Abstract
We present a genome assembly from an individual male Lochmaea crataegi (the hawthorn leaf beetle; Arthropoda; Insecta; Coleoptera; Chrysomelidae). The genome sequence is 891.3 megabases in span. Most of the assembly is scaffolded into 16 chromosomal pseudomolecules, including the X and Y sex chromosomes. The mitochondrial genome has also been assembled and is 18.32 kilobases in length.
Keywords: Lochmaea crataegi, hawthorn leaf beetle, genome sequence, chromosomal, Coleoptera
Species taxonomy
Eukaryota; Opisthokonta; Metazoa; Eumetazoa; Bilateria; Protostomia; Ecdysozoa; Panarthropoda; Arthropoda; Mandibulata; Pancrustacea; Hexapoda; Insecta; Dicondylia; Pterygota; Neoptera; Endopterygota; Coleoptera; Polyphaga; Cucujiformia; Chrysomeloidea; Chrysomelidae; Galerucinae; Galerucini; Lochmaea; Lochmaea crataegi ( Forster, 1771) (NCBI:txid1143063).
Background
The Hawthorn Leaf Beetle, Lochmaea crataegi, is a distinctive leaf beetle about 4–5 mm long. It can be recognised by the glabrous dorsal surface, reddish colour, four elongate black marks on the elytra and pair of black spots on pronotum. The taxonomic history of the species is complex, with at least six junior synonyms with type localities in Central Europe ( Bezděk, 2004).
Lochmaea Weise, 1883 is a genus of leaf beetle widely distributed in Europe and Asia with about a dozen species divided in groups or subgenera. They are phytophagous beetles that use several plant families as host, including Betulaceae, Salicaceae, Rosaceae, Fagaceae, Ericaceae, and Cucurbitaceae ( Jolivet & Hawkeswood, 1995). The species Lochmaea crataegi ( Forster, 1771) is broadly distributed in Europe, Siberia and east Asia ( Bezděk, 2004; Warchałowski, 2003) with recent records for Taiwan ( Lee, 2019). The species was originally described from England by the pastor and naturalist Johann Reinhold Forster (1729–1798).
As the common name suggests, it is associated with common hawthorn, Crataegus monogyna Jacq. (Rosaceae), with both adults and larvae feeding on it and in some cases affecting the plant’s viability ( Bezděk, 2004; Warchałowski, 2003). Adults appear shortly before the flowers open, upon which time they begin feeding on the pollen, occasionally feeding on pollen of other species. The larvae feed exclusively on the developing fruits, developing throughout the summer before dropping to the soil to pupate. Adults eclose during the late summer to autumn and overwinter in this stage. It occurs across a wide range of habitats, anywhere where the host may be found.
The genomic information on L. crataegi will allow to disentangle the taxonomy and systematics of the species as well as the genus and promote insect-host adaptation studies, being the insect and the host both common and widely distributed in Europe.
Genome sequence report
The genome was sequenced from one male Lochmaea crataegi ( Figure 1) collected from | Wytham Woods, Oxfordshire, UK (51.77, –1.34). A total of 35-fold coverage in Pacific Biosciences single-molecule HiFi long reads was generated. Primary assembly contigs were scaffolded with chromosome conformation Hi-C data. Manual assembly curation corrected 66 missing joins or mis-joins and removed 8 haplotypic duplications, reducing the assembly length by 0.45% and the scaffold number by 29.38%.
Figure 1. Photograph of the Lochmaea crataegi (icLocCrat2) specimen used for genome sequencing.
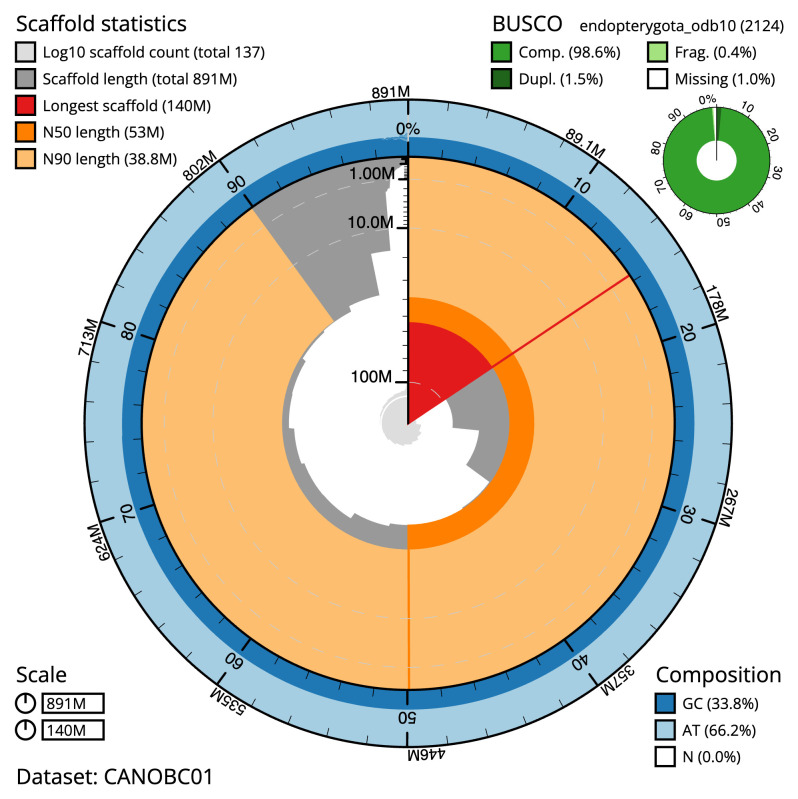
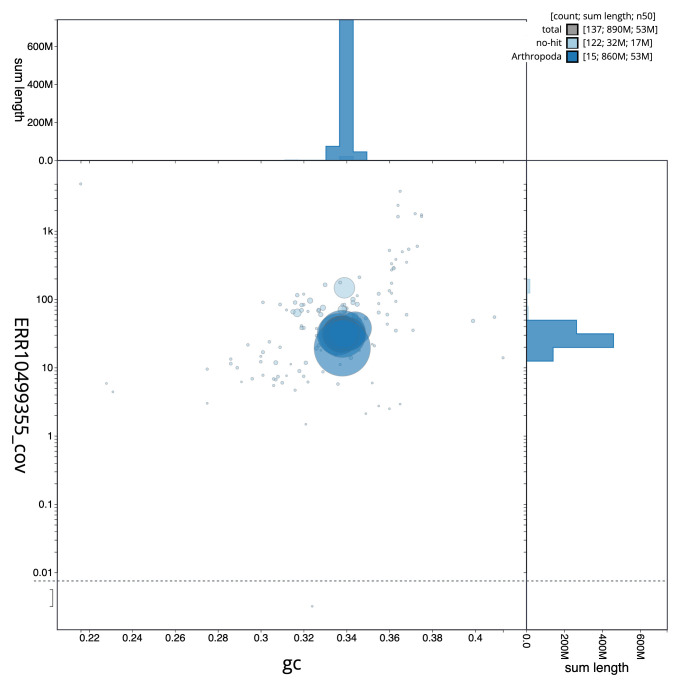
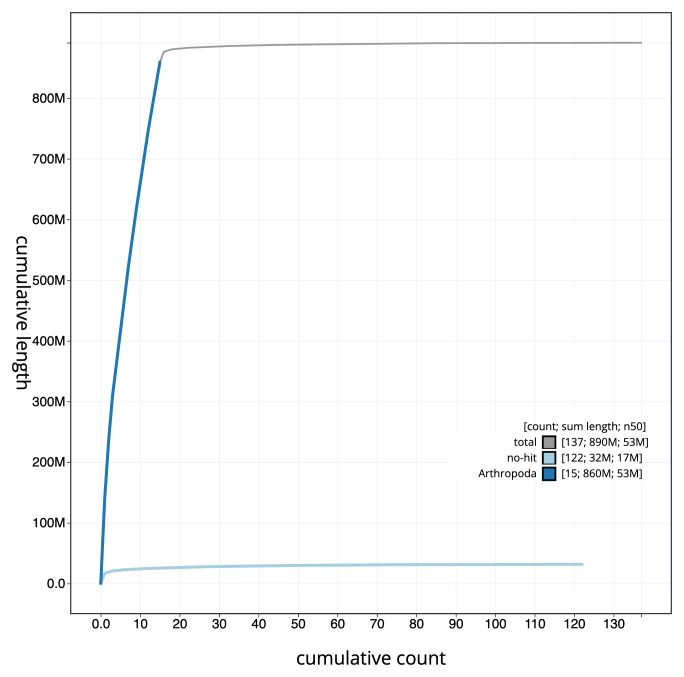
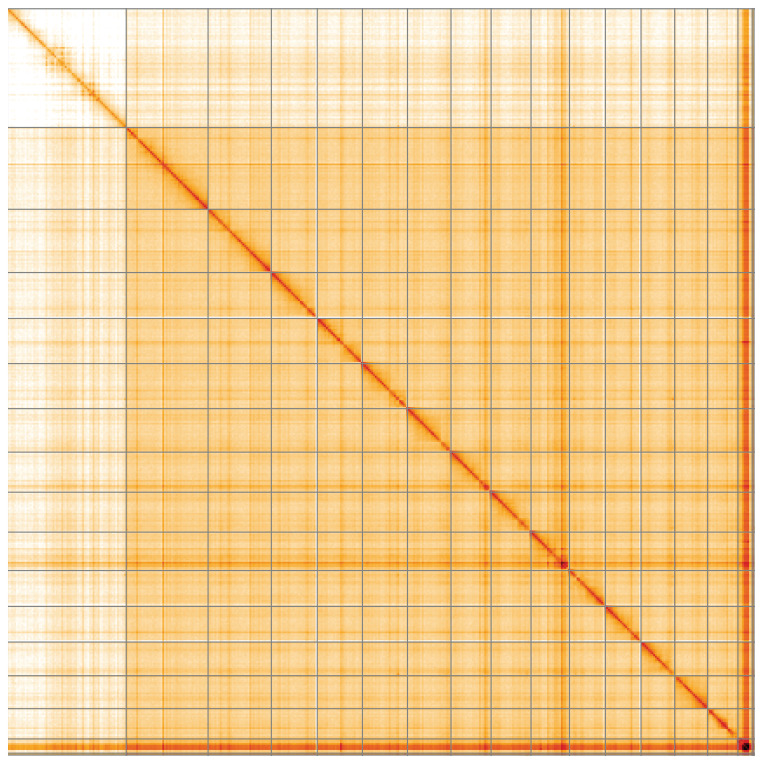
The final assembly has a total length of 891.3 Mb in 136 sequence scaffolds with a scaffold N50 of 53.0 Mb ( Table 1). The snailplot in Figure 2 provides a summary of the assembly statistics, while the distribution of assembly scaffolds on GC proportion and coverage is shown in Figure 3. The cumulative assembly plot in Figure 4 shows curves for subsets of scaffolds assigned to different phyla. Most (98.32%) of the assembly sequence was assigned to 16 chromosomal-level scaffolds, representing 14 autosomes and the X and Y sex chromosomes. Chromosome-scale scaffolds confirmed by the Hi-C data are named in order of size ( Figure 5; Table 2). The following regions of this assembly are of undetermined order and orientation: Chromosome 9 region 37.4 Mbp to the end, and the whole Y chromosome. While not fully phased, the assembly deposited is of one haplotype. Contigs corresponding to the second haplotype have also been deposited. The mitochondrial genome was also assembled and can be found as a contig within the multifasta file of the genome submission.
Figure 2. Genome assembly of Lochmaea crataegi, icLocCrat2.1: metrics.
The BlobToolKit Snailplot shows N50 metrics and BUSCO gene completeness. The main plot is divided into 1,000 size-ordered bins around the circumference with each bin representing 0.1% of the 891,301,393 bp assembly. The distribution of scaffold lengths is shown in dark grey with the plot radius scaled to the longest scaffold present in the assembly (139,762,297 bp, shown in red). Orange and pale-orange arcs show the N50 and N90 scaffold lengths (53,024,378 and 38,778,232 bp), respectively. The pale grey spiral shows the cumulative scaffold count on a log scale with white scale lines showing successive orders of magnitude. The blue and pale-blue area around the outside of the plot shows the distribution of GC, AT and N percentages in the same bins as the inner plot. A summary of complete, fragmented, duplicated and missing BUSCO genes in the endopterygota_odb10 set is shown in the top right. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CANOBC01/dataset/CANOBC01/snail.
Figure 3. Genome assembly of Lochmaea crataegi, icLocCrat2.1: BlobToolKit GC-coverage plot.
Sequences are coloured by phylum. Circles are sized in proportion to sequence length. Histograms show the distribution of sequence length sum along each axis. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CANOBC01/dataset/CANOBC01/blob.
Figure 4. Genome assembly of Lochmaea crataegi, icLocCrat2.1: BlobToolKit cumulative sequence plot.
The grey line shows cumulative length for all sequences. Coloured lines show cumulative lengths of sequences assigned to each phylum using the buscogenes taxrule. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CANOBC01/dataset/CANOBC01/cumulative.
Figure 5. Genome assembly of Lochmaea crataegi, icLocCrat2.1: Hi-C contact map of the icLocCrat2.1 assembly, visualised using HiGlass. Chromosomes are shown in order of size from left to right and top to bottom. An interactive version of this figure may be viewed at https://genome-note-higlass.tol.sanger.ac.uk/l/?d=FAZ9iekhQ6-CeJrt7SmoIg.
Table 1. Genome data for Lochmaea crataegi, icLocCrat2.1.
| Project accession data | ||
|---|---|---|
| Assembly identifier | icLocCrat2.1 | |
| Species | Lochmaea crataegi | |
| Specimen | icLocCrat2 | |
| NCBI taxonomy ID | 1143063 | |
| BioProject | PRJEB57666 | |
| BioSample ID | SAMEA110451579 | |
| Isolate information | icLocCrat2, male: whole organism (DNA sequencing)
icLocCrat1, whole organism (Hi-C scaffolding) |
|
| Assembly metrics * | Benchmark | |
| Consensus quality (QV) | 60.4 | ≥ 50 |
| k-mer completeness | 100.0% | ≥ 95% |
| BUSCO ** | C:98.6%[S:97.2%,D:1.5%],
F:0.4%,M:1.0%,n:2,124 |
C ≥ 95% |
| Percentage of assembly mapped to chromosomes | 98.32% | ≥ 95% |
| Sex chromosomes | XY | localised homologous pairs |
| Organelles | Mitochondrial genome: 18.32 kb | complete single alleles |
| Raw data accessions | ||
| PacificBiosciences SEQUEL II | ERR10499355 | |
| Hi-C Illumina | ERR10501013 | |
| Genome assembly | ||
| Assembly accession | GCA_947563755.1 | |
| Accession of alternate haplotype | GCA_947563735.1 | |
| Span (Mb) | 891.3 | |
| Number of contigs | 738 | |
| Contig N50 length (Mb) | 2.8 | |
| Number of scaffolds | 136 | |
| Scaffold N50 length (Mb) | 53.0 | |
| Longest scaffold (Mb) | 139.76 | |
* Assembly metric benchmarks are adapted from column VGP-2020 of “Table 1: Proposed standards and metrics for defining genome assembly quality” from Rhie et al. (2021).
** BUSCO scores based on the endopterygota_odb10 BUSCO set using version 5.3.2. C = complete [S = single copy, D = duplicated], F = fragmented, M = missing, n = number of orthologues in comparison. A full set of BUSCO scores is available at https://blobtoolkit.genomehubs.org/view/CANOBC01/dataset/CANOBC01/busco.
Table 2. Chromosomal pseudomolecules in the genome assembly of Lochmaea crataegi, icLocCrat2.
| INSDC
accession |
Chromosome | Length
(Mb) |
GC% |
|---|---|---|---|
| OX387423.1 | 1 | 96.32 | 34.0 |
| OX387424.1 | 2 | 74.48 | 33.5 |
| OX387425.1 | 3 | 54.01 | 33.5 |
| OX387426.1 | 4 | 53.19 | 34.0 |
| OX387427.1 | 5 | 53.02 | 34.0 |
| OX387428.1 | 6 | 51.23 | 33.5 |
| OX387429.1 | 7 | 47.18 | 34.0 |
| OX387430.1 | 8 | 46.99 | 34.0 |
| OX387431.1 | 9 | 45.26 | 34.5 |
| OX387432.1 | 10 | 42.22 | 33.5 |
| OX387433.1 | 11 | 41.92 | 34.0 |
| OX387434.1 | 12 | 39.66 | 34.0 |
| OX387435.1 | 13 | 38.78 | 34.0 |
| OX387436.1 | 14 | 35.63 | 34.0 |
| OX387422.1 | X | 139.76 | 34.0 |
| OX387437.1 | Y | 16.76 | 34.0 |
| OX387438.1 | MT | 0.02 | 22.0 |
The estimated Quality Value (QV) of the final assembly is 60.4 with k-mer completeness of 100.0%, and the assembly has a BUSCO v5.3.2 completeness of 98.6% (single = 97.2%, duplicated = 1.5%), using the endopterygota_odb10 reference set ( n = 2,124).
Metadata for specimens, barcode results, spectra estimates, sequencing runs, contaminants and pre-curation assembly statistics are given at https://links.tol.sanger.ac.uk/species/1143063.
Methods
Sample acquisition and nucleic acid extraction
A male Lochmaea crataegi (specimen ID Ox002129, ToLID icLocCrat2) was collected from Wytham Woods, Oxfordshire (biological vice-county Berkshire), UK (latitude 51.77, longitude –1.34) on 2022-04-28 by beating. The specimen was collected and identified by Liam Crowley (University of Oxford). The specimen used for Hi-C sequencing (specimen ID Ox001430, ToLID icLocCrat1) was collected from the same location on 2021-05-25 by beating. The specimen was collected and identified by Mark Telfer (independent researcher). Both specimens were snap-frozen on dry ice.
The workflow for high molecular weight (HMW) DNA extraction at the Wellcome Sanger Institute (WSI) includes a sequence of core procedures: sample preparation; sample homogenisation, DNA extraction, fragmentation, and clean-up. In sample preparation, the icLocCrat2 sample was weighed and dissected on dry ice ( Jay et al., 2023). Tissue from the whole organism was homogenised using a PowerMasher II tissue disruptor ( Denton et al., 2023a). HMW DNA was extracted using the Automated MagAttract v1 protocol ( Sheerin et al., 2023). DNA was sheared into an average fragment size of 12–20 kb in a Megaruptor 3 system with speed setting 30 ( Todorovic et al., 2023). Sheared DNA was purified by solid-phase reversible immobilisation ( Strickland et al., 2023): in brief, the method employs a 1.8X ratio of AMPure PB beads to sample to eliminate shorter fragments and concentrate the DNA. The concentration of the sheared and purified DNA was assessed using a Nanodrop spectrophotometer and Qubit Fluorometer and Qubit dsDNA High Sensitivity Assay kit. Fragment size distribution was evaluated by running the sample on the FemtoPulse system.
Protocols developed by the WSI Tree of Life laboratory are publicly available on protocols.io ( Denton et al., 2023b).
Sequencing
Pacific Biosciences HiFi circular consensus DNA sequencing libraries were constructed according to the manufacturers’ instructions. DNA sequencing was performed by the Scientific Operations core at the WSI on a Pacific Biosciences SEQUEL II instrument. Hi-C data were also generated from the whole organism tissue of icLocCrat1 using the Arima2 kit and sequenced on the Illumina NovaSeq 6000 instrument.
Genome assembly, curation and evaluation
Assembly was carried out with Hifiasm ( Cheng et al., 2021) and haplotypic duplication was identified and removed with purge_dups ( Guan et al., 2020). The assembly was then scaffolded with Hi-C data ( Rao et al., 2014) using YaHS ( Zhou et al., 2023). The assembly was checked for contamination and corrected as described previously ( Howe et al., 2021). Manual curation was performed using HiGlass ( Kerpedjiev et al., 2018) and Pretext ( Harry, 2022). The mitochondrial genome was assembled using MitoHiFi ( Uliano-Silva et al., 2023), which runs MitoFinder ( Allio et al., 2020) or MITOS ( Bernt et al., 2013) and uses these annotations to select the final mitochondrial contig and to ensure the general quality of the sequence.
A Hi-C map for the final assembly was produced using bwa-mem2 ( Vasimuddin et al., 2019) in the Cooler file format ( Abdennur & Mirny, 2020). To assess the assembly metrics, the k-mer completeness and QV consensus quality values were calculated in Merqury ( Rhie et al., 2020). This work was done using Nextflow ( Di Tommaso et al., 2017) DSL2 pipelines “sanger-tol/readmapping” ( Surana et al., 2023a) and “sanger-tol/genomenote” ( Surana et al., 2023b). The genome was analysed within the BlobToolKit environment ( Challis et al., 2020) and BUSCO scores ( Manni et al., 2021; Simão et al., 2015) were calculated.
Table 3 contains a list of relevant software tool versions and sources.
Table 3. Software tools: versions and sources.
| Software
tool |
Version | Source |
|---|---|---|
| BlobToolKit | 4.1.7 |
https://github.com/blobtoolkit/
blobtoolkit |
| BUSCO | 5.3.2 | https://gitlab.com/ezlab/busco |
| Hifiasm | 0.16.1-r375 |
https://github.com/chhylp123/
hifiasm |
| HiGlass | 1.11.6 | https://github.com/higlass/higlass |
| Merqury | MerquryFK |
https://github.com/thegenemyers/
MERQURY.FK |
| MitoHiFi | 2 |
https://github.com/marcelauliano/
MitoHiFi |
| PretextView | 0.2 |
https://github.com/wtsi-hpag/
PretextView |
| purge_dups | 1.2.3 |
https://github.com/dfguan/purge_
dups |
| sanger-tol/
genomenote |
v1.0 |
https://github.com/sanger-tol/
genomenote |
| sanger-tol/
readmapping |
1.1.0 |
https://github.com/sanger-tol/
readmapping/tree/1.1.0 |
| YaHS | 1.1a.2 | https://github.com/c-zhou/yahs |
Wellcome Sanger Institute – Legal and Governance
The materials that have contributed to this genome note have been supplied by a Darwin Tree of Life Partner. The submission of materials by a Darwin Tree of Life Partner is subject to the ‘Darwin Tree of Life Project Sampling Code of Practice’, which can be found in full on the Darwin Tree of Life website here. By agreeing with and signing up to the Sampling Code of Practice, the Darwin Tree of Life Partner agrees they will meet the legal and ethical requirements and standards set out within this document in respect of all samples acquired for, and supplied to, the Darwin Tree of Life Project.
Further, the Wellcome Sanger Institute employs a process whereby due diligence is carried out proportionate to the nature of the materials themselves, and the circumstances under which they have been/are to be collected and provided for use. The purpose of this is to address and mitigate any potential legal and/or ethical implications of receipt and use of the materials as part of the research project, and to ensure that in doing so we align with best practice wherever possible. The overarching areas of consideration are:
Ethical review of provenance and sourcing of the material
Legality of collection, transfer and use (national and international)
Each transfer of samples is further undertaken according to a Research Collaboration Agreement or Material Transfer Agreement entered into by the Darwin Tree of Life Partner, Genome Research Limited (operating as the Wellcome Sanger Institute), and in some circumstances other Darwin Tree of Life collaborators.
Data availability
European Nucleotide Archive: Lochmaea crataegi (hawthorn leaf beetle). Accession number PRJEB57666; https://identifiers.org/ena.embl/PRJEB57666 ( Wellcome Sanger Institute, 2022). The genome sequence is released openly for reuse. The Lochmaea crataegi genome sequencing initiative is part of the Darwin Tree of Life (DToL) project. All raw sequence data and the assembly have been deposited in INSDC databases. The genome will be annotated using available RNA-Seq data and presented through the Ensembl pipeline at the European Bioinformatics Institute. Raw data and assembly accession identifiers are reported in Table 1.
Author information
Members of the University of Oxford and Wytham Woods Genome Acquisition Lab are listed here: https://doi.org/10.5281/zenodo.7125292.
Members of the Darwin Tree of Life Barcoding collective are listed here: https://doi.org/10.5281/zenodo.4893703.
Members of the Wellcome Sanger Institute Tree of Life Management, Samples and Laboratory team are listed here: https://doi.org/10.5281/zenodo.10066175.
Members of Wellcome Sanger Institute Scientific Operations: Sequencing Operations are listed here: https://doi.org/10.5281/zenodo.10043364.
Members of the Wellcome Sanger Institute Tree of Life Core Informatics team are listed here: https://doi.org/10.5281/zenodo.10066637.
Members of the Tree of Life Core Informatics collective are listed here: https://doi.org/10.5281/zenodo.5013541.
Members of the Darwin Tree of Life Consortium are listed here: https://doi.org/10.5281/zenodo.4783558.
Funding Statement
This work was supported by Wellcome through core funding to the Wellcome Sanger Institute [206194, <a href=https://doi.org/10.35802/206194>https://doi.org/10.35802/206194</a>] and the Darwin Tree of Life Discretionary Award [218328, <a href=https://doi.org/10.35802/218328>https://doi.org/10.35802/218328 </a>].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- Abdennur N, Mirny LA: Cooler: Scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics. 2020;36(1):311–316. 10.1093/bioinformatics/btz540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allio R, Schomaker-Bastos A, Romiguier J, et al. : MitoFinder: Efficient automated large-scale extraction of mitogenomic data in target enrichment phylogenomics. Mol Ecol Resour. 2020;20(4):892–905. 10.1111/1755-0998.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt M, Donath A, Jühling F, et al. : MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 2013;69(2):313–319. 10.1016/j.ympev.2012.08.023 [DOI] [PubMed] [Google Scholar]
- Bezděk J: A review of the Lochmaea crataegi (Forster, 1771) species group from Asia Minor, Near East and Caucasus (Coleoptera: Chrysomelidae: Galerucinae). Annales Zoologici. 2004;54(3):561–566. Reference Source [Google Scholar]
- Challis R, Richards E, Rajan J, et al. : BlobToolKit - interactive quality assessment of genome assemblies. G3 (Bethesda). 2020;10(4):1361–1374. 10.1534/g3.119.400908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, et al. : Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–175. 10.1038/s41592-020-01056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton A, Oatley G, Cornwell C, et al. : Sanger Tree of Life Sample Homogenisation: PowerMash. protocols.io. 2023a. 10.17504/protocols.io.5qpvo3r19v4o/v1 [DOI] [Google Scholar]
- Denton A, Yatsenko H, Jay J, et al. : Sanger Tree of Life Wet Laboratory Protocol Collection V.1. protocols.io. 2023b. 10.17504/protocols.io.8epv5xxy6g1b/v1 [DOI] [Google Scholar]
- Di Tommaso P, Chatzou M, Floden EW, et al. : Nextflow enables reproducible computational workflows. Nat Biotechnol. 2017;35(4):316–319. 10.1038/nbt.3820 [DOI] [PubMed] [Google Scholar]
- Forster JR: Novae species Insectorum. Londini: Centuria I. T. Davies et B. White,1771. Reference Source [Google Scholar]
- Guan D, McCarthy SA, Wood J, et al. : Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36(9):2896–2898. 10.1093/bioinformatics/btaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry E: PretextView (Paired REad TEXTure Viewer): A desktop application for viewing pretext contact maps. 2022; [Accessed 19 October 2022]. Reference Source
- Howe K, Chow W, Collins J, et al. : Significantly improving the quality of genome assemblies through curation. GigaScience. Oxford University Press,2021;10(1): giaa153. 10.1093/gigascience/giaa153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay J, Yatsenko H, Narváez-Gómez JP, et al. : Sanger Tree of Life Sample Preparation: Triage and Dissection. protocols.io. 2023. 10.17504/protocols.io.x54v9prmqg3e/v1 [DOI] [Google Scholar]
- Jolivet P, Hawkeswood TJ: Host-plants of Chrysomelidae of the World: An Essay about the Relationships between the Leaf-beetles and their Food-plants. Leiden: Backhuys Publishers,1995. Reference Source [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, et al. : HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018;19(1): 125. 10.1186/s13059-018-1486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CF: The genus Lochmaea Weise, 1883 in Taiwan: results of taxonomic expeditions by citizen scientists (Coleoptera, Chrysomelidae, Galerucinae). ZooKeys. 2019;856:75–100. 10.3897/zookeys.856.30838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, et al. : BUSCO update: Novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 2021;38(10):4647–4654. 10.1093/molbev/msab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SSP, Huntley MH, Durand NC, et al. : A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–1680. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, et al. : Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021;592(7856):737–746. 10.1038/s41586-021-03451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, et al. : Merqury: Reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1): 245. 10.1186/s13059-020-02134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin E, Sampaio F, Oatley G, et al. : Sanger Tree of Life HMW DNA Extraction: Automated MagAttract v.1. protocols.io. 2023. 10.17504/protocols.io.x54v9p2z1g3e/v1 [DOI] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, et al. : BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- Strickland M, Cornwell C, Howard C: Sanger Tree of Life Fragmented DNA clean up: Manual SPRI. protocols.io. 2023. 10.17504/protocols.io.kxygx3y1dg8j/v1 [DOI] [Google Scholar]
- Surana P, Muffato M, Qi G: sanger-tol/readmapping: sanger-tol/readmapping v1.1.0 - Hebridean Black (1.1.0). Zenodo. 2023a. 10.5281/zenodo.7755665 [DOI] [Google Scholar]
- Surana P, Muffato M, Sadasivan Baby C: sanger-tol/genomenote (v1.0.dev). Zenodo. 2023b. 10.5281/zenodo.6785935 [DOI] [Google Scholar]
- Todorovic M, Sampaio F, Howard C: Sanger Tree of Life HMW DNA Fragmentation: Diagenode Megaruptor®3 for PacBio HiFi. protocols.io. 2023. 10.17504/protocols.io.8epv5x2zjg1b/v1 [DOI] [Google Scholar]
- Uliano-Silva M, Ferreira JGRN, Krasheninnikova K, et al. : MitoHiFi: a python pipeline for mitochondrial genome assembly from PacBio high fidelity reads. BMC Bioinformatics. 2023;24(1): 288. 10.1186/s12859-023-05385-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasimuddin Md, Misra S, Li H, et al. : Efficient Architecture-Aware Acceleration of BWA-MEM for Multicore Systems.In: 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS).IEEE,2019;314–324. 10.1109/IPDPS.2019.00041 [DOI] [Google Scholar]
- Warchałowski A: Chrysomelidae. The leaf-beetles of Europe and the Mediterranean area. Warszawa: Natura Optima Dux Foundation,2003. Reference Source [Google Scholar]
- Wellcome Sanger Institute: The genome sequence of the hawthorn leaf beetle, Lochmaea crataegi (Forster, 1771). European Nucleotide Archive.[dataset], accession number PRJEB57666,2022.
- Zhou C, McCarthy SA, Durbin R: YaHS: yet another Hi-C scaffolding tool. Bioinformatics. 2023;39(1): btac808. 10.1093/bioinformatics/btac808 [DOI] [PMC free article] [PubMed] [Google Scholar]