Abstract
Background
Duchenne muscular dystrophy (DMD) is a progressive muscle condition starting in childhood, leading to severe disability and a shortened life span. It is due to severe deficiency of the protein dystrophin which performs both structural and signalling roles within skeletal and cardiac myocytes. Calcium accumulates in dystrophic muscle cells and plays a role in cell damage. It has been hypothesised that use of calcium antagonists might reduce this calcium load and its toxic effect on muscle cells. This is an updated review, in which no new trials were found.
Objectives
To evaluate the effects of calcium antagonists on muscle function and muscle strength in people with DMD.
Search methods
We searched the Cochrane Neuromuscular Disease Group Trials Register (July 2010), MEDLINE (from January 1950 to July 2010) and EMBASE (from January 1947 to July 2010). We also searched bibliographies in reports of any trials.
Selection criteria
All randomised or quasi‐randomised controlled trials of any calcium antagonist in people with DMD.
Data collection and analysis
Both authors assessed all identified trials for inclusion in the study on the basis of whether they fulfilled the selection criteria. Both authors extracted data from the trials and assessed the methodological quality. Had there been more than one trial of the same intervention and outcome of sufficient methodological quality, we had planned to undertake a meta‐analysis.
Main results
Five randomised or quasi‐randomised double‐blind trials fulfilled the selection criteria, but were not sufficiently comparable to undertake a meta‐analysis. The drugs studied were verapamil (8 participants), diltiazem (56 participants), nifedipine (105 participants) and flunarizine (27 participants). There were limitations in the description of blinding and randomisation, and definition of outcome measures. One trial, using verapamil, showed a difference between groups in muscle force measured by ergometry, but also revealed cardiac side effects. The numbers of people included in the trials were low, and so the studies may not have included enough people for sufficient power to detect small differences in muscle force or function between placebo and control groups. In addition, calcium antagonists were in an early stage of development and some of the second generation drugs that have a better side effect profile, such as amlodipine, have not been studied.
Authors' conclusions
There is no evidence to show a significant beneficial effect of calcium antagonists on muscle function in DMD.
Keywords: Humans; Calcium Channel Blockers; Calcium Channel Blockers/therapeutic use; Diltiazem; Diltiazem/therapeutic use; Flunarizine; Flunarizine/therapeutic use; Muscle Strength; Muscle Strength/drug effects; Muscle, Skeletal; Muscle, Skeletal/drug effects; Muscular Dystrophy, Duchenne; Muscular Dystrophy, Duchenne/drug therapy; Nifedipine; Nifedipine/therapeutic use; Randomized Controlled Trials as Topic; Verapamil; Verapamil/therapeutic use
Plain language summary
Calcium channel blocking drugs for Duchenne muscular dystrophy
Duchenne muscular dystrophy is a progressive wasting condition of muscles which starts in early childhood, leads to dependence on a wheelchair by the age of thirteen and respiratory failure by late teens. The condition is due to absence of dystrophin, a large muscle protein that has several functions within muscle cells. We know that calcium molecules build up in the muscle cells of people with Duchenne muscular dystrophy and this is associated with cell death. The rationale behind this review was to ascertain whether randomised controlled trials using drugs that block calcium entry into muscle would result in a reduction in progression of the condition. Although these trials were conducted over ten years ago a systematic review was not done at that time, and so a potential effect of calcium blocking drugs (antagonists) on the course of DMD may have been missed. If it were to exist, calcium antagonists might be an effective treatment in their own right or, more likely, could be used in combination with newer treatments such as corticosteroids or potential treatments such as gene related therapies.
This is an updated review. In the original review original eight studies were identified and five were high enough quality to be included. In this update no new trials were identified and so five were still included in the review. These studies were of different types of calcium channel blocking drugs and measured a variety of outcomes such as muscle strength, scales of muscle function, biochemical changes in muscle and electrocardiographic findings. Only one study showed a beneficial effect, which was an increase in muscle strength, but in this study the drug used was also associated with cardiac side effects. Adverse effects noted were mostly known side effects of calcium channel blockers. Limitations of the review were that a meta‐analysis could not be done as the trials used different calcium channel blockers and measured different outcomes, and all but one of the trials included a low number of patients. In conclusion, the review did not find calcium antagonists to have a useful effect.
Background
Duchenne muscular dystrophy (DMD) is an X‐linked recessive neuromuscular disorder, characterised by progressive muscle weakness. Presentation is usually between two and four and half years (Mohamed 2000), children become wheelchair dependent by their thirteenth birthday and the mean age for loss of ambulation being nine and half years (Van Essen 1997). In 90% of people death is directly related to chronic respiratory insufficiency (Rideau 1983). Untreated, respiratory failure leads to death by late teenage years, however, with non‐invasive ventilation, the mean age at death has increased to 25.3 years (Eagle 2002). By 14 years of age, cardiac abnormalities are detected in 30% of children and by 18 years of age, 100% have cardiomyopathy (Gilroy 1963; Nigro 1990). Symptomatic cardiac failure is rare, probably because of the severe immobility caused by the condition. Cardiomyopathy is a cause of premature death in 20% of people with DMD (Eagle 2002). Glucocorticoid corticosteroids (steroids) have been shown to improve muscle strength and function (Manzur 2008). Long‐term open studies suggest that steroid treatment preserves respiratory function (Biggar 2006) and may delay the onset of cardiomyopathy (Biggar 2006; Markham 2008). Steroids are given routinely to children with DMD from a young age (five to seven years). This form of treatment, however, has significant side effects including osteoporosis, weight gain, growth failure, cataracts, gastric ulceration, hypertension and glucose intolerance. There is a need to find alternative therapies that are effective in delaying progression of the disease.
In 1987 the gene that encodes the protein dystrophin was cloned (Koenig 1987). Dystrophin, which is absent or severely reduced in DMD (Bonilla 1988), forms part of a chain of proteins that link the actin filaments of the myofibril to the extracellular matrix. Dystrophin interacts with actin at its amino terminus and with the dystrophin associated protein complex (DAPC), which is embedded in the sarcolemma (plasma membrane of a muscle fibre), at the carboxyl terminal domain (Zubrzycka‐Gaarn 1988; Ibraghimov 1992).
Dystrophin has several roles. It serves a structural role within both skeletal and cardiac muscle cells, as its absence destabilises the DAPC and causes the sarcolemma to be susceptible to degeneration when put under mechanical stress (Moens 1993; Petrof 1993; Danialou 2001). A weakened cytoskeleton may be more prone to damage with repeated contraction and relaxation, which is a cause for continuing muscle damage. It also appears to have a signalling role in association with the DAPC, the exact nature of which is still being determined, and affects calcium homeostasis. Calcium accumulates in muscle fibres of patients with DMD (Cullen 1975; Bodensteiner 1978) and the calcium permeability of the sarcolemma is increased in dystrophin deficient muscle (Fong 1990; Vandebrouck 2002; Iwata 2003), which appears to be due to influx through mechanically activated ion channels. A study of mitogen‐activated protein (MAP) kinase signalling pathways in mdx mice muscle fibres before the onset of muscle necrosis has shown increased activation of these pathways in response to mechanical stretch and associated with increased influx of calcium ions and an increase in compliance of muscle cells (Kumar 2004). In that study pretreatment of cells with nifedipine resulted in a marginal decrease in channel activation.
Regulation of calcium in normal skeletal muscle is complex, and can be affected by several mechanisms. Intracellular accumulation of calcium is toxic to cells, causing activation of proteases and triggering of apoptosis and there is extensive evidence to demonstrate that calcium plays an important role in the aetiology of muscle damage in normal muscle and in muscle disease (Oberc 1977; Jackson 1991; Mallouk 2000).
The rationale behind use of calcium antagonists as a putative treatment for DMD is that they may reduce intracellular calcium and thus its toxic effect in the muscle cells. However the system involved is complex as calcium antagonists will have differing effects on the heart, peripheral vascular system and intracellular calcium metabolism. The accumulation of calcium within the cell is just one of many factors that occur in the chain of events that lead to muscle cell degeneration and cell death.
Calcium antagonists are a heterogeneous group of drugs that cause blockade of voltage dependent calcium channels and so may prevent accumulation of calcium in cells. There are three different classes of calcium antagonist drugs (dihydropyridine, benzodiazepine and phenylalkylamine). The dihydropyridine class (typified by nifedipine) have a predominantly vascular effect with cardiac sparing, whilst the other two classes have a significant negative inotropic effect through their action on nodal tissue. All classes can cause a reduction in myocardial oxygen demand. There are differing effects in other areas, for instance amlodipine inhibits platelet aggregation (Hernandez 1999) and has antioxidant properties (Mason 1999). There has also been debate as to whether calcium antagonists may actually increase calcium accumulation in muscle, rather than decrease it, through an effect on voltage insensitive calcium leak channels (Turner 1991). This may also cause a beneficial effect, at least in the short‐term, through potentiation of muscle force (Roed 1991), but at the expense of a toxic effect on the muscle cell. Studies of myoblast cell lines suggest reduction in intracellular calcium by verapamil, dantrolene and nifedipine, which inhibit myoblast differentiation (Porter 2002).
In vitro or cell culture studies of the action of calcium antagonists on normal muscle show that calcium antagonists inhibit protein efflux from muscle (Anand 1980). Previous in vivo studies of the functional effects of calcium antagonists using animal models of muscular dystrophy have been inconclusive (Hudecki 1984) or have not shown a protective effect on skeletal muscle (Jones 1984). In conclusion a number of previous studies indicate a protective effect of calcium antagonists on skeletal muscle, although these are far from conclusive. Therefore the purpose of this review is to evaluate the effects of calcium antagonists on muscle function and muscle force in people with DMD. This is an updated review, and no new studies have been found that add to the findings of the original review.
Objectives
The objective of this review is to evaluate the effects of calcium antagonists on muscle function and muscle force in people with Duchenne muscular dystrophy.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomised controlled trials (RCTs) and quasi‐randomised trials and reports involving the use of calcium antagonists in DMD.
Types of participants
Ambulant and non‐ambulant DMD patients without any other (neurological) disorder affecting muscle function were included.
Types of interventions
We considered use of calcium antagonists, such as verapamil and nifedipine, compared with placebo, no treatment or any other agent.
Types of outcome measures
Primary outcomes
Ambulant DMD patients: change in muscle function after six months assessed by timed functional testing; walking a certain distance as fast as possible.
Non‐ambulant DMD patients: change in forced vital capacity (FVC).
Secondary outcomes
1.Change in muscle function after six months:
climbing four standardised stairs;
rising up from the floor to a standing position without assistance.
2. Change in muscle force after six months:
quantitative change (dynamometry)
manual muscle strength testing.
3. adverse events (any adverse events noted during the treatment or follow‐up period).
We will include a summary of findings table in future updates to this review if the data allow. The summary of findings table will include the following outcomes:
Timed walk (in studies where participants are ambulant)
Change in forced vital capacity (FVC)
Timed Gower’s manoeuvre (rising from lying position on floor to a standing position, without assistance of others)
Timed get up and go (TUG) test
Any degree of heart block on ECG, as an adverse effect
Symptomatic hypotension
Change in muscle force of quadriceps, measured by dynamometry or manual muscle testing
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Disease Group Trials Register (July 2010) for reports of treatment using the following terms: 'calcium antagonists',or 'calcium channel blocker' and 'verapamil', or 'flunarizine', 'nifedipine', 'diltiazem' dantrolene, amlodipine or nicardipine and 'muscular dystrophy Duchenne'. We adapted this strategy to search MEDLINE (from January 1950 to July 2010) and EMBASE (from January 1947 to July 2010).
See Appendix 1 and Appendix 2.
Searching other resources
We checked the bibliographies in reports of the trials and contacted their authors and other experts in the field, to identify (un)published data.
Data collection and analysis
Selection of studies
Both authors independently searched the titles and abstracts retrieved by the initial search of all the databases and reference lists to identify citations with potential relevance to the review. The full text of all potentially relevant studies was obtained for assessment. Two authors, MP and RQ, decided which trials fitted the inclusion criteria and graded their methodological quality. Disagreement about inclusion criteria was resolved by discussion between the authors. The same two authors assessed the quality of trials included in the review independently.
Data extraction and management
Two authors, MP and RQ, independently performed data extraction onto a data extraction form. We would have sought full reports from authors of unpublished trials, had any been identified, or where any trials identified had been published in abstract form, presented at meetings or presented as posters. Had any missing data been identified the authors would have attempted to contact the trial authors.
Assessment of risk of bias in included studies
We independently assessed risk of bias in the included studies using the Cochrane Collaboration's risk of bias tool, described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008), addressing six risk of bias domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and 'other sources of bias'. We judged the adequacy of each study in relation to each domain, where 'Yes' indicated a low risk of bias, 'No' a high risk of bias and 'Unclear' an unclear or unknown risk of bias.
Measures of treatment effect
Had suitable data been available for analysis, we would have entered and analysed them using the Review Manager (RevMan) software. Where appropriate, we would have pooled estimates from individual studies to obtain overall estimates and 95% confidence intervals (95% CI). For dichotomous data we would have used risk ratios (RR) with 95% CI. For continuous data we would have used weighted mean differences (WMD) with 95% CI.
Assessment of heterogeneity
The possibility of heterogeneity of treatment effect differences among studies would have been investigated with appropriate tests. If evidence for heterogeneity had been found the possibility of it having arisen due to variation in study quality or patient type would have been investigated by a stratified meta‐analysis of the studies. If this did not explain the heterogeneity then we had planned to look for a weighted treatment effect using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Subgroups of interest were ambulant versus non‐ambulant DMD people.
Non‐randomised literature describing adverse events from calcium antagonists and indications of possible treatment effects was considered in the Discussion section of the full review.
Results
Description of studies
For this update a total of 114 references were identified (27 from MEDLINE, 86 from EMBASE, 1 from the Cochrane Neuromuscular Disease Group Trials Register) but no further studies were identified for closer review. Eight studies of calcium antagonists have been identified to this point. Three studies were excluded on methodological grounds because of a lack of randomisation or blinding, or both (Bertorini 1988; Garcia 1990; Bertorini 1991). These studies are described in more detail in the Table Characteristics of excluded studies. Of the five included studies, the manuscript of one study of verapamil, was obtained directly from the author (Emery 1983), another study, which used diltiazem, was written in German, (Toifl 1991) and was translated by a native German speaker. The remaining three studies suitable for inclusion used diltiazem, nifedipine and flunarizine (Dick 1986; Moxley 1987; Pernice 1988). Some of the studies were undertaken prior to the identification of dystrophin. In these studies the participants were accepted as having DMD based upon the accepted diagnostic criteria for the time. Accepted diagnostic criteria for DMD were: male, onset of weakness before five years of age, proximal weakness, serum creatine kinase at least 10 times upper limit of normal at some stage in the illness and dystrophic appearances on muscle biopsy. Exclusion criteria included the presence of ptosis, extraocular muscle weakness, skin rash suggestive of dermatomyositis, absence of dystrophic features on muscle biopsy examination, normal electromyography (EMG) or with major evidence of denervation, affected female family members.
Risk of bias in included studies
The risk of bias summary assessment for included studies is summarised in Figure 1, and further details are given in the Characteristics of included studies table. None of the trials described an intention‐to‐treat analysis. There were two trials which studied the effects of diltiazem. Unfortunately, the outcome measures were sufficiently different to prevent a meta‐analysis of the data.
1.
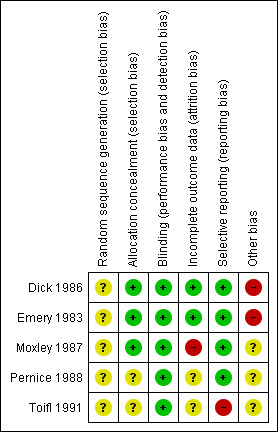
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The risk of bias varied within and across studies. The trial of flunarizine (Dick 1986) and that of verapamil (Emery 1983) both appeared to have an overall unclear risk of bias, with the domain of sequence generation being unclear, and those of allocation concealment, blinding, incomplete outcome data and selective reporting being low. A further source of bias in both was that the geographical area participants were recruited from was limited to that near the researchers. The trial of nifedipine (Moxley 1987) had a high risk of bias as there was incomplete reporting of outcomes relating to functional grades and pulmonary function tests, and numbers of those completing all the various timed tests were not given. In addition the risk of bias in sequence generation was also unclear as the best of 20 randomisations was chosen by hand. The diltiazem trial by Pernice et al. (Pernice 1988) had an unclear risk of bias, with the sequence generation and allocation concealment not being described, and with the numbers of those completing the various outcomes not being described. The other diltiazem trial (Toifl 1991) had a high risk of bias due to selective outcome reporting, with the results of electrocardiography, computerised tomography of specific muscle groups and pulmonary function tests not being given, confirmation that the complete dataset was not given for muscle strength testing; and with allocation concealment, sequence generation and blinding not being described.
Overall, the risk of bias across studies was unclear. Sequence generation was a particular area where there was a lack of clarity, as was the geographical area participants were recruited from, but these, and perhaps some of the other unclear areas, may be consistent with the reporting standards of studies in that era (1983 ‐1991). Incomplete outcome data, and selective reporting of outcome data which may have been associated with this in those studies in which it occurred may be one of the major factors that could lead to an underestimation of the effect of calcium antagonists, especially as those studies in which reporting was complete were those of smaller numbers of participants, and so may have been underpowered.
Effects of interventions
It was not possible to perform a meta‐analysis because the included studies used different agents and reported different outcomes. Their results are summarised below.
Verapamil
Emery (Emery 1983) conducted an initial open label phase study prior to a RCT, the purpose of which was to assess safety. The open part of the study comprised four participants who were given 40 mg verapamil for a few months, the precise duration of treatment not being specified, following which eight new participants aged between four and nine years were randomised into the full study. Participants were recruited on the basis of living within easy travelling distance of the hospital, being of normal intelligence, cooperative and ambulant. Allocation concealment was not fully described, but was performed by a hospital pharmacist, which may be an indication of robust allocation (Schulz 2002). The study was described as randomised and double‐blind, but the methods of randomisation and blinding were not discussed. The two groups were matched as closely as possible in terms of age, although the method of doing this was not described. Three participants were given verapamil and five placebo. The diagnosis of DMD had been established by clinical findings, serum enzyme studies, muscle biopsy demonstrating dystrophic features but not including dystrophin analysis and, in some, EMG. These methods of diagnosis would have been the acceptable at the time of the study. Verapamil was given orally at a dose of 40 mg twice daily for 12 months. Outcome measures included functional measures of strength including the Vignos score (Archibald 1959), Hammersmith Motor Ability Score (Scott 1982), muscle power in ten major muscle groups (sum of measurements on both sides of the body) as measured by both manual testing using MRC grading (MRC 1976) and ergometry; and biochemical measures including serum creatine kinase (CK), urinary creatinine and creatine excretion rates. Investigations to monitor for side effects included clinical examination, ocular slit lamp examination, electrocardiography (ECG), serum and urinary calcium. Differences between treatment and placebo groups were assessed using the Mann Whitney U test. There were no withdrawals or dropouts, and follow‐up was complete at 12 months, when the trial ended.
One of the subjects taking verapamil developed a prolonged PR interval of 0.24 seconds after 12 months. This returned to normal once the drug was stopped and two of the four subjects in the preliminary open label study developed a prolonged PR interval, one after eight months, the other after 18 months. The PR interval returned to normal after stopping the drug.
There was a significant difference between the groups as measured by muscle ergometry, with the verapamil treated group having the greater muscle power, with a rise in power in both groups initially that was attributed to a training effect. The actual values were not given, but the probabilities were. The probability of a difference in muscle ergometry values was 0.036 at 3, 6, 9 and 12 months; the probability of a difference in the Vignos score was 0.036 at 3 months and 0.072 at 6 and 12 months; and in the Hammersmith Motor Ability score was 0.142 at 6 months and 0.25 at 12 months.
Nifedipine
A RCT of nifedipine, 0.75 mg/kg/day for six months and 1.5 to 2 mg/kg/day for six months in three divided doses, was undertaken by Moxley 1987 and included 105 patients aged between three and 27 years of age attending four muscle clinics. A total of 97 subjects completed the trial. The diagnostic criteria for DMD were clearly defined and acceptable for the time of the study, however, one participant aged 27 years was considered to be unusual for DMD and could either have had an intermediate dystrophinopathy or limb girdle muscular dystrophy. The randomisation procedure was performed by a statistician and was designed to balance the groups on the basis of age, weight, average muscle strength score, treatment with placebo or L‐leucine in a previous trial, average joint contracture score and functional grade for lower limbs. Siblings received the same treatment. The power of the study to detect a difference between groups was calculated using decrease of average muscle strength over time as the primary outcome measure, and the data from a previous natural history study, with detection of a 50% difference in decline being possible with power of 90%. Except for ensuring siblings had the same treatment, a description of allocation concealment was not given. Plasma levels of nifedipine were evaluated to ensure the study drug was being taken, and whether it was reaching therapeutic levels.
The outcome measures used were described in detail in a separate publication (Brooke 1981) and included muscle strength using a modified MRC scale which employed a system of measuring against a 'reference muscle', which was the stronger of two weak predefined muscles, in upper and lower limbs and which was designated as grade 4. The scale was further modified and included subdivisions that added scores of 5‐, 4S, 4, 4W, 3+, 3‐ to the usual 0‐5 scale. Sixteen muscle groups were assessed bilaterally and 2 unilaterally (cervical flexion and extension). The muscles were described as 'individual', but appeared to be muscle groups in the methodological paper. A mean muscle score was calculated. Passive range of joint motion was measured in degrees for shoulder abduction, wrist extension and flexion, hip and knee extension, iliotibial bands and ankle dorsiflexion. An average contracture score was given which was calculated as the sum of the contracture measurement expressed as a percentage of the theoretical maximum possible contractures. Functional grades were given for hips, legs and arms, the hip and leg score was a modification of the functional mobility score used by Vignos (Archibald 1959), the arm score was a 1‐6 ordinal scale of ability to raise arms and shoulders. There were timed functional tests for travelling 30 feet, rising from supine, climbing from standard chair, cutting out a 3 x 3 inch square, with a maximum time of 120 seconds. Pulmonary function was assessed by FVC, maximum voluntary ventilation and maximum expiratory pressure. Cardiovascular tests including: pulse, standing and supine blood pressure. Laboratory tests included CK, glutamic oxalacetic transaminase (SGOT), serum glutamic pyruvate transaminase (SGPT), lactic acid dehydrogenase (LDH), complete blood count, blood urea nitrogen (BUN), creatinine, uric acid, alkaline phosphatase, glucose, total protein.
Average muscle strength was used as a primary outcome measure for evaluation of the study's power. Analysis was performed as a cross‐sectional graphical analysis of specific measurements for all patients at all visits plotted against age. Cross‐sectional measures at the start of the trial were compared with those at the end of the trial, the statistical test used was not described. Analysis was also performed by plotting scores for specific measures longitudinally for each patient and variability of that measurement over time was assessed by an unstated statistical method, except for functional tests where Student's t‐test was used. Four participants dropped out and four died of respiratory complications before study completion, with no deaths thought to be connected with nifedipine.
Side effects noted were a significantly slower pulse rate in the nifedipine group (95.8 beats per minute compared with 98.3, P < 0.02) and a higher standing systolic blood pressure. Only symptomatic side effects noted as severe were described, and these were 20 episodes in 14 patients, including shortness of breath, swelling of legs, flushing, headache and racing pulse, each only in one or two participants.
The results showed no difference in muscle strength or in rate of decline between the groups. Muscle strength was compared as a mean score, for each of the muscle groups and by a sub‐analysis on 83 patients without severe weakness. The mean difference for summated MRC score was ‐0.09 for the nifedipine group and ‐0. 20 for the placebo group at 6 months; ‐0.51 for the nifedipine group and ‐0.59 for the placebo group at completion (18 months). Confidence intervals for the difference were not given. There was no difference in contracture score, functional grades, pulmonary function tests, timed tests or biochemical tests. Values were not given for these.
Flunarizine
Dick et al. (Dick 1986) undertook a RCT of flunarizine which included 27 people aged five to fifteen years attending a muscle clinic. Selection was on the basis of living within 40 miles of the hospital and exclusion criteria included a lack of clinical or electrocardiographic evidence of cardiac disease. Randomisation was undertaken by the drug manufacturers although no methodology was given. The placebo and control groups were meant to be matched as pairs on the basis of stage of disease, age and weight, but an error led to them being matched as groups. It was stated that the assessing team were separate to the treating team to assist in blinding, and that none of the researchers were aware of the drug allocation, with the capsules appearing identical.
An estimation of the power to detect a difference in outcomes suggested the trial could have detected a 50% change in muscle score and a 25% change in myometry score if either were present.
Subjects were given either placebo or flunarizine at a dose of 5 mg on alternate days if body weight was less than 25 kg; 5 mg once daily if body weight was between 25 and 40 kg; or 5 mg twice daily if body weight was over 40 kg. The duration of treatment was 12 months. After an initial five days in hospital for observation visits were at monthly intervals for 12 months. The outcome measures used were: muscle strength as assessed by the MRC scale (MRC 1976), modified using the system of reference muscles and muscle groups described in the paper by Moxley 1987 (Moxley 1987) and with addition of grades 4+ and 4‐, so making it a 0 ‐ 7 scale; myometry in 7 muscle groups on the non‐dominant side. Muscle function using timed tasks including rising from supine to standing; run, walk or propel a wheelchair for 11 metres; climb 6 standard stairs. A functional grade for upper limbs included the same ordinal scale and adaptation of the Vignos scale used by Moxley 1987(Moxley 1987). Locomotor ability was assessed using the Hammersmith motor ability scale (Scott 1982) and contractures were measured at the shoulder, hip, knee and ankle using a goniometer. Forced vital capacity (FVC) was measured to assess respiratory function. Biochemical measures included serum calcium, alkaline phosphatase, phosphate, myoglobin and CK.
There was enquiry about side effects at each visit and monitoring of heart rate, blood pressure, ECG and weight. The ECG parameters measured were PR interval, QT interval and QRS width. The participants were divided into 13 matched pairs for analysis, on the basis of their age and initial locomotor score. Initial values for the parameters measured were compared with final values, with the initial values calculated from the mean of the two visits prior to the start of treatment, and the final values the mean of the final three visits. Paired t‐tests were used. Measurements stopped when the drugs were discontinued. There were no drop‐outs recorded but one participant was not included in the analysis as there was no participant in the other group to pair him with. Three boys could not cooperate with muscle testing or myometry due to low IQ, and only four were able to perform all the timed tests as the others were not ambulant. If one of a pair could not perform a test the other was not included. This meant there were some missing values. There were no side effects, nor electrocardiographic changes reported.
There was no significant difference on comparing measures before and after one year of treatment for impairment or functional measures. Mean difference in combined muscle MRC score was ‐0.15, 95% CI ‐0.42 to 0.11; in combined myometry score mean difference 0.52, 95% CI ‐3.35 to 4.38; in FVC ‐0.1 litres , 95% CI ‐0.21 to 0.01 litres; in Hammersmith motor ability score ‐2.11, 95% CI ‐5.0 to 0.78; in time to propel wheelchair 11 metres 9.14 seconds, 95% CI ‐4.0 to 22.3 seconds. There was a significant difference in rate of decline of functional grading in the lower limbs, with the flunarizine group declining more rapidly, P < 0.03, but this rate of decline was not recorded in the paper.
Diltiazem
Pernice et al. (Pernice 1988) studied 26 boys with DMD aged three to ten years who were given placebo or diltiazem at a dose of 5 mg/kg and for one year. Thereafter, the placebo group was also given diltiazem in an open label phase of the trial and all participants were followed for a further two years. This larger group was compared with 46 patients attending the department who did not receive diltiazem. The trial was described as randomised and double‐blind but the methods of allocation concealment, randomisation and blinding were not described.
The outcome measures included timed functional tests: climbing 18 steps, getting up five times from a chair, lifting a weight of 500 g five times and keeping arms outstretched as long as possible, and grip strength; muscle function score consisting of ordinal scales of gait, climbing stairs, arising from a chair and sitting to standing (Cornelio 1984). Cardiological assessments included ECG measuring PQ time, depth of Q amplitude and right bundle branch block, and echocardiography measuring LV ejection & shortening fraction. Computerised Tomography (CT) scans of quadriceps and gastrocnemius were undertaken to determine X ray density. Biochemical measures included: CK, Creatine kinase ‐MB (CK‐MB), glutamic oxalacetic transaminase (GOT), glutamic pyruvate transaminase (GPT), LDH, aldolase. Muscle biopsy for percentage of calcium positive muscle fibres was performed and included analysis of magnesium and calcium content measured by atomic absorption spectroscopy. There was no indication that any people dropped out. The results reported (except for side effects) were those analysed at one year for placebo versus treatment group, both n = 13.
Side effects reported included a rash in one, gingival hyperplasia in five participants after three years of therapy and lowering of blood pressure in one child. There were no ECG nor echocardiographic changes in either group. Analysis of data was undertaken using Wilcoxon signed ranks test. The results showed a lower percentage of calcium positive fibres in the muscle biopsies of the diltiazem group, but no statistical analysis was performed. There was a change in the calcium/magnesium ratio between the groups being lower in the diltiazem group, although the significance of this was not given. There was no difference in any other test, with the difference between the group mean in muscle function score being reported as 11.76 in the diltiazaem group and 11.47 in the control group, no confidence interval given. The results of timed tests were not given.
Toifl et al. (Toifl 1991) performed a randomised placebo controlled trial of diltiazem. Thirty people aged five to 18 years were given either placebo or diltiazem once daily, the dose adjusted according to body weight was: 90 mg /day for weight less than 30 kg, for body weight 30 to 40 kg the dose was 180 mg/day; for body weight 40 to 50 kg the dose was 270 mg/day and for body weights greater than 50 kg the dose was 360 mg/day. The treatment group comprised 17 people and there were 13 people in the placebo group. There was no description of selection criteria, randomisation or blinding. The duration of the trial was 12 months.
The outcome measures used were: muscle strength measured with a myometer and included: arm adductors, fist, hip flexion and hip adduction and a 0‐5 grading of 'muscle state' was devised for cervical spine, hips, upper body, knee, foot, toes, scapulae, shoulder, elbow, wrist, fingers, thumbs. There was no description of or reference provided for this 0‐5 grading. The Vignos scale (Archibald 1959) was used to assess function. Functional assessments were performed at 0, 6 and 12 months. Pulmonary function was measured by vital capacity (VC), forced expiratory volume in one second (FEV1), maximum expiratory flow at 50% of vital capacity (MEF50) at 0, 6 and 12 months and an ECG was performed every three months. A CT scan of the paraspinal muscles, gluteus, diaphragm, mid thigh was performed at 0 and 6 months. Biochemical measures performed every two months included serum CK, CKMB, myoglobin, GOT, GPT, aldolase, LDH, gamma GT, creatinine, calcium, adenosine triphosphatase, urea and electrolytes every two months. Statistical analysis was performed using the Wilcoxon signed rank test. There were some drop outs: three patients left the trial at the start, but with no reason given. One member of the placebo group died one month after the start of the study. No adverse effects were reported. There were no differences between placebo and diltiazem groups in muscle power, 'muscle state', Vignos scale, serum myoglobin or CK. The mean Vignos score before treatment was 5.82 (range 1 to 8) and after treatment was 5.88 (range 2 to 8) in the diltiazem group, 5.46 (range 2 to 9) before and 5.84 (range 3 to 9) after in the placebo group. ECG and pulmonary function results were not commented on.
Discussion
Duchenne muscular dystrophy is a severe progressive disorder resulting in muscle wasting and weakness, and leads to a significantly reduced life expectancy. Calcium plays a vital role in muscle function, as it does in many other cell types. Regulation of calcium in muscle is complex and affected by several mechanisms. Intracellular accumulation of calcium is toxic to cells and there is extensive evidence regarding the role of calcium in muscle damage in normal muscle and in muscle disease (Oberc 1977; Jackson 1991; Mallouk 2000). For this reason it seems reasonable to hypothesise that calcium antagonists might be beneficial in DMD.
Only one of five randomised controlled trials, verapamil, demonstrated a significant improvement in muscle strength but also had a high incidence of cardiac side effects (Emery 1983). Any future studies of verapamil would need to include careful and detailed monitoring of cardiac conduction and function. The remainder showed no benefit and could not be directly compared because of use of different calcium antagonists and different outcome measures. The trial by Moxley et al. (Moxley 1987) used an unpaired analysis and it may have been more appropriate to use a paired analysis so that before and after changes in individuals could be compared. Three treatment studies could not be included for methodological reasons, and these are discussed in more detail below.
The trial that showed a difference in strength used verapamil (Emery 1983) and this difference was shown in ten muscle groups, as measured by a force transducer and analysed using a paired non‐parametric analysis. However, there were limitations in the study methodology, with small groups, assessors who were not independent and with no description of blinding. There were significant side effects with reversible prolongation of PR interval in one of three boys taking verapamil in the double‐blind trial and two of four in the preliminary open label study, resulting in discontinuation of the drug.
The three studies that could not be included were those by Garcia et al.(Garcia 1990) and two by Bertorini et al. (Bertorini 1988; Bertorini 1991). Further details regarding these are in the table Characteristics of excluded studies.
The other trials did not show any differences in effectiveness for diltiazem, nifedipine and flunarizine. Diltiazem appeared to be the most promising from animal studies but those effects have not translated into humans. The study by Pernice et al. (Pernice 1988) did demonstrate an effect of diltiazem on the calcium content of muscle, but there was no evidence for clinical improvement. However there were no adverse cardiac effects noted over the three year period of the study. The use of diltiazem has also been studied in a pilot trial of patients with facioscapulohumeral muscular dystrophy (FSHD) (Elsheikh 2007) as a result of individual reports by patients that it seemed to lead to improvement. This was an open label study of 20 ambulant patients with an AB design. The primary outcome measures were an average muscle score calculated from manual muscle testing results in 39 muscle groups (MMT) and maximum voluntary isometric contraction testing performed on four muscle groups; secondary outcome measures were DEXA determined muscle mass and the time to walk 30 feet. There were no side effects, ECGs were not performed, but the risk for developing cardiomyopathy in FSHD is much lower than DMD. Analysis was paired, and the study was powered to detect a change from baseline of 0.13 MMT units. No difference in muscle strength or function was seen.
There are two case reports that give further indications of the possible side effects of calcium antagonists. One concerned acute respiratory failure following the use of intravenous verapamil (Zalman 1983) administered to a 17 year old person with DMD who presented with atrial flutter and a rapid ventricular rate (160 beats per minute). The young man had a respiratory arrest immediately after administration of 6 mg of verapamil. He was resuscitated with endotracheal intubation and the cardiac arrhythmia was terminated by cardioversion followed by intravenous procainamide to maintain sinus rhythm. Unfortunately, he could not be extubated because of reduced ventilatory effort. The procainamide was stopped one week later following some concern that it may interfere with neuromuscular transmission, there was some improvement in respiratory function, but not sufficient to allow extubation and he died one month later. The other case report (Melacini 1986) concerned a 10 year old boy who was given 60 mg twice daily of diltiazem in a therapeutic trial. Five months after starting the drug he developed retrosternal chest pain, and ECG indicated a myocardial infarction.
Two animal studies of calcium antagonists suggest that affecting calcium levels in the cell may have a beneficial effect resulting in reduced muscle degeneration. One, in the nematode Caenorhabditis elegans, investigated muscle degeneration in double mutants with a mutation of the dystrophin gene, dys‐1, and either a partial loss or gain of function allele for the major voltage gated calcium channel egl‐19 (Mariol 2001). This resulted in an increase in muscle degeneration associated with the gain of function mutation and a reduction associated with the loss of function mutation. The other study was of verapamil, diltiazem and nifedipine in BIO‐14.6 dystrophic hamsters (Johnson 1993). Outcome measures were relative soleus and cardiac hypertrophy, mortality, CK, LDH, aldolase, excessive intracellular calcium accumulation (EICA) in muscle and muscle pathology. The drugs were given for six months to different groups of ten to eleven hamsters. There was a significant reduction in EICA in the myocardium and rectus femoris in verapamil treated hamsters, but not in the other two treatments, and similarly for CK and LDH. There was a significant difference in mortality between diltiazem and verapamil treated hamsters with no fatalities in the diltiazem group but a 45% mortality in the verapamil treated group. A comparison of the age of start of treatment and the length of treatment was considered in order to compare with humans, and it was thought that this had been equivalent to starting at an age of 2.75 years and continuing for 14 years in humans.
There are three sites where variations in action of calcium antagonists influence their overall effect in muscle disease. These are their effects on skeletal muscle, neuromuscular transmission (Chang 1988; Ozkul 2007) and the cardiovascular system (Sun 1995). The differing actions on skeletal muscle and the neuromuscular junction could result in a potential difference in efficacy, if the drugs did have an influence on muscle strength or function, and could alter their side effect profile, for instance, they could significantly contribute to muscle weakness if transmission across the neuromuscular junction is affected in an individual who already has muscle weakness.
The differences in effect on the cardiovascular system would be especially important in the side effect profile of the drug, but may also affect any protective or detrimental effects on cardiac function, especially as this group of patients have the potential to develop a dilated cardiomyopathy (Gilroy 1963; Nigro 1990), and have differences in their peripheral vascular system (Miyatake 1989; Turturro 2005 ). An animal study, using verapamil in mice deficient in ß‐sarcoglycan and in mdx mice, restored normal cardiac muscle morphology and normal cardiac troponin T levels in the sarcoglycan deficient mice but not the mdx mice, which reverted when verapamil was stopped (Cohn 2001), but these results may not necessarily translate to humans because of the differences in the mdx mouse model from DMD in humans. Studies of calcium antagonists for idiopathic or non‐ischaemic dilated cardiomyopathy in people without DMD, have indicated possible protective effects for diltiazem in the diltiazem in dilated cardiomyopathy (DiDi) trial (Figulla 1996) and no adverse effects, but no overall benefit with verapamil (Neglia 2000). Using amlodipine, a second generation calcium antagonist, there is a lack of association with raised neurohormone levels and oxidative stress (Wijeysundera 2003), in contrast to first generation calcium antagonists which have negative inotropic effects causing sympathetic activation. Amlodipine was also associated with a delay in time to first hospitalisation (O'Connor 1999) and a reduction in combined risk of fatal cardiac events and admission with cardiac events (Packer 1996). Both of these findings were from the prospective randomised amlodipine survival evaluation (PRAISE) trial.
Authors' conclusions
Implications for practice.
There is no evidence from RCTs for significant benefit from calcium antagonists in DMD. However, there is evidence of harmful cardiac side effects.
Implications for research.
There is a theoretical basis for use of calcium antagonists in DMD, due to a myoprotective effect in vitro, although there remains uncertainty because of the differing effects in vivo of calcium antagonists on intracellular calcium concentration. Any possible effect may be small, but could act synergistically with other modes of treatment. Further trials should avoid diltiazem and verapamil, because of their effects on sinus node function and atrioventricular conduction and consider drugs such as amlodipine because of its possible cardioprotective effect and lack of association with sympathetic activation. They should be sufficiently powered to detect a difference in function (activity) and not just in muscle strength, and should specifically record cardiovascular parameters, such as conduction block and left ventricular function by use of ECG and echocardiography, both to detect specific side effects and to detect any cardioprotective effect. As any effect would be protective, this suggests that trials starting at a young age would show the greatest benefit. If a minor effect were shown, where clinical significance were uncertain, a combination trial with other drugs affecting outcome, such as corticosteroids, could be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 15 February 2012 | Review declared as stable | Information added to Published notes about the updating of this review. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 15 September 2010 | New search has been performed | This review has been updated, with a new search on 22/07/10, but no new trials identified. |
| 12 August 2008 | Amended | Converted to new review format. |
Notes
The conclusions of this review are unlikely to change with the addition of new information and the intervention has been superseded. T he review is therefore no longer being updated.
Acknowledgements
Professor A. Emery for providing a copy of the paper concerning his study of verapamil published in Cardiomyology. Editorial support from the Cochrane Neuromuscular Disease Group was funded by the TREAT NMD European Union Grant 036825.
Appendices
Appendix 1. MEDLINE search strategy
1. Muscular Dystrophy, Duchenne/ 2. (pseudohypertrophic muscular dystrophy or duchenne).mp. 3. (calcium antagonist$ or calcium channel blocker$).mp. 4. Calcium Channel Blockers/ 5. Verapamil.mp. 6. Flunarizine.mp. 7. Nifedipine.mp. 8. Dantrolene.mp. 9. Diltiazem.mp. 10. Nicardipine.mp. 11. Nimodipine.mp. 12. Amlodipine.mp. 13. Dihydropyridines.mp. 14. Felodipine.mp. 15. 1 or 2 16. or/3‐14 17. 15 and 16
Appendix 2. EMBASE search strategy
1. Duchenne Muscular Dystrophy/ 2. (pseudohypertrophic muscular dystrophy or duchenne).mp. 3. (calcium antagonist$ or calcium channel blocker$).mp. 4. Calcium Channel Blockers/ 5. Verapamil.mp. 6. Flunarizine.mp. 7. Nifedipine.mp. 8. Dantrolene.mp. 9. Diltiazem.mp. 10. Nicardipine.mp. 11. Nimodipine.mp. 12. Amlodipine.mp. 13. Dihydropyridines.mp. 14. Felodipine.mp. 15. 1 or 2 16. or/3‐14 17. 15 and 16
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dick 1986.
| Methods | Double‐blind parallel group design with stratified random sampling. Random allocation was by drug manufacturer, dispensed via the hospital pharmacy | |
| Participants | 27 boys with DMD aged 5 to 14 years. 12 were ambulant. Diagnosed according to high CK and muscle biopsy | |
| Interventions | Flunarizine 5 mg alternate days < 25 kg; 5 mg once daily 25 to 40 kg; 5 mg twice daily > 40 kg. In total varied between 0.1 to 0.25 mg/kg/day or placebo for 12 months | |
| Outcomes | Heart Rate; Blood Pressure; ECG; weight; Modified MRC scale (0 to 7, due to subdivision of grade 4 to ‐4, 4, +4) with system of 18 muscle groups, including 8 reference muscle groups suggested by Brooke, on dominant side; myometry in 7 muscle groups on non‐dominant side; timed tasks (rise from supine to standing; run, walk or propel a wheelchair for 11 metre; climb 6 standard stairs); functional grade for upper and lower limbs; locomotor ability score (Scott et al); FVC; serum calcium, alkaline phosphatase, phosphate, myoglobin, CK | |
| Notes | There was a retrospective allocation to 13 matched pairs on the basis of age and weight, with 1 omitted. The intention had been to do this prospectively but, due to error, they were allocated to matched groups retrospectively. Some outcomes were not measured due to low IQ and physical ability. Retrospective power calculation suggested 80% power to detect a slowing of 25% in the decline in mean muscle or myometry score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ‘Boys were then randomly allotted by the drug manufacturers to the active drug group (13 boys) or a placebo group, on the basis of the initial assessment of the stage of their disease and their age and weight’, page 350 Comment: No details given of how randomisation numbers generated |
| Allocation concealment (selection bias) | Low risk | Quote: ‘Boys were then randomly allotted by the drug manufacturers’ (same as quotation above) Comment: this suggests a central allocation, although not how allocation was communicated to the investigators |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: ‘The medical team had two parts ‐ team A ... was responsible for issuing coded prescriptions, monitoring side effects and continuing clinical care, and Team B ...was responsible only for the regular assessment...’., ’the teams operated completely independently, and their records were completely separate and were never discussed’ Comment: Probable that Team B was not unblinded as they were not assessing side effects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ‘One boy was unpaired, his results were omitted from the analysis.’ Comment: although this allowed pairs to be matched it resulted in loss of data from a relatively small sample, analysis of the two groups rather than matched pairs would have allowed this, and other data, to be used Quote: ‘The variables were not all measured in both boys of each pair; for example three boys with low intelligence were unable to cooperate with formal muscle testing or myometry....Two boys became unable to walk .. leaving only 4 ambulant pairs for ..time to run 11m, climb 6 standard stairs .. to rise from supine to standing’ Comment: these were good reasons for omissions from outcome data, although suggesting that outcome measures could have been matched more closely to the likely ability of the participants when the study was devised |
| Selective reporting (reporting bias) | Low risk | Comment: no indication of selective outcome reporting |
| Other bias | High risk | Quote: 'Those living within a radius of 40 miles were invited to take part.' Comment: this may have introduced bias because of differences in socioeconomic status and in ability to travel |
Emery 1983.
| Methods | Double‐blind parallel group with stratified random sampling | |
| Participants | 8 boys with DMD aged 4 years 8 months to 9 years 6 months. Diagnosed according to clinical findings, serum enzymes, muscle biopsy; and EMG in some | |
| Interventions | Verapamil 40 mg twice daily or placebo for 12 months | |
| Outcomes | Vignos score (0 to 10). Motor ability (0 to 40). Muscle force in 10 bilateral muscle groups by MRC scale and ergometry. CK. 24 hr creatine. 24 hr creatinine. Assessed as difference from baseline | |
| Notes | 1 in verapamil arm developed prolonged PR interval. Study preceded by a trial of verapamil with 4 participants, of whom 2 developed prolonged PR interval | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ‘..then randomly assigned ‘. Comment: does not describe how randomly assigned |
| Allocation concealment (selection bias) | Low risk | Quote: ‘then randomly assigned to either the active drug ... or placebo by a pharmacist..who was not involved in assessing the effects of the drug’ Comment: suggests a central pharmacy allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: ‘ The effects of treatment were assessed ..........without any knowledge of the results of previous assessments or of any of the biochemical findings’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome data were complete |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective reporting |
| Other bias | High risk | Quote: ‘the criteria of selection were that they should live within easily travelling distance of this hospital...’ Comment: ‘this may have introduced bias because of differences in socioeconomic status and in ability to travel |
Moxley 1987.
| Methods | Double‐blind parallel group trial with stratified random sampling on basis of age, weight, previous treatment, mean muscle strength score, contracture score, functional lower limb test. Any siblings allocated to same group. Process done by statistician using a randomisation procedure designed to balance the groups on these variables. This produced different randomisations of which the best 20 were examined by hand and one chosen as 'best' | |
| Participants | 105 participants with DMD from 4 clinics, from 3 to 27 years of age Diagnostic criteria used: male, onset of weakness before 5, presence of proximal weakness, CK at least 10 times upper limit of normal at some stage in illness. The diagnosis was further refined by excluding the following: ptosis or extraocular muscle weakness present; skin rash suggestive of dermatomyositis; sensory abnormalities; normal muscle biopsy; muscle biopsy suggestive of denervation, glycogen storage disease, lipid storage disease, congenital myopathy, inflammatory myopathy; normal EMG or EMG with major evidence of denervation; girls in family who fulfil these criteria (except that of being male) | |
| Interventions | Nifedipine 0.75 mg/kg/day in 3 doses for 6 months then 1.5 to 2 mg/kg/day for final 12 months or placebo. Checked by plasma levels ‐ where levels not as high as seen in adults and with end of dose dips noted | |
| Outcomes | Muscle strength in 34 individual muscles (cited as individual but description was of muscle groups); range of motion in shoulder abduction, wrist extension and flexion, hip and knee extension, iliotibial bands and ankle dorsiflexion (presumed by reviewers to be passive not active range of movement, but not stated); functional grade for hip, leg and arm; timed functional tests for travelling 30 feet, rising from supine, climbing from standard chair, cutting out 3 x 3 inch square; pulmonary function by forced vital capacity, maximum voluntary ventilation, maximum expiratory pressure; laboratory tests: CK, SGOT, SGPT, LDH, complete blood count, BUN, creatinine, uric acid, alkaline phosphatase, glucose, total protein | |
| Notes | Significantly lower pulse rate in nifedipine group, and higher standing systolic blood pressure. Usual side effects of nifedipine experienced. Compliance assessed as good but checked by weighing medication bottles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ‘Randomization procedure designed to balance the composition of the two groups. Over 100 randomizations into two study groups were generated by computer, and for each, a Wilk’s lambda statistic was computed to measure the balance between the two groups with respect to age, weight..., average muscle strength score, prior treatment (placebo or L‐leucine), average score for joint contractures, functional muscle test for the legs. The best 20 randomizations were examined by hand and one was finally chosen as ‘best’.’ Comment: the choosing of the best randomisation by hand may have introduced bias |
| Allocation concealment (selection bias) | Low risk | Quote: ‘Assignment of patients to placebo or nifedipine .... was performed by a statistician prior to the start of the trial’ Comment: central allocation, although no details on how communicated to investigators |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: ‘Siblings were randomised to receive the same therapeutic agent. This was to prevent comparisons between nifedipine and placebo by patients and their family members.’ and (in results) ‘the clinical evaluators did not interview the parents of patients to determine the presence of side effects. This was the responsibility of the physician investigators who were not directly involved in the measurements of performance...’ Comment: blinding probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ‘Of the 105 patients ...97 patients completed the 18 months...’ Comment: reasons for the eight who dropped out were given Quote: ‘At the start of the study, there were 14 patients in the nifedipine‐treated group and 16 patients in the placebo group who were able to stand from the supine position’ Comment: this means a very small proportion of the sample were able to perform this outcome measure. Numbers were not given for the other timed functional tests It was not confirmed that the dataset was full for any of the outcome measures. Functional grade and pulmonary function test results were commented on, but descriptive statistics not given |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Quote: ‘One hundred and five patients with DMD from four different clinics participated in ..’ Comment: no further description given regarding how these patients were ascertained from the clinics |
Pernice 1988.
| Methods | Double‐blind randomised placebo controlled study. Method of randomisation not described | |
| Participants | 26 participants with DMD aged 3 to 10 years. Diagnosed on 'usual criteria' ‐ reference given for this is same as study 3 | |
| Interventions | Diltiazem 5 mg/kg and placebo for 1 year. After 1 year the whole group was given diltiazem and followed for a further 2 years. This larger group was compared with 46 patients in the department who did not receive diltiazem | |
| Outcomes | Muscle function according to Cornelio Timed tests: 18 steps, getting up 5 times from chair, lifting weight of 500g 5 times, keeping arms outstretched as long as possible. Done bilaterally. Grip strength, bilateral. CK, CKMB, GOT, GPT, LDH, aldolase. ECG: PQ time, depth of Q amplitude and right bundle branch block. Echocardiography measuring left ventricular ejection and shortening fraction. CT of quadriceps and gastrocnemius to determine X ray density. Muscle biopsy for percentage of calcium positive muscle fibres. muscular magnesium and calcium content measured by atomic absorption spectroscopy | |
| Notes | Well tolerated ‐ rash in one, transient low BP in one, gingival hyperplasia in 5 after 3 years. No changes in echocardiography between groups or over time. No difference in ECG changes, although mean heart rate in diltiazem patients was 97 and controls 93 ‐ but was not significant. Therapy group had fewer calcium positive muscle fibres than controls. Evaluated in 7 receiving diltiazem and 6 controls. The others could not be assessed as muscle biopsies consisted of fat and connective tissue. Muscular X‐ray density was less in both groups, but the reduction appeared greater in the diltiazem group for thigh and rectus femoris, but this was not significant. The control group had higher initial rectus femoris values but not thigh values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: ‘ ...outpatient controls (context suggests that 'controls' means assessment) were carried out by two independent physicians...’. Comment: above statement and study description suggests blinding adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Numbers not confirmed for those completing outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported on |
| Other bias | Unclear risk | Comment: ascertainment not described |
Toifl 1991.
| Methods | Randomised double‐blind placebo controlled trial. No details given about randomisation or blinding | |
| Participants | 30 participants with 'Advanced DMD' (definition of advanced not given, but age range would suggest not different in nature from other studies) 5 to 18 years, not stated how chosen. 3 patients left trial at the start no reason given. 1 member of placebo group died 1 month after start | |
| Interventions | Diltiazem once daily: < 30 kg 90 mg/day; 30 to 40 kg 180 mg/day; 40 to 50 kg 270 mg/day; > 50 kg 360 mg/day in 17 patients. 13 in placebo group | |
| Outcomes | Muscle strength in arm adductors, fist, hip flexion,hip adduction by 'mechanical power measure' ‐ details of measuring device not stated. Vignos scale at baseline and 12 months. 'Muscle state' by 0‐5 grading for cervical spine, hips, upper body, knee, foot, toes, scapulae, shoulder, elbow, wrist, finger, thumbs. Tested at 0, 6 and 12/12. CK, CKMB, myoglobin, GOT, GPT, aldolase, LDH, gammaGT, Creatinine, Ca, ATPase, U&E. CT of back muscles, gluteus, diaphragm, mid thigh 0 & 12/12. VC, FEV1, MEF50 ‐ 6 &12/12. ECG per 3/12 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: not commented on except in description of study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Descriptive statistics and confirmation that dataset complete not given for muscle strength testing. Incomplete myoglobin and CPK data noted from information given in tables 3 and 4. Stated that one patient in placebo group died one month after starting trial |
| Selective reporting (reporting bias) | High risk | Comment: No results given for electrocardiography, computerised tomography performed of specific muscle groups and lung function tests |
| Other bias | Unclear risk | Comment: ascertainment not described |
Abbreviations used in table: ECG ‐ electrocardiogram, FVC ‐ forced vital capacity, CK ‐ creatine kinase, (S)GOT ‐ (serum) glutamic oxalacetic transaminase, (S)GPT ‐ (serum) glutamic pyruvate transaminase , LDH ‐ lactic acid dehydrogenase, BUN ‐ blood urea nitrogen, VC ‐ vital capacity , FEV1 ‐ forced expiratory volume in one second, MEF50 ‐ Maximum expiratory flow at 50% of vital capacity, CT ‐ computed tomography.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bertorini 1988 | There was no randomisation or quasi‐randomisation. 22 participants with DMD aged six to 18 years were paired according to age and functional activity. One of the pair received diltiazem, and one received placebo. Allocation concealment was insufficient because no provision was made for siblings to receive the same treatment. Assessments were undertaken at 0 and 32 months, and included functional assessment scales and manual muscle testing, using a similar protocol to Brooke 1981. Analysis of muscle biopsies was performed after 12 months. Deterioration of lower extremity, but not upper extremity, muscle function was significantly reduced in the treatment group compared with controls. No significant adverse effects were reported, although blood pressure increased in both control and treatment groups, but to a lesser extent in the diltiazem group |
| Bertorini 1991 | There was no randomisation or quasi‐randomisation, nor allocation concealment. Two types of design were used: one a case control design where 7 boys with DMD were treated with dantrolene and matched with 7 non treated historical controls; and the other an AB design where the 7 boys treated had a 2 year without treatment phase followed by a two year treatment phase. Dantrolene was given in a dose of 2 mg/kg increasing to 8 mg/kg as tolerated. ECG, CK, aldolase, vital signs, functional activity and manual muscle testing according to Brooke 1981 were recorded. There was no mention of blinding and, if the design was known to the investigator who performed measurements, that person would not have been blinded to treatment. One child did not tolerate the 8 mg/kg dose which resulted in a reported increase in muscle weakness, but the child did tolerate the 6 mg/kg dose; otherwise no side effects were reported. There was no significant difference in functional or manual muscle testing. There was a significant difference in CK levels when comparing the second year without treatment with the first year with treatment, and the age matched controls against the first year of treatment |
| Garcia 1990 | 17 patients were allocated to receive verapamil at a dose of 40 to 60 mg per day and 7 to receive no treatment. ages ranged from to 13 years. This was an open trial, with no randomisation or blinding of treatment. The control group did not receive a placebo but instead had no treatment. Clinical evaluation consisted of manual muscle testing by two independent evaluators. Red cell ghost protein levels were evaluated together with serum creatine kinase and LDH. The authors reported the drug to be well tolerated without side effects, but they would not have identified a prolonged PR interval had it occurred because they did not perform ECGs. No significant improvement was noted after four years of treatment |
ECG ‐ electrocardiogram, CK ‐ creatine kinase, LDH ‐ lactic acid dehydrogenase
Differences between protocol and review
RCT filter not used in MEDLINE and EMBASE searches due to few results.
Contributions of authors
MFP and RQ both performed independent searches, short‐listed studies, made a joint decision on studies to be included and excluded and wrote the review.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Dick 1986 {published data only}
- Dick D, Gardner‐Medwin D, Gates P, Gibson M, Simpson J, Walls T. A trial of flunarizine in the treatment of Duchenne Muscular Dystrophy. Muscle & Nerve 1986;9:349‐54. [DOI] [PubMed] [Google Scholar]
Emery 1983 {published data only}
- Emery A, Skinner R. Double blind controlled trial of a calcium blocker in Duchenne Muscular Dystrophy. Cardiomyology 1983;2:13‐23. [Google Scholar]
- Emery AEH, Skinner R, Howden LC, Matthews MB. Verapamil in Duchenne muscular dystrophy. Lancet 1982;319(8271):559. [DOI] [PubMed] [Google Scholar]
Moxley 1987 {published data only}
- Moxley RT, Brooke MH, Fenichel GM, Mendell J, Griggs R, Miller P, et al. Clinical investigation in Duchenne dystrophy. VI Double blind controlled trial of nifedipine. Muscle & Nerve 1987;10(1):22‐33. [DOI] [PubMed] [Google Scholar]
Pernice 1988 {published data only}
- Pernice W, Beckmann R, Ketelsen U‐P, Frey M, Schmidt‐Redemann B, Haap KP, et al. A double blind placebo controlled trial of diltiazem in Duchenne dystrophy. Klinische Wochenschrift 1988;66(13):565‐70. [DOI] [PubMed] [Google Scholar]
Toifl 1991 {published data only}
- Toifl K, Presterl E, Graninger W. Lack of effect of diltiazem in the treatment of Duchenne's muscular dystrophy: a double blind placebo controlled study [Fehlende Wirksamkeit von Diltiazem bei Duchenne‐Muskeldystrophie: eine placebo‐kontrollierte Doppelblindstudie]. Wiener klinische Wochenschrift 1991;103(8):232‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Bertorini 1988 {published data only}
- Bertorini TE, Palmieri GMA, Griffin JW, Igarishi M, McGee J, Brown R, et al. Effect of chronic treatment with calcium antagonist diltiazem in Duchenne muscular dystrophy. Neurology 1988;38(4):609‐13. [DOI] [PubMed] [Google Scholar]
Bertorini 1991 {published data only}
- Bertorini TE, Palmieri GMA, Griffin J, Igarishi M, Hinton A, Karas JG. Effect of dantrolene in Duchenne muscular dystrophy. Muscle & Nerve 1991;14(6):503‐7. [DOI] [PubMed] [Google Scholar]
Garcia 1990 {published data only}
- Garcia AM, Goldemberg AL, Fernandez H, Fortunato M, Ricci L, Trucco RE. Effect of chronic administration of verapamil in Duchenne muscular dystrophy. General Pharmacology 1990;21(6):939‐42. [DOI] [PubMed] [Google Scholar]
Additional references
Anand 1980
- Anand R, Emery AE. Calcium‐stimulated enzyme efflux from human skeletal muscle. Research communications in chemical pathology and pharmacology 1980;28(3):541‐50. [PubMed] [Google Scholar]
Archibald 1959
- Archibald KC, Vignos PJ. A study of contractures in muscular dystrophy. Archives of Physical Medicine 1959;40(4):150‐7. [PubMed] [Google Scholar]
Biggar 2006
- Biggar WD, Harris VA, Eliasoph L, Alman B. Long‐term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscular Disorders 2006;16:249‐55. [DOI] [PubMed] [Google Scholar]
Bodensteiner 1978
- Bodensteiner JB, Engel AG. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies. Neurology 1978;28(5):439‐46. [DOI] [PubMed] [Google Scholar]
Bonilla 1988
- Bonilla E, Samitt CE, Miranda AF, Hays AP, Salviati G, DiMauro S, et al. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell 1988;54(4):447‐52. [DOI] [PubMed] [Google Scholar]
Brooke 1981
- Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle & Nerve 1981;4(3):186‐97. [DOI] [PubMed] [Google Scholar]
Chang 1988
- Chang CC, Lin SO, Hong SJ, Chiou LC. Neuromuscular block by verapamil and diltiazem and inhibition of acetylcholine release. Brain Research 1988;454(1‐2):332‐9. [DOI] [PubMed] [Google Scholar]
Cohn 2001
- Cohn RD, Durbeej M, Moore SA, Coral‐Vazquez R, Prouty S, Campbell KP. Prevention of cardiomyopathy in mouse models lacking the sarcoglycan‐sarcospan complex. The Journal of Clinical Investigation 2001;107(2):153‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornelio 1984
- Cornelio F, Dworzak F, Morandi L. Therapeutic trials in Duchenne Muscular Dystrophy. Italian Journal of Neurological Sciences 1984;S3:133‐6. [DOI] [PubMed] [Google Scholar]
Cullen 1975
- Cullen MJ, Fulthorpe JJ. Stages in fibre breakdown in Duchenne muscular dystrophy: an electron‐microscopic study. Journal of the Neurological Sciences 1975;24(2):179‐200. [DOI] [PubMed] [Google Scholar]
Danialou 2001
- Danialou G, Comtois AS, Dudley R, Karpati G, Vincent G, Rosiers C, et al. Dystrophin deficient cardiomyocytes are abnormally vulnerable to mechanical stress‐induced contractile failure and injury. FASEB Journal 2001;15(9):1655‐7. [DOI] [PubMed] [Google Scholar]
Eagle 2002
- Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscular Disorders 2002;12(10):926‐9. [DOI] [PubMed] [Google Scholar]
Elsheikh 2007
- Elsheikh BH, Bollman E, Peruggia M, King W, Galloway G, Kissel JT. Pilot trial of diltiazem in facioscapulohumeral muscular dystrophy. Neurology 2007;68(17):1428‐9. [DOI] [PubMed] [Google Scholar]
Figulla 1996
- Figulla HR, Gietzen F, Zeymer U, Raiber M, Hegselmann J, Soballa R, et al. Diltiazem improves cardiac function and exercise capacity in patients with idiopathic dilated cardiomyopathy. Results of the diltiazem in dilated cardiomyopathy trial. Circulation 1996;94(3):346‐52. [DOI] [PubMed] [Google Scholar]
Fong 1990
- Fong PY, Turner PR, Denetclaw WF, Steinhardt RA. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science 1990;250(4981):673‐6. [DOI] [PubMed] [Google Scholar]
Gilroy 1963
- Gilroy J, Cahalan JL, Berman R, Newman M. Cardiac and pulmonary complications in Duchenne's progressive muscular dystrophy. Circulation 1963;27(4 Pt 1):484‐93. [DOI] [PubMed] [Google Scholar]
Hernandez 1999
- Hernandez‐Hernandez R. Effects of amlodipine and enelapril on platelet function in patients with mild to moderate hypertension. International journal of clinical pharmacology and therapeutics 1999;37(7):323‐31. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Hudecki 1984
- Hudecki MS, Pollina CM, Heffner RR. In vivo effects of three calcium blockers on chickens with inherited muscular dystrophy. Experimental Neurology 1984;84(3):512‐23. [DOI] [PubMed] [Google Scholar]
Ibraghimov 1992
- Ibraghimov‐Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin‐associated glycoproteins linking dystrophin to the extracellular matrix. Nature 1992;355(6362):696‐702. [DOI] [PubMed] [Google Scholar]
Iwata 2003
- Iwata Y, Katanosaka y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+‐permeable growth factor regulated channel. Journal of Cell Biology 2003;161(5):957‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 1991
- Jackson MJ, McArdle A, Edwards RHT. Free radicals, calcium and damage in dystrophic and normal skeletal muscle. In Calcium free radicals and tissue damage. Cambridge: Cambridge University Press, 1991:pp139‐48.. [Google Scholar]
Johnson 1993
- Johnson PL, Bhattacharya SK. Regulation of membrane‐mediated chronic muscle degeneration in dystrophic hamsters by calcium channel blockers: diltiazem, nifedipine and verapamil. Journal of the Neurological Sciences 1993;115(1):76‐90. [DOI] [PubMed] [Google Scholar]
Jones 1984
- Jones DA, Jackson MJ, McPhail G, Edwards RHT. Experimental mouse muscle damage: the importance of external calcium. Clinical Science 1984;66(3):317‐22. [DOI] [PubMed] [Google Scholar]
Koenig 1987
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987;50(3):509‐17. [DOI] [PubMed] [Google Scholar]
Kumar 2004
- Kumar A, Khandelwal N, Malya R, Reid MB. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibres. The FASEB journal 2004;18(1):102‐13. [DOI] [PubMed] [Google Scholar]
Mallouk 2000
- Mallouk N, Jacquemond V, Allard B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibres detected with Ca2+ activated K+ channels. Proceedings of the National Academy of Sciences of the United States of America 2000;97(9):4950‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manzur 2008
- Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Mariol 2001
- Mariol M‐C, Segalat L. Muscular degeneration in the absence of dystrophin is a calcium‐dependent process. Current biology 2001;11(21):1691‐4. [DOI] [PubMed] [Google Scholar]
Markham 2008
- Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe L. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscular Disorders 2008;18:365‐70. [DOI] [PubMed] [Google Scholar]
Mason 1999
- Mason RP, Walter MF, Trumbore MW, Olmstead EG jr, Mason PE. Membrane antioxidant effects of the charged dihydropyridine calcium antagonist amlodipine. Journal of molecular and cellular cardiology 1999;31(1):275‐81. [DOI] [PubMed] [Google Scholar]
Melacini 1986
- Melacini P, Fasoli G, Mammola C, Angelini C, Dalla Volta S. Cardiac complications during a trial with a calcium antagonist in a boy with Duchenne muscular dystrophy. Cardiomyology 1986;5(1):39‐47. [Google Scholar]
Miyatake 1989
- Miyatake M, Miike T, Zhao JE, Yoshioka K, Uchino M, Tuki G. Possible systemic smooth muscle layer dysfunction due to a deficiency of dystrophin in Duchenne muscular dystrophy. Journal of the Neurological Sciences 1989;93(1):11‐17. [DOI] [PubMed] [Google Scholar]
Moens 1993
- Moens P, Baatsen PH, Marechal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. Journal of Muscle Research and Cell motility 1993;14(4):446‐51. [DOI] [PubMed] [Google Scholar]
Mohamed 2000
- Mohamed K, Appleton R, Nicolaides P. Delayed diagnosis of Duchenne muscular dystrophy. European Journal of Paediatric Neurology 2000;4(5):219‐23. [DOI] [PubMed] [Google Scholar]
MRC 1976
- Medical Research Council. Aids to the investigation of peripheral nerve injuries. London: HMSO, 1976. [Google Scholar]
Neglia 2000
- Neglia D, Sambuceti G, Giorgetti A, Bartoli M, Salvadori P, Sorace O, et al. Effects of long‐term treatment with verapamil on left ventricular function and myocardial bloodflow in patients with dilated cardiomyopathy without overt heart failure. Journal of Cardiovascular Pharmacology 2000;36(6):744‐50. [DOI] [PubMed] [Google Scholar]
Nigro 1990
- Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. International Journal of Cardiology 1990;26(3):271‐7. [DOI] [PubMed] [Google Scholar]
O'Connor 1999
- O'Connor CM, Radensky PW, Unger AN, Martin BC. Hospital use and costs among patients with nonischemic cardiomyopathy in the first prospective randomized amlodipine survival evaluation study. Clinical Therapeutics 1999;21(7):1254‐65. [DOI] [PubMed] [Google Scholar]
Oberc 1977
- Oberc MA, Engel WK. Ultrastructural localization of calcium in normal and abnormal skeletal muscle. Laboratory Investigation 1977;36(6):566‐77. [PubMed] [Google Scholar]
Ozkul 2007
- Ozkul Y. Influence of calcium channel blocking drugs in neuromuscular transmission. Clinical Neurophysiology 2007;118(9):2005‐8. [DOI] [PubMed] [Google Scholar]
Packer 1996
- Packer M, O'Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. New England Journal of Medicine 1996;335(15):1107‐14. [DOI] [PubMed] [Google Scholar]
Petrof 1993
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the National Academy of Sciences of the USA 1993;90(8):3710‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porter 2002
- Porter GA, Makuck RF, Rivkees SA. Reduction in intracellular calcium levels inhibits myoblast differentiation. Journal of Biological Chemistry 2002;277(32):28942‐7. [DOI] [PubMed] [Google Scholar]
Rideau 1983
- Rideau Y. Prolongation of life in Duchenne's muscular dystrophy. Acta Neurologica 1983;5(2):118‐24. [PubMed] [Google Scholar]
Roed 1991
- Roed A. Selective potentiation of subtetanic and tetanic contractions by the calcium‐channel antagonist nifedipine in the rat diaphragm preparation. General Pharmacology 1991;22(2):313‐18. [DOI] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359(9306):614‐8. [DOI] [PubMed] [Google Scholar]
Scott 1982
- Scott OM, Hyde SA, Goddard C, Dubowitz V. Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle & Nerve 1982;5(4):291‐301. [DOI] [PubMed] [Google Scholar]
Sun 1995
- Sun J, Triggle DJ. Calcium channel antagonists: cardiovascular selectivity of action. Journal of pharmacology and experimental therapeutics 1995;274(1):419‐26. [PubMed] [Google Scholar]
Turner 1991
- Turner PR, Fong PY, Denetclaw WF, Steinhardt RA. Increased calcium influx in dystrophic muscle. Journal of Cell Biology 1991;115(6):1701‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turturro 2005
- Turturro F, Rocca B, Gumina S, Cristofaro R, Mangiola F, Maggiano N, et al. Impaired primary hemostasis with normal platelet function in Duchenne muscular dystrophy during highly‐invasive spinal surgery. Neuromuscular Disorders 2005;15(8):532‐40. [DOI] [PubMed] [Google Scholar]
Van Essen 1997
- Essen AJ, Verhij JBGM, Reefhuis J, Fidler V, Begeer JH, Visser M, et al. The natural history of Duchenne muscular dystrophy. Analysis of data from Dutch survey and review of age related events [PhD thesis]. Vol. Chapter 3, 1997:49‐68. [ISBN 90 367 0711] [Google Scholar]
Vandebrouck 2002
- Vanderbrouck C, Martin D, Colson‐Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. Journal of Cell Biology 2002;158(6):1089‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijeysundera 2003
- Wijeysundera HC, Hansen MS, Stanton E, Cropp AS, Hall C, Dhalla NS, et al. Neurohormones and oxidative stress in nonischemic cardiomyopathy: relationship to survival and the effect of treatment with amlodipine. American Heart Journal 2003;146(2):291‐7. [DOI] [PubMed] [Google Scholar]
Zalman 1983
- Zalman F, Perloff JK, Durant NN, Campion DS. Acute respiratory failure following intravenous verapamil in Duchenne's muscular dystrophy. American Heart Journal 1983;105(3):510‐11. [DOI] [PubMed] [Google Scholar]
Zubrzycka‐Gaarn 1988
- Zubrzycka‐Gaarn EE, Bulman DE, Karpati G, Burghes AH, Belfall B, Klamut HJ, et al. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature 1988;333(6172):466‐9. [DOI] [PubMed] [Google Scholar]


