiography assessments (Supplemental digital content 5: Table showing sensitivity analysis). When analyzing only the prospective studies which include echocardiography assessments, the incidence of cardiac dysfunction tended to be lower than that reported in the retrospective studies (13% and 26%, respectively).
ECG abnormalities
The incidence of ECG abnormalities were reported in nine studies including 689 participants (Supplemental digital content 6: Table showing the incidence of ECG abnormalities)21,29,31,33,36,39,42–44. Two studies43,44 reported only ECG abnormalities without troponin or echocardiographic evaluation and were excluded from the meta-analysis. Five studies29,31,33,39,42 reported ECG abnormalities with both troponin and echocardiographic evaluation, one study21 reported ECG and only troponin, and one study reported ECG and only echocardiography36. The reported criteria for ECG abnormalities varied greatly among studies. Only four studies 29,36,43,44 pre-specified the definition of ECG abnormalities. ST segment abnormality were used in six studies 33,36,39,42–44, T wave inversion was used in six studies 29,33,36,39,43,44, and abnormal QT interval was used in eight studies 29,31,33,36,39,42–44. Arrhythmia was reported in five studies 21,31,36,43,44. Since there was heterogeneity in the definition of ECG abnormalities and timing of ECG tests among studies, the incidence of ECG abnormalities following TBI varied between 37% - 88%. Moreover, ECG abnormalities are less specific for myocardial injury. Therefore, we did not include ECG abnormalities in the meta-analysis of the incidence of cardiac dysfunction.
Publication bias
The risk of publication bias was visualized across the 17 studies reporting myocardial injury after TBI. The Doi plot analysis and LFK index of the incidence of myocardial injury and cardiac dysfunction estimates are shown in supplemental digital content 7.
The Doi plot for myocardial injury demonstrated major asymmetry (LFK index, −3.08). For subgroup analysis, the Doi plot for the studies which included only patients with isolated TBI demonstrated no asymmetry (LFK index, 0.73) while the Doi plot for the studies among patients with moderate-severe TBI demonstrated major asymmetry (LFK index, −3.06). The risk of publication bias for the 9 studies reporting cardiac dysfunction using echocardiographic evaluation showed no asymmetry (LFK index, 0.22). For subgroup analysis, both the Doi plots for the studies which included only patients with isolated TBI and patients with moderate-severe TBI demonstrated minor asymmetry (LFK index, −1.45 and −1.28, respectively).
DISCUSSION
This systematic review and meta-analysis found that myocardial injury (elevated cardiac troponin level) occurred in approximately one-third of the patients and cardiac dysfunction (LVEF < 50% or RWMA on echocardiogram) occurred in about 16% of patients following moderate-severe TBI. Myocardial injury was associated with increased mortality. Given the high presence of myocardial injury and cardiac dysfunction in TBI patients and possible impact on poor patient outcomes, further research is needed to optimize the recognition and management strategies of patients with cardiac complications following moderate-severe TBI.
Traumatic brain injury is associated with single- and multi-organ dysfunction, which may be related to autonomic dysfunction following widespread catecholamine release via the hypothalamic-pituitary-adrenal axis and activation of the sympathetic nervous system45–47. Initially adaptive, persistent sympathetic activation becomes maladaptive, resulting in multi-organ dysfunction. Specific to the cardiovascular system, high sympathetic tone and excess catecholamine levels result in vasoconstriction and increase in cardiac afterload and myocardial workload, and, subsequently, to increased myocardial oxygen demand. Due to simultaneous coronary vasoconstriction, a mismatch in cardiac oxygen delivery may lead to cardiac injury48 which presents as contraction band necrosis under light microscopy49. The resulting myocardial injury may be ascertained clinically through elevated cardiac troponin levels and may potentially lead to the development of cardiac dysfunction presenting as low LVEF or RWMAs. In addition to autonomic dysfunction, the robust dysregulated inflammatory cascade following TBI may also have a role in organ remodeling and subsequent dysfunction50.
Given the high estimates of myocardial injury and cardiac dysfunction in moderate-severe TBI and its association with poorer immediate23,29,31 and long-term outcomes38,40, there may be a need for early cardiac monitoring in TBI patients. From this systematic review, seven studies reported troponin elevation on admission with a pooled incidence of myocardial injury of 25%, suggesting that cardiac injury may occur early after TBI. Meanwhile, the pooled incidence of myocardial injury was higher (38%) when serial troponin levels were obtained, indicating that more patients with moderate-severe TBI developed myocardial injury later during their hospital admission. Hence, continuous cardiac monitoring might be considered. However, the higher incidence of myocardial injury when serial troponin levels were obtained could be due to other factors such as sepsis51, renal failure52, or surgery51 which complicated TBI during the hospital course.
High heterogeneity among the available studies was observed, including in TBI severity, presence of associated injury, and timing of the cardiac evaluations. Therefore, it was not possible to rigorously examine the characteristics of those patients at risk for development of myocardial injury. Most of the studies were conducted in patients with moderate-severe TBI; therefore, the identified incidence of myocardial injury in patients with moderate-severe TBI was similar to the overall incidence. There may be specific patient subgroups who may benefit from increased cardiovascular monitoring, including patients with pre-existing cardiovascular comorbidities, and older patients who are at risk for hemodynamic instability, poorer outcomes and greater mortality, which should be further investigated. Currently, the guidelines for managing TBI patients recommend maintenance of systolic blood pressure above 100mmHg (50–69 years of age) and 110mmHg (others) to reduce mortality and improve outcomes53 as a component the cardiovascular recommendations, without regard to the status of cardiac function.
In this analysis, cardiac monitoring was mainly conducted using serum cardiac troponin levels or echocardiography. Cardiac troponin was elevated in more patients (32%) compared to echocardiogram findings of reduced LVEF or RMWA (16%). However, it is important to interpret elevated troponin levels with respect to the sensitivity and specificity of the particular troponin assays used. There are no specific criteria for diagnosis of myocardial injury in patients with TBI or other neurologic injury. In this review, both troponin I and high-sensitivity troponin T were included and the cut-off points for troponin elevation based on the original studies were used, which may have resulted in the heterogeneity of the pooled incidence. Overestimation of the incidence of myocardial injury using elevated troponin level compared to cardiac dysfunction evidenced by echocardiographic evaluation may occur for the following reasons. First, the lower cut-off for elevated troponin may potentially create a false-positive result and lead to an over-estimation of myocardial injury54,55. In the study by Lippi et al 34, two detection limits (10 ng/L vs. 40 ng/L or 99th percentile) were used and troponin elevation was reported in 68% and 21%, respectively. Second, troponin elevation reported in some of the studies may have been due to polytrauma since troponin elevation has been observed in trauma patients without TBI 56,57.Moreover, the presence of low serum troponin concentration in healthy individuals has also been reported58. Therefore, careful interpretation of troponin as a biomarker for myocardial injury following TBI should be made in the context of other cardiac parameters, in clinical practice or future research.
Nine studies21,22,26,29,31,33,39,42 included using ECG as part of the overall cardiac monitoring parameters assessed. However, ECG abnormalities do not necessarily correlate with troponin elevation21,26 or abnormal echocardiography findings31,33. Since ECG abnormalities are less specific for the diagnosis of cardiac dysfunction, we did not include ECG abnormality in this meta-analysis. Various factors other than injury to the myocytes may also cause ECG abnormalities in TBI patients, including pain, electrolyte abnormalities, and hypotension. Moreover, it is difficult to determine whether the ECG abnormalities were newly developed or pre-existing. Therefore, ECG abnormalities should be interpreted in conjunction with either troponin or cardiac imaging for the diagnosis of TBI-related cardiac dysfunction.
Although heterogeneity between studies was observed, our secondary analysis suggested an association between myocardial injury and in-hospital mortality following TBI. Elevated cardiac troponin levels have been reported in other neurological diseases, such as subarachnoid hemorrhage and stroke, and have been demonstrated to be associated with increased mortality regardless of the presence of coronary artery disease. Since cardiac troponin is a sensitive biomarker for cardiac injury and has a prognostic benefit for mortality, guidelines recommend routine assessment of serum troponin in patients with acute stroke59. A meta-analysis El-Menyar et al.60 also reported an association between elevated cardiac troponin and mortality in both TBI and non-TBI brain injury. Although patients with TBI generally carry lower risk of cardiovascular disease, serum troponin may potentially be a biomarker for cardiac injury after TBI.
While enhanced cardiac evaluation in TBI patients may improve clinical management, there remain significant areas for future research. Our analysis found various methods used and different timing for the initiation of cardiac evaluation. Therefore, the optimal timing for initiation and duration for monitoring troponin and echocardiography following TBI is unknown. Although there has been increased recognition of myocardial injury following TBI in recent years, few studies have evaluated the hemodynamic consequences and its relationship to cardiac dysfunction. In this analysis, the pooled incidence of myocardial injury was higher than the incidence of cardiac dysfunction. Although myocardial injury was associated with increased mortality, it was challenging to demonstrate whether death was related to cardiovascular impairment. In this analysis, six studies reported both troponin elevation and cardiac dysfunction using echocardiography, but none reported a relationship between troponin elevation and clinical or subclinical cardiac dysfunction. Future studies should examine the effects of myocardial injury to the patients, the association between troponin elevation and cardiac dysfunction, and how the recognition of cardiac dysfunction may modify hemodynamic management to optimize cerebral perfusion following TBI, in order to improve outcomes.
There are some limitations to this study. First, different troponin assays were used among the included studies with variations in threshold for troponin elevation; this created heterogeneity of the results. Troponin elevation can also occur due to reasons other than TBI, especially if troponin > 99th reference value is used as the threshold. Cardiac troponins may be falsely elevated due to known cross-reactivities with alkaline phosphatase or rheumatoid factor55. Moreover, troponin elevation has also been reported in trauma patients without TBI so troponin elevation in some of the studies may have been the result of polytrauma. This may explain the difference between the incidence of myocardial injury measured by cardiac troponin and the incidence of cardiac dysfunction assessed by echocardiography and have overestimated the incidence of myocardial injury. Second, it is impractical to have baseline troponin and echocardiographic evaluations before TBI, and developing a suitable control group for TBI patients may be challenging. Third, there are methodological confounders in the included studies. There was inconsistent timing of troponin and echocardiography assessments amongst studies. Given the potentially transient nature of cardiac dysfunction, LVEF and RWMA abnormalities may not be comparable if the echocardiogram is not conducted in the same timeframe (e.g., within 24 h of admission). Some studies also included ECGs, though many used them differently. For example, one study21 used T-wave changes in ECG as screening criteria, while another39 measured ECG as part of cardiac monitoring. These methodological differences across these studies limit their overall comparability. Fourth, some studies conducted echocardiograms after vasopressor infusion despite the potential of vasopressors to mask reduced LVEF or RWMAs. Of note, in one study29, echocardiograms were only undertaken when clinically indicated (rather than all patients), resulting in selection bias. Moreover, we focused echocardiographic-defined cardiac dysfunction primarily on LV systolic function and, hence, failed to include patients who might have had abnormal diastolic function or impaired right ventricular function. Finally, the analysis may be susceptible to publication bias, as the Doi plot showed asymmetry, since only studies with positive results were more likely to be published.
CONCLUSION
Despite significant variation in patient selection, methods, and timing of cardiac evaluation between studies, which may result in bias and over-estimation, this meta-analysis found that myocardial injury occurred in about one-third and cardiac dysfunction in approximately one-sixth of adult patients following moderate-severe TBI. The association between myocardial injury and cardiac dysfunction after TBI remains unclear and should be further investigated. However, myocardial injury was associated with increased mortality.
Supplementary Material
Supplemental digital content 1
Table showing search terms and results for MEDLINE and EMBASE
Supplemental digital content 2
Table showing patient characteristics of the included studies.
Supplemental digital content 3
Table summarizing the Joanna Briggs Institute Critical Appraisal Checklist for studies reporting prevalence data.
Supplemental digital content 4
Table showing criteria used for risk of bias grading of each domain of the Joanna Briggs Institute Critical Appraisal Checklist.
Supplemental digital content 5
Table showing sensitivity analysis.
Supplemental digital content 6
Table showing studies reporting the incidence of ECG abnormalities in patients with traumatic brain injury.
Supplemental digital content 7
Figure showing Doi plot analysis and LFK index of the incidence of myocardial injury and cardiac dysfunction estimates.
Funding source:
NINDS K23NS109274 (Krishnamoorthy)
Footnotes
Authors report no conflicts of interest.
REFERENCES
- 1.Krishnamoorthy V, Temkin N, Barber J, et al. Association of Early Multiple Organ Dysfunction With Clinical and Functional Outcomes Over the Year Following Traumatic Brain Injury: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury Study. Crit Care Med 2021;49:1769–78. doi: 10.1097/CCM.0000000000005055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Menyar A, Goyal A, Latifi R, Al-Thani H, Frishman W. Brain-Heart Interactions in Traumatic Brain Injury. Cardiol Rev 2017;25:279–88. 10.1097/CRD.0000000000000167 [DOI] [PubMed] [Google Scholar]
- 3.Miller JD, Sweet RC, Narayan R, Becker DP. Early Insults to the Injured Brain. JAMA 1978;240:439–42. doi: 10.1001/jama.1978.03290050029011 [DOI] [PubMed] [Google Scholar]
- 4.Byer E, Ashman R, Toth LA. Electrocardiograms with large, upright T waves and long Q-T intervals. Am Heart J 1947;33:796–806. doi: 10.1016/0002-8703(47)90025-2 [DOI] [PubMed] [Google Scholar]
- 5.Cropp GJ, Manning GW. Electrocardiographic changes simulating myocardial ischemia and infarction associated with spontaneous intracranial hemorrhage. Circulation 1960;22:25–38. doi: 10.1161/01.cir.22.1.25 [DOI] [PubMed] [Google Scholar]
- 6.Mascia L, Sakr Y, Pasero D, et al. Extracranial complications in patients with acute brain injury: a post-hoc analysis of the SOAP study. Intensive Care Med 2008;34:720–7. doi: 10.1007/s00134-007-0974-7. [DOI] [PubMed] [Google Scholar]
- 7.Murthy SB, Shah S, Rao CP, Bershad EM, Suarez JI. Neurogenic Stunned Myocardium Following Acute Subarachnoid Hemorrhage: Pathophysiology and Practical Considerations. J Intensive Care Med 2015;30:318–25. doi: 10.1177/0885066613511054 [DOI] [PubMed] [Google Scholar]
- 8.Gao L, Smielewski P, Czosnyka M, Ercole A. Early Asymmetric Cardio-Cerebral Causality and Outcome after Severe Traumatic Brain Injury. J Neurotrauma 2017;34:2743–52. doi: 10.1089/neu.2016.4787. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamoorthy V, Rowhani-Rahbar A, Chaikittisilpa N, et al. Association of Early Hemodynamic Profile and the Development of Systolic Dysfunction Following Traumatic Brain Injury. Neurocrit Care 2017;26:379–87. doi: 10.1007/s12028-016-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015;13:147–53. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 13.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Furuya-Kanamori L, Barendregt JJ, Doi SA. A new improved graphical and quantitative method for detecting bias in meta-analysis. JBI Evidence Implementation 2018;16:195–203. doi: 10.1097/XEB.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 15.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health 2013;67:974–8. doi: 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods 2017;8:5–18. . doi: 10.1002/jrsm.1230 [DOI] [PubMed] [Google Scholar]
- 20.Krishnamoorthy V, Rowhani-Rahbar A, Gibbons EF, et al. Early Systolic Dysfunction Following Traumatic Brain Injury: A Cohort Study. Crit Care Med 2017;45:1028–36. doi: 10.1097/CCM.0000000000002404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baffoun N, Lakhdhar R, Baccar K, Djebari K, Kaddour C. Cardiac injury in traumatic subarachnoid hemorrhagea: prospective study in 35 patients. Tunis Med 2011;89:184–7. [PubMed] [Google Scholar]
- 22.Bender M, Stein M, Schoof B, Kolodziej MA, Uhl E, Schöller K. Troponin I as an early biomarker of cardiopulmonary parameters during the first 24 h of intensive care unit treatment in isolated traumatic brain injury patients. Injury 2020;51:1189–95. doi: 10.1016/j.injury.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 23.Cai SS, Bonds BW, Hu PF, Stein DM. The role of cardiac troponin I in prognostication of patients with isolated severe traumatic brain injury. J Trauma Acute Care Surg 2016;80:477–83. doi: 10.1097/ta.0000000000000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaikittisilpa N, Vavilala MS, Lele AV, Moore AE, Bethel J, Krishnamoorthy V. Early cardiovascular function and associated hemodynamics in adults with isolated moderate-severe traumatic brain injury: A pilot study. J Clin Neurosci 2019;69:97–103. doi: 10.1002/jnr.24100 [DOI] [PubMed] [Google Scholar]
- 25.Cuisinier A, Maufrais C, Payen JF, Nottin S, Walther G, Bouzat P. Myocardial function at the early phase of traumatic brain injury: a prospective controlled study. Scand J Trauma Resusc Emerg Med 2016;24:129. doi: 10.1186/s13049-016-0323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixit S, Castle M, Velu RP, Swisher L, Hodge C, Jaffe AS. Cardiac involvement in patients with acute neurologic disease: Confirmation with cardiac troponin I. Archives of Internal Medicine 2000;160:3153–8. doi: 10.1001/archinte.160.20.3153 [DOI] [PubMed] [Google Scholar]
- 27.Dou LW, Du Z, Zhu JH, Wang TB. Changes and significance of serum troponin in trauma patients: A retrospective study in a level I trauma center. World Journal of Emergency Medicine 2022;13:136–40. doi: 10.5847/wjem.j.1920-8642.2022.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Menyar A, Asim M, Ramzee AF, et al. Bio-Shock Index: Proposal and Rationale for a New Predictive Tool for In-Hospital Mortality in Patients with Traumatic Brain Injury. World Neurosurg 2019;132:e169–e77. doi: 10.1016/j.wneu.2019.08.229 [DOI] [PubMed] [Google Scholar]
- 29.Gibbons PW, Goldberg RJ, Muehlschlegel S. A pilot study evaluating a simple cardiac dysfunction score to predict complications and survival among critically-ill patients with traumatic brain injury. J Crit Care 2019;54:130–5. doi: 10.1016/j.jcrc.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamdi E, Taema K, Shehata M, Radwan W. Predictive value of cardiac troponin I in traumatic brain injury. Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2012;49:365–73. [Google Scholar]
- 31.Hasanin A, Kamal A, Amin S, et al. Incidence and outcome of cardiac injury in patients with severe head trauma. Scand J Trauma Resusc Emerg Med 2016;24:58. . doi: 10.1186/s13049-016-0246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnamoorthy V, Manley GT, Jain S, et al. Incidence and Clinical Impact of Myocardial Injury Following Traumatic Brain Injury: A Pilot TRACK-TBI Study. Journal of Neurosurgical Anesthesiology 2022;34:233–7. doi: 10.1097/ANA.0000000000000772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee M, Oh JH, Lee KB, et al. Clinical and Echocardiographic Characteristics of Acute Cardiac Dysfunction Associated With Acute Brain Hemorrhage - Difference From Takotsubo Cardiomyopathy. Circ J 2016;80:2026–32. doi: 10.1253/circj.CJ-16-0395 [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Buonocore R, Mitaritonno M, Cervellin G. Cardiac Troponin I is increased in patients with polytrauma and chest or head trauma. Results of a retrospective case-control study. Journal of Medical Biochemistry 2016;35:275–81. doi: 10.1515/jomb-2016-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prathep S, Sharma D, Hallman M, et al. Preliminary report on cardiac dysfunction after isolated traumatic brain injury. Crit Care Med 2014;42:142–7. doi: 10.1097/CCM.0b013e318298a890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Praveen R, Jayant A, Mahajan S, et al. Perioperative cardiovascular changes in patients with traumatic brain injury: A prospective observational study. Surgical Neurology International 2021;12. doi: 10.25259/SNI_5_2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rimaz S, Ashraf A, Marzban S, et al. Significance of cardiac troponin i elevation in traumatic brain injury patients. Anesthesiology and Pain Medicine 2019;9. doi: 10.5812/aapm.90858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salim A, Hadjizacharia P, Brown C, et al. Significance of troponin elevation after severe traumatic brain injury. Journal of Trauma - Injury, Infection and Critical Care 2008;64:46–52. doi: 10.1097/TA.0b013e31815eb15a [DOI] [PubMed] [Google Scholar]
- 39.Serri K, El Rayes M, Giraldeau G, Williamson D, Bernard F. Traumatic brain injury is not associated with significant myocardial dysfunction: an observational pilot study. Scand J Trauma Resusc Emerg Med 2016;24:31. doi: 10.1186/s13049-016-0217-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasaiah B, Muthuchellappan R, Ganne Sesha UR. A prospective observational study of electrocardiographic and echocardiographic changes in traumatic brain injury–effect of surgical decompression. British Journal of Neurosurgery 2022;1–6. doi: 10.1080/02688697.2021.2024497 [DOI] [PubMed] [Google Scholar]
- 41.Stewart D, Waxman K, Brown CA, et al. B-type natriuretic peptide levels may be elevated in the critically injured trauma patient without congestive heart failure. J Trauma 2007;63:747–50. doi: 10.1097/01.ta.0000240458.46050.38. [DOI] [PubMed] [Google Scholar]
- 42.Venkata C, Kasal J. Cardiac Dysfunction in Adult Patients with Traumatic Brain Injury: A Prospective Cohort Study. Clin 2018;16:57–65. doi: 10.3121/cmr.2018.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamila M, Hussein K, Ismail MF, Kamal A. Prevalence of Electrocardiographic Changes in Patients with Traumatic Brain Injury: A Prospective Hospital-based Study. Open Access Macedonian Journal of Medical Sciences 2022;10:408–12. . doi: 10.3889/oamjms.2022.8313 [DOI] [Google Scholar]
- 44.Lenstra JJ, Kuznecova-Keppel Hesselink L, la Bastide-van Gemert S, et al. The Association of Early Electrocardiographic Abnormalities With Brain Injury Severity and Outcome in Severe Traumatic Brain Injury. Front Neurol 2020;11:597737. doi: 10.3389/fneur.2020.597737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koiv L, Merisalu E, Zilmer K, Tomberg T, Kaasik AE. Changes of sympatho-adrenal and hypothalamo-pituitary-adrenocortical system in patients with head injury. Acta Neurol Scand 1997;96:52–8. doi: 10.1111/j.1600-0404.1997.tb00238.x [DOI] [PubMed] [Google Scholar]
- 46.Grundy PL, Harbuz MS, Jessop DS, Lightman SL, Sharples PM. The hypothalamo-pituitary-adrenal axis response to experimental traumatic brain injury. J Neurotrauma 2001;18:1373–81. doi: 10.1093/bjaceaccp/mkr058 [DOI] [PubMed] [Google Scholar]
- 47.Rosner MJ, Newsome HH, Becker DP. Mechanical brain injury: the sympathoadrenal response. J Neurosurg 1984;61:76–86. doi: 10.3171/jns.1984.61.1.0076 [DOI] [PubMed] [Google Scholar]
- 48.Gregory T, Smith M. Cardiovascular complications of brain injury. Continuing Education in Anaesthesia Critical Care & Pain 2012;12:67–71. doi: 10.1093/bjaceaccp/mkr058 [DOI] [Google Scholar]
- 49.Samuels MA. The brain-heart connection. Circulation 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995 [DOI] [PubMed] [Google Scholar]
- 50.McKee CA, Lukens JR. Emerging Roles for the Immune System in Traumatic Brain Injury. Front Immunol 2016;7:556. doi: 10.3389/fimmu.2016.00556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zochios V, Valchanov K. Raised cardiac troponin in intensive care patients with sepsis, in the absence of angiographically documented coronary artery disease: A systematic review. J Intensive Care Soc 2015;16:52–7. doi: 10.1177/1751143714555303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee D, Perrett C, Banerjee A. Troponins, Acute Coronary Syndrome and Renal Disease: From Acute Kidney Injury Through End-stage Kidney Disease. Eur Cardiol 2019;14:187–90. doi: 10.15420/ecr.2019.28.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017;80:6–15. doi: 10.1227/NEU.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 54.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231–64. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 55.Chaulin AM. False-Positive Causes in Serum Cardiac Troponin Levels. Journal of Clinical Medicine Research 2022;14:80–7. doi: 10.14740/jocmr4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crewdson K, Thompson J, Thomas M. Cardiac troponin T is associated with mortality in patients admitted to critical care in a UK major trauma centre: a retrospective database analysis. J Intensive Care Soc 2019;20:132–7. doi: 10.1177/1751143718767782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Decavele M, Gault N, Gauss T, et al. Cardiac troponin I as an early prognosis biomarker after trauma: a retrospective cohort study. Br J Anaesth 2018;120:1158–64. . doi: 10.1016/j.bja.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 58.de Lemos JA. Increasingly sensitive assays for cardiac troponins: a review. JAMA 2013;309:2262–9. doi: 10.1001/jama.2013.5809 [DOI] [PubMed] [Google Scholar]
- 59.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 60.El-Menyar A, Sathian B, Wahlen BM, Al-Thani H. Serum cardiac troponins as prognostic markers in patients with traumatic and non-traumatic brain injuries: A meta-analysis. Am J Emerg Med 2019;37:133–42. doi: 10.1016/j.ajem.2018.10.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content 1
Table showing search terms and results for MEDLINE and EMBASE
Supplemental digital content 2
Table showing patient characteristics of the included studies.
Supplemental digital content 3
Table summarizing the Joanna Briggs Institute Critical Appraisal Checklist for studies reporting prevalence data.
Supplemental digital content 4
Table showing criteria used for risk of bias grading of each domain of the Joanna Briggs Institute Critical Appraisal Checklist.
Supplemental digital content 5
Table showing sensitivity analysis.
Supplemental digital content 6
Table showing studies reporting the incidence of ECG abnormalities in patients with traumatic brain injury.
Supplemental digital content 7
Figure showing Doi plot analysis and LFK index of the incidence of myocardial injury and cardiac dysfunction estimates.


