Abstract
Background
Patients with primary nephrotic syndrome mostly need immunosuppression to achieve remission, but many of them either relapse after immunosuppression therapy or resistant to it. On the other hand, immunosuppression therapy could increase the adverse effect. Huangqi and Huangqi type formulations have been used to treat nephrotic syndrome for years in China, however the effects and safety of these formulations have not been systematically reviewed. This is an update of a review first published in 2008.
Objectives
To assess the benefits and harms of Huangqi and Huangqi type formulations in treating nephrotic syndrome in any age group, either as sole agents or in addition to other drug therapies.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Chinese Biomedicine Database (CBM), CNKI, VIP and reference lists of articles. There was no language restriction. Date of search: April 2011.
Selection criteria
All randomised controlled trials (RCTs) assessing the use of Huangqi or Huangqi type formulations in treating nephrotic syndrome in adults and children, either as sole agents or in addition to other drug therapies.
Data collection and analysis
Two authors independently assessed study quality and extracted data. For dichotomous outcomes results were expressed as relative risk (RR) and 95% confidence intervals (CI). Continuous outcomes were expressed as mean difference (MD) with 95% CI.
Main results
Nine studies were identified. One was judged to be at high risk of bias for random sequence, the rest were judged to be at low risk of bias. All studies had high risk of bias for allocation concealment and performance bias; unclear risk for detection bias and low risk for attrition bias. Two studies had unclear risk reporting bias and the rest had low risk. No other potential threats to validity were found. Compared to control interventions, Huangqi type formulations had a positive effect on plasma albumin (MD 6.41 g/dL, 95% Cl 4.24 to 8.59), urine albumin excretion (‐0.57 g/24 h, 95% CI ‐1.04 to ‐0.10), cholesterol (MD ‐1.70 mmol/L, 95% Cl ‐2.60 to ‐1.13) and triglycerides (‐0.33 mmol/L, 95% CI ‐0.63 to ‐0.03); and more patients showed improvement at three months (RR 0.41, 95% CI 0.20 to 0.84). There was no significant difference between Huangqi type formulations and control interventions for complete (RR 1.59, 95% CI 0.29 to 8.65) or partial remission (RR 1.22, 95% CI 0.57 to 2.58). While some formulations showed improvement in the number of patients achieving complete or partial remission, the number of studies (usually one per formulation), and the number patients (ranging from 38 to 78) were small. Relapse was reported at varying time points, ranging from three months to three years, and therefore these results were not pooled. Complications of nephrotic syndrome and adverse events were only reported by two studies; Only one study reported complications of nephrotic syndrome (infection) and another reported adverse reactions to treatment (Cushing's syndrome, steroid withdrawal syndrome, respiratory tract infection, and upper gastrointestinal haemorrhage). Both studies reported those treated with Huangqi type formulations had significantly less complications or adverse reactions.
Authors' conclusions
Huangqi and Huangqi type formulations may have some positive effects in treating nephrotic syndrome by increasing plasma albumin and reducing urine albumin excretion, blood cholesterol and triglycerides, and decreasing the number who don't show improvement at three months. Some formulations showed an increase in the number of patients achieving complete or partial remission, however study and participant numbers were small.
Keywords: Adult; Child; Humans; Phytotherapy; Albumins; Albumins/metabolism; Chemistry, Pharmaceutical; Drugs, Chinese Herbal; Drugs, Chinese Herbal/adverse effects; Drugs, Chinese Herbal/therapeutic use; Nephrotic Syndrome; Nephrotic Syndrome/blood; Nephrotic Syndrome/drug therapy; Recurrence
Plain language summary
Chinese herbal medicine Huangqi type formulations for nephrotic syndrome
Heavy proteinuria (protein in the urine), hypoalbuminaemia (low blood albumin levels), oedema (a build‐up of fluid, resulting in swelling) and hypercholesterolaemia (high blood cholesterol) are the major characteristics of nephrotic syndrome. At present, the primary drugs for nephrotic syndrome are corticosteroids, alkylating agents and cyclosporin. However there are many adverse effects associated with their use. This review identified nine studies (461 participants) comparing Huangqi type formulations with control drugs. The results of this review suggest that Huangqi type formulations may have a positive effect on nephrotic syndrome by increasing plasma albumin and reducing urine albumin excretion, blood cholesterol and triglycerides. Huangqi type formulation may reduce some adverse effects of other drugs used for treating nephrotic syndrome, however these were only reported in two studies. The methodological quality of the nine included studies was poor and was the major limitation of this review. The types of pathology, sex and age of the patients, as well as the duration and dosage of the Huangqi type formulations could not be analysed.
Background
Nephrotic syndrome is a condition that is often caused by any disease that damages the kidneys. Heavy proteinuria (> 3.5 g/d), hypoalbuminaemia (serum albumin < 2.5 g/dL), oedema and hypercholesterolaemia are the main characteristics (Cohen 2011; UMMC 2009). It can be divided into two types ‐ primary and secondary nephrotic syndrome. Primary nephrotic syndrome may occur in association with a diverse array of glomerular disorders including;
minimal change disease (MCD) responsible for about 80% of nephrotic syndrome in children, and about 20% in adults,
focal segmental glomerulosclerosis (FSGS) responsible for about 8% of nephrotic syndrome in children, and about 15% in adults,
membranous glomerulonephritis (MGN) responsible for about 1% of nephrotic syndrome in children, and about 25% in adults,
membranoproliferative glomerulonephritis (MPGN) responsible for about 5% of nephrotic syndrome in children, and about 12% in adults (UMMC 2009; Ye 2003).
Normally, proteins are restricted by a charge‐selective barrier and a size‐selective barrier (McCarthy 2012). But in nephrotic syndrome, the barriers are damaged, which let proteins leak into the urine (Cohen 2011). As a result, plasma protein and colloid osmotic pressure (COP) decrease, which result in the shift of fluid from the blood vessels into the body tissues causing oedema. Sodium retention causes a greater shift in fluid and thus an increase in oedema and occurs in some patients with nephrotic syndrome (Koomans 2003). Hypoalbuminaemia is caused not only by urinary loss of albumin but also results from increased catabolism, decreased synthesis, and increased gastrointestinal loss (Lane 2011). Hypercholesterolaemia (high levels of cholesterol, VLDL, IDL, LDL, lipoprotein (a) (Lp (a)) and triglyceride) is thought to be the consequence of both increased synthesis and decreased catabolism of lipoprotein. Abnormal function of enzymes or regulatory proteins such as lecithin‐cholesterol acyltransferase, lipoprotein lipase, and cholesteryl ester transfer protein also contribute to the hypercholesterolaemia (Doucet 2000; Saland 2002).
Immunosuppression therapy is the most important treatment for the primary nephrotic syndrome. However, many patients may relapse or resistant after the therapy. For example, while up to 90% of adults with MCD will respond to initial therapy with prednisone, approximately one‐third of these same patients will relapse within 6 months and require further immunosuppression(Palmer 2008; Waldman 2007).With diseases such as idiopathic membranous nephropathy (IMN) and FSGS, for which first‐line therapies produce substantially lower response rates than for MCD, physicians are often compelled to use second‐, third‐, and even fourth‐line therapies to achieve remission(Cattran 1999; Cattran 2007; Segarra 2009).There is no standard therapy for patients with frequent relapsing, steroid‐dependent or resistant nephrotic syndrome. Prolonged or repeated steroid therapy can lead to a variety of serious side effects. Achieving remission is an important goal that predicts an excellent long‐term prognosis (Das 2009). Antibiotics may be needed to control infections. Angiotensin converting enzyme inhibitors, diuretic medications and a low‐protein diet are also used to treat nephrotic syndrome. Treatment depends on the underlying disorder which has caused nephrotic syndrome. Though many of these drugs are effective on the treatment of primary nephrotic syndrome, they also cause many adverse effects including infection, osteoporosis, suppression of bone marrow and liver damage. All in all, the lack of efficacy and safety of existing treatment protocols make the treatment of nephrotic syndrome difficult (Meyrier 2004; Orth 1998; Ye 2003).
In China, traditional Chinese herbal medicines are commonly used in the treatment of nephrotic syndrome (Wang 2001a). Most of the physicians consider them could increase the remission rate and reduce the adverse effect. Huangqi and Huangqi type formulations have been used to treat nephrotic syndrome for years in China, such as Huangqi intravenous injection, Huangqi oral solution, Yiqibushen soup, Shenkanglin doction and Huangqi‐Danggui mixture. Huangqi with Danggui mixture could increase the synthesis of liver protein on the mRNA level, and decrease the blood lipid level (Tong 2003). Huangqi, Taizishen and Shanyao have an effect on strengthening spleen, supplementing qi, inducing diuresis for removing oedema (Lan 2005). Huangqi could improve anaemia, and also the status of water‐sodium retention (Liu 2001a). Huangqi is a one of the traditional Chinese herbal medicines. It is the dried root of Huangqi membranaceus (Fisch.) Bge. Var. mongholicus (Bge.) Hsiao or Huangqi membranaceus (Fisch.) Bge. Or Hedysarum polybotrys Hand.‐Mazz (fam. Leguminosae) (Deng 1998). Huangqi contains many active components, including calycosin 7‐O‐beta‐D‐glucoside, formononetin 7‐O‐beta‐D‐glucoside, (6 alpha R, 11 alpha R) 3‐hydroxy‐9,10‐dimethoxypterocarpan‐3‐O‐beta‐D‐glucoside, 7,2'‐dihydroxy‐3',4'‐dimethoxyisoflavan‐7‐O‐beta‐D‐glucoside, calycosin and formononetin (Wu 2005). Studies in patients and experimental animals suggest that Huangqi reduces proteinuria, hypoalbuminaemia, hyperlipidaemia and acts as a diuretic (Peng 2005). Huangqi may reduce proteinuria by:
protecting both the charge‐selective and size‐selective barrier (Bao 2003),
correct hypoalbuminaemia through promoting the transcription of albumin gene, enhancing the synthesis of albumin in the liver (Wang 2004b),
alleviate nephrotic hyperlipidaemia by up‐regulating the expression of hepatic LDL‐R gene and increasing the activities of serum LPL and LCAT (Li 2000).
As a result, degradation of VLDL and reverse transportation of cholesterol are accelerated, which is beneficial to the decrease of serum VLDL. These effects are favourable for preventing further kidney injury caused by hyperlipidaemia (Li 2000). The diuretic effect of Huangqi relieves the oedema resulting from water and sodium retention (Su 2000b; Wang 2002).
Although Huangqi and its formulations have been widely used for nephrotic syndrome in China, the effectiveness and adverse effect have not been reviewed systematically.
Objectives
To assess the benefits and harms of Huangqi and Huangqi type formulations in treating nephrotic syndrome in adults and children, either as sole agents or in addition to other drug therapies.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) assessing the use of Huangqi and Huangqi type formulations in treating nephrotic syndrome in adults and children, either as sole agents or in addition to other drug therapies. The first period of randomised crossover studies were also included.
Types of participants
Adults and children with primary nephrotic syndrome. In the absence of an explicit definition of nephrotic syndrome, the diagnosis of nephrotic syndrome in adults was based on the excretion of large amount of protein in the urine/d (> 3.5 g/24 h urine) and low serum protein (< 30 g/L), and in children with proteinuria > 3+ on dipstick, urinary protein‐creatinine ratio > 0.2 g/mmol, > 40 mg/m²/h or > 50 mg/kg/d.
Patients with secondary nephrotic syndrome were excluded as it is in the majority of cases a renal manifestation of a systemic general illness. The common causes of secondary nephrotic syndrome are diabetes mellitus, lupus erythematosus, viral infection, amyloidosis and paraproteinemias, and malignant tumours.
Types of interventions
Huangqi or Huangqi type formulations versus other drugs, formulations or placebo.
Huangqi or Huangqi type formulations in addition to other drugs versus other drugs.
Types of outcome measures
Primary outcomes
Mortality
Complete remission at three months: urine protein (nil), proteinuria ≤ 0.2 g/24 h, plasma albumin ≥ 35 g/L, normal kidney function, disappearance of all the nephrotic syndrome symptoms (e.g. oedema, hypertension)
Partial remission at three months: urine protein decreased, proteinuria < 3.0 g/24 h, improved plasma albumin, improved kidney function
Urinary protein excretion (g/24 h)
Plasma albumin
Secondary outcomes
Triglycerides
Total cholesterol
Oedema remission (days to remission)
No improvement in nephrotic syndrome at three months: urine protein unimproved, plasma albumin unimproved, nephrotic syndrome symptoms do not disappear, kidney function unchanged. Complete remission and partial remission constitute improvement.
The number and proportion of patients developing hypertension, chronic kidney disease (CKD) or end‐stage kidney disease (ESKD)
The number and proportion of patients who relapse. Relapse was defined as urine protein changing from negative to positive longer than two weeks within three months after complete remission
The duration of remission
Complications of nephrotic syndrome: infection, thrombosis, acute kidney injury
Adverse effects
Traditional Chinese Medicine (TCM) outcomes: the tongue picture, pulse picture and symptoms
Cost
Search methods for identification of studies
Electronic searches
We used the following data‐bases to search all relevant studies (Appendix 1 ‐Electronic search strategies).
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 2, 2011).
MEDLINE (June 2006 to May 2011)
EMBASE (June 2006 to May 2011)
The Chinese Biomedicine Database (CBM) (June 2006 to May 2011).
CNKI (June 2006 to May 2011)
VIP (June 2006 to May 2011)
The MEDLINE search strategy was modified as required to search other databases.
We also searched for ongoing studies in the National Research Register, Meta‐Register of Controlled Trials, Medical Research Council Clinical Trials Directory and the Cochrane Complimentary Medicine Field's trials register.
Searching other resources
We checked the references of published studies to identify additional studies. We contacted study authors to identify any unpublished papers, but were not able to contact pharmaceutical companies who produced relevant products. There was no language restriction.
Data collection and analysis
Selection of studies
The search strategy was used to obtain citations that may be relevant to the review. The titles and abstracts of all retrieved citations were screened independently by two authors who discarded citations that were not applicable. All citations for studies and reviews that might report relevant data or information on available studies were retained initially. The same two authors independently assessed retrieved abstracts and, if necessary, the full text of these studies, to determine which studies satisfied the inclusion criteria. Two authors telephoned the original authors of Chinese articles to identify the randomisation procedure and other methodological issues to insure the included studies were RCTs. If the required information was not available, the articles were added to Studies awaiting classification. Reasons for exclusion from the review were recorded to Characteristics of excluded studies. Disagreements during the study selection were resolved in consultation with a third author.
Data extraction and management
The quality of the studies included was assessed independently by two authors by means of using a piloted data extraction form. There were no disagreements. We extracted the formulation contents of the included studies (Table 1 ‐ Preparation and composition of the herbal medicines in the included studies). Where more than one publication of one study exists, reports were grouped together and the publication with the most complete data was used in the analyses.
1. Preparation and composition of the herbal medicines in the included studies.
| Study ID | Herbs (composition) | Preparation |
| Chang 2002a | Huangqi with Danggui mixture: Huangqi and Danggui |
|
| Hu 2002 | Huangqi intravenous injection: Huangqi | Produced by Chengdu Didao Jiuhong pharmaceutical factory |
| Yuan 2004 | Huangqi with Hongzao mixture: Huangqi and Hongzao | Not described in detail |
| Wang 2006a | Huangqi oral solution: Huangqi | Produced by Jiangshu Yangzi Jiang pharmaceutical factory |
| Zhou 2010 | Huangqi granules: Huangqi | Produced by Sichuan Baili pharmaceutical factory |
| Luo 2008 | Shenzongerjia soup: Huangqi, Taizishen, Fulin, fried Baishu, Shudi, Guiban, Biejia, Danshen, Chuanqiong, Fangfeng, Chantui, Jiangchan, Dilong, Yimucao, Bai maogeng | Not described in detail |
| Ai 2008 | Shenkanglin decoction: Huangqi, Shendi, Shanyu, Huaishan, Zexie, Fulin, Taizishen, Zhimu, Danshen, Shanlizhi, Xiuhuazhen | Not described in detail |
| Lin 2008 | Huangqi intravenous injection: Huangqi | Produced by Chengdu Didao Jiuhong pharmaceutical factory |
| Zou 1997 | Ci Wu Jia with Huangqi mixture: Ci Wu Jia and Huangqi | Not described in detail |
Assessment of risk of bias in included studies
For this update the following items were independently assessed by five authors using the risk of bias assessment checklist (Higgins 2011) (seeAppendix 2). Disagreements during the assessment of risk of bias in included studies were resolved by discussion between all authors.
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (mortality, relapse, complications, adverse effects, no improvement at three months, the duration of remission, the number and proportion of patients developing hypertension, CKD or ESKD), results were expressed as risk ratios (RR) and 95% confidence intervals (CI). RR and 95% CI within individual studies were calculated from the number of events and numbers of participants at risk extracted from each included study. For continuous outcomes (urine albumin excretion, triglycerides, cholesterol, plasma albumin, oedema remission), results were expressed as mean difference (MD) with 95% CI.
Data synthesis
Heterogeneity was analysed, where applicable, using a chi‐squared test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% corresponded to low, medium and high levels of heterogeneity. The sensitivity analysis was done, and there was no statistical significance between the fixed‐effects and random‐effects model in the data synthesis. So the random‐effect model in the studies was used in the study. Data for each study were analysed and expressed as RRs and mean difference. Subgroup analysis was planned to explore the heterogeneity in different interventions. We used a meta‐analysis to calculate the pooled effect size of these studies.
Results
Description of studies
Results of the search
Initial review
A total of 188 studies were retrieved that claimed to be randomised. We successfully contacted 93 study authors by telephone. Of these studies, 87 were excluded, because the study authors misunderstood true random allocation. Ninety five studies were listed in Studies awaiting classification as we could not locate the original study authors to identify the randomisation method. Six reports were identified as true RCTs. Of these, three were excluded. The data for Chang 2002b (two reports) was not in accordance with the study description, and the outcomes reported in Lin 2006 (L‐10) were not relevant to this review. Three studies were included (Chang 2002a; Hu 2002; Yuan 2004). The total number of patients randomised was 128. All the studies were conducted in China.
Review update
In this updated review, a total of 551 studies were retrieved that claimed to be randomised (Figure 1). After title and abstract screening, 320 were excluded and 233 potentially eligible studies were retrieved for further assessment. Through the full text screening, 222 studies were excluded because: the study authors misunderstood true random allocation; the articles were retrospective studies; the interventions did meet our inclusion criteria; secondary nephrotic patients were not excluded; or they were not clinical trials. Five studies are awaiting assessment (Studies awaiting classification) as we could not locate the original study authors to identify the randomisation method. Six studies were identified as true RCTs in and have been included in this update (Ai 2008; Lin 2008; Luo 2008; Wang 2006a; Zhou 2010; Zou 1997). In the original review, 95 studies were listed in Studies awaiting classification. During this update, we successfully contacted 86 study authors by telephone. Of these 86 studies, 85 studies were excluded because the study authors misunderstood true random allocation; the articles were retrospective studies; the inclusion criteria were not in accordance with our protocol; and one study was identified as a true RCT (Wang 2006a) which had already been included in this new update. A total of 14 studies are listed in Studies awaiting classification in this update. This update has identified an additional six studies (333 participants) (Ai 2008; Lin 2008; Luo 2008; Wang 2006a; Zhou 2010; Zou 1997). This brings the total number of studies included in this review to nine (461 participants). All the studies were conducted in China.
1.
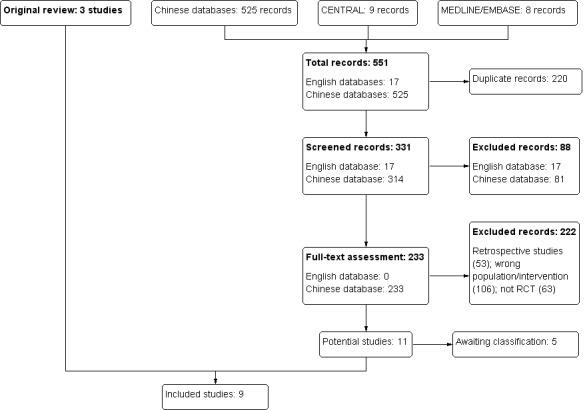
Study flow diagram.
Included studies
Study characteristics are shown in the table Characteristics of included studies.
Chang 2002a compared Huangqi‐Danggui mixture with control drugs (N = 30). The article did not describe pathology, baseline kidney function of the patients and course of disease. The control group received prednisone, dipyridamole and heparin. The experimental group received Huangqi with Danggui mixture in addition to the drugs the control group received.
Hu 2002 compared Huangqi intravenous injection with control drugs (N = 38). The article did not describe pathology, baseline kidney function of the patients and course of disease. The control group received corticosteroid, anticoagulants and diuretics. The experimental group received Huangqi intravenous injection in addition to the drugs the control group received.
Yuan 2004 compared Huangqi and Hongzao with control drugs (N = 60). The study only included patients with refractory nephrotic syndrome (mesangial proliferative glomerulonephritis). The article did not describe baseline kidney function of the patients and course of disease. The control group received prednisone and best support care. The experimental group received Huangqi with Hongzao mixture in addition to the drugs the control group received.
Ai 2008 compared Shenkanglin decoction with control drugs (N = 68). The study only included frequent relapse nephropathy of children. The average course of disease in the experiment group was 1.42 ± 0.67 years, and that in the control group was 1.32 ± 0.44 years. The article did not describe pathology and baseline kidney function of the patients. The control group received prednisone, when prednisone was inefficacy or partial efficacy, MMF or CsA was used. The experimental group received Shenkanglin decoction in addition to the drugs the control group received.
Lin 2008 compared Huangqi intravenous injection with control drugs (N = 81). The article did not describe pathology, baseline kidney function of the patients and course of disease. The control group received prednisone and anti‐inflammation therapy. The experimental group received Huangqi intravenous injection in addition to the drugs the control group received.
Luo 2008 compared Shenzongerjia soup with control drugs (N = 78). The study only included patients with refractory nephrotic syndrome. The average course of disease in the experiment group was 3.1 years, and that in the control group was 2.9 years. The article did not describe pathology and baseline kidney function of the patients. The control group received prednisone, anticoagulation therapy, low salt diet, decrease blood lipid, control blood pressure, diuretics and calcium supplement. The experimental group received Shenzongerjia soup in addition to the drugs the control group received.
Wang 2006a compared Huangqi oral solution with control drugs (N = 30). The article did not describe pathology, baseline kidney function of the patients and course of disease. The control group received prednisone and symptomatic treatment. The experimental group received Huangqi oral solution in addition to the drugs the control group received.
Zhou 2010 compared Huangqi granules with control drugs (N = 46). The article did not describe pathology or baseline declining kidney function of the patients. The control group received prednisone, symptomatic and supportive therapy. The experimental group received Huangqi granules in addition to the drugs the control group received.
Zou 1997 compared Ciwujia with Huangqi mixture with control drugs (N = 30). The article did not describe pathology or baseline declining kidney function of the patients. The control group received steroid and best support care. The experimental group received Ciwujia with Huangqi mixture in addition to the drugs the control group received.
Risk of bias in included studies
See Figure 2 and Figure 3 for summary of risk of bias assessment
2.
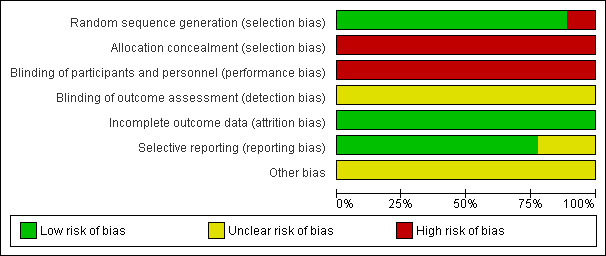
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
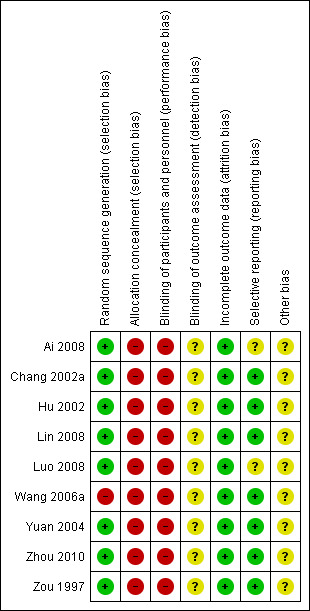
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All nine studies mentioned "randomly allocating the participants", and one study described the use of a random number table (Zhou 2010). After telephoning the other eight study authors, we determined that five used random number tables (Chang 2002a; Ai 2008; Luo 2008; Lin 2008; Zou 1997), one used computer software (Yuan 2004), one used minimised imbalance index distribution (Hu 2002), and one drew lots (Wang 2006a).
All nine studies did not provide any information about allocation concealment. After telephoning the study authors, we established all the studies had a high risk of allocation concealment (investigator knew the intervention group before eligible participants entered in the study).
Blinding
In terms of performance bias, single blinding (patients were blinded) was used in two studies (Chang 2002a; Yuan 2004). The remaining seven studies did not use blinding (Hu 2002; Ai 2008; Luo 2008; Lin 2008; Wang 2006a; Zhou 2010; Zou 1997). All studies were determined to be at high risk of bias because it was highly likely that the patients knew if they were taking Chinese herbal medicine.
Blinding of outcome assessors was not reported in any of the included studies.
Incomplete outcome data
There were no withdrawals or dropouts from any of the included studies. All data were reported.
Selective reporting
In two studies (Ai 2008; Luo 2008) not all pre‐defined outcomes were reported (complete blood count, urinary routine examination). All the clinically relevant and reasonably expected outcomes were reported in the other seven studies.
Other potential sources of bias
No other potential threats to validity were found in the nine studies.
Effects of interventions
All studies compared Huangqi type compounds versus control drugs.
Mortality
No study reported mortality.
Complete remission
There was no significant difference in the number of patients achieving complete remission between Huangqi type compounds and the control drugs (Analysis 1.1 (3 studies, 176 participants): RR 1.59, 95% Cl 0.29 to 8.65; I² = 97%) (Luo 2008; Hu 2002; Yuan 2004).
1.1. Analysis.
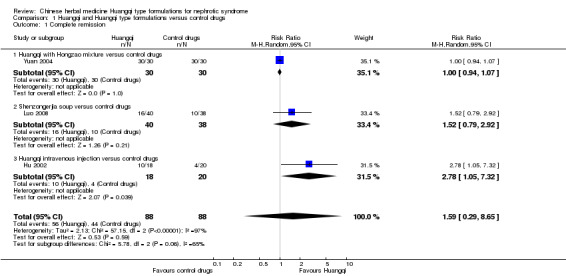
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 1 Complete remission.
Huangqi with Hongzao versus control drugs
One study reported all patients achieved complete remission in both the Huangqi with Hongzao treatment group and the control drugs group (Analysis 1.1.1 (1 study, 60 participants): RR 1.00, 95% Cl 0.94 to 1.07) (Yuan 2004).
Shenzongerjia soup versus control drugs
One study reported no significant difference in complete remission between Shenzongerjia soup and the control drugs (Analysis 1.1.2 (1 study, 78 participants): RR 1.52, 95% Cl 0.79 to 2.92) (Luo 2008).
Huangqi intravenous injection versus control drugs
One study reported Huangqi intravenous injection significantly increased the number of patients achieving complete remission compared to the control drugs (Analysis 1.1.3 (1 study, 38 participants): RR 2.78, 95% Cl 1.05 to 7.32) (Hu 2002).
Partial remission
There was no significant difference in the number of patients achieving partial remission between the Huangqi type compounds and the control drugs (Analysis 1.2 (2 studies, 116 participants): RR 1.22, 95% Cl 0.57 to 2.58; I² = 0%) (Hu 2002; Luo 2008).
1.2. Analysis.
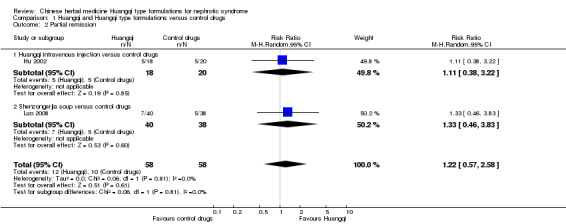
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 2 Partial remission.
Huangqi intravenous injection versus control drugs
One study reported no significant difference in the number of patients achieving partial remission between Huangqi intravenous injection and the control drugs (Analysis 1.2.1 (1 study, 38 participants): RR 1.11, 95% Cl 0.38 to 3.22) (Hu 2002).
Shenzongerjia soup versus control drugs
One study reported no significant difference in partial remission between Shenzongerjia soup and the control drugs (Analysis 1.2.2 (1 study, 78 participants): RR 1.33, 95% Cl 0.46 to 3.83) (Luo 2008).
Urinary protein excretion
Huangqi type formulations significantly decreased urinary protein excretion when compared to the control drugs (Analysis 1.3 (3 studies, 176 participants): MD ‐0.57 g/24 h, 95% CI ‐1.04 to ‐0.10; I² = 85%) (Ai 2008; Luo 2008; Wang 2006a).
1.3. Analysis.
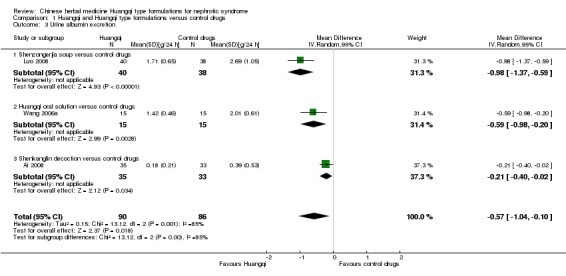
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 3 Urine albumin excretion.
Shenzongerjia soup versus control drugs
One study reported Shenzongerjia soup significantly decreased urinary protein excretion when compared to the control drugs (Analysis 1.3.1 (1 study, 78 participants): MD ‐0.98 g/24 h, 95% Cl ‐1.37 to ‐0.59) (Luo 2008).
Huangqi oral solution versus control drugs
One study reported Huangqi oral solution significantly decreased urinary protein excretion when compared to the control drugs (Analysis 1.3.2 (1 study, 30 participants): MD ‐0.59 g/24 h, 95% Cl ‐0.98 to ‐0.20) (Wang 2006a).
Shenkanglin decoction versus control drugs
One study reported Shenkanglin decoction significantly decreased urinary protein excretion when compared to the control drugs (Analysis 1.3.3 (1 study, 68 participants): MD ‐0.21 g/24 h, 95% Cl ‐0.40 to ‐0.02) (Ai 2008).
Plasma albumin
Huangqi type formulations significantly increased plasma albumin compared to the control drugs (Analysis 1.4 (5 studies, 295 participants): MD 6.41 g/dL, 95%CI 4.24 to 8.59; I² = 68%) (Ai 2008; Hu 2002; Lin 2008; Luo 2008; Wang 2006a).
1.4. Analysis.
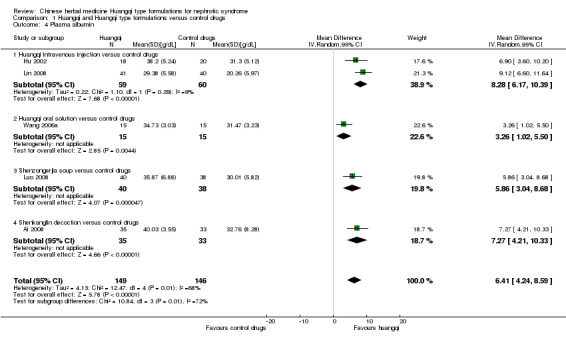
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 4 Plasma albumin.
Huangqi intravenous injection versus control drugs
Huangqi intravenous injection significantly increased plasma albumin compared to the control drugs (Analysis 1.4.1 (2 studies, 199 participants): MD 8.28 g/dL, 95% Cl 6.17 to 10.39; I² = 9%) (Hu 2002; Lin 2008).
Huangqi oral solution versus control drugs
One study reported Huangqi oral solution significantly increased plasma albumin compared to the control drugs (Analysis 1.4.2 (1 study, 30 participants): MD 3.26 g/dL, 95% Cl 1.02 to 5.50) (Wang 2006a).
Shenzongerjia soup versus control drugs
One study reported Shenzongerjia soup significantly increased plasma albumin compared to the control drugs (Analysis 1.4.3 (1 study, 78 participants): MD 5.86 g/dL, 95% Cl 3.04 to 8.68) (Luo 2008).
Shenkanglin decoction versus control drugs
One study reported Shenkanglin decoction significantly increased plasma albumin compared to the control drugs (Analysis 1.4.4 (1 study, 68 participants): MD 7.27, 95% Cl 4.21 to 10.33) (Ai 2008).
Triglycerides
Huangqi type formulations significantly decreased triglycerides compared to the control drugs (Analysis 1.5 (4 studies, 217 participants): MD ‐0.33 mmol/L, 95%CI ‐0.63 to ‐0.02; I² = 41%) (Ai 2008; Chang 2002a; Hu 2002; Lin 2008).
1.5. Analysis.
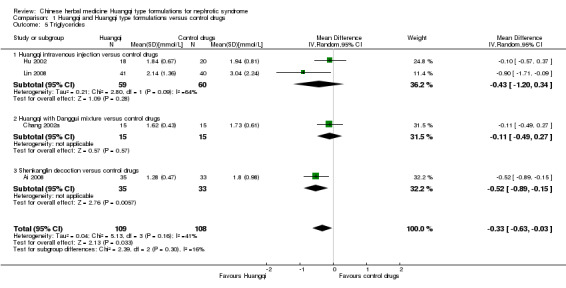
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 5 Triglycerides.
Huangqi intravenous injection versus control drugs
There was no significant difference in triglycerides between Huangqi intravenous injection and the control drugs (Analysis 1.5.1 (2 studies, 119 participants): MD ‐0.43 mmol/L, 95% Cl ‐1.20 to 0.34; I² = 64%) (Hu 2002; Lin 2008).
Huangqi with Danggui mixture versus control drugs
One study reported no significant difference in triglycerides between Huangqi with Danggui mixture and the control drugs (.Analysis 1.5.2 (1 study, 30 participants): MD ‐0.11 mmol/L, 95% Cl ‐0.49 to 0.27) (Chang 2002a).
Shenkanglin decoction versus control drugs
One study reported Shenkanglin decoction significantly decreased triglycerides when compared to control drugs (Analysis 1.5.3 (1 study, 68 participants): MD ‐0.52 mmol/L, 95% Cl ‐0.89 to ‐0.15) (Ai 2008).
Cholesterol
Huangqi type formulation significantly decreased cholesterol when compared to control drugs (Analysis 1.6 (4 studies, 217 participants): MD ‐1.70 mmol/L, 95%CI ‐2.26 to ‐1.13; I² = 43%) (Ai 2008; Chang 2002a; Hu 2002; Lin 2008).
1.6. Analysis.
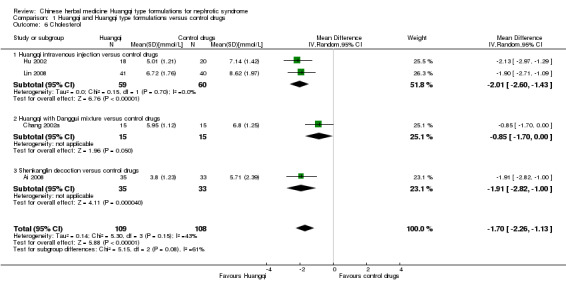
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 6 Cholesterol.
Huangqi intravenous injection versus control drugs
Huangqi intravenous injection significantly decreased cholesterol when compared to control drugs (Analysis 1.6.1 (2 studies, 119 participants): MD ‐2.01 mmol/L, 95% Cl ‐2.60 to ‐1.43; I² = 0%) (Hu 2002; Lin 2008).
Huangqi with Danggui mixture versus control drugs
One study reported a decrease in cholesterol when Huangqi with Danggui was compared to control drugs (Analysis 1.6.2 (1 study, 30 participants): MD ‐0.85 mmol/L, 95% Cl ‐1.70 to 0.00) (Chang 2002a).
Shenkanglin decoction versus control drugs
One study reported a significant decrease cholesterol when Shenkanglin decoction was compared to control drugs (Analysis 1.6.3 (1 study, 68 participants): MD ‐1.91 mmol/L, 95% Cl ‐2.82 to ‐1.00) (Ai 2008).
Oedema remission
One study reported Huangqi oral solution significantly reduced the number of days to oedema remission (Analysis 1.7.1 (1 study, 30 participants): MD ‐5.00 days, 95% Cl ‐6.62 to ‐3.38) (Wang 2006a).
1.7. Analysis.
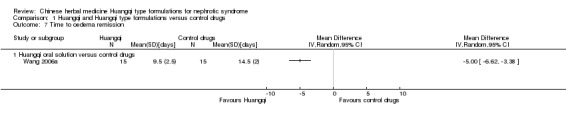
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 7 Time to oedema remission.
No improvement at three months
Huangqi type formulation significantly improved clinical and physical symptoms at three months (without complete or partial remission) when compared to control drugs (Analysis 1.8 (3 studies, 176 participants): MD ‐0.41, 95%CI ‐0.20 to ‐0.84; I² = 0%) (Hu 2002; Luo 2008; Yuan 2004).
1.8. Analysis.
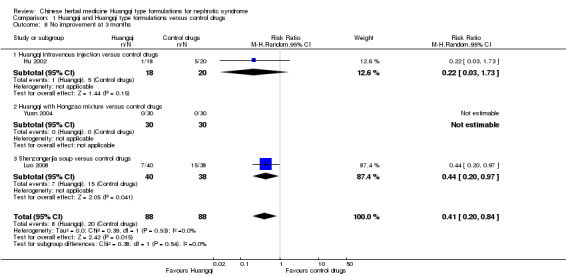
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 8 No improvement at 3 months.
Huangqi intravenous injection versus control drugs
One study reported no significant improvement in clinical and physical symptoms at three months (without complete or partial remission) when Huangqi oral solution was compared to control drugs (Analysis 1.8.1 (1 study 38 participants): RR 0.22 95% Cl 0.03 to 1.73) (Hu 2002).
Huangqi with Hongzao mixture versus control drugs
One study reported all patients achieved complete remission in both the Huangqi with Hongzao mixture group and the control drugs group (Yuan 2004).
Shenzongerjia soup versus control drugs
One study reported a significant improvement in clinical and physical symptoms at three months (without complete or partial remission) when Shenzongerjia soup was compared to control drugs (Analysis 1.8.3 (1 study, 78 participants): RR 0.44, 95% Cl 0.20 to 0.97) (Luo 2008).
Hypertension, CKD or ESKD
The number developing hypertension, CKD or ESKD were not reported.
Relapse
The studies reporting relapse were not pooled as each measured relapse at different time points.
Huangqi with Hongzao versus control drugs (12 and 24 months)
One study reported no significant difference in the number who relapsed between Huangqi with Hongzao and the control drugs at 12 months (Analysis 1.9.1 (1 study 60 participants): RR 0.75, 95% 0.18 to 3.07) and 24 months (Analysis 1.9.2 (1 study, 60 participants): RR 0.08, 95% 0.00 to 1.31) (Yuan 2004).
1.9. Analysis.
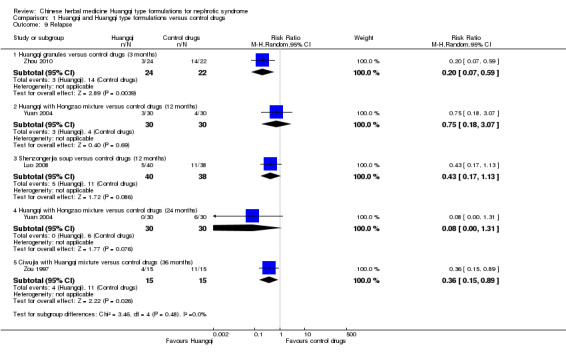
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 9 Relapse.
Huangqi granules versus control drugs (3 months)
One study reported a significant decrease in the number who relapsed when Huangqi granules were compared to control drugs at three months (Analysis 1.9.3 (1 study, 46 participants): RR 0.2, 95% Cl 0.07 to 0.59) (Zhou 2010).
Shenzongerjia soup versus control drugs (12 months)
One study reported no significant difference in relapse between Shenzongerjia soup and the control drugs at 12 months (Analysis 1.9.4 (1 study, 78 participants): RR 0.43, 95% 0.17 to 1.13) (Luo 2008).
Ciwujia with Huangqi mixture versus control drugs (36 months)
One study reported a significant decrease in the number who relapsed when Ci Wu Jia with Huangqi mixture were compared to control drugs at 36 months (Analysis 1.9.5 (1 study, 30 participants): RR 0.36, 95% Cl 0.15 to 0.89) (Zou 1997).
Complications
One study reported a significant decrease in infection when Huangqi granules was compared to control drugs (Analysis 1.10 (1 study, 46 participants): RR 0.61, 95% Cl 0.39 to 0.95) (Zhou 2010).
1.10. Analysis.

Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 10 Complications.
Adverse reactions
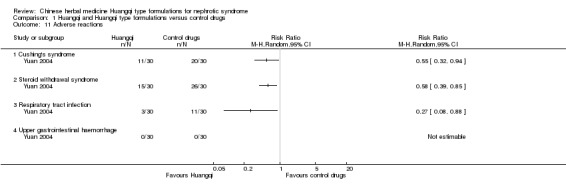
Only one study reported adverse reactions(Yuan 2004). Huangqi with Hongzao mixture significantly reduced the occurrence of Cushing's syndrome (Analysis 1.11.1 (1 study, 60 participants): RR 0.55, 95% Cl 0.32 to 0.94), steroid withdrawal syndrome (Analysis 1.11.2 (1 study, 60 participants): RR 0.58 95% Cl 0.39 to 0.85) and respiratory tract infection (Analysis 1.11.3 (1 study, 60 participants): RR 0.27, 95% Cl 0.08 to 0.88) when compared to control drugs. This study also reported that the use of Huangqi with Hongzao mixture did not increase upper gastrointestinal haemorrhage.
1.11. Analysis.
Comparison 1 Huangqi and Huangqi type formulations versus control drugs, Outcome 11 Adverse reactions.
TCM outcomes
TCM outcomes (the tongue picture, pulse picture, symptoms) were not reported.
Cost
Cost was not reported.
Discussion
Summary of main results
Based on nine studies enrolling 461 participants conducted in China, Huangqi type formulations may have a beneficial effect on increasing plasma albumin, and reducing urine albumin excretion, triglycerides and cholesterol, and increasing the number reporting improvement in clinical and physical symptoms at three months. Huangqi type formulations may also reduce the number to days to oedema remission, number of infections and the adverse reactions of other drugs. There was insufficient evidence to demonstrate if Huangqi type formulations improve complete or partial remission, or reduce or delay the number of patients who relapse. No adverse events of Huangqi type formulation were reported. However, studies of Huangqi type formulations for nephrotic syndrome lacked sufficient power to provide reliable estimates of their effectiveness and adverse effects, due to poor study design and methodological quality.
Overall completeness and applicability of evidence
We were unable to determine the benefits and harms of Huangqi and Huangqi type formulations in treating nephrotic syndrome in any age group, either as sole agents or in addition to other drug therapies. All nine studies were conducted in China. Eight studies involved patients with all types of nephrotic syndrome (Chang 2002a; Hu 2002; Zhou 2010; Ai 2008; Lin 2008; Luo 2008; Wang 2006a; Zou 1997) and did not mention the histological subtype of nephrotic syndrome; Yuan 2004 only involved patients with mesangial proliferative glomerulonephritis type nephrotic syndrome. Different histological subtype of primary nephrotic syndrome may lead to different responds to the same therapy. We could not perform any subgroup analyses to investigate this based on the limited data.
Three studies included both adults and children but none reported the number of adults or children (Chang 2002a; Hu 2002; Yuan 2004). Five studies only included children (Ai 2008; Lin 2008; Wang 2006a; Zhou 2010; Zou 1997), and Luo 2008 included only adults. Children are more likely to respond to therapy than adults, even when adults have minimal change disease, and this may have had an influence on the results. Again, we could not do subgroup analysis because of limited data.
Patient diagnoses also varied. Five studies included nephrotic syndrome patients who had not been previously treated (Chang 2002a; Hu 2002; Lin 2008; Wang 2006a; Zou 1997), and two studies (Ai 2008; Yuan 2004) only included relapsing nephrotic syndrome patients. Two studies (Luo 2008; Zhou 2010) enrolled both patients presenting for the first time with nephrotic syndrome and those with relapsing nephrotic syndrome, however data were not presented separately. The therapeutic effect may be totally different between these two groups although they received the same therapy, so this may influence the results.
Four studies (Chang 2002a; Hu 2002; Lin 2008; Zhou 2010) used Huangqi as the intervention, and five studies used Huangqi type formulation (Ai 2008; Luo 2008; Wang 2006a; Yuan 2004; Zou 1997). Complete remission (three studies), partial remission (two studies), urine albumin excretion (three studies), plasma albumin (five studies), triglycerides (four studies), cholesterol (four studies), time to oedema remission (one study), no improvement at three months (three studies), relapse (four studies), complications (one study) and adverse reactions (1 study) were reported. Mortality, number and proportion of patients developing hypertension, CKD or ESKD, the duration of remission, TCM outcome and cost were not reported. The timing of outcome measurements were not clearly reported in six studies (Chang 2002a; Hu 2002; Yuan 2004; Luo 2008; Lin 2008; Wang 2006a). Three studies reported when the outcomes were assessed, however each study measured these at different time points. Zhou 2010 evaluated the effects of therapy after one month treatment; Zou 1997 measured the immune index after six weeks treatment and evaluated relapse after three years; and Ai 2008 assessed the outcomes after three months of treatment. None of the studies reported who measured these outcomes.
The standard first line medication for nephrotic syndrome is prednisolone or prednisone. However, many patients may relapse or are resistant to therapy. While Chinese herbal medicine is not widely used in treating nephrotic syndrome patients outside China, many hospitals in China use immunosuppression combined with Chinese herbal medicine, such as Huangqi to treat nephrotic syndrome with the aim of increasing the efficacy and safety of the immunosuppression therapy. In our review, Huangqi type formulations may have some positive effects in treating nephrotic syndrome by increasing plasma albumin and reducing urine albumin excretion, blood cholesterol and triglycerides. It may also decrease the number of days to oedema remission, the number of patients who relapse, the number of patients with no improvement at three months, infection and adverse events. There were no significant differences between Huangqi type formulations and control drugs on complete or partial remission.
Quality of the evidence
The methodological quality of the included studies was poorly reported. One study described the use of a random number table to create the random sequence (Zhou 2010); one stated they used simple randomisation (Ai 2008); and seven studies did not describe random sequence generation. We telephoned the authors of these seven studies and established that they were all RCTs. Overall, six studies used a random number table (Chang 2002a; Lin 2008; Luo 2008; Zou 1997; Zhou 2010; Ai 2008), one used computer software (Yuan 2004), one used minimised imbalance index distribution (Hu 2002) and one used simple randomisation (drawing lots) (Wang 2006a). Allocation concealment and blinding were also not mentioned in any of the included studies. By telephoning the authors, we were able to confirm that no study used allocation concealment; single blinding was used in two studies (Chang 2002a; Yuan 2004) and no blinding was used in the other seven studies (Ai 2008; Hu 2002Lin 2008; Luo 2008; Wang 2006a; Zhou 2010; Zou 1997). These three characteristics may lead to selection, performance and detection bias and may result in false positive findings. All nine studies reported the outcome data of the included participants, and the risk of attrition bias was assessed to be low. Luo 2008 and Ai 2008 did not report all the pre‐defined outcomes, such as complete blood count, routine urine examination and kidney function. This may lead to both selection and reporting bias.
Potential biases in the review process
This systematic review involved a comprehensive search strategy and only included RCTs. We searched English and Chinese language databases to identify all possible RCTs. We acknowledge that there may be studies of Huangqi type formulation published in other languages, however by searching CENTRAL (which contains over 500,000 reports of studies from indexed, non‐indexed and handsearched journals and conference proceedings in many languages) we do not believe we have missed any major study. Data extraction, analysis and methodological quality assessments were performed by two or more authors. We found a large number of clinical trials investigating Huangqi type formulation for the treatment of nephrotic syndrome. We contacted authors to clarify how the study was conducted; this resulted in the exclusion of 363 reports. Most investigators misunderstood true random allocation resulting in poor methodological quality and ineligible study design.
Agreements and disagreements with other studies or reviews
Two systematic reviews investigating Huangqi or Huangqi type formulation for the treatment of nephrotic syndrome have been previously published (Li 2006f; Zhou 2009d). Li 2006f included four studies (Chen 2001a; Wu 1998b; Zhao 1999c; Zhang 1998) and concluded Buyanghuanwu soup (Huangqi, Guiwei, Chishao, Dilong, Chuanqiong, Taoren, Honghua) improved the effective rate and safety of primary nephrotic syndrome. In our review we excluded these four studies. On phoning the authors we determined that these four studies were not randomised. Zhou 2009d included 20 studies (Chen 2008b; Dai 2006; Deng 2003; Dong 2001; Kang 2005; Li 1999a; Li 2003b; Li 2007g; Lin 2007; Ning 2002; Shi 2004; Wang 1997; Wang 2001c; Wang 2002f; Wang 2006a; Wu 2005a; Xu 2000a; Yu 2001; Yu 2003; Zhang 2001c). This review concluded that Radix astragali could increase the therapeutic effect of prednisone and immunosuppression for primary nephrotic syndrome and reduce its recurrence. Radix Astragali also increased plasma albumin and decreased 24 hour proteinuria and plasma cholesterol. Again on phoning the authors of these 20 studies we excluded 19 for the same reasons as for Li 2006f ‐ the studies were not randomised. The reason for this obvious difference in study selection might be that these two review authors did not telephone the study authors to confirm study design, and in particular random sequence generation. Five studies in our review enrolled children (Ai 2008; Lin 2008; Wang 2006a; Zou 1997; Zhou 2010), however only one of the four studies published before the completion of Zhou 2009d was included (Wang 2006a). We performed a comprehensive search using a well‐defined search strategy and contacted all authors of potentially eligible studies.
Authors' conclusions
Implications for practice.
Huangqi and Huangqi type formulations may have a positive effect on nephrotic syndrome. However, limited by the small number of poorly‐designed RCTs enrolling small number of participants, there is currently insufficient evidence to support the use of Huangqi type formulations for the treatment of nephrotic syndrome.
Implications for research.
Large, properly randomised, controlled and double blind studies are needed to evaluate the effect of Huangqi type formulations. The following factors should be considered for future studies.
sample size should be calculated before commencement of the study
randomisation and allocation concealment procedures should be reported
studies should be blinded and this should be described in detail
baseline characteristics of the participants should be described in detail
the histological subtype of participants should be described in detail
the name and dose of all the drugs used in the control groups should be described in detail
outcomes should be clearly defined
the composition, dosage and course of the drugs (intervention and control) should be clearly described
describe the outcomes (methods and units of measure) and the time to measure in detail
long‐term follow‐up is needed to evaluate the benefits and harms of Huangqi type formulations.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2013 | New citation required and conclusions have changed | Six new studies added; new interventions and outcomes available |
| 18 March 2013 | New search has been performed | Review methodology updated, risk of bias has replaced quality assessment checklist |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | Contact details updated. |
| 12 May 2008 | Amended | Converted to new review format. |
Acknowledgements
We wish to thank the referees for their valuable comments and suggestions during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL | #1. MeSH descriptor Nephrotic Syndrome explode all trees #2. MeSH descriptor Nephrosis, Lipoid explode all trees #3. MeSH descriptor Glomerulonephritis, Membranous explode all trees #4. MeSH descriptor Glomerulosclerosis, Focal explode all trees #5. MeSH descriptor Glomerulonephritis, Membranoproliferative explode all trees #6. minimal change disease #7. (MCD or MPGN or FSGS) #8. nephrotic syndrome #9. lipoid nephrosis #10. membrano* and glomerul* #11. focal near glomerul* #12. minimal change glomerul* #13. (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14. MeSH descriptor Huangqi Plant explode all trees #15. MeSH descriptor Fabaceae explode all trees #16. MeSH descriptor Drugs, Chinese Herbal explode all trees #17. MeSH descriptor Phytotherapy explode all trees #18. MeSH descriptor Plants, Medicinal explode all trees #19. MeSH descriptor Plant Roots explode all trees #20. MeSH descriptor Plant Extracts explode all trees #21. astragal* #22. hedysarum polybotrys #23. huangqi or (huang next qi) #24. milkvetch or (milk next vetch) #25. (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) #26. (#13 AND #25) |
| MEDLINE | 1. Nephrotic Syndrome/ 2. Nephrosis, Lipoid/ 3. Glomerulonephritis, Membranous/ 4. Glomerulosclerosis, Focal/ 5. Glomerulonephritis, Membranoproliferative/ 6. minimal change disease.tw. 7. (MCD or MPGN or FSGS).tw. 8. nephrotic syndrome$.tw. 9. lipoid nephrosis.tw. 10. membrano$ glomerul$.tw. 11. (focal adj2 glomerul$).tw. 12. minimal change glomerul$.tw. 13. or/1‐12 14. exp Huangqi Plant/ 15. Fabaceae/ 16. Drugs, Chinese Herbal/ 17. Phytotherapy/ 18. Plants, Medicinal/ 19. Plant Roots/ 20. Plant Extracts/ 21. astragal$.tw. 22. hedysarum polybotrys.tw. 23. (huangqi or huang qi).tw. 24. (milk vetch or milkvetch).tw. 25. or/14‐24 26. 13 and 25 |
| EMBASE | 1. Nephrotic Syndrome/ 2. Lipoid Nephrosis/ 3. Membranous Glomerulonephritis/ 4. Minimal Change Glomerulonephritis/ 5. Membranoproliferative Glomerulonephritis/ 6. Focal Glomerulonephritis/ 7. minimal change disease.tw. 8. (MCD or MPGN or FSGS).tw. 9. nephrotic syndrome$.tw. 10. lipoid nephrosis.tw. 11. membrano$ glomerul$.tw. 12. (focal adj2 glomerul$).tw. 13. minimal change glomerul$.tw. 14. or/1‐13 15. Huangqi plant/ 16. Huangqi Membranaceus/ 17. Huangqi Membranaceus Extract/ 18. Huangqi mongholicus extract/ 19. Chinese Medicine/ or Herbal Medicine/ 20. Chinese Drug/ 21. Medicinal Plant/ 22. Plant Root/ 23. Plant Extract/ 24. Phytotherapy/ 25. astragal$.tw. 26. hedysarum polybotrys.tw. 27. (huangqi or huang qi).tw. 28. (milkvetch or milk vetch).tw. 29. or/15‐28 30. and/14,29 |
Appendix 2. Risk of bias checklist
Randomisation
High risk: Methods of allocation that appeared to be biased, for instance, coin tossing, sequence of seeing doctor, alternation, assignment based on date of birth, case record number and date of presentation or draw straws will be considered inadequate if it took place in front of the participants.
Unclear risk: Randomisation stated but no information on method used is available.
Low risk: Random method was described using one of the following approaches: random number tables, computer‐generated random numbers.
Allocation concealment
High risk: The randomisation number and schedule must be concealed from all care providers, ward physicians, and other research personnel before entering the study by using random number tables, computer‐generated random numbers, opaque and sealed envelopes, or similar.
Unclear risk: Did not report the method of allocation concealment.
Low risk: Concealed allocation that reported an approach that did not fall into one of the categories in high risk.
Blinding
Blinding of investigators: Yes/no/not stated
Blinding of participants: Yes/no/not stated
Blinding of outcome assessor: Yes/no/not stated
Blinding of data analysis: Yes/no/not stated
Blinding of manuscript writers: Yes/no/not stated
When considering the risk of bias from lack of blinding it is important to consider specifically:
who was and was not blinded
risk of bias in actual outcomes due to lack of blinding during the study (e.g. due to co‐intervention or differential behaviour)
risk of bias in outcome assessments (considering how subjective or objective an outcome is).
Incomplete outcome data
High risk: A difference in the proportion of incomplete outcome data across groups is of concern if the availability of outcome data is determined by the participants’ true outcomes. For example, if participants with poorer clinical outcomes are more likely to drop out due to adverse effects, and this happens mainly in the experimental group, then the effect estimate will be biased in favour of the experimental intervention.
Unclear risk: The numbers randomised into each intervention group are not clearly reported.
Low risk: To conclude that there are no missing outcome data, review authors should be confident that the participants included in the analysis are exactly those who were randomised into the trial. Participants randomised but subsequently found not to be eligible need not always be considered as having missing outcome data.
Selective outcome reporting
Low risk: Pre‐defined or clinically relevant and reasonably expected outcomes were reported.
Unclear risk: Not all pre‐defined or clinically relevant and reasonably expected outcomes were reported, or they were not reported fully, or it is unclear whether data on these outcomes were recorded.
High risk: One or more clinically relevant and reasonably expected outcomes was not reported, and the data on these outcomes were likely to have been recorded.
Other potential threats to validity
Design‐specific risks of bias
Early stopping
Baseline imbalance
Blocked randomisation in unblinded trials
Differential diagnostic activity
others: The conduct of the study is affected by interim results. There is deviation from the study protocol in a way that does not reflect clinical practice. There is pre‐randomization administration of an intervention that could enhance or diminish the effect of a subsequent, randomised, intervention. Inappropriate administration of an intervention. Occurrence of ‘null bias’ due to interventions being insufficiently well delivered or overly wide inclusion criteria for participants. An insensitive instrument is used to measure outcomes. Inappropriate influence of funders.
Data and analyses
Comparison 1. Huangqi and Huangqi type formulations versus control drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 3 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.29, 8.65] |
| 1.1 Huangqi with Hongzao mixture versus control drugs | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.94, 1.07] |
| 1.2 Shenzongerjia soup versus control drugs | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.79, 2.92] |
| 1.3 Huangqi intravenous injection versus control drugs | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.05, 7.32] |
| 2 Partial remission | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.57, 2.58] |
| 2.1 Huangqi intravenous injection versus control drugs | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.38, 3.22] |
| 2.2 Shenzongerjia soup versus control drugs | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.46, 3.83] |
| 3 Urine albumin excretion | 3 | 176 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.04, ‐0.10] |
| 3.1 Shenzongerjia soup versus control drugs | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.37, ‐0.59] |
| 3.2 Huangqi oral solution versus control drugs | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.98, ‐0.20] |
| 3.3 Shenkanglin decoction versus control drugs | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.02] |
| 4 Plasma albumin | 5 | 295 | Mean Difference (IV, Random, 95% CI) | 6.41 [4.24, 8.59] |
| 4.1 Huangqi intravenous injection versus control drugs | 2 | 119 | Mean Difference (IV, Random, 95% CI) | 8.28 [6.17, 10.39] |
| 4.2 Huangqi oral solution versus control drugs | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 3.26 [1.02, 5.50] |
| 4.3 Shenzongerjia soup versus control drugs | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 5.86 [3.04, 8.68] |
| 4.4 Shenkanglin decoction versus control drugs | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 7.27 [4.21, 10.33] |
| 5 Triglycerides | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.63, ‐0.03] |
| 5.1 Huangqi intravenous injection versus control drugs | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.20, 0.34] |
| 5.2 Huangqi with Danggui mixture versus control drugs | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.49, 0.27] |
| 5.3 Shenkanglin decoction versus control drugs | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.89, ‐0.15] |
| 6 Cholesterol | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.26, ‐1.13] |
| 6.1 Huangqi intravenous injection versus control drugs | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐2.60, ‐1.43] |
| 6.2 Huangqi with Danggui mixture versus control drugs | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.70, ‐0.00] |
| 6.3 Shenkanglin decoction versus control drugs | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐2.82, 1.00] |
| 7 Time to oedema remission | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Huangqi oral solution versus control drugs | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 No improvement at 3 months | 3 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.20, 0.84] |
| 8.1 Huangqi intravenous injection versus control drugs | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.73] |
| 8.2 Huangqi with Hongzao mixture versus control drugs | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Shenzongerjia soup versus control drugs | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.20, 0.97] |
| 9 Relapse | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Huangqi granules versus control drugs (3 months) | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.59] |
| 9.2 Huangqi with Hongzao mixture versus control drugs (12 months) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.18, 3.07] |
| 9.3 Shenzongerjia soup versus control drugs (12 months) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.13] |
| 9.4 Huangqi with Hongzao mixture versus control drugs (24 months) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.31] |
| 9.5 Ciwujia with Huangqi mixture versus control drugs (36 months) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.15, 0.89] |
| 10 Complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Adverse reactions | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.1 Cushing's syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Steroid withdrawal syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Respiratory tract infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Upper gastrointestinal haemorrhage | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ai 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We interviewed the author by telephone, a random number table was used for generating the allocation sequence |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. Investigator knew the intervention group before eligible participants entered in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not used in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data for all 68 patients were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐defined outcomes were reported, such as complete blood count, routine urine exam and kidney function. |
| Other bias | Unclear risk | The study did not show any interest. |
Chang 2002a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We interviewed the author by telephone, a random number table was used for generating the allocation sequence |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. Investigator knew the intervention group before eligible participants entered in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Simple blinding was used, but the doctors and data analysts knew who took the Huangqi‐Danggui mixture. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data for all 30 patients were reported. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | The study did not show any interest. |
Hu 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We interviewed the author by telephone, they used simple randomisation. |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. Investigator knew the intervention group before eligible participants entered in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not used in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data for all 38 patients were reported. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | The study did not show any interest. |
Lin 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We interviewed the author by telephone, a random number table was used for generating the allocation sequence |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. Investigator knew the intervention group before eligible participants entered in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not used in the study. The patients in control group and doctors knew they did not receive the Huangqi injection. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data for all 81 patients were reported. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | The study did not show any interest. |
Luo 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We interviewed the author by telephone, a random number table was used for generating the allocation sequence. |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. Investigator knew the intervention group before eligible participants entered in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not used in the study. The patients in control group and doctors knew they did not receive the traditional Chinese medicine. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data for all 78 patients were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐defined outcomes were reported, such as complete blood count and routine urine exam. |
| Other bias | Unclear risk | The study did not show any interest. |
Wang 2006a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | We interviewed the author by telephone, drawing lots was used for generating the allocation sequence. |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. Investigator knew the intervention group before eligible participants entered in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not used in the study. The patients in control group and doctors knew they did not receive the traditional Chinese medicine. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data all 30 patients were reported. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | The study did not show any other bias. |
Yuan 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We interviewed the author by telephone, computer generated random numbers sequence was used. |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. Investigator knew the intervention group before eligible participants entered in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Simple blinding was used, but the doctors and data analysts knew who took the Huangqi (25 g) and 15 g Hongzao. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data all 60 patients were reported. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | The study did not show any interest. |
Zhou 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers sequence was used. |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. The participants involved in the clinical trial could not know the sequence before the patients entering in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not used in the study. The patients in control group and doctors knew they did not take the Huangqi granules. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data all 46 patients were reported. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | The study did not show any interest. |
Zou 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers sequence was used. |
| Allocation concealment (selection bias) | High risk | The random number table was produced by themselves. Investigator knew the intervention group before eligible participants entered in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not used in the study. The patients in control group and doctors knew they did not receive the traditional Chinese medicine. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data all 30 patients were reported. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | The study did not show any interest. |
NS: not stated
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bai 2004 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0564‐3338406 |
| Bai 2010 | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 13700201192 |
| Bie 2006 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0898‐65343033 |
| Cao 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 15633142939 |
| Chang 2002b | The data is not in accord with the study description. |
| Chen 1998 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0917‐8957075 |
| Chen 1999 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. |
| Chen 2001a | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0539‐2254441 |
| Chen 2001b | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0396‐5039693 |
| Chen 2001c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 13305521186 |
| Chen 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0731‐4327001 |
| Chen 2003 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0595‐22771677 |
| Chen 2004 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order of the patients came to hospital, odd number to one group and even number to another. Telephone number: +86 0731‐4327001 |
| Chen 2005 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0595‐86869356 |
| Chen 2006a | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0662‐3527122 |
| Chen 2006b | The patients in the treatment group did not receive the Huangqi. |
| Chen 2006c | The patients in the treatment group received the danshen injection, but the control group did not receive. |
| Chen 2007 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Chen 2008a | The article is the review, not the RCT. |
| Chen 2008b | The patients were allocated into two groups according to the medical order. |
| Chen 2009a | The secondary nephrotic syndrome patients were not excluded in the study. |
| Chen 2009b | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 0738‐5212590 |
| Chen 2010a | The treatment group did not use the Huangqi. |
| Chen 2010b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Chen 2010c | The patients in the treatment group receive the dexamethasone, but the control group did not receive it. The intervention was not in accord with the criteria. |
| Chen 2010d | We telephoned the author in June 2011.
The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 023-68755114 |
| Chen 2011 | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical data. Telephone number: +86 0319‐2233383. |
| Cheng 2004 | The patients in the treatment group did not receive the Huangqi. |
| Dai 2006 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0591‐83947272 |
| Deng 2003 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 020‐81048142 |
| Deng 2008 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0745‐7622223 |
| Ding 2008 | The patients were allocated into two groups according to the medical order. |
| Dong 2001 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0551‐2237306 |
| Dong 2005 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 0432‐2166109 |
| Dong 2009 | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical data. Telephone number: +86 0731‐84917863. |
| Du 2006a | The treatment group used both Huangqi and danshen injection, and the control group did not use the danshen injection. |
| Du 2006b | The patients in the treatment group receive the Danshen injection. |
| Duan 2006 | The patients were allocated into two groups according to the medical order. |
| Fan 2001 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 13103309866 |
| Feng 2007 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Feng 2010 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0710‐3440874 |
| Fu 2008 | The patients in the treatment group did not receive the Huangqi. |
| Gao 2001 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups as the patients wish. Telephone number: +86 0718‐8295122 |
| Gao 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0912‐5641830 |
| Gong 2007 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 0519‐88104931 |
| Gong 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0633‐2252750 |
| Gong 2010 | The study was a retrospective study. The author summarized the past medical records. |
| Gu 1998 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author numbered the patients, odd numbers were assigned to one group and even numbers to the other. Telephone number: +86 010‐66385849 |
| Guo 1997 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0471‐6921203 |
| Guo 1999 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0571‐85827888 |
| Guo 2006 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0760‐8802802 |
| Guo 2008a | The patients were allocated into the two groups according to the medical order. |
| Guo 2008b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Guo 2010 | We telephoned the author in June 2011. The study was retrospective study. The author summarized the past medical records. Telephone number: +86 0827‐7330108 |
| Hao 2007 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 022‐27432299 |
| He 2008 | The intervention was not in accord with the criteria. |
| He 2010 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Hong 2001 | The study was not a double‐blind RCT. The author divided patients into groups using random sampling. |
| Hu 2005 | The patients did not receive the Huangqi in the treatment group. |
| Hu 2006a | The patients in the treatment group did not receive the Huangqi. |
| Hu 2006b | The study was not a RCT, the patients were not divided into two groups randomly. |
| Hu 2009 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Hua 2010 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Huang 2004a | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 020‐81048142 |
| Huang 2004b | The patients in the treatment group did not receive the Huangqi formulation. |
| Huang 2006 | The patients in the treatment group did not receive the Huangqi. |
| Huang 2007 | The patients in the treatment group did not receive the Huangqi. |
| Huang 2009a | The patients were allocated into two groups according to the medical order. |
| Huang 2009b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Huang 2010 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Ji 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0370‐2701366 |
| Jia 2010 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0313‐7219062. |
| Jiang 1997 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the pathogenetic condition. Telephone number: +86 0519‐8132511 |
| Jiang 2001a | We telephoned the author in August 2006 in order to identify the randomisation procedure and other methodological issues, but the author refused further information. We couldn't determine whether it is an RCT. Telephone number: +86 0451‐53740549 |
| Jiang 2001b | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 023‐85381656 |
| Jiang 2001c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0535‐6691999 |
| Jiang 2008a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 13038228715 |
| Jiang 2008b | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 0459‐5805065 |
| Jin 2001 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0377‐ 63200081 |
| Jin 2007 | The study did not exclude the secondary nephrotic syndrome patients. |
| Kang 2005 | The secondary nephrotic syndrome patients were not excluded in the study. |
| Kong 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 010‐69314902 |
| Lai 2006 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0798‐8417496. |
| Lan 2005 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the patients' opinion. Telephone number: +86 13028500622 |
| Lan 2007 | The study did not exclude the secondary nephrotic syndrome patients. |
| Lei 2006 | The patients in the treatment group received the losartan, but the patients in the control group did not receive it. |
| Li 1988 | The study was a case report. |
| Li 1994 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the pathogenetic condition. Telephone number: +86 0431‐86816453 |
| Li 1997 | The study was not a RCT, there was no control group. |
| Li 1999a | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the pathogenetic condition. Telephone number: +86 0543‐3201274 |
| Li 1999b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 13679015009 |
| Li 2003a | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0411‐83168514 |
| Li 2003b | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 0932‐6622418 |
| Li 2004a | We telephoned the author in June 2011. The study was a retrospective study. Telephone number: +86 0734‐8279011 |
| Li 2004b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0392‐7256007 |
| Li 2005 | The patients in the treatment group received the lower molecular heparin, but the patients in the control group did not receive it. |
| Li 2006a | The patients in the treatment group did not receive the Huangqi. |
| Li 2006b | The patients in the treatment group did not receive the Huangqi. |
| Li 2006c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the patient's opinion. Telephone number: +86 0736‐2857719 |
| Li 2006d | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0730‐6222684 |
| Li 2006e | We telephoned the author in June 2011. The study was a retrospective study. Telephone number: +86 0312‐2322220‐8076 |
| Li 2007a | The patients in the treatment group did not use the Huangqi. The intervention was not in accord with the criteria. |
| Li 2007b | The secondary nephrotic syndrome patients were included in the study. |
| Li 2007c | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Li 2007d | We telephoned the author in June 2011. The study was not a double‐blind RCT. Patients were divided into groups at their discretion. Telephone number: +86 029‐33320873 |
| Li 2007e | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 0372‐2957248 |
| Li 2007g | The secondary nephrotic syndrome patients were not excluded in the study. |
| Li 2008a | The patients in the control group did not use the erigeron. The intervention was not in accord with the criteria. |
| Li 2008b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Li 2008c | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Li 2008d | The patients were allocated into two groups according to the medical order. |
| Li 2008e | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 0411‐83168346 |
| Li 2009 | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 0451‐55653086 |
| Li 2010a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Li 2010b | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 0875‐5137574. |
| Li 2011a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Li 2011b | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 13907385605 |
| Li 2011c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 13359757979 |
| Liang 2005 | The patients in the control group received the hormone, but the patients in the treatment group did not receive it. |
| Lin 2006 | The outcomes reported in were not relevant to this review (L‐10). |
| Lin 2007 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0714‐6348622 |
| Liu 1990 | The study was not a RCT, there was no control group. |
| Liu 1994 | Some patients did not receive the Huangqi. |
| Liu 1999a | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0355‐2024990 |
| Liu 1999b | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order of the patients came to hospital, odd number into one group and even number into another. Telephone number: +86 0539‐8226999 |
| Liu 2001a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0554‐3320706 |
| Liu 2001b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0931‐7616126 |
| Liu 2002 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0774‐8282011 |
| Liu 2003 | The study was not a RCT, there was no control group. |
| Liu 2006 | We telephoned the author in June 2011. The study was a retrospective study. Telephone number: +86 0710‐3449468 |
| Liu 2007a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 022‐23051087 |
| Liu 2007b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0971‐8247545 |
| Liu 2008a | The patients were allocated into two groups according to the medical order. |
| Liu 2008b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Liu 2008c | We telephoned the author in June 2011. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 13914564298 |
| Liu 2008d | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0536‐8652150 |
| Liu 2008e | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0432‐64661278 |
| Liu 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0993‐2858573 |
| Liu 2010a | The patients in the treatment group receive the sodium ferulate for Injection and Huangqi formulation, but the control group did not receive both. The intervention was not in accord with the criteria. |
| Liu 2010b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the patients' opinion. Telephone number: +86 13973956628 |
| Lu 2001 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the day of the patients came to hospital, odd numbered days to one group and even numbered days to the other. Telephone number: +86 0773‐2838977 |
| Lu 2004 | The patients in the treatment group did not receive the Huangqi. |
| Lu 2007 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Lu 2010 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Luo 2002a | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0579‐4133053 |
| Luo 2002b | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order of the patients came to hospital, such as, 1‐3 into one group and 4‐6 into another. Telephone number: +86 0310‐2115089 |
| Luo 2009 | The patients in the treatment group received tripterygium glycosides. The intervention was not in accord with the criteria. |
| Luo 2010 | The intervention therapy was not equal in the two groups. The intervention was not in accord with the criteria. |
| Lv 2001 | The patients in the treatment group received the nao ming injection, but the patients in the control group did not receive it. |
| Lv 2006 | The patients in the treatment group received the sodium ferulate, but the patients in the control group did not receive it. |
| Lv 2007a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Lv 2007b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Ma 2006 | The patients in the treatment group did not receive the Huangqi. |
| Ma 2007 | The study was not a clinical trial. |
| Mao 2006 | The patients in the treatment group received the five leaf gynostemma herb, but the patients in the control group did not receive it. |
| Min 2008a | The experimental group did not use the Huangqi. The intervention was not in accord with the criteria. |
| Min 2008b | The patients in the treatment group did not use the Huangqi. The intervention was not in accord with the criteria. |
| Mo 2004a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0759‐2387421 |
| Mo 2004b | The secondary nephrotic syndrome patients were not excluded in the study. |
| Mo 2010 | We telephoned the author in June 2011. The study was a retrospective study. The author summarized the past medical records. Telephone number: +86 0775‐7222155 |
| Ni 2007 | The patient in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Ni 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0731—28290022 |
| Nie 1985 | The study was not a RCT, there was no control group. |
| Ning 2002 | The study is a retrospective study. |
| Niu 1999 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0372‐5119119 |
| Ou 2003 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0731‐4762681 |
| Pang 2010 | The patients were allocated into two groups according to the medical order. |
| Peng 2008 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Peng 2010 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Qi 1998 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0378‐5956174 |
| Qian 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the pathogenetic condition. Telephone number: +86 0523‐6361348 |
| Qin 2009 | The patients in the treatment group did not use the Huangqi. The intervention was not in accord with the criteria. |
| Qin 2010 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Qiu 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0538‐3212864 |
| Qiu 2006 | The study did not exclude the secondary nephrotic syndrome patients. |
| Qiu 2010 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Qu 2008 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0532‐87895264 |
| Ren 1999 | The patients in the treatment group received the fu fang dan shen injection, but the patients in the control group did not receive it. |
| Shan 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the time of the patients came to hospital, in one period of time patients we assigned to one group and the next period of time to the other. Telephone number: +8613333352318 |
| Shen 2002 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0871‐3638663. |
| Shen 2003 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0745‐2261533 |
| Shen 2005 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +8613305756080 |
| Shi 2001 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0573‐88022799. |
| Shi 2003a | We telephoned the author in August 2006. The author had written protocol before commencing the study. In the protocol the author obtained serial numbers using a random numbers table, but did not strictly follow the protocol. Telephone number: +86 0756‐2528723 |
| Shi 2003b | The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. |
| Shi 2004 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0313‐8046904. |
| Shi 2007 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0731‐84917778 |
| Shi 2010 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0515‐81608138 |
| Song 1999a | The patients in the treatment group did not receive the Huangqi. |
| Song 1999b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0373‐2022300. |
| Song 2003 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0539‐5221030 |
| Sun 2004a | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0538‐3159235 |
| Sun 2004b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number:+86 0718‐8240995 |
| Sun 2009a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Sun 2009b | We telephoned the author in June 2006. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0313‐4323450 |
| Sun 2010 | The patients were allocated into two groups by medical order. |
| Tan 2010 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0772‐4362154. |
| Tang 2000a | The author divided patients into groups according to the order of the patients came to hospital, the experimental group number is twice as much as the control group. |
| Tang 2000b | The patients in the treatment group received dan shen injection, but the patients in the control group did not receive it. |
| Tang 2006 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0816‐2222566. |
| Tang 2007 | The patients in the treatment group received the tripterygium glycosides and Huangqi, but the control group did not receive both. The intervention was not in accord with the criteria. |
| Tong 2003 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0352‐5556001. |
| Wan 2000 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 022‐25868010. |
| Wang 1992 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 027‐83662142. |
| Wang 1997 | Not an RCT |
| Wang 1999 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0451‐82576141 |
| Wang 2000a | We telephoned the author in August 2006. The author hadn't written the protocol beforehand. The author divided patients in groups by allocating when they came to hospital. Telephone number: +86 0311‐87027951 |
| Wang 2001a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0351‐4988999. |
| Wang 2001b | The patients in the control group received the CTX, but the patients in the treatment group did not receive it. |
| Wang 2001c | The treatment group did not use the hormone. The control group used the hormone. |
| Wang 2002a | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0830‐3165341 |
| Wang 2002b | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0854‐8261502 |
| Wang 2002c | Not randomised |
| Wang 2002d | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0351‐4988999. |
| Wang 2002e | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 13975503269. |
| Wang 2002f | The study did not use the random sequence. |
| Wang 2003 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 027‐85726187 |
| Wang 2004a | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0903‐2050231 |
| Wang 2004b | The patients in the treatment group received the losartan, but the patients in the control group did not receive it. |
| Wang 2006b | The patients were allocated into two groups by medical order. |
| Wang 2006c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0854‐8223218. |
| Wang 2007a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Wang 2007b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Wang 2008a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 022‐26220505 |
| Wang 2008b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0873‐7653257. |
| Wang 2009a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Wang 2010a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Wang 2010b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Wang 2010c | The diagnostic criteria were not according to the protocol. |
| Wang 2010d | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0530‐5925757 |
| Wang 2010e | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0396‐2926207. |
| Wang 2011 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0717‐6486471 |
| Wei 1999 | We couldn't determine who received Huangqi. |
| Wei 2000a | We couldn't determine who received Huangqi. |
| Wei 2000b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0750‐3509898. |
| Wei 2000c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0772‐2592557. |
| Wei 2000d | Some patients in the treatment group did not receive the Huangqi. |
| Wei 2002a | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0511‐5345556 |
| Wei 2002b | The study was not a double‐blind RCT. The author divided patients into groups according to the order of the patients came to hospital. |
| Wei 2003 | The patients in the control received warfarin sodium, but the patients in the control group did not receive it. |
| Wei 2004 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0377‐65973416 |
| Wei 2005 | The study was not a double‐blind RCT. The author divided patients into groups according to the order of the patients came to hospital. |
| Wei 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0776‐5822395 |
| Wen 2005 | We telephoned the author in June 2011. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 13076877015. |
| Wen 2007 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Wu 1998b | The patients in the control group received the aspirin and dipyridamole, but the patients in the treatment group did not receive them. |
| Wu 1998c | The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital. |
| Wu 1999 | We couldn't determine who received Huangqi. |
| Wu 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0571‐63345410. |
| Wu 2005a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the medical data. Telephone number: +86 0713‐5282130. |
| Wu 2009a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Wu 2009b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0527‐83552038. |
| Xi 2003 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0511‐5345556. |
| Xiang 2007 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 15997643773 |
| Xiao 2000 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the day the patients came to hospital, odd numbered days to one group and even numbered days to the other. Telephone number: +86 0536‐8068883 |
| Xie 2008 | The Huangqi was not totally used in the treatment group. The intervention was not in accord with the criteria. |
| Xie 2010 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0716‐8217707 |
| Xiu 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 0452‐2739726. |
| Xu 1999 | The patients in the treatment group received the β‐sodium aescinate, but the patients in the control group did not receive it. |
| Xu 2000a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 023‐63632756. |
| Xu 2002a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 0574‐83870243. |
| Xu 2002b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0752‐3385263. |
| Xu 2004 | The patients in the treatment group received the chuan qiong qin injection, but the patients in the control group did not receive it. |
| Xu 2006 | The patients in the control group received the prednisone, but the patients in the treatment group did not receive it. |
| Xu 2008a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0825‐6621105. |
| Xu 2008b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0431‐85595114 |
| Xu 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0776‐2513120 |
| Xue 2004b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0951‐4091488. |
| Xue 2006 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 13839288161 |
| Xue 2007 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 13610076366 |
| Yan 2008 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0743‐6612369 |
| Yang 2000 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0571‐82621086‐2347. |
| Yang 2002a | The patients in the control group received the dan shen, but the patients in the treatment group did not receive it. |
| Yang 2002b | We telephoned the author in June 2011. The study was not a RCT, the author summarized the past medical data. Telephone number: +86 0396‐2922028. |
| Yang 2004 | The patients in the control group received the aspirin, but the patients in the treatment group did not receive it. |
| Yang 2005 | We telephoned the author in August 2006 in order to identify the randomisation procedure and other methodological issues, but the author refused further information. We couldn't determine whether it is an RCT. Telephone number: +86 0775‐4200288 |
| Yang 2006a | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the patients' wishes. Telephone number: +86 0351‐2272164 |
| Yang 2006b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0762‐3185586 |
| Yang 2007 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Yang 2008 | The secondary nephrotic syndrome patients were allocated in the study. |
| Yang 2010 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0370‐2629955. |
| Yao 2010 | The study did not exclude the secondary nephrotic syndrome. |
| Ye 1999 | The patients in the control group received the persantin, but the patients in the treatment group did not receive it. |
| Ye 2002 | We telephoned the author in August 2006 in order to identify the randomisation procedure and other methodological issues, but the author refused further information. We couldn't determine whether it is an RCT. Telephone number: +86 0576‐6207776 |
| Yi 2006 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0717‐6486797. |
| Yin 2000 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized past medical data. Telephone number: +86 0530‐5328667. |
| Yin 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0539‐5222878 |
| Yu 2000 | The patients in the treatment group did not receive the Huangqi. |
| Yu 2001 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 020‐81048888. |
| Yu 2003 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized past medical data. Telephone number: +86 13660299035. |
| Yu 2005a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0351‐3365921. |
| Yu 2005b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 029‐87213310. |
| Yu 2005c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0952‐2013270. |
| Yu 2006 | The patient in the treatment group did not receive the Huangqi. |
| Yu 2007 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Yu 2008 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Yu 2009 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0711‐3222091 |
| Yuan 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0634‐6279175. |
| Yuan 2006 | The patients were allocated into two groups according to the medical order. |
| Yuan 2008a | The patients in the treatment group received the Huangqi and danshen injection, but the control group did not receive both. The intervention was not in accord with the criteria. |
| Yuan 2008b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0537‐2253431 |
| Yue 2006 | The patients in the treatment group received the Ci Wu Jia, but the patients in the control group did not receive it. |
| Zang 2007 | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Zeng 2008 | We telephoned the author in June 2011.
The study is a retrospective clinical analysis. The author summarized the past medical data. Telephone number: +86 0774‐2036883 |
| Zhang 1993 | The study was not a RCT, there was no control group. |
| Zhang 1994 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0536‐3272121 |
| Zhang 1997 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. 0532‐88555437 |
| Zhang 1998 | The intervention in the treatment group was not same. |
| Zhang 2001a | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 010‐88276527 |
| Zhang 2001b | The study is semi‐randomised. |
| Zhang 2001c | The study did not use the random sequence. |
| Zhang 2002a | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 020‐81081947. |
| Zhang 2002b | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 010‐88276527 |
| Zhang 2004 | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0558‐2516001‐2077 |
| Zhang 2005a | We telephoned the author in August 2006. The study was not a double‐blind RCT. Patients were divided into groups at the author's discretion. Telephone number: +86 0396‐2959317 |
| Zhang 2005b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0854‐8261265. |
| Zhang 2006a | The patients in the treatment group received the bailing capsule, but the control group did not receive it. |
| Zhang 2006b | The patients in the treatment group did not receive the Huangqi. |
| Zhang 2007a | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Zhang 2007b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Zhang 2007c | The inclusion criteria were in not accordance with the protocol. |
| Zhang 2007d | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Zhang 2007e | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the day of the patients came to the hospital, odd numbered days to one group and even numbered days to the other. Telephone number: +86 0393‐4416620 |
| Zhang 2008a | The study used the quasi‐RCT. |
| Zhang 2008b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Zhang 2008c | We telephoned the author in June 20116. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0759‐2633663 |
| Zhang 2008d | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the patients' opinion. Telephone number: +86 0516‐83956312 |
| Zhang 2008e | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0315‐6612587 |
| Zhang 2009a | The patients were allocated into two groups according to the medical order. |
| Zhang 2009b | The patients were allocated into two groups according to the medical order. |
| Zhang 2009c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the day of the patients came to the hospital, odd numbered days to one group and even numbered days to the other. Telephone number: +86 13568150066 |
| Zhang 2010a | The patients were allocated into the two groups according to the time sequence of seeing doctor. |
| Zhang 2010b | The patients in the treatment group did not receive the Huangqi. The intervention was not in accord with the criteria. |
| Zhang 2010c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical records. Telephone number: +86 022‐27285222 |
| Zhang 2010d | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0758‐7765408 |
| Zhang 2010e | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the day of the patients came to the hospital, odd numbered days to one group and even numbered days to the other. Telephone number: +86 0530‐8211111 |
| Zhao 1999a | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 0871‐3211101. |
| Zhao 1999c | The inclusion criteria were not in accordance with the protocol. |
| Zhao 2001a | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the day of the patients came to the hospital, odd numbered days to one group and even numbered days to the other. Telephone number: +86 0536‐8068883 |
| Zhao 2001b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0319‐3286194. |
| Zhao 2001c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 0746‐8413510. |
| Zhao 2010 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 072‐82433165. |
| Zhi 2010 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 010‐68489948 |
| Zhong 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author summarized medical records. Telephone number: +86 0754‐8258290. |
| Zhong 2006 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the pathogenetic condition. Telephone number: +86 0750‐8860123 |
| Zhong 2008 | The patients in the treatment group did not receive the Huangqi. |
| Zhong 2009 | The treatment group did not use the Huangqi. |
| Zhong 2011 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 15842672222 |
| Zhou 2000 | The control group did not receive the Danshen injection. |
| Zhou 2003 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0511‐5345556. |
| Zhou 2004 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical record. Telephone number: +86 0518‐83229682 |
| Zhou 2006 | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical record. Telephone number: +86 0790‐6651018 |
| Zhou 2007a | The intervention therapy was not equal in the two groups. |
| Zhou 2007b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical record. Telephone number: +86 0739‐8244825 |
| Zhou 2008 | The patients in the treatment group did not receive the Huangqi. |
| Zhou 2009a | The patients in the treatment group did not receive the Huangqi. |
| Zhou 2009c | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 13396169118 |
| Zhu 2001 | The patients in the treatment group received the cordate houttuynia, but the control group did not receive it. |
| Zhu 2002 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the pathogenetic condition. Telephone number: +86 021‐58670283 |
| Zhu 2004 | We telephoned the author in August 2006. The study was not a double‐blind RCT. The author divided patients into groups according to the order the patients came to hospital, odd numbers were assigned to one group and even numbers into the other. Telephone number: +86 0511‐5345556 |
| Zhu 2006 | The treatment group did not use the Huangqi. |
| Zhu 2010a | The treatment group used the Huangqi and shuxuetong injection. But the control group did not use the shuxuetong injection. |
| Zhu 2010b | We telephoned the author in June 2011. The study was not a double‐blind RCT. The author summarized the past medical data. Telephone number: +86 0394‐8279742 |
Characteristics of studies awaiting assessment [ordered by study ID]
Li 2007f.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Liu 1998.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Ma 2001.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Su 2000a.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Tang 2005.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Wan 2009.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Wang 2000b.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Wu 1998a.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Xue 2004a.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Zhang 2010f.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Zhao 1999b.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Zhao 2003.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Zhou 2009b.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Zou 2008.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
CTX ‐ cyclophosphamide
Differences between protocol and review
Risk of bias assessment tool has replaced the quality assessment checklist.
Contributions of authors
FM, YW and TW were responsible for development of protocol and review, FM and ZRZ was responsible for searching for references and data extraction, interviewing the authors of trials. All five authors contributed to quality assessment of the trials.
Sources of support
Internal sources
Chinese Cochrane Center, Chinese Centre of Evidence‐Based Medicine, West China Hospital of Sichuan University, China.
External sources
China Medical Board of New York, USA.
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ai 2008 {published data only}
- Ai S, Zhen J. The clinical research on nuclear factor‐kappa B, TXBA2, 6‐keto‐PGF1a in children with frequent relapse nephrotic syndrome by treatment of decoction Shenkangling by tonifying the kidney and promoting blood circulation. Master Thesis of Fu Jian College of Traditional Chinese Medicine 2008:1‐33.
Chang 2002a {published data only}
- Chang ZJ. Effect of Huangqi and Danggui on the function of endothelial cell of nephrotic syndrome patients. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2002;2(6):648‐9. [Google Scholar]
Hu 2002 {published data only}
- Hu GC, Tang SY, Zhang XJ. Clinical observation on Huangqi injection for primary nephrotic syndrome. Chengde Yixueyuan Xuebao [Chengde Medical College] 2002;19(1):21‐2. [Google Scholar]
Lin 2008 {published data only}
- Lin FJ, Huang JC, Cui MX. The effect of Huangqi injection on the blood biochemistry and immunoglobulin of children nephrotic syndrome. Zhongguo Zhongziyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2008;9(1):72‐3. [Google Scholar]
Luo 2008 {published data only}
- Luo J, Shu HQ, Yi XY, Zhen S. Clinical observation of refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Sichuan Zhongyi [Journal of Sichuan of Traditional Chinese Medicine] 2008;26(9):82‐3. [Google Scholar]
Wang 2006a {published data only}
- Wang Y. Study on the effect of Huangqi injection for children with nephrotic syndrome. Shiyong Yixue Zazhi [Journal of Practical Medicine] 2006;22(11):1328‐9. [Google Scholar]
Yuan 2004 {published data only}
- Yuang HZ, Xie F, Zhang CT. The effect of Huangqi and red Chinese date in the process of corticosterone reduction in refractory primary nephrotic syndrome with mesangial proliferative glomerulonephritis. Zhongguo Jiehe Yixue Zazhi [Chinese Journal of Integrated Medicine] 2004;5(7):413‐4. [Google Scholar]
Zhou 2010 {published data only}
- Zhou JJ, Kang GG, Zhang Q, Chen HQ, Kang YQ. The effect of Huangqi granules on the serum and urine IFN‐γ, IL‐13,TGF‐β1 in the primary nephrotic syndrome patients. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2010;11(3):242‐3. [Google Scholar]
Zou 1997 {published data only}
- Zou HQ, Wang YQ. Mechanism that Chinese traditional medicine reduces relapses of idiopathic nephrotic syndrome (INS) in children [abstract]. Nephrology 1997:S124. [Google Scholar]
References to studies excluded from this review
Bai 2004 {published data only}
- Bai YW. Curative observation of the treatment of primary nephrotic syndrome with Huangqi injection. Anhui Yixue [Anhui Medical Journal] 2004;25(4):299‐300. [Google Scholar]
Bai 2010 {published data only}
- Bai J, Zhu HH, Zeng XR, Wu J. The 56 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2010;33(5):51‐2. [Google Scholar]
Bie 2006 {published data only}
- Bie FY, Lu BS. 30 case observation on the treatment of primary nephrotic syndrome with lisinopril, astragalus and angelica. Shiyong Zhenduan yu Zhiliao [Journal of Practical Diagnosis and Therapy] 2006;20(10):752‐3. [Google Scholar]
Cao 2009 {published data only}
- Cao XM, An WF. Clinical observation of 56 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Shandong Yiyao [Shandong Medical Journal] 2010;50(16):103. [Google Scholar]
Chang 2002b {published data only}
- Chang ZJ. Effect of Huangqi and Danggui on blood fat and apolipoprotein in nephrotic syndrome. Zhonghua Xiandai Linchuang Yixue Zazhi [Chinese Journal of Current Clinical Medicine] 2002;3(9):66‐7. [Google Scholar]
- Chang ZJ, Huang X. Effect of Huangqi and Danggui on NO in nephrotic syndrome patients. Zhonghua Xiandai Linchuang Yixue Zazhi [Chinese Journal of Current Clinical Medicine] 2002;3(3):76‐7. [Google Scholar]
Chen 1998 {published data only}
- Chen WQ, Xue CX. Curative observation of hormone, Huangqi and Fufangdanshen for primary nephrotic syndrome. Xibei Guofang Yixue Zazhi [Medical Journal of National Defending Forces in Northwest China] 1998;19(4):276‐7. [Google Scholar]
Chen 1999 {published data only}
- Chen Y, Liu ZQ, Ding YH. Clinical observation on the treatment of the recrudescent hormone sensitive nephrotic syndrome with Huangqi. Linchuang Huicui [Clinical Focus] 1999;14(5):274‐5. [Google Scholar]
Chen 2001a {published data only}
- Chen SB, Yu J. Curative observation of the treatment of primary nephrotic syndrome with Longshoudan above other drugs. Shizhen Guoyi Guoyao [Lishizhen Medicine and Materia Medica Research] 2001;12(9):815. [Google Scholar]
Chen 2001b {published data only}
- Chen K, Wang WP, Liu H. Triple therapy for primary nephrotic syndrome 35 case. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2001;2(11):668‐9. [Google Scholar]
Chen 2001c {published data only}
- Chen WD, Jia P, Zhang Y. Curative effect observation of the treatment of primary nephrotic syndrome with Huangqi. Bengbu Yixueyuan Xuebao [Journal of Bengbu Medical College] 2001;26(3):202‐4. [Google Scholar]
Chen 2002 {published data only}
- Chen LP, Zhou QL, Yang TG, Liu ZC, Wang JW. Clinical observation on united use Huangqi therapy on elderly primary nephrotic syndrome. Zhongguo Yixue Gongcheng [China Medical Engineering] 2002;10(6):295‐6. [Google Scholar]
Chen 2003 {published data only}
- Chen JH, Huang RG, Liu GJ, Li YT, Lin WH. Clinical observation of the treatment of nephrotic syndrome with Huangqi injection and molecular. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2003;4(8):484‐5. [Google Scholar]
Chen 2004 {published data only}
- Chen LP, Zhou Ql, Yang JH, Ou YC. Protective effects of Huangqi injection on tubular in patients with primary nephrotic syndrome. Zhong Nan Da Xue Xue Bao Yi Xue Ban [Journal of Central South University (Medical Sciences)] 2004;9(2):152‐3. [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen ML, Huang XD. Effective observation of the treatment of primary nephrotic syndrome with Huangqi injection above other drugs. Fujian Yiyao Zazhi [Fujian Medical Journal] 2005;27(3):125‐6. [Google Scholar]
Chen 2006a {published data only}
- Chen TY. 52 case curative observation of the treatment of primary nephrotic syndrome with integrated traditional and western medicine. Linchuang Neike Zazhi [Journal of Clinical Internal Medicine] 2006;15(8):840‐1. [Google Scholar]
Chen 2006b {published data only}
- Chen ZX. The observational study of 36 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Gansu Zhongyi [Gansu Journal of Tradition Chinese Medicine] 2006;19(11):30. [Google Scholar]
Chen 2006c {published data only}
- Chen ZX. The clinical observation of 80 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Heilongjiang Zhongyiyao [Heilongjiang Journal of Traditional Chinese Medicine] 2006, (6):19‐20. [Google Scholar]
Chen 2007 {published data only}
- Chen H. The 34 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2007;23(3):169. [Google Scholar]
Chen 2008a {published data only}
- Chen XF. The study advance of the refractory nephrotic syndrome treated with integrated Chinese and western medicine. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2008;9(10):938‐40. [Google Scholar]
Chen 2008b {published data only}
- Chen J, Chen SQ. Clinical observation of preventing infection in nephrotic syndrome children treated with Huangqi granules. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2008;28(5):467‐9. [Google Scholar]
Chen 2009a {published data only}
- Chen YS, Zhang LQ, Huang JH. The 50 cases of nephrotic syndrome patients treated with traditional Chinese medicine and aftercare treatment. Zhongyi Zazhi [Journal of Traditional Chinese Medicine] 2009;50:198‐9. [Google Scholar]
Chen 2009b {published data only}
- Chen XP, Liu AH. The clinical observation of 32 cases of hormone dependent refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyiyao Daobao [Guiding Journal of Traditional Chinese Medicine] 2009;15(8):22‐3. [Google Scholar]
Chen 2010a {published data only}
- Chen ZQ, Kang JH, Liang JQ. Effect observation of traditional Chinese medicine and western medicine on the treatment of nephrotic syndrome. Yiyao Dao Bao [Herald of Medicine] 2010;7(18):57‐8. [Google Scholar]
Chen 2010b {published data only}
- Chen XF. The study of 70 cases refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyi Zazhi [Journal of Traditional Chinese Medicine] 2010;51(6):533‐4. [Google Scholar]
Chen 2010c {published data only}
- Chen XF. The clinical observation of 30 cases of adults with frequently relapsing nephrotic syndrome patients treated with traditional Chinese medicine and double shock therapy. Xinzhongyi [Journal of New Chinese Medicine] 2010;42(3):33‐4. [Google Scholar]
Chen 2010d {published data only}
- Chen F, Li YY, Huang YJ, Yuan FH. Clinical observation of combined treatment with traditional Chinese medicine and western medicine for refractory nephrotic syndrome. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2010;19(19):2343‐4. [Google Scholar]
Chen 2011 {published data only}
- Chen GQ, Zhao AJ, Shen SL, Wang G. The clinical observation of 48 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Sichuan Zhongyi [Journal of Sichuan of Traditional Chinese Medicine] 2011;29(1):85‐6. [Google Scholar]
Cheng 2004 {published data only}
- Cheng M. Integrated traditional and western medicine for primary nephrotic syndrome 40 case. Xiandai Zhongyiyao [Modern Traditional Chinese Medicine] 2004;25(12):1081‐2. [Google Scholar]
Dai 2006 {published data only}
- Dai QF. Clinical observation on the treatment of children nephrotic syndrome with integrated traditional and western medicine. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2006;22(7):431. [Google Scholar]
Deng 2003 {published data only}
- Deng Y, Yu L, Wong ZY, Huang YH, Zhang YX, Zhuo MY. The effect of Huangqi injection on urine protein and plasma protein in children with nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2003;4(10):578‐9. [Google Scholar]
Deng 2008 {published data only}
- Deng ZH. Clinical observation of 48 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyiyao Daobao [Guiding Journal of Traditional Chinese Medicine] 2008;14(2):30‐1. [Google Scholar]
Ding 2008 {published data only}
- Ding SY, Xiao GX, Ma SY. The 25 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyi Zazhi [Journal of Traditional Chinese Medicine] 2008;49(8):720‐1. [Google Scholar]
Dong 2001 {published data only}
- Dong Y. Huangqi in treatment of secondary immunodeficiency. Zhongguo Zhongyi Jichu Yixue [Chinese Journal of Basic Medicine in Traditional Chinese Medicine] 2001;7(5):37‐8. [Google Scholar]
Dong 2005 {published data only}
- Dong H. Clinical observation of the treatment of nephrotic syndrome with Huangqi injection combine other drugs. Zhonghua Linchuang Yixue Yanjiu Zazhi [Chinese Magazine of Clinical Medicinal Professional Research] 2005;11(1):4‐6. [Google Scholar]
Dong 2009 {published data only}
- Dong YZ, Peng SJ, Zhang WJ. The clinical observation of nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Minzu Minjian Yiyao [Chinese Journal of Ethnomedicine and Ethnopharmacy] 2009, (3):63‐4. [Google Scholar]
Du 2006a {published data only}
- Du J. The effect of Huangqi and Fufangdanshen injection for nephrotic syndrome. Hei Long Jiang Yi Yao Ke Xue [Heilongjiang Medicine and Pharmacy] 2006;29(1):60. [Google Scholar]
Du 2006b {published data only}
- Du WX, Zhang YJ. The 35 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Henan Zhongyi [Henan Traditional Chinese Medicine] 2006;26(9):62‐3. [Google Scholar]
Duan 2006 {published data only}
- Duan ZX, Chen BM, Liu YL, Wei L, Deng HT, Cai CM. The clinical observation of children recurrent nephrotic syndrome treated with integrated Chinese and western medicine. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2006;22(12):754‐5. [Google Scholar]
Fan 2001 {published data only}
- Fan RF. Clinical observation of the effect of Huangqi on high blood coagulate state in nephrotic syndrome patient. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2001;2(11):672‐3. [Google Scholar]
Feng 2007 {published data only}
- Feng LH. The 58 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Minjian Laofa [China's Naturopathy] 2007;15(12):4‐5. [Google Scholar]
Feng 2010 {published data only}
- Feng HH, Shu ZE. Clinical observation of primary nephrotic syndrome treated with integrated Chinese and western medicine. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2010;26(8):550‐1. [Google Scholar]
Fu 2008 {published data only}
- Fu YS, Zhang HY. The nephrotic syndrome in children treated with integrated traditional Chinese and western medicine in stages. Shiyong Yiyao Zazhi [Practical Journal of Medicine & Pharmacy] 2008;25(10):1196‐7. [Google Scholar]
Gao 2001 {published data only}
- Gao F, Zhou XG. Curative effect observation of Chinese traditional medicine for the adverse reaction of hormone therapy in nephrotic syndrome. Hubei Minzu Xueyuan Xuebao (Yixueban) [Journal of Hubei Institute for Nationalities (Medical Edition)] 2001;18(4):28‐30. [Google Scholar]
Gao 2002 {published data only}
- Gao X, Hu WH, Sun WH. Huangqi Yimutang and hormone for nephrotic syndrome 30 case. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2002;23(10):884‐5. [Google Scholar]
Gong 2007 {published data only}
- Gong SF, Feng X, Wang FF. Clinical observation of 90 cases of primary nephrotic syndrome treated with Huangqi and prednisone. Suzhou Yixueyuan Xuebao (Yixue Ban) [Suzhou University Journal of Medical Science] 2007;27(5):772‐4,754. [Google Scholar]
Gong 2009 {published data only}
- Gong LH. Clinical observation of nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Wuzhenxue Zazhi [Chinese Journal of Misdiagnostics] 2009;19(10):2355‐6. [Google Scholar]
Gong 2010 {published data only}
- Gong ML. The clinical observation of nephrotic syndrome treated with integrated Chinese and western medicine. Zhongguo Baojian Yingyang [China Health & Nutrition] 2010;8:115. [Google Scholar]
Gu 1998 {published data only}
- Gu LY, Xie TZ. Integrated Chinese and western medicine for nephrotic syndrome. Junyi Jinxiu Xueyuan Xuebao [Academic Journal of PLA Postgraduate Medical School] 1998;19(2):146‐8. [Google Scholar]
Guo 1997 {published data only}
- Guo XY, Li L, Wang Y. Integrated traditional and western medicine for refractory nephrotic syndrome 33 case. Zhongyiyao Xuekan [Correspondence Journal of Traditional Chinese Medicine] 1997;16(4):29. [Google Scholar]
Guo 1999 {published data only}
- Guo YF, Ge XP. Clinical observation of Huangqixianlingpitang and Shuizhijiaowan for high coagulation in nephrotic syndrome. Zhongyi Zazhi [Journal of Traditional Chinese Medicine] 1999;40(5):281‐4. [Google Scholar]
Guo 2006 {published data only}
- Guo NT, Yang J, Li YL, Deng P. Huangqi hushen decoction together with hormone in treating 30 cases of primary nephrotic syndrome. Henan Zhongyi [Henan Traditional Chinese Medicine] 2006;26(1):49‐50. [Google Scholar]
Guo 2008a {unpublished data only}
- Guo XX, Gong JH, Fu ZC. The observation of the 50 child primary nephrotic syndrome treated with integrated traditional Chinese and western medicine in primary hospital. 2008 national integrated traditional Chinese and western medicine kidney disease in Nanjing BBS paper 2008:259‐62.
Guo 2008b {published data only}
- Guo XX, Gong JH, Fu ZC. Experience of combining traditional Chinese and western medicine treating babies with nephrotic syndrome. Liaoning Zhongyi Xueyuan Xuebao [Journal of Liaoning University of Traditional Chinese Medicine] 2008;10(12):120‐2. [Google Scholar]
Guo 2010 {published data only}
- Guo XD, Wang XQ, Jing JS, Shi JH. The clinical study of refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyiyao Daobao [Guiding Journal of Traditional Chinese Medicine] 2010;16(11):28‐30. [Google Scholar]
Hao 2007 {published data only}
- Hao RF. 27 cases of child hormone sensitive primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyiyao Xinxi [Information on Traditional Chinese Medicine] 2007;14(7):66‐7. [Google Scholar]
He 2008 {published data only}
- He M, Xue S, Wang R. Clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Hubei Zhongyi Zazhi [Hubei Journal of Traditional Chinese Medicine] 2008;30(12):27‐8. [Google Scholar]
He 2010 {published data only}
- He S, Li CJ, Shi X. The clinical study of 34 cases children nephrotic syndrome treated with integrated Chinese and western medicine. Zhongguo Fuyou Baojian [Maternal and Child Health Care of China] 2010;25(36):5541‐2. [Google Scholar]
Hong 2001 {published data only}
- Hong JD, Li SG, Guo QM, He SJ, Peng WL. Clinical study on the treatment of primary nephrotic syndrome with Astragalus Angelica mixture above other medicine. Fujian Yiyao Zazhi [Fujian Medical Journal] 2001;23(3):112‐3. [Google Scholar]
Hu 2005 {published data only}
- Hu SJ, Fang Q, Liu JS, Zhang L, Cao EZ. Clinical study on intervention of Liuweidihuang pill on hormonotherapy in treating nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2005;25(2):107‐10. [PubMed] [Google Scholar]
Hu 2006a {published data only}
- Hu JJ. The study of 30 refractory nephrotic syndrome patients treated with integrated traditional Chinese and western medicine. Zhejiang Zhongxiyi Jiehe Zazhi [Zhejiang Journal of Integrated Traditional Chinese and Western Medicine] 2006;45(1):58. [Google Scholar]
Hu 2006b {published data only}
- Hu GH, Dang XQ, Wang JH, Yao JC, Wang YC. Clinical effect of Shenbing mistura combined with glucocorticoid on recurrent nephrotic syndrome in children and levels of interleukin‐6 and tumor necrosis factor‐a in blood and urine. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2006;26(10):892‐5. [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu FT. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyiyao Xinxi [Information on Traditional Chinese Medicine] 2009;16(3):73. [Google Scholar]
Hua 2010 {published data only}
- Hua SS. 48 adult primary nephrotic syndrome patients treated with integrated traditional Chinese and western medicine. Changchun Zhongyiyao Daxue Xuebao [Journal of Changchun University of Traditional Chinese Medicine] 2010;26(3):380‐1. [Google Scholar]
Huang 2004a {published data only}
- Huang YH, Deng Y, Weng ZY, Yu L. The study of the effect of Huangqi injection on urinary protein in children with nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2004;13(12):1562‐3. [Google Scholar]
Huang 2004b {published data only}
- Huang YY, Liu Y, Liu CM. Renal tubular protection of Shenqifuzheng injection in treating primary nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2004;24(12):1091‐3. [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang CM. The effect observation of refractory nephrotic syndrome treated with integrated Chinese and western medicine. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2006;7(9):540‐1. [Google Scholar]
Huang 2007 {published data only}
- Huang WQ, Chen JH. The study of refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2007;8(2):110‐1. [Google Scholar]
Huang 2009a {published data only}
- Huang ZD. The observation study of 30 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Hunan Zhongyi Zazhi [Hunan Journal of Traditional Chinese Medicine] 2009;25(1):16‐7. [Google Scholar]
Huang 2009b {published data only}
- Huang YX, Peng JJ, Song QL. The clinical study of 67 cases children primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Xinzhongyi [Journal of New Chinese Medicine] 2009;41(8):67‐9. [Google Scholar]
Huang 2010 {published data only}
- Huang Q, Liu XL. Primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyi Zazhi [Journal of Traditional Chinese Medicine] 2010;17(29):76,81. [Google Scholar]
Ji 2009 {published data only}
- Ji HL. 48 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyi Yanjiu [Traditional Chinese Medicinal Research] 2009;22(8):31‐2. [Google Scholar]
Jia 2010 {published data only}
- Jia J. The clinical observation of 53 cases of hormone dependent nephrotic syndrome patients treated with integrated Chinese and western medicine. Hebei Beifang Xueyuan Xuebao (Yixueban) [Journal of Hebei North University (Medical Edition)] 2010;27(4):30‐1. [Google Scholar]
Jiang 1997 {published data only}
- Jiang HQ. Jianpiwenshenhuoxuelishuifa for primary nephrotic syndrome 26 case. Zhejiang Zhongyi Xueyuan Xuebao [Journal of Zhejiang University of Traditional Chinese Medicine] 1997;32(4):161‐2. [Google Scholar]
- Jiang HQ. Zinijianpihuoxueyushentang for primary nephrotic syndrome 34 case. Jilin Zhongyiyao [Jilin Journal of Traditional Chinese Medicine] 1997;17(4):10‐1. [Google Scholar]
Jiang 2001a {published data only}
- Jiang WY, Jin XY. Curative effect observation of the treatment of nephrotic syndrome with Huangqi injection. Heilongjiang Yiyao [Heilongjiang Medicine Journal] 2001;14(5):385. [Google Scholar]
Jiang 2001b {published data only}
- Jiang XH, Xia QH. Clinical observation of the treatment of primary nephrotic syndrome with integrated traditional and western medicine. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2001;2(4):240‐1. [Google Scholar]
Jiang 2001c {published data only}
- Jiang XH, Wang JM, Tang NB. Clinical observation of compound Huangqi and prednisone for nephrotic syndrome. Zhongguo Zhongyao Zazhi [China Journal of Chinese Materia Medica] 2001;26(9):643‐5. [Google Scholar]
Jiang 2008a {published data only}
- Jiang Y. The clinical observation of primary nephrotic syndrome patients treated with Huangqi injection. Sichuan Yixue [Sichuan Medical Journal] 2008;29(2):164‐5. [Google Scholar]
Jiang 2008b {published data only}
- Jiang GH, Song RX. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Hei Long Jiang Yi Yao Ke Xue [Heilongjiang Medicine and Pharmacy] 2008;31(4):47‐8. [Google Scholar]
Jin 2001 {published data only}
- Jin L, Ren DS, Tao YF, Hui S. Clinical study on the treatment of refractory nephrotic syndrome with Huangqi and cyclophosphamide. Zhongguo Zonghe Linchuang [Clinical Medicine of China] 2001;17(8):597‐8. [Google Scholar]
Jin 2007 {published data only}
- Jin CH, Zhang PQ. Clinical observation of refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2007;8(3):168‐9. [Google Scholar]
Kang 2005 {published data only}
- Kang GG. Preventive effect of huangqi particle on complicated infection of children with nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2005;6(12):718‐9. [Google Scholar]
Kong 2009 {published data only}
- Kong LX. Clinical research of combination of traditional Chinese medicine and western medicine on the treatment of primary nephrotic syndrome. Hebei Zhongyi [Hebei Journal of Traditional Chinese Medicine] 2009;31(5):707‐9. [Google Scholar]
Lai 2006 {published data only}
- Lai LW, Hou KZ. Clinical analysis of the treatment of primary nephrotic syndrome with Huangqi with Danggui mixture. Gannan Yixueyuan Xuebao [Journal of Gannan Medical University] 2006;26(3):415. [Google Scholar]
Lan 2005 {published data only}
- Lan CF, Duan XP. Supplementing Qi reinforcing kidney broth and hormone for nephrotic syndrome 43 case. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2005;26(4):306. [Google Scholar]
Lan 2007 {published data only}
- Lan HQ, Kuang HT, Zhou K, Wang XZ. Clinical observation on treating 45 cases of nephrotic syndrome with combination of traditional Chinese and western medicine. Zhongyiyao Daobao [Guiding Journal of Traditional Chinese Medicine] 2007;13(6):21‐2. [Google Scholar]
Lei 2006 {published data only}
- Lei H. Clinical observation of the treatment of primary nephrotic syndrome with losartan and Huangqi with Danggui mixture. Zhonghua Shiyong Zhongxiyi Zazhi [Chinese Journal of the Practical Chinese with Modern Medicine] 2006;19(7):780‐1. [Google Scholar]
Li 1988 {published data only}
- Li BH. Huangqi gruel for spleen deficiency type nephrotic syndrome. Jiangxi Zhongyiyao [Jiangxi Journal of Traditional Chinese Medicine] 1988, (3):54. [Google Scholar]
Li 1994 {published data only}
- Li Y, Li XM, Li XX, Luan LY, Li XL, Zhang BL. Clinical observation of the treatment of nephrotic syndrome with Shuqitang 98 case. Zhongguo Xiangcun Yiyao [Chinese Rural Medicine] 1994;22(2):37. [Google Scholar]
Li 1997 {published data only}
- Li L, Huang RZ, Li LN. Reinforcing Qi nourishing Yin, activating blood circulation to dissipate blood stasis and hormone for refractory nephrotic syndrome 30 case. Fujian Zhongyiyao [Fujian Journal of Traditional Chinese Medicine] 1997;28(2):6. [Google Scholar]
Li 1999a {published data only}
- Li HM, Li JH, Wang F. The appliance of Huangqi injection in nephrotic syndrome. Zhongxiyi Jiehe Shiyong Linchuang Zazhi [Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care] 1999;6(4):162. [Google Scholar]
Li 1999b {published data only}
- Li Z, Li XY. Curative effect observation of Huangqi injection for nephrotic syndrome. Luzhou Yixueyuan Xuebao [Journal of Luzhou Medical College] 1999;22(6):529. [Google Scholar]
Li 2003a {published data only}
- Li Z, Meng H, Hong J. Clinical observation of 37 nephrotic syndrome patients treated with Quadri‐combination therapy combined with Tripterygium wilfordii and Huangqi. Zhongguo Quanke Yixue [Chinese General Practice] 2003;6(3):248. [Google Scholar]
Li 2003b {published data only}
- Li SY. The effect of Huangqi on plasma albumin in children with nephrotic syndrome. Gansu Zhongyi Xueyuan Xuebao [Journal of Gansu College of Traditional Chinese Medicine] 2003;20(3):41‐2. [Google Scholar]
Li 2004a {published data only}
- Li T, Wang H. Clinical curative effect observation of Huangqi injection and prednisone for primary nephrotic syndrome. Hunan Zhongyi Xueyuan Xuebao [Journal of Traditional Chinese Medicine University of Hunan] 2004;24(1):45‐6. [Google Scholar]
Li 2004b {published data only}
- Li XK, Feng WX, Wu LJ, Gu BQ, Zhu YB, Meng XH, et al. 32 case effective observation on the treatment of primary nephrotic syndrome with Huangqi and Danggui. Zhongyuan Yikan [Central Plains Medical Journal] 2004;31(6):10‐11. [Google Scholar]
Li 2005 {published data only}
- Li NQ, Li ZS, Liu GC, Chao Q. Clinical study of low‐molecular‐weight heparin calcium and radix astragali combine use for the treatment of refractory nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2005;6(2):92‐4. [Google Scholar]
Li 2006a {published data only}
- Li SR, Gui Q. Curative effect of therapy integrated Chinese and western medicine for nephrotic syndrome and its nursing care. Shijie Zhongxiyi Jiehe Zazhi [World Journal of Integrated Traditional and Western Medicine] 2006;1(3):168‐70. [Google Scholar]
Li 2006b {published data only}
- Li W. The clinical observation of 52 cases of primary nephrotic syndrome patients treated with traditional medicine and hormone. Zhongguo Shiyong Xiangcun Yisheng Zazhi [Chinese Practical Journal of Rural Doctor] 2006;13(10):47. [Google Scholar]
Li 2006c {published data only}
- Li JM. The 60 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhonghua Yixue Yanjiu Zazhi [Journal of Chinese Medicine Research] 2006;6(10):1152‐3. [Google Scholar]
Li 2006d {published data only}
- Li NJ, Li YX. The clinical analysis of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Ziran Yixue Zazhi [Chinese Journal of Natural Medicine] 2006;8(1):20‐2. [Google Scholar]
Li 2006e {published data only}
- Li WS, Xiao SQ, Hu LX, Cao HZ, Hu XR, Li HQ, Zhang LH. Clinical observation of combined Chinese and western medicine on 52 patients with primary nephrosis syndrome. Hebei Zhongyi [Hebei journal of traditional Chinese medicine] 2006;28(1):54‐5. [Google Scholar]
Li 2007a {published data only}
- Li YX, Dong W. The effect observation of 78 primary nephrotic syndrome patients treated with Chinese traditional medicine to correct the side effect of hormones. Shanxi Yiyao Zazhi [Shanxi Medicine Journal] 2007;36(5):466‐7. [Google Scholar]
Li 2007b {published data only}
- Li YD, Ding ZS, Li GH, Tang CY, Huang LL. Clinical result observation of treating nephric tubule dysfunction of nephrotic syndrome by TCM and modern medicine. Zhonghua Shiyong Zhongxiyi Zazhi [Chinese Journal of the Practical Chinese with Modern Medicine] 2007;20(17):1522‐4. [Google Scholar]
Li 2007c {published data only}
- Li DH, Xu YW, Zhao B, Wang Y. The 30 cases of primary nephrotic syndrome patients treated with traditional Chinese medicine and western medicine. Changchun Zhongyiyao Daxue Xuebao [Journal of Changchun University of Traditional Chinese Medicine] 2007;23(6):49. [Google Scholar]
Li 2007d {published data only}
- Li M. The clinical observation of 30 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Beijing Zhongyi [Beijing Journal of Traditional Chinese Medicine] 2007;26(5):289‐90. [Google Scholar]
Li 2007e {published data only}
- Li YP. Primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Xiandai Yaowu Yinyong [Chinese Journal of Modern Drug Application] 2007;1(3):49. [Google Scholar]
Li 2007g {published data only}
- Li BE, Kang GG. The effect of Huangqi granules on the immune function of simple type nephrotic syndrome children. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2007;8(7):413‐4. [Google Scholar]
Li 2008a {published data only}
- Li H. Clinical observation of Huangqi and Dengzhanhua in treatment of 46 patients with idiopathic nephrotic syndrome. Zhongguo Xiandai Yisheng [China Modern Doctor] 2008;46(19):80‐1. [Google Scholar]
Li 2008b {published data only}
- Li Y, He Q. The observation of 28 refractory nephrotic syndrome patients treated with cytotoxic drugs and traditional Chinese medicine. Zhongguo Quanke Yixue [Chinese General Practice] 2008;11(7B):1303‐4. [Google Scholar]
Li 2008c {published data only}
- Li GP. The clinical observation of 36 cases of refractory nephrotic syndrome treated with integrated Chinese and western medicine. Zhongyiyao Daobao [Guiding Journal of Traditional Chinese Medicine] 2008;14(5):43,57. [Google Scholar]
Li 2008d {published data only}
- Li XQ, Geng XY, Chen XT, Ye LZ, Shen ZX, Wang H. Effects of Buyanghuanwu decoctions on microalbuminuria in nephrotic syndrome. Zhongyiyao Xuekan [Chinese Archives of Traditional Chinese Medicine] 2008;26(3):647‐8. [Google Scholar]
Li 2008e {published data only}
- Li Z, Zhou Y, Sun MJ, Meng H, Shu L. 26 cases of nephrotic syndrome patients treated with Huangqi injection. Shijie Zhongyiyao [World Chinese Medicine] 2008;3(4):223. [Google Scholar]
Li 2009 {published data only}
- Li LY, Ma BY. The clinical analysis of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyiyao Xinxi [Information on Traditional Chinese Medicine] 2009;26(4):50‐1. [Google Scholar]
Li 2010a {published data only}
- Li RJ. Effective observation on treating nephrotic syndrome in the integrative medicine. Zhongyi Linchuang Yanjiu [Clinical Journal of Chinese Medicine] 2010;2(11):45‐6, 48. [Google Scholar]
Li 2010b {published data only}
- Li J. The clinical observation of 40 adults primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Yunnan Zhongyi Zhongyao Zazhi [Yunnan Journal of Traditional Chinese Medicine and Materia Medica] 2010;31(7):22‐4. [Google Scholar]
Li 2011a {published data only}
- Li JP. The clinical observation of nephrotic syndrome patients treated with integrated Chinese and western medicine. Hubei Zhongyi Zazhi [Hubei Journal of Traditional Chinese Medicine] 2011;33(2):16‐7. [Google Scholar]
Li 2011b {published data only}
- Li HG. The clinical observation of primary nephrotic syndrome patients treated with Huangqi injection. Yixue Xinxi [Medical Information] 2011;24(4):1989‐90. [Google Scholar]
Li 2011c {published data only}
- Li J. 30 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu [TCM Modern Distance Education of China] 2011;9(2):83. [Google Scholar]
Liang 2005 {published data only}
- Liang RQ, Lin KL, Liang D, Fan GH, Huang DX. Curative effect observation of kidney‐supplementing and blood‐flow‐promoting for refractory nephrotic syndrome. Xinzhongyi [Journal of New Chinese Medicine] 2005;37(8):30‐1. [Google Scholar]
Lin 2006 {published data only}
- Lin FJ, Gao Y, Chen SM, Zhao JZ, Huang JC, Cui MX. Effect of Huangqi on serum L10 in children with primary nephrotic syndrome. Yiyao Dao Bao [Herald of Medicine] 2006;25(5):415‐7. [Google Scholar]
Lin 2007 {published data only}
- Lin JR, Deng CE. Effect of astragalus injection in treating children with primary nephrotic syndrome. Shizhen Guoyi Guoyao [Lishizhen Medicine and Materia Medica Research] 2007;18(1):169‐70. [Google Scholar]
Liu 1990 {published data only}
- Liu JF. Huangqidihaungdang for children with nephrotic syndrome. Sichuan Zhongyi [Journal of Sichuan of Traditional Chinese Medicine] 1990;8(9):19‐20. [Google Scholar]
Liu 1994 {published data only}
- Liu BH, Dai EL, Cao TM, Zhang YH. Study on efficacy of adult primary nephrotic syndrome with TCM‐WM therapy and its hemorrheologic effects. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 1994;14(11):658‐60. [PubMed] [Google Scholar]
Liu 1999a {published data only}
- Liu JH, Zhang YP. 61 case clinical analyse of the treatment of nephrotic syndrome in adult with high dose of Huangqi. Shanxi Linchuang Yiyao [Shanxi Clinical Medicine] 1999;8(5):344‐5. [Google Scholar]
Liu 1999b {published data only}
- Liu JS, Shi J, Wu SJ, Xu YQ. Curative effect observation of the treatment of children primary nephrotic syndrome with integrated traditional and western medicine. Shantou Daxue Yixueyuan Xuebao [Shantou University Medical College] 1999;12(2):58‐9. [Google Scholar]
Liu 2001a {published data only}
- Liu Z, Qi XL, Yuan L. Curative effect observation of Huangqi and Danggui for nephrotic syndrome. Zhongguo Jiceng Yiyao [Chinese Journal of Primary Medicine and Pharmacy] 2001;8(5):397‐8. [Google Scholar]
Liu 2001b {published data only}
- Liu ML. Clinical experience of the treatment of primary nephrotic syndrome with Huangqi injection combine other drugs. Hangkong Hangtian Yiyao [Aerospace Medicine] 2001;12(1):29‐30. [Google Scholar]
Liu 2002 {published data only}
- Liu YF. The curative effect observation of cyclophosphamide, prednisone and Huangqi for refractory nephrotic syndrome. Huaxia Yixue [Acta Medicinae Sinica] 2002;15(3):333‐4. [Google Scholar]
Liu 2003 {published data only}
- Liu LS, Wang YM, Guo Q. Effect of Huangqi injection and cyclophosphamide on bone marrow and the blood system in nephrotic syndrome patient. Shanxi Yixue Zazhi [Shanxi Medical Journal] 2003;32(12):1095‐7. [Google Scholar]
Liu 2006 {published data only}
- Liu DW, Hu SH. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Guangming Zhongyi [Guangming Traditional Chinese Medicine] 2006;21(11):62‐3. [Google Scholar]
Liu 2007a {published data only}
- Liu YS. Clinical observation of 35 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Shiyong Zhongyi Neike Zazhi [Journal of Practical Traditional Chinese Internal Medicine] 2007;21(3):52. [Google Scholar]
Liu 2007b {published data only}
- Liu YL. Clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Shandong Zhongyi Zazhi [Shandong Journal of Traditional Chinese Medicine] 2007;26(6):405‐6. [Google Scholar]
Liu 2008a {published data only}
- Liu XB. The 55 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Xiandai Yaowu Yinyong [Chinese Journal of Modern Drug Application] 2008;2(14):67. [Google Scholar]
Liu 2008b {published data only}
- Liu LC, Liu X, Feng MJ, Wang WF, He XP. The clinical observation of 30 cases primary refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Xinzhongyi [Journal of New Chinese Medicine] 2008;40(2):28‐9. [Google Scholar]
Liu 2008c {published data only}
- Liu YP, Zhu PJ. The clinical observation of 40 nephrotic syndrome patients treated with Huangqi injection. Yunnan Zhongyi Zhongyao Zazhi [Yunnan Journal of Traditional Chinese Medicine and Materia Medica] 2008;29(9):8‐9. [Google Scholar]
Liu 2008d {published data only}
- Liu XX, Li XL, Wang JL, Shi HX, Zhang LL, Xiao Q. Observation on effect of integrated Chinese and western medicine in treating refractory nephrotic syndrome. Weifang Yixueyuan Xuebao [Acta Academiae Medicinae Weifang] 2008;30(1):33‐5. [Google Scholar]
Liu 2008e {published data only}
- Liu B. 35 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Changchun Zhongyiyao Daxue Xuebao [Journal of Changchun University of Traditional Chinese Medicine] 2008;24(6):690. [Google Scholar]
Liu 2009 {published data only}
- Liu C, Yang XP, Li YP, Zhang JP, Zhao J, Chen H. The clinical effect of radix astragali on primary nephritic syndrome. Nong Hen Yi Xue [Journal of Nongken Medicine] 2009;31(5):407‐9. [Google Scholar]
Liu 2010a {published data only}
- Liu XY. The 30 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Shiyong Zhongxiyi Jiehe Linchuang [Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine] 2010;10(4):22‐3. [Google Scholar]
Liu 2010b {published data only}
- Liu Z. The clinical observation of 42 nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyiyao Daobao [Guiding Journal of Traditional Chinese Medicine] 2010;16(4):35‐6. [Google Scholar]
Lu 2001 {published data only}
- Lu. Observation of the treatment of nephrotic syndrome with Huangqi and Danggui. Huaxia Yixue [Acta Medicinae Sinica] 2001;14(3):277‐8. [Google Scholar]
Lu 2004 {published data only}
- Lu H, Wang YY, Guo MZ, Wang CL. Effect of kidney warming and astringent therapy on plasma endothelin and interleukin 2 receptor in patients with nephrotic syndrome. Zhong Xiyi Jiehe Xuebao [Journal of Chinese Integrative Medicine] 2004;2(1):17‐9. [DOI] [PubMed] [Google Scholar]
Lu 2007 {published data only}
- Lu GP, Pang XS, Hu JP. The 30 cases of nephrotic syndrome patients treated with Zi yin jiang huo of traditional Chinese medicine. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2007;28(7):834‐5. [Google Scholar]
Lu 2010 {published data only}
- Lu LM. The clinical study of child nephrotic syndrome treated with integrated Chinese and western medicine. Zhongguo Xiandai Yaowu Yinyong [Chinese Journal of Modern Drug Application] 2010;4(14):139‐40. [Google Scholar]
Luo 2002a {published data only}
- Luo M, Liu JH. Clinical observation of the effect of Huangqi injection on urine protein in nephrotic syndrome. Shiyong Zhongyi Neike Zazhi [Journal of Practical Traditional Chinese Internal Medicine] 2002;16(1):22. [Google Scholar]
Luo 2002b {published data only}
- Luo SF, Yao LY, Zhang ZF, Ye JH, Liu J, An XL. Curative effect observation of the treatment of primary nephrotic syndrome with astragalus‐angelica mixture. Linchuang Huicui [Clinical Focus] 2002;17(6):316‐7. [Google Scholar]
Luo 2009 {published data only}
- Luo B. The clinical observation of 36 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu [TCM Modern Distance Education of China] 2009;7(5):97. [Google Scholar]
Luo 2010 {published data only}
- Luo SY, Zhou G, Lu EF, Fang RM. The observation of the low risk patients with idiopathic membranous nephropathy treated with Danggui Huangqi soup and candesartan cilexetil. Xinan Guofang Yiyao [Medical Journal of National Defending Forces in Southwest China] 2010;20(3):277‐9. [Google Scholar]
Lv 2001 {published data only}
- Lv BF, Qiao YC, Chen K. 35 case analysis of the treatment of primary nephrotic syndrome with triple therapy. Zhongguo Zhong‐Xiyi Jiehe Jijiu Zazhi [Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care] 2001;8(6):365‐6. [Google Scholar]
Lv 2006 {published data only}
- Lv ML. Clinical observation on primary nephrotic syndrome treated with Huangqi and Aweisuanna. DI Shijiuci Quanguo Zhongyi Shenbing Xueshu Jiaoliuhui [The 19th National Academic Communication about Traditional Chinese Medicine of Nephrology] 2006:12‐4. [Google Scholar]
Lv 2007a {published data only}
- Lv. The clinical observation of 20 cases of children nephrotic syndrome treated with integrated Chinese and western medicine. Zhejiang Zhongyiyao Daxue Xuebao [Journal of Zhejiang Chinese Medical University] 2007;31(2):163‐4. [Google Scholar]
Lv 2007b {published data only}
- Lv YX, Fu ZJ, Hou YL. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongyi Jizhen [Journal of Emergency in Traditional Chinese Medicine] 2007;16(6):665‐6. [Google Scholar]
Ma 2006 {published data only}
- Ma JW, Zhang Z. The clinical analysis of refractory nephrotic syndrome treated with integrated Chinese and western medicine. Liaoning Zhongyi Xueyuan Xuebao [Journal of Liaoning University of Traditional Chinese Medicine] 2006;8(4):100‐1. [Google Scholar]
Ma 2007 {published data only}
- Ma WP. The research progress of nephrotic syndrome treated with Huangqi. Shanxi Zhongyi Xueyuan Xuebao [Journal of Shanxi College of Traditional Chinese Medicine] 2007;8(3):56‐8. [Google Scholar]
Mao 2006 {published data only}
- Mao YY, Cai YH, Chen J. An observation on Gynostemma pentaphyllum (Thunb) Makino and Huangqi in preventing recrudescence of children suffering from primary nephrotic syndrome. Zhongguo Fuyou Baojian [Maternal and Child Health Care of China] 2006;21:1358‐9. [Google Scholar]
Min 2008a {published data only}
- Min CY, Liu HQ. Integrated traditional Chinese and western medicine treatment of nephrotic syndrome. Zhongyiyao Xuekan [Chinese Archives of Traditional Chinese Medicine] 2008;26(3):565‐6. [Google Scholar]
Min 2008b {published data only}
- Min CY, Liu HQ. Integrated traditional Chinese and western medicine treatment of nephrotic syndrome. Zhongyiyao Xuekan [Chinese Archives of Traditional Chinese Medicine] 2008;26(3):565‐6. [Google Scholar]
Mo 2004a {published data only}
- Mo ZY, Liang D, Si TY, Huang PP, Chen XW. Effect of Huangqi on oxidative stress status in primary nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2004;5(4):209‐11. [Google Scholar]
Mo 2004b {published data only}
- Mo YM. Curative observation of the treatment of nephrotic syndrome with Huangqi and Danggui mixture. Zhongguo Linchuang Yixue [Clinical Medical Journal of China] 2004;3(12):31. [Google Scholar]
Mo 2010 {published data only}
- Mo GP. The clinical observation of refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Shiyong Yiyao [China Practical Medicine] 2010;5(5):131‐2. [Google Scholar]
Ni 2007 {published data only}
- Ni Z. The clinical observation of children refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Paper Assembly of the Chinese Medicine Pediatric Academic Seminar 2007:543‐6.
Ni 2009 {published data only}
- Ni XR. The clinical observation of 35 nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyiyao Daobao [Guiding Journal of Traditional Chinese Medicine] 2009;15(4):26‐7. [Google Scholar]
Nie 1985 {published data only}
- Nie LF. The experience of Huangqidishentang for nephrotic syndrome. Liaoning Zhongyi Zazhi [Liaoning Journal of Traditional Chinese Medicine] 1985, (5):22‐3. [Google Scholar]
Ning 2002 {published data only}
- Ning F, Wang YW, Tang C. 40 case of Huangqi injection above other medicine for children with nephrotic syndrome. Zhongyiyao Xuebao [Acta Chinese Medicine and Pharmacology] 2002;30(2):31‐2. [Google Scholar]
Niu 1999 {published data only}
- Niu HX, Zhai GY, Li XX, Xu Q. Huangqi injection as the main drug for nephrotic syndrome 27 cases. Hunan Zhongyi Zazhi [Hunan Journal of Traditional Chinese Medicine] 1999;14(1):58. [Google Scholar]
Ou 2003 {published data only}
- Ou CL, Cheng MC, Peng YM. Clinical study of the treatment of primary nephrotic syndrome with integrated traditional and western medicine. Zhongguo Linchuang Yixue Yanjiu Zazhi [Chinese Magazine of Clinical Medicinal Professional Research] 2003, (80):9‐11. [Google Scholar]
Pang 2010 {published data only}
- Pang L, Xu XS, Chen JP. The 46 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Xiandai Zhongyiyao [Modern Traditional Chinese Medicine] 2010;30(3):26‐7. [Google Scholar]
Peng 2008 {published data only}
- Peng SQ, Zhu LX. The clinical observation of refractory nephrotic syndrome treated with integrated Chinese and western medicine. Paper Assembly of Kidney Disease Branch Association of Chinese Medicine 2008:462‐4.
Peng 2010 {published data only}
- Peng R, Qiu GP, Zhong XY. The 92 cases of nephrotic syndrome patients treated with traditional Chinese medicine. Zhongguo Xiandai Yisheng [China Modern Doctor] 2010;48(1):55‐6. [Google Scholar]
Qi 1998 {published data only}
- Qi KQ. Curative effect observation of Huangqi injection for nephrotic syndrome. Linchuang Yixue [Clinical Medicine] 1998;18(11):21. [Google Scholar]
Qian 2002 {published data only}
- Qian XH. Curative effect observation of triptoryium wilfordii hook for primary nephrotic syndrome. Zhongguo Jiaotong Yixue Zazhi [Medical Journal of Communications] 2002;16(6):620‐1. [Google Scholar]
Qin 2009 {published data only}
- Qin WL, Liu GF. The study of the primary nephrotic syndrome patients treated with integrated traditional Chinese and western medicine. Zhongguo Shiyong Yiyao [China Practical Medicine] 2009;4(35):125‐6. [Google Scholar]
Qin 2010 {published data only}
- Qin YJ, Du L. The 59 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Liaoning Zhongyi Zazhi [Liaoning Journal of Traditional Chinese Medicine] 2010;37(9):1775‐6. [Google Scholar]
Qiu 2002 {published data only}
- Qiu L, Fan PJ, Han CH. Use Huangqi injection and Ligustrazine treat nephrotic syndrome above other drugs 22 case. Yiyao Dao Bao [Herald of Medicine] 2002;21(6):365‐7. [Google Scholar]
Qiu 2006 {published data only}
- Qiu X, Pan CM. Clinical observation of nephrotic syndrome patients treated with Huangqi injection. Handan Yixue Gaodeng Zhuanke Xuexiao Xuebao [Journal of Handan Medical College] 2006;19(5):406‐7. [Google Scholar]
Qiu 2010 {published data only}
- Qiu DX, Zheng LM. The clinical analysis of children nephrotic syndrome treated with integrated Chinese and western medicine. Zhejiang Zhongyiyao Daxue Xuebao [Journal of Zhejiang Chinese Medical University] 2010;34(5):739,742. [Google Scholar]
Qu 2008 {published data only}
- Qu ZJ, Wang XT. Clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Nei Meng Gu Zhong Yi Yao [Traditional Chinese Medicine and Medicinals of Inner Mongolia] 2008, (8):26‐7. [Google Scholar]
Ren 1999 {published data only}
- Ren W, Yang M, Chai SR. Huangqi injection and compound injection of red sage root for refractory nephrotic syndrome 47 case. Zhongyiyao Linchuang Zazhi [Clinical Journal of Traditional Chinese Medicine] 1999;11(2):76‐7. [Google Scholar]
Shan 2002 {published data only}
- Shan FJ, Qi K, Chen GL, Ma L, Zhou ZL. Observation of Huangqi injection for nephrotic syndrome. Renmin Junyi [People's Military Surgeon] 2002;45(12):716‐7. [Google Scholar]
Shen 2002 {published data only}
- Shen Y, Zhang JL, Yuan HL, Zhao S, Chen F. Clinical observation of the treatment of nephrotic syndrome with Huangqi injection. Zhongguo Zhongyao Zazhi [China Journal of Chinese Materia Medica] 2002;23(1):23. [Google Scholar]
Shen 2003 {published data only}
- Shen J. 68 case generalization of integrated traditional and western medicine for primary nephrotic syndrome. Hunan Zhongyi Zazhi [Hunan Journal of Traditional Chinese Medicine] 2003;19(2):16‐7. [Google Scholar]
Shen 2005 {published data only}
- Shen SJ, Hu ZX, Wang T. Clinical effective observation of Huangqi injection and prednisone for primary nephrotic syndrome. Anhui Yiyao [Anhui Medical and Pharmaceutical Journal] 2005;26(5):409‐11. [Google Scholar]
Shi 2001 {published data only}
- Shi L, Zhou XR. 57 case of integrated traditional and western medicine for nephrotic syndrome. Hebei Yixue [Hebei Medicine] 2001;33(2):31‐2. [Google Scholar]
Shi 2003a {published data only}
- Shi JL, Xie Y, He ZG, Chen KZ. 80 case clinical observation of chaiqidaipaoji and corticosteroid for primary nephrotic syndrome. Zhongyi Zazhi [Journal of Traditional Chinese Medicine] 2003;44(8):601‐2. [Google Scholar]
Shi 2003b {published data only}
- Shi P, Lin WM, Fu ZG. Clinical study on the treatment of nephrotic syndrome with high dose Huangqi injection. Zhongshan Yike Daxue [New Chinese Medicine] 2003;2(12):30‐1. [Google Scholar]
Shi 2004 {published data only}
- Shi W, Liang GZ, Guo AZ, Li XZ. The effect of Huangqi on plasma protein and blood fat in children with nephrotic syndrome. Beijing Zhongyi [Beijing Journal of Traditional Chinese Medicine] 2004;11(3):36‐7. [Google Scholar]
Shi 2007 {published data only}
- Shi Q, Mo YL. The observation of 30 cases of refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Hunan Zhongyi Zazhi [Hunan Journal of Traditional Chinese Medicine] 2007;23(1):26‐7. [Google Scholar]
Shi 2010 {published data only}
- Shi L, Chen X, Zhou LN. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Liaoning Zhongyi Xueyuan Xuebao [Journal of Liaoning University of Traditional Chinese Medicine] 2010;12(1):151‐2. [Google Scholar]
Song 1999a {published data only}
- Song HB, Chen WQ. Integrated traditional and western medicine for nephrotic syndrome 30 case. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 1999;20(3):146. [Google Scholar]
Song 1999b {published data only}
- Song SY, Wang GF. Huangqi combine other drugs for nephrotic syndrome 91 case. Shiyong Erke Linchuang Zazhi [Journal of Applied Clinical Pediatrics] 1999;14(5):301. [Google Scholar]
Song 2003 {published data only}
- Song SR, Duan LY. Observation of the treatment of nephrotic syndrome with integrated traditional and western medicine 40 cases. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2003;19(4):199. [Google Scholar]
Sun 2004a {published data only}
- Sun XY, Li GM. Curative observation of the treatment of primary nephrotic syndrome with Huangqi injection. Zhonghua Xiandai Linchuang Yixue Zazhi [Chinese Journal of Current Clinical Medicine] 2004;2(3):234‐5. [Google Scholar]
Sun 2004b {published data only}
- Sun ZJ. Integrated traditional and western medicine for nephrotic syndrome 40 case. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2004;25(4):306‐7. [Google Scholar]
Sun 2009a {published data only}
- Sun X. The clinical study of 46 cases children primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyi Erke Zazhi [Journal of Pediatrics of Traditional Chinese Medicine] 2009;5(5):35‐7. [Google Scholar]
Sun 2009b {published data only}
- Sun ZL. The 40 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Shandong Zhongyi Zazhi [Shandong Journal of Traditional Chinese Medicine] 2009;28(8):564‐5. [Google Scholar]
Sun 2010 {published data only}
- Sun QK. The clinical study of 36 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Yunnan Zhongyi Zhongyao Zazhi [Yunnan Journal of Traditional Chinese Medicine and Materia Medica] 2010;31(10):18‐9. [Google Scholar]
Tan 2010 {published data only}
- Tan GL. Clinical observation of 28 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2010;26(2):96‐7. [Google Scholar]
Tang 2000a {published data only}
- Tang SF, Hong QG. Clinical observation on the treatment of the nephrotic syndrome with Shenfukang. Guangzhou Zhongyiyao Daxue Xuebao [Journal of Guangzhou University of Traditional Chinese Medicine] 2000;17(2):134‐6. [Google Scholar]
Tang 2000b {published data only}
- Tang ZQ. Primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2000;1(4):235‐6. [Google Scholar]
Tang 2006 {published data only}
- Tang ZQ. Primary nephrotic syndrome patients treated with integrated Chinese and western medicine. The Fourth International Conference of Combining Traditional Chinese and Western Medicine Kidney Paper Assembly 2006:227‐8.
Tang 2007 {published data only}
- Tang SR. The 38 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Sichuan Zhongyi [Journal of Sichuan of Traditional Chinese Medicine] 2007;25(5):38‐9. [Google Scholar]
Tong 2003 {published data only}
- Tong DG, Zhang XM. Clinical observation of the treatment of nephrotic syndrome with Huangqi and Danggui mixture. Shiyong Yiji Zazhi [Journal of Practical Medical Techniques] 2003;10(6):613‐4. [Google Scholar]
Wan 2000 {published data only}
- Wan YL, Zhang JR. Curative observation on the treatment of nephrotic syndrome with integrated traditional and western medicine. Hebei Zhongyi [Hebei Journal of Traditional Chinese Medicine] 2000;22(8):626. [Google Scholar]
Wang 1992 {published data only}
- Wang YQ, Zou HQ, Gao JC, Xu QR, Wu HS. Study on the effect of Huangqi and Ciwujia on children with refractory nephrotic syndrome. Huazhong Keji Daxue Xuebao (Yixueban) [Acta Medicinae Universitatis Scientiae et Technologiae Huazhong] 1992;30(3):137‐8. [Google Scholar]
Wang 1997 {published data only}
- Wang SH. Clinical observation of Huangqi for children nephrotic syndrome patients. Journal of Qiannan Medical College for Nationalities 1997;10(2):37‐8. [Google Scholar]
Wang 1999 {published data only}
- Wang XM, Guo W, Wang L, Wang J, Wang L, Zhao HL, et al. Clinical observation of Huangqi injection for nephrotic syndrome 50 case. Heilongjiang Yi Xue [Heilongjiang Medical Journal] 1999;6:20. [Google Scholar]
Wang 2000a {published data only}
- Wang XF, Li H. Clinical observation of the treatment of nephrotic syndrome with integrated traditional and western medicine. Hebei Zhongyi [Hebei Journal of Traditional Chinese Medicine] 2000;22(2):149‐50. [Google Scholar]
Wang 2001a {published data only}
- Wang QY, Wang XY, Li YX, Qing L, Wang RL. Clinical study on the treatment of primary nephrotic syndrome with Fufangsheqi decoction. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2001;2(6):337‐8. [Google Scholar]
Wang 2001b {published data only}
- Wang JS, Sun SL. Integrated traditional and western medicine for nephrotic syndrome 40 case. Shandong Zhongyi Zazhi [Shandong Journal of Traditional Chinese Medicine] 2001;20(6):359‐60. [Google Scholar]
Wang 2001c {published data only}
- Wang YJ. Clinical observation of primary nephrotic syndrome in children treated with Huangqi injection. Jilin Zhongyiyao [Jilin Journal of Traditional Chinese Medicine] 2001, (4):21. [Google Scholar]
Wang 2002a {published data only}
- Wang ZW, Zhang F, Wen XQ. A forward effect study on Huangqi heji for minimizing the recurring of kidney disease syndrome. Zhonghua Shiyong Zhongxiyi Zazhi [Chinese Journal of the Practical Chinese with Modern Medicine] 2002, (1):59. [Google Scholar]
Wang 2002b {published data only}
- Wang SH. Clinical observation on the treatment of nephrotic syndrome in children with Huangqi. Zhonghua Yixue Xiezuo Zazhi [Chinese Journal of Medical Writing] 2002;9(8):618‐9. [Google Scholar]
Wang 2002c {published data only}
- Wang YF, Shi WZ. Clinical observation on the treatment of nephrotic syndrome in children. Zhonghua Yixue Xiezuo Zazhi [Chinese Journal of Medical Writing] 2002;9(8):618‐9. [Google Scholar]
Wang 2002d {published data only}
- Wang QY, Wang XY, Ji J. Clinical study on the treatment of primary nephrotic syndrome with Huangqi injection and western medicine. Zhonghua Linchuang Yiyao Zazhi [Journal of Chinese Clinical Medicine] 2002;3(20):49‐50. [Google Scholar]
Wang 2002e {published data only}
- Wang YF, Shi WZ. Curative effect observation of Huangqi injection for primary nephrotic syndrome. Chenzhou Yixue Gaodeng Zhuanke Xuexiao Xuebao [Journal of Chenzhou Medical College] 2002;4(4):32‐4. [Google Scholar]
Wang 2002f {published data only}
- Wang ZG, Ye SY, Tao YH. Clinical observation of nephrotic syndrome in children treated with Huangqi granules. Huaxi Yixue [West China Medical Journal] 2002;17(4):552. [Google Scholar]
Wang 2003 {published data only}
- Wang MJ, Liu JS, Zhu ZH, Fan XJ. The effect of Huangqi with Danggui mixture on primary nephrotic syndrome. Linchuang Shenzangbing Zazhi [Journal of Clinical Nephrology] 2003;3(4):177‐8. [Google Scholar]
Wang 2004a {published data only}
- Wang HY, Li ZL, Qin ZH. The effect of Huangqi and Xuesaitong on haemorrheology in patients with nephrotic syndrome. Beijing Daxue Xuebao (Yixueban) [Journal of Peking University (Health Sciences)] 2004;5(11):74. [Google Scholar]
Wang 2004b {published data only}
- Wang MJ, Zhu ZH, Chen ZH, Yuan H. The effect of Huangqi with Danggui mixture and losartan on idiopathic nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2004;5(5):270‐2. [Google Scholar]
Wang 2006b {published data only}
- Wang YL, Shi SL. The observational study of 65 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Shiyong Zhongyi Neike Zazhi [Journal of Practical Traditional Chinese Internal Medicine] 2006;20(6):629. [Google Scholar]
Wang 2006c {published data only}
- Wang SH. Clinical observation of children simple nephrotic syndrome treated with integrated Chinese and western medicine. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2006;15(18):2505‐6. [Google Scholar]
Wang 2007a {published data only}
- Wang WF, Chen XH. The 36 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Jiangxi Zhongyiyao [Jiangxi Journal of Traditional Chinese Medicine] 2007;38(294):53‐4. [Google Scholar]
Wang 2007b {published data only}
- Wang ZM. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongyiyao Xinxi Zazhi [Chinese Journal of Information on Traditional Chinese Medicine] 2007;14(2):66‐7. [Google Scholar]
Wang 2008a {published data only}
- Wang Y. The study of 40 cases refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Shandong Zhongyi Zazhi [Shandong Journal of Traditional Chinese Medicine] 2008;27(12):829‐30. [Google Scholar]
Wang 2008b {published data only}
- Wang YR, Zhang Z, Xiao H. Clinical observation of 48 primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Yunnan Zhongyi Zhongyao Zazhi [Yunnan Journal of Traditional Chinese Medicine and Materia Medica] 2008;29(2):22‐3. [Google Scholar]
Wang 2009a {published data only}
- Wang QZ. The 20 cases of primary nephrotic syndrome patients treated with medicine of the prescription and hormone. Zhongguo Dangdai Yiyao [China Modern Medicine] 2009;16(10):79‐80. [Google Scholar]
Wang 2010a {published data only}
- Wang ZL. The clinical observation of refractory nephrotic syndrome treated with integrated Chinese and western medicine. Yatai Chuantong Yiyao [Asia‐Pacific Traditional Medicine] 2010;6(2):40‐1. [Google Scholar]
Wang 2010b {published data only}
- Wang XT, Liu X, Qu Y, Meng QT. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Wuzhenxue Zazhi [Chinese Journal of Misdiagnostics] 2010;10(4):788‐9. [Google Scholar]
Wang 2010c {published data only}
- Wang F. Chinese and Western medicine treatment of children with primary nephrotic syndrome of clinical observation. Master Dissertation of Hubei University of Chinese Medicine 2010:1‐26.
Wang 2010d {published data only}
- Wang HR, Xue AQ, Liu S. The clinical observation of nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Minzu Minjian Yiyao [Chinese Journal of Ethnomedicine and Ethnopharmacy] 2010, (10):134‐5. [Google Scholar]
Wang 2010e {published data only}
- Wang FQ. Clinical effect of 42 cases of children nephrotic syndrome patients treated with hormone and traditional Chinese medicine. Zhongguo Yiliao Qianyan [National Medical Frontiers of China] 2010;5(17):50,54. [Google Scholar]
Wang 2011 {published data only}
- Wang Q, Hou XQ, Cui XJ. The effect on the immune state of the primary nephrotic syndrome patients treated with the Huangqi and Baihuasheshecao tang. Shiyong Yiyao Zazhi [Practical Journal of Medicine & Pharmacy] 2011;51(9):69‐70. [Google Scholar]
Wei 1999 {published data only}
- Wei LB, Zhang ZF, Li ZJ. Clinical observation on integrated treatment of western medicine and traditional Chinese medicine in senile primary nephrotic syndrome. Nanfang Yeie Daxue Xuebao [Journal of Southern Medical University] 1999;19(41):41‐3. [Google Scholar]
Wei 2000a {published data only}
- Wei LB, Lu YY, Ye RG, Luan T. Clinical observation on integrated treatment of western medicine and traditional Chinese medicine in adult hormone dependent primary nephrotic syndrome. Nanjing Zhongyiyao Daxue Xuebao (Ziran Kexue Ban) [Journal of Nanjing University of Traditional Chinese Medicine] 2000;16(4):209‐11. [Google Scholar]
Wei 2000b {published data only}
- Wei F. The effect of Huangqi with Danggui mixture on blood fat and apolipoprotein in nephrotic syndrome. Hainan Yixue [Hainan Medical Journal] 2000;11(2):63‐4. [Google Scholar]
Wei 2000c {published data only}
- Wei MM. Clinical observation on the treatment of nephrotic syndrome with Huangqi. Linchuang Huicui [Clinical Focus] 2000;15(18):833‐4. [Google Scholar]
Wei 2000d {published data only}
- Wei LB, Ye RG, Chen XH, Li ZJ, Lv RH, Luan T. Clinical observation of elderly idiopathic nephrotic syndrome treated with integrated traditional Chinese and western medicine. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2000;20(2):99‐101. [PubMed] [Google Scholar]
Wei 2002a {published data only}
- Wei XJ, Zhu PJ. The effect of Huangqi on coagulation and fibrinolysis in refractory nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2002;3(12):709‐10. [Google Scholar]
Wei 2002b {published data only}
- Wei XM. 62 case of Leidan Siwu Tang and corticosteroid for nephrotic syndrome. Zhongyi Yanjiu [Traditional Chinese Medicinal Research] 2002;15(3):48‐9. [Google Scholar]
Wei 2003 {published data only}
- Wei XM. 40 case of the treatment of nephrotic syndrome with benefit kidney, reinforce spleen and betake sputum. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2003;24(4):306‐8. [Google Scholar]
Wei 2004 {published data only}
- Wei HL, Fan G. 75 case clinical observation of the treatment of nephrotic syndrome with Huangqi injection. Zhongyuan Yikan [Central Plains Medical Journal] 2004;31(9):35‐6. [Google Scholar]
Wei 2005 {published data only}
- Wei HY. Analysis of Huangqi injection for primary nephrotic syndrome 30 case. Zhonghua Zonghe Yixue Zazhi [Chinese Journal of Synthetic Medicine] 2005;6(6):562‐3. [Google Scholar]
Wei 2009 {published data only}
- Wei YZ. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Yiyao Zhinan [Guide of China Medicine] 2009;7(23):60‐1. [Google Scholar]
Wen 2005 {published data only}
- Wen GY, Huang HQ. Clinical study on the treatment of primary nephrotic syndrome with integrated traditional and western medicine. Jiangsu Zhongyi [Jiangsu Journal of Traditional Chinese Medicine] 2005;26(2):16‐8. [Google Scholar]
Wen 2007 {published data only}
- Wen WB. The study of 60 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Shijie Zhongxiyi Jiehe Zazhi [World Journal of Integrated Traditional and Western Medicine] 2007;2:138‐9. [Google Scholar]
Wu 1998b {published data only}
- Wu YS, Yang JH, Jiang LP, Wang ZK. Clinical study on improving effect of Bu Yang Huan Wu decoction on plasma thromboxane B2, 6‐keto prostaglandin F1a, endothelin and calcitonin gene related peptide in primary nephrotic syndrome patients. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 1998;18(12):715‐7. [PubMed] [Google Scholar]
Wu 1998c {published data only}
- Wu XX, Zhang X. The development and effect on nephrotic syndrome of Shenkang capsule. Zhongyaocai [Journal of Chinese Medicinal Materials] 1998;27(3):161‐162. [Google Scholar]
Wu 1999 {published data only}
- Wu JY, Ye RG. Clinical control observation on treatment of primary nephrotic syndrome with adrenocortical hormone and the syndrome differentiation method. Guangxi Zhongyiyao [Guangxi Journal of Traditional Chinese Medicine] 1999;22(3):1‐4. [Google Scholar]
Wu 2002 {published data only}
- Wu SJ. Clinical study on the treatment of primary nephrotic syndrome with Huangqi and Danshen apozem. Zhejiang Zhongyi Xueyuan Xuebao [Journal of Zhejiang University of Traditional Chinese Medicine] 2002;26(6):29‐30. [Google Scholar]
Wu 2005a {published data only}
- Wu HB. The effect analysis of the treatment of children nephrotic syndrome with Huangqi injection above other medicine. Yixue Xinzhi Zazhi [Journal of New Medicine] 2005;15(2):69. [Google Scholar]
Wu 2009a {published data only}
- Wu MA. Clinical observation of 38 cases of steroid‐ resistant nephrotic syndrome treated with integrated Chinese and western medicine. Shijie Zhongxiyi Jiehe Zazhi [World Journal of Integrated Traditional and Western Medicine] 2009;14(2):120‐2. [Google Scholar]
Wu 2009b {published data only}
- Wu YJ. Clinical observation of nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Shiyong Yiyao Zazhi [Chinese Journal of Practical Medicine] 2009;4(11):42‐3. [Google Scholar]
Xi 2003 {published data only}
- Xi HM, Zhu PJ. The effect of Huangqi injection immunologic function in nephrotic syndrome. Zhejiang Zhongxiyi Jiehe Zazhi [Zhejiang Journal of Integrated Traditional Chinese and Western Medicine] 2003;13(12):746‐8. [Google Scholar]
Xiang 2007 {published data only}
- Xiang QY. The clinical observation of primary nephrotic syndrome adult patients treated with integrated Chinese and western medicine. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2007;16(35):5233, 5238. [Google Scholar]
Xiao 2000 {published data only}
- Xiao Q, Zhao XL, Guo M, Zhang Y. Assessment on application of Chinese herbs in process of corticosteroid reduction in nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2000;20(10):725‐6. [PubMed] [Google Scholar]
Xie 2008 {unpublished data only}
- Xie GB. The experience of 20 hormone resistance nephrotic syndrome patients treated with combining traditional Chinese and western medicine. The 2008 Annual Meeting of Gansu Province Traditional Chinese Medical Association 2008:145‐6.
Xie 2010 {published data only}
- Xie XL, Peng Y. The clinical observation of prevent recurrent infections of nephrotic syndrome patients treated with Huangqi. Hubei Zhongyi Zazhi [Hubei Journal of Traditional Chinese Medicine] 2010;32(5):9‐10. [Google Scholar]
Xiu 2002 {published data only}
- Xiu JH, Xue LF, Cong YH, Xu XY, Shi CH. Clinical observation of the effect of Huangqi on serum immune globulin serum protein in children with nephrotic syndrome. Zhonghua Shiyong Zhongxiyi Zazhi [Chinese Journal of the Practical Chinese with Modern Medicine] 2002, (3):306. [Google Scholar]
Xu 1999 {published data only}
- Xu KL, Fan HC. 30 case of Huangqi and Qiyezaodaina injection for nephrotic syndrome. Zhongcheng Yao [Chinese Traditional Patent Medicine] 1999;21(2):75‐6. [Google Scholar]
Xu 2000a {published data only}
- Xu PJ, Li Q. The effect of Huangqi on the serum globulins and haemocyanin on children with nephrotic syndrome. Shiyong Erke Linchuang Zazhi [Journal of Applied Clinical Pediatrics] 2000;15(5):278‐9. [Google Scholar]
Xu 2002a {published data only}
- Xu JF, Guo QZ, Song JG, Li YY. Clinical analysis on treating primary nephrotic syndrome with Huangqi and Chuanqiongqin. Neimenggu Minzu Daxue Xuebao [Journal of Inner Mongolia University for Nationalities] 2002;17(2):57‐9. [Google Scholar]
Xu 2002b {published data only}
- Xu JH, Zhou R. Effect of Huangqi injection on platelet activation in nephrotic syndrome patient. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2002;3(4):225. [Google Scholar]
Xu 2004 {published data only}
- Xu AH, Wang F. Curative observation of the treatment of refractory nephrotic syndrome with integrated traditional Chinese medicine and western medicine. Zhonghua Shiyong Zhongxiyi Zazhi [Chinese Journal of the Practical Chinese with Modern Medicine] 2002, (3):306. [Google Scholar]
Xu 2006 {published data only}
- Xu JJ, Zhang YM. The effect of Shenkangsan on nephrotic syndrome and haemorrheology. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2006;27(8):924‐6. [Google Scholar]
Xu 2008a {published data only}
- Xu ZY. 40 cases of primary nephrotic syndrome patients treated with Huangqi. Zhongwai Yiliao [China Foreign Medical Treatment] 2008, (25):46. [Google Scholar]
Xu 2008b {published data only}
- Xu YW. Primary nephrotic syndrome patients treated with integrated Chinese and western medicine. 21st Association of Chinese Medicine Academic Conference Papers Assembly Branch of Kidney Disease 2008:296‐7.
Xu 2009 {published data only}
- Xu ZK. Clinical analysis of 37 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Youjiang Yixue [Youjiang Medical Journal] 2009;37(6):712‐3. [Google Scholar]
Xue 2004b {published data only}
- Xue N, Pang YB. Huangqi injection for refractory nephrotic syndrome. Zhongwai Yiliao [China and Foreign Medical Treatment] 2004;2(12):59‐61. [Google Scholar]
Xue 2006 {published data only}
- Xue XJ, Chai HY, Wei YL. The effect of Huangqi with Danggui mixture on primary nephrotic syndrome. Jiankang Dashiye: Yiyao Xueshuban [Medical Academic Forum] 2006;14(6):102‐3. [Google Scholar]
Xue 2007 {published data only}
- Xue ZH, Huang BT. The clinical observation of nephrotic syndrome patients treated with integrated Chinese and western medicine. Xiandai Yiyao Weisheng [Modern Medicine Health] 2007;23(18):2715‐6. [Google Scholar]
Yan 2008 {published data only}
- Yan WY. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Xiandai Yiyao Weisheng [Modern Medicine Health] 2008;24(6):854‐5. [Google Scholar]
Yang 2000 {published data only}
- Yang AH, Sun Y. Curative effect analysis of Huangqi and corticosteroid for primary nephrotic syndrome 35 case. Nongken Yixue [Agricultural Reclamation Medicine] 2000;22(6):395‐6. [Google Scholar]
Yang 2002a {published data only}
- Yang B, Yang H. The effect of Huangqi injection on immunologic function and urine protein in nephrotic syndrome patient. Zhongguo Yaoye [China Pharmaceuticals] 2002;11(7):74‐5. [Google Scholar]
Yang 2002b {published data only}
- Yang XD, Shan HY, Tian YJ, Lai SF, Du XC, Ding GY, et al. Clinical observation of the treatment of primary nephrotic syndrome with Huangqi injection. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2002;3(8):482. [Google Scholar]
Yang 2004 {published data only}
- Yang WM. Curative observation on the treatment of nephrotic syndrome with corticosteroid, Huangqi and Danshen injection. Guizhou Yiyao [Guizhou Medical Journal] 2004;28(8):728‐9. [Google Scholar]
Yang 2005 {published data only}
- Yang XY. The use of Huangqi in nephrotic syndrome. She Zhi [Journal of Snake] 2005;17(1):34‐5. [Google Scholar]
Yang 2006a {published data only}
- Yang YT. Effective observation on the treatment of adult refractory nephrotic syndrome with Huangqi and Danggui mixture. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2006;7(7):423. [Google Scholar]
Yang 2006b {published data only}
- Yang BJ. The clinical observation of 60 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Linchuang He Shiyan Yixue Zazhi [Journal of Clinical and Experimental Medicine] 2006;5(6):753‐4. [Google Scholar]
Yang 2007 {published data only}
- Yang XM, Liu H. The 16 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Shiyong Zhongyi Neike Zazhi [Journal of Practical Traditional Chinese Internal Medicine] 2007;21(9):51. [Google Scholar]
Yang 2008 {published data only}
- Yang J. The 50 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Guangming Zhongyi [Guangming Traditional Chinese Medicine] 2008;23(12):1989. [Google Scholar]
Yang 2010 {published data only}
- Yang H. The 30 cases of primary syndrome patients treated with integrated Chinese and western medicine. Zhongyi Yanjiu [Traditional Chinese Medicinal Research] 2010;23(12):44‐5. [Google Scholar]
Yao 2010 {published data only}
- Yao JB. 30 cases of spleen kidney yang xu nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyi Yanjiu [Traditional Chinese Medicinal Research] 2010;23(8):41‐3. [Google Scholar]
Ye 1999 {published data only}
- Ye XL, Ye J, Zhang GH, Xu LP, Wei MY. Clinical discuss of Danbailing chongji for nephrotic syndrome. Zhongguo Weixunhuan [Journal of Chinese Microcirculation] 1999;3(4):252. [Google Scholar]
Ye 2002 {published data only}
- Ye XB. Clinical observation of the treatment of nephrotic syndrome with Huangqi and Danshen injection. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2002;12(7):425‐6. [Google Scholar]
Yi 2006 {published data only}
- Yi Q, Cui XJ, Pan L. Huangqi and Baihuasheshecao for primary nephrotic syndrome. Hubei Zhongyi Zazhi [Hubei Journal of Traditional Chinese Medicine] 2006;28(9):11‐2. [Google Scholar]
Yin 2000 {published data only}
- Yin XB. Chuanqiongqin and Huangqi injection combine other drugs for nephrotic syndrome. Jinggangshan Yizhuan Xuebao [Journal of Jinggangshan Medical College] 2000;7(1):61‐2. [Google Scholar]
Yin 2009 {published data only}
- Yin K. The clinical observation comparison of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Minzu Minjian Yiyao [Chinese Journal of Ethnomedicine and Ethnopharmacy] 2009;18(10):96. [Google Scholar]
Yu 2000 {published data only}
- Yu KY, Huang XH, Li WL, Hu JP, Li HD, Pan CS, et al. Clinical observation on treatment of pediatric intractable nephropathy with modified taohongsiwu decoction. Zhongguo Zhongxiyi Jiehe Zqzhi [Chinese Journal of Integrated Traditional and Western Medicine] 2000;20(11):831‐3. [PubMed] [Google Scholar]
Yu 2001 {published data only}
- Yu L, Wong ZY, Huang YH, Zhang YX, Zhuo MY. Effect of Huangqi injection on cytokines production and gene expression of in children with nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2001;2(9):523‐5. [Google Scholar]
Yu 2003 {published data only}
- Yu L. Curative analysis of the effect of Huangqi injection on nephrotic syndrome in children. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2003;12(6):572‐3. [Google Scholar]
Yu 2005a {published data only}
- Yu WM, Liu GY. Curative observation of the treatment of adult refractory nephrotic syndrome with Huangqi injection. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2005;6(3):41‐2. [Google Scholar]
Yu 2005b {published data only}
- Yu XY, Tian Y, Shi J. Curative observation of the treatment of refractory nephrotic syndrome with integrated traditional and western medicine 30 case. Xinzhongyi [Journal of New Chinese Medicine] 2005;37(3):47‐8. [Google Scholar]
Yu 2005c {published data only}
- Yu P, Wang LF, Wang FX. Curative effect observation of Huangqi on blood fat in nephrotic syndrome patient. Ningxia Yixue Zazhi [Ningxia Medical Journal] 2005;27(1):62. [Google Scholar]
Yu 2006 {published data only}
- Yu M, Yu J, Tian Q, Shi YX, Wang AN. The clinical observation of steroid‐dependent nephrotic syndrome treated with integrated Chinese and western medicine. Zhongyiyao Xuekan [Chinese Archives of Traditional Chinese Medicine] 2006;24(12):2224‐5. [Google Scholar]
Yu 2007 {published data only}
- Yu CH. The clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Journal of Xianning College 2007;21(6):505‐6. [Google Scholar]
Yu 2008 {published data only}
- Yu WJ. The 40 cases of refractory nephrotic syndrome patients treated with integrated Chinese and western medicine. Shiyong Zhongyi Neike Zazhi [Journal of Practical Traditional Chinese Internal Medicine] 2008;22(5):56. [Google Scholar]
Yu 2009 {published data only}
- Yu WZ, Gao GS, He XS, Jin XB. A clinical study of the effect of astragale injection on serum lipids in nephrotic syndrome patients. Assembly of 10th National Integrated Traditional Chinese and Western Academic Nephrotic Conference 2009:677‐9.
Yuan 2002 {published data only}
- Yuan ML, Cao WM. Clinical observation of Huangqi injection for nephrotic syndrome 32 case. Shandong Yiyao [Shandong Medical Journal] 2002;35:327‐8. [Google Scholar]
Yuan 2006 {published data only}
- Yuan YH, Zhou YQ, Bao GX, Shen J, Yuan Y. The clinical observation of the 60 primary nephrotic syndrome patients treated with integrated traditional Chinese and western medicine. Heilongjiang Yiyao [Heilongjiang Medicine Journal] 2006, (4):10‐2. [Google Scholar]
Yuan 2008a {published data only}
- Yuan BR. The clinical observation of nephrotic syndrome patients treated with integrated Chinese and western medicine. Yiyao Dao Bao [Herald of Medicine] 2008;5(28):43‐4. [Google Scholar]
Yuan 2008b {published data only}
- Yuan XT. 25 cases children simple nephrotic syndrome patients treated with integrated Chinese and western medicine. Shiyong Zhongyi Neike Zazhi [Journal of Practical Traditional Chinese Internal Medicine] 2008;22(8):38‐9. [Google Scholar]
Yue 2006 {published data only}
- Yue YT, Wang QM, Song ZM. Ci Wu Jia with Huangqi injection. Yiyao Luntan Zazhi [Journal of Medical Forum] 2006;27(1):62‐3. [Google Scholar]
Zang 2007 {published data only}
- Zang XL. The 40 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Shiyong Yiyao [China Practical Medicine] 2007;2(29):74. [Google Scholar]
Zeng 2008 {published data only}
- Zeng H. Clinical observation on the treatment of renal syndrome with traditional Chinese medicine and western medicine. Zhongguo Redai Yixue [China Tropical Medicine] 2008;8(5):761‐2. [Google Scholar]
Zhang 1993 {published data only}
- Zhang JL, Wang GZ. The integrated traditional and western medicine for nephrotic syndrome 41 case. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 1993;14(4):158. [Google Scholar]
Zhang 1994 {published data only}
- Zhang FS, Liu YH. Integrated Chinese and western medicine for refractory nephrotic syndrome 44 case. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 1994;15(4):153. [Google Scholar]
Zhang 1997 {published data only}
- Zhang M, Sun SH, Wang W. Kangshentang for refractory nephrotic syndrome 46 case. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 1997;13(3):4‐5. [Google Scholar]
Zhang 1998 {published data only}
- Zhang SR, Zhang WH. 41 cases of primary nephrotic syndrome patients treated with Buyanghuanwu soup. Zhongguo Xiangcun Yiyao [Chinese Rural Medicine] 1998;26(6):44. [Google Scholar]
Zhang 2001a {published data only}
- Zhang CY, Zhang JR, Ding T, Geng YQ. Huangqi with Danggui mixture and western medicine for nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2001;2(9):224. [Google Scholar]
Zhang 2001b {published data only}
- Zhang WP, Hu XY, Song GW, Xie RZ. Clinical observation on the treatment of children nephrotic syndrome with reinforce Qi active blood agent. Xinzhongyi [Journal of New Chinese Medicine] 2001;33(2):31‐2. [Google Scholar]
Zhang 2001c {published data only}
- Zhang GH, Shen ZW, Sun YL, Huang YJ. Clinical observation of nephrotic syndrome in children treated with Huangqi. Linchuang Huicui [Clinical Focus] 2001;16(21):991. [Google Scholar]
Zhang 2002a {published data only}
- Zhang MZ, Yu L. Clinical observation of the treatment of nephrotic syndrome in children with Huangqi and hormone. Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional and Western Medicine] 2002;3(4):300‐2. [Google Scholar]
Zhang 2002b {published data only}
- Zhang CY, Zhang JR, Ding T, Geng YQ. Huangqi with Danggui mixture and western medicine for nephrotic syndrome 50 case. Wujing Yixue [Medical Journal of the Chinese People's Armed Police Forces] 2002;13(4):224. [Google Scholar]
Zhang 2004 {published data only}
- Zhang J. Curative observation of the treatment of nephrotic syndrome with Yijitai. Zhonghua Yixue Xiezuo Zazhi [Chinese Journal of Medical Writing] 2004;11(8):1544‐5. [Google Scholar]
Zhang 2005a {published data only}
- Zhang JH, Xiao BL. Clinical observation of Huangqi for primary nephrotic syndrome with Huangqi injection. Zhonghua Xiandai Yixue Yu Linchuang [China Modern Medical and Clinical] 2005;2(9):64‐5. [Google Scholar]
Zhang 2005b {published data only}
- Zhang P, Liang JS. The effect of Huangqi with Danggui mixture and low molecular weight heparin on refractory nephrotic syndrome. Zhongguo Zhongyao Zazhi [China Journal of Chinese Materia Medica] 2005;33(17):1371‐2,1384. [Google Scholar]
Zhang 2006a {published data only}
- Zhang JP, Xie FP, Long YC. Clinical efficacy analysis of Huangqi and Bailting on preventing childrens initial nephrotic syndrome. Hainan Yixue [Hainan Medical Journal] 2006;17(10):34‐5. [Google Scholar]
Zhang 2006b {published data only}
- Zhang LY, Liu Y, Zhu TC, Guo MH. The traditional and western medicine treatment of 40 patients with frequent relapses nephrotic syndrome. Xinxiang Yixueyuan Xuebao [Journal of Xinxiang Medical College] 2006;23(6):613‐5. [Google Scholar]
Zhang 2007a {published data only}
- Zhang T. The study of primary nephrotic syndrome patients treated with integrated traditional Chinese and western medicine. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2007;16(27):3969‐70. [Google Scholar]
Zhang 2007b {published data only}
- Zhang QH. The 39 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2007;16(35):5234. [Google Scholar]
Zhang 2007c {published data only}
- Zhang L, Zhen J. The effect of decoction Shenkangling on urinary retinal‐binding protein,a1‐microglobulin,N‐acetyl‐beta‐d‐glucosaminidase of children with frequently relapsing nephrotic syndrome. Master Thesis of Fujian Traditional Chinese Medicine in 2007 2007:1‐27.
Zhang 2007d {published data only}
- Zhang WL, Chen F, Zheng J. The clinical study on the treatment of nephrotic syndrome patients with integrated Chinese and western medicine. Zhongguo Zhongyi Jizhen [Journal of Emergency in Traditional Chinese Medicine] 2007;16(2):165‐6. [Google Scholar]
Zhang 2007e {published data only}
- Zhang RZ, Cai HQ. 50 cases of primary hydremic nephritis with the traditional Chinese medicine. Henan Zhongyi Xueyuan Xuebao [Journal of Henan University of Chinese Medicine] 2007;22(129):53. [Google Scholar]
Zhang 2008a {published data only}
- Zhang HG. The clinical research on the children with primary nephrotic syndrome with the Shu Feng Qing Re Jie Du theory of traditional Chinese medicine. Master Thesis of Guangzhou University of Chinese Medicine 2008:1‐31.
Zhang 2008b {published data only}
- Zhang ZQ, Zhang WJ, Zhang SJ. The study of refractory nephrotic syndrome treated with integrated Chinese and western medicine. Zhongguo Zhongyiyao Xinxi Zazhi [Chinese Journal of Information on Traditional Chinese Medicine] 2008;15(9):51‐2. [Google Scholar]
Zhang 2008c {published data only}
- Zhang J, Chen WM. The clinical effect of 42 cases primary nephrotic syndrome patients treated with Zhangyaoyishen soup and hormone. Zhongguo Baojian Yingyang [China Health & Nutrition] 2008;17(18):19‐20. [Google Scholar]
Zhang 2008d {published data only}
- Zhang L, Wang HX, Chen MJ. The clinical observation of adults refractory primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongyi Jizhen [Journal of Emergency in Traditional Chinese Medicine] 2008;17(10):1378‐9, 1387. [Google Scholar]
Zhang 2008e {published data only}
- Zhang JH, Song XY. Clinical observation of 40 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Hebei Zhongyi [Hebei Journal of Traditional Chinese Medicine] 2008;30(9):956. [Google Scholar]
Zhang 2009a {published data only}
- Zhang MP, Zhang Q, Luo YB. The effect observation of the edema phase of the primary nephrotic syndrome treated by combining traditional Chinese and Western medicine. Liaoning Zhongyi Zazhi [Liaoning Journal of Traditional Chinese Medicine] 2009;36(4):593‐4. [Google Scholar]
Zhang 2009b {published data only}
- Zhang XP. The clinical study of nephrotic syndrome in children treated with integrated traditional Chinese and western medicine. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2009;18(26):3190‐1. [Google Scholar]
Zhang 2009c {published data only}
- Zhang MP, Zhang Q, Luo YB. Clinical observation of edema phase of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Liaoning Zhongyi Zazhi [Liaoning Journal of Traditional Chinese Medicine] 2009;36(4):593‐4. [Google Scholar]
Zhang 2010a {published data only}
- Zhang LH. Treating children PNS in TCM. Zhongyi Linchuang Yanjiu [Clinical Journal of Chinese Medicine] 2010;2(13):107‐8. [Google Scholar]
Zhang 2010b {published data only}
- Zhang AF. The observational study of 51 adult primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Xiandai Yaowu Yinyong [Chinese Journal of Modern Drug Application] 2010;4(18):178‐9. [Google Scholar]
Zhang 2010c {published data only}
- Zhang XH. The 43 cases of refractory primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2010;26(4):24,26. [Google Scholar]
Zhang 2010d {published data only}
- Zhang HQ, Huang JF. The clinical observation of 80 cases nephrotic syndrome patients treated with integrated Chinese and western medicine. Yunnan Zhongyi Zhongyao Zazhi [Yunnan Journal of Traditional Chinese Medicine and Materia Medica] 2010;31(7):15‐7. [Google Scholar]
Zhang 2010e {published data only}
- Zhang XW. Clinical observation of primary nephrotic syndrome patients treated with Huangqi injection and hormone. Zhongguo Wuzhenxue Zazhi [Chinese Journal of Misdiagnostics] 2010;10(21):5144. [Google Scholar]
Zhao 1999a {published data only}
- Zhao CL, Gao XZ, Zhang X. Binary assaulting integrated traditional and western medicine for refractory nephrotic syndrome 30 case. Qinghai Yiyao Zazhi [Qinghai Medical Journal] 1999;29(12):59. [Google Scholar]
Zhao 1999c {published data only}
- Zhao DG, Zhao X, Zhang JD. Clinical observation of the hypercoagulable state in nephrotic syndrome patients treated with Buyanghuanwu soup. Beijing Zhongyi [Beijing Journal of Traditional Chinese Medicine] 1999;18(1):21. [Google Scholar]
Zhao 2001a {published data only}
- Zhao XL, Lin SJ. Huangqi with Danggui mixture and western medicine for primary nephrotic syndrome. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2001;2(1):23‐5. [Google Scholar]
Zhao 2001b {published data only}
- Zhao HM. Curative effect observation of the treatment of primary nephrotic syndrome with Huangqi and hormone. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2001;10(4):310‐1. [Google Scholar]
Zhao 2001c {published data only}
- Zhao H. Integrated traditional and western medicine for refractory nephrotic syndrome 30 case. Shanxi Zhongyi [Shanxi Journal of Traditional Chinese Medicine] 2001;22(4):204‐5. [Google Scholar]
Zhao 2010 {published data only}
- Zhao W, Chen CH. The clinical study of child primary nephrotic syndrome patients treated with traditional Chinese medicine retention enema mixture. Yiyao Dao Bao [Herald of Medicine] 2010;7(12):118‐9. [Google Scholar]
Zhi 2010 {published data only}
- Zhi Y, Zhang WL. Clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongyi Xuebao [Journal of Chinese Medicine] 2010;25(146):133‐4. [Google Scholar]
Zhong 2002 {published data only}
- Zhong ZH, Lu JJ, Cai CD. Clinical analysis of the treatment of refractory nephrotic syndrome with hormone and triptolide. Zhongguo Zonghe Linchuang [Clinical Medicine of China] 2002;18(5):435‐6. [Google Scholar]
Zhong 2006 {published data only}
- Zhong CH. Clinical observation of Danshen and Huangqi injection for nephrotic syndrome. Hebei Yixue [Hebei Medicine] 2006;12(1):71‐2. [Google Scholar]
Zhong 2008 {published data only}
- Zhong HL, Ou WN. The clinical study of 40 cases of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Dangdai Yixue [China Contemporary Medicine] 2008, (141):90‐1. [Google Scholar]
Zhong 2009 {published data only}
- Zhong JC. The clinical research on the children with primary nephrotic syndrome with the Shu Feng Qing Re Jie Du theory of traditional Chinese medicine. Master thesis of Guangzhou University of Chinese Medicine 2009:1‐27.
Zhong 2011 {published data only}
- Zhong Y, Zhong A. The clinical observation of primary nephrotic syndrome patients treated with Huangqi and Danggui mixture. Yixue Xinxi [Medical Information] 2011, (1):216. [Google Scholar]
Zhou 2000 {published data only}
- Zhou QF, Zhang YB, Yue CL. Curative observation on the treatment of nephrotic syndrome with Huangqi and Danshen injection. Shijie Jinri Yixue Zazhi [World Journal of Medicine Today] 2000;1(5):532‐3. [Google Scholar]
Zhou 2003 {published data only}
- Zhou X, Hua XY. Observation of the effect of Huangqi injection and nourishing kidney accommodation immune on T lymphocyte subsets in nephrotic syndrome. Jiangsu Daxue Xuebao: Yixueban [Journal of Jiangsu University Medicine Edition] 2003;13(3):250‐1. [Google Scholar]
Zhou 2004 {published data only}
- Zhou JZ. The curative observation on the treatment of nephrotic syndrome with Huangqi and Danggui mixture. Hei Long Jiang Yi Yao Ke Xue [Heilongjiang Medicine and Pharmacy] 2004;27(6):50‐1. [Google Scholar]
Zhou 2006 {published data only}
- Zhou Q. Clinical analysis of nephrotic syndrome patients treated with integrated Chinese and western medicine. The Fourth International Conference of Combining Traditional Chinese and Western Medicine Kidney Paper Assembly 2006:213‐4.
Zhou 2007a {published data only}
- Zhou ZX. 42 primary nephrotic syndrome patients treated with the Chinese herb drugs and hormones in stage. Guangxi Zhongyiyao [Guangxi Journal of Traditional Chinese Medicine] 2007;30(3):20‐1. [Google Scholar]
Zhou 2007b {published data only}
- Zhou L. Clinical observation of 23 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Yixue Linchuang Yanjiu [Journal of Clinical Research] 2007;24(5):868‐9. [Google Scholar]
Zhou 2008 {published data only}
- Zhou Y, Jiang HJ. The observational study of 20 cases of nephrotic syndrome patients treated with integrated Chinese and western medicine. Binzhou Yixueyuan Xuebao [Journal of Binzhou Medical University] 2008;31(6):473‐4. [Google Scholar]
Zhou 2009a {published data only}
- Zhou Z, Dai W, Huang J. The observational study of 28 adults relapsing primary nephrotic syndrome treated with integrated traditional Chinese and western medicine. Yunnan Zhongyi Zhongyao Zazhi [Yunnan Journal of Traditional Chinese Medicine and Materia Medica] 2009;30(12):22‐3. [Google Scholar]
Zhou 2009c {published data only}
- Zhou XR, Fu BC. The clinical observation of 40 cases nephrotic syndrome patients treated with integrated Chinese and western medicine. Shanxi Zhongyi Xueyuan Xuebao [Journal of Shanxi College of Traditional Chinese Medicine] 2009;10(3):46‐7. [Google Scholar]
Zhu 2001 {published data only}
- Zhu W, Zhu J, Wang GZ, Gao LL, Gao Y. 35 case curative experience of children primary nephrotic syndrome. Henan Yike Daxue Xuebao [Journal of Henan Medical College] 2001;36(2):211‐2. [Google Scholar]
Zhu 2002 {published data only}
- Zhu XP. Clinical observation of the treatment of refractory nephrotic syndrome with Chinese traditional medicine and hormone. Shanghai Zhongyiyao Zazhi [Shanghai Journal of Traditional Chinese Medicine] 2002;36(9):18‐9. [Google Scholar]
Zhu 2004 {published data only}
- Zhu PJ, Wei XJ, Zhou X, Liu YP, Zhao H. Clinical study of the effect of Huangqi injection on blood fat and plasma albumin in nephrotic syndrome. Linchuang Shenzangbing Zazhi [Journal of Clinical Nephrology] 2004;4(1):30‐2. [Google Scholar]
Zhu 2006 {published data only}
- Zhu PJ, Zhou X, Zhao H. Curative effect of prednisone combined with traditional Chinese medicine on child with hydropigenous nephritis according to stage and symptom. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2006;15(14):1870‐2. [Google Scholar]
Zhu 2010a {published data only}
- Zhu LY. The observational study of 26 primary nephrotic syndrome patients treated with Huangqi injection and Shuxuetong. Zhejiang Zhongxiyi Jiehe Zazhi [Zhejiang Journal of Integrated Traditional Chinese and Western Medicine] 2010;45(6):425. [Google Scholar]
Zhu 2010b {published data only}
- Zhu JN. The 32 cases nephrotic syndrome patients treated with integrated Chinese and western medicine. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu [TCM Modern Distance Education of China] 2010;8(7):44. [Google Scholar]
References to studies awaiting assessment
Li 2007f {published data only}
- Li M. Clinical observation of primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Beijing Zhongyi [Beijing Journal of Traditional Chinese Medicine] 2007;26(5):289‐90. [Google Scholar]
Liu 1998 {published data only}
- Liu H, Wang YL. Curative effect observation of Huangqi injection for nephrotic syndrome. Hebei Zhongxiyi Jiehe Zazhi [Hebei Journal of Integrated Traditional and Western Medicine] 1998;7(10):1597. [Google Scholar]
Ma 2001 {published data only}
- Ma AQ, Ma SZ. Curative observation on the treatment of nephrotic syndrome with Huangqi and Yimuzao. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2001;2(9):60. [Google Scholar]
Su 2000a {published data only}
- Su LJ, Zhang YZ. Integrated traditional and western medicine for children simplex nephrotic syndrome 30 case. Zhongguo Zhongyao Zazhi [China Journal of Chinese Materia Medica] 2000;21(6):19‐20. [Google Scholar]
Tang 2005 {published data only}
- Tang JH, Zhu GH, Fang MJ, Yu QS. Primary study on the precaution recrudescent nephrotic syndrome with Huangqi above other medicine. Zhongguo Quanke Yixue [Chinese General Practice] 2005;8(14):1136‐7. [Google Scholar]
Wan 2009 {published data only}
- Wan MG, Shi W. Primary nephrotic syndrome patients treated with Huangqi and Danggui. Zhongguo Minjian Laofa [China's Naturopathy] 2009;17(10):32. [Google Scholar]
Wang 2000b {published data only}
- Wang LS. The effect observation on assisted treating adult primary nephrotic syndrome by Huangqi injection. Zhonghua Shiyong Zhongxiyi Zazhi [Chinese Journal of the Practical Chinese with Modern Medicine] 2000;13(7):1451. [Google Scholar]
Wu 1998a {published data only}
- Wu WB, Fan ZH. Protective effects of astragali injection on tubular in patients with primary nephrotic syndrome. Zhongyao Xinyao Yu Linchuang Yaoli [Traditional Chinese Drug Research & Clinical Pharmacology] 1998;14(5):33‐4. [Google Scholar]
Xue 2004a {published data only}
- Xue XJ. Curative analysis of the treatment of primary nephrotic syndrome with astragalus‐angelica mixture. Zhongguo Linchuang Yixue [Clinical Medical Journal of China] 2004;3(7):67‐8. [Google Scholar]
Zhang 2010f {published data only}
- Zhang Y, Zhang H. Therapeutic effects of Huangqi combined with routine therapy with glucocorticosteroid for treating 55 children with nephritic syndrome. Zhongguo Shiyong Yiyao Zazhi [Chinese Journal of Practical Medicine] 2010;5(8):13‐4. [Google Scholar]
Zhao 1999b {published data only}
- Zhao Y, Yuan SH. 20 case clinical observation on the treatment of the primary nephrotic syndrome in adult with Huangqi and corticosteroid. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 1999;8(12):1961. [Google Scholar]
- Zhao Y, Yuan SH. Huangqi and corticosteroid for primary nephrotic syndrome. Shoudu Yiyao [Capital Medicine] 1999;6(11):38. [Google Scholar]
Zhao 2003 {published data only}
- Zhao SY. The curative clinical observation on the treatment of primary nephrotic syndrome with Astragalus Angelica mixture. Guiyang Zhongyi Xueyuan Xuebao [Journal of Guiyang College of Traditional Chinese Medicine] 2003;25(2):17‐8. [Google Scholar]
Zhou 2009b {published data only}
- Zhou WH. The clinical analysis of nephrotic syndrome through combined treatment of traditional Chinese medicine and western medicine. Zhonghua Zhongxi Yixue Zazhi [China and Foreign Medical Journal] 2009;7(11):7‐8. [Google Scholar]
Zou 2008 {published data only}
- Zou MH. Clinical observation of 55 cases primary nephrotic syndrome patients treated with integrated Chinese and western medicine. Association of Chinese Medicine of the 21 Nephropathy Academic Conference Papers Assembly 2008:240‐1.
Additional references
Bao 2003
- Bao XI, Zhou ZL, Lin L, Wu ZP, Yu P. Western medicine with Astragalus Injection for nephritic proteinuria. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2003;19(3):132‐3. [Google Scholar]
Cattran 1999
- Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, et al. A randomized trial of cyclosporine in patients with steroid‐resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney International 1999;56(6):2220–6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cattran 2007
- Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, et al. Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: workshop recommendations. Kidney International 2007;72(12):1429–47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 2011
- Cohen EP, Sujeet K. Nephrotic syndrome. http://emedicine.medscape.com/article/244631‐overview (accessed March 2013).
Das 2009
- Das U, Dakshinamurty KV, Prasad N. Ponticelli regimen in idiopathic nephrotic syndrome. Indian Journal of Nephrology 2009;19(2):48‐52. [EMBASE: 2009390537 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deng 1998
- Deng WL, Wang YS, Xue CS. Pharmacology and applications of Chinese Materia Medica. 2nd Edition. Beijing: People's Medical Publishing House, 1998:982‐1004. [Google Scholar]
Doucet 2000
- Doucet C, Mooser V, Gonbert S, Raymond F, Chapman J, Jacobs C, et al. Lipoprotein(a) in the nephrotic syndrome: molecular analysis of lipoprotein(a) and apolipoprotein(a) fragments in plasma and urine. Journal of the American Society of Nephrology 2000;11(3):507‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration1, 2011. Available from www.cochrane‐handbook.org.
Koomans 2003
- Koomans HA. Pathophysiology of edema in idiopathic nephrotic syndrome. Nephrology Dialysis Transplantation 2003;18 Suppl 6:vi30‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lane 2011
- Lane JC. Pediatric nephrotic syndrome. http://emedicine.medscape.com/article/982920‐overview (accessed March 2013).
Li 2000
- Li J, Yu L, Li N, Wang H. Astragalus mongholicus and Angelica sinensis compound alleviates nephrotic hyperlipidemia in rats. Chinese Medical Journal 2000;113(4):310‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Li 2006f
- Li KJ. Systematic review of the primary nephrotic syndrome treated with Buyanghuanwu soup. Liaoning Zhongyi Xueyuan Xuebao [Journal of Liaoning University of Traditional Chinese Medicine] 2006;8(6):63‐4. [Google Scholar]
McCarthy 2012
- McCarthy KJ, Wassenhove‐McCarthy DJ. The glomerular basement membrane as a model system to study the bioactivity of heparan sulfate glycosaminoglycans. Microscopy & Microanalysis 2012; Vol. 18, issue 1:3‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Meyrier 2004
- Meyrier A. Nephrotic focal segmental glomerulosclerosis in 2004: an update. Nephrology Dialysis Transplantation 2004;19(10):2437‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orth 1998
- Orth SR, Ritz E. The nephrotic syndrome. New England Journal of Medicine 1998;338(17):1202‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2008
- Palmer SC, Nand K, Strippoli GF. Interventions for minimal change disease in adults with nephrotic syndrome. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD001537.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2005
- Peng A, Gu Y, Lin SY. Herbal treatment for renal diseases. Annals of the Academy of Medicine, Singapore 2005;34(1):44‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Saland 2002
- Saland JM, Ginsberg H, Fisher EA. Dyslipidemia in pediatric renal disease: epidemiology, pathophysiology, and management. Current Opinion in Pediatrics 2002;14(2):197‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Segarra 2009
- Segarra A, Praga M, Ramos N, Polanco N, Cargol I, Gutierrez‐Solis E, et al. Successful treatment of membranous glomerulonephritis with rituximab in calcineurin inhibitor‐dependent patients. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(6):1083–8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2000b
- Su L, Chen XJ, Hu JD, Zhou SG, Mao JC. Comparisons different doses of Astragalus membranaceus and Salvia miltiorrhiza in rats proteinuria. Zhongguo Xinyao Yu Linchuang Zazhi [Chinese Journal of New Drugs and Clinical Remedies] 2000;3:205‐8. [Google Scholar]
UMMC 2009
- Smith JF. Nephrotic syndrome ‐ overview. http://www.umm.edu/ency/article/000490.htm (accessed March 2013).
Waldman 2007
- Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, et al. Adult minimal‐change disease: clinical characteristics, treatment, and outcomes. Clinical Journal of The American Society of Nephrology: CJASN 2007;2(3):445–53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2002
- Wang HY, Li JZ, Pan JS, Zou WZ, Li XM, Zhang YK, et al. The effect of Astragali and Angelica on nephrotic syndrome and its mechanisms of action. Beijing Daxue Xuebao (Yixueban) [Journal of Peking University (Health Sciences)] 2002;34:542‐52. [Google Scholar]
Wu 2005
- Wu T, Annie Bligh SW, Gu LH, Wang ZT, Liu HP, Cheng XM, et al. Simultaneous determination of six isoflavonoids in commercial Radix Astragali by HPLC‐UV. Fitoterapia 2005;76(2):157‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ye 2003
- Ye RG, Yang X, Liu GX, Xu HS, Fang JG, Cui JG, et al. Integration of Chinese and western medicine. 1st Edition. Beijing: People's Medical Publishing House, 2003. [Google Scholar]
Zhou 2009d
- Zhou LJ. Systematic review of primary nephrotic syndrome in children treated with Huangqi and western medicine. Shandong Zhongyiyao Daxue Xuebao [Journal of Shandong University of Traditional Chinese Medicine] 2009:1‐49. [Google Scholar]
References to other published versions of this review
Yuan 2007
- Yuan W, Wang J, Wu T. Chinese herbal medicine Huangqi type formulation for nephrotic syndrome. Cochrane Database of Systematic Reviews 1, Issue 2007. [DOI: 10.1002/14651858.CD006335] [DOI] [PubMed] [Google Scholar]
Yuan 2008
- Yuan W, Wang J, Wu T. Chinese herbal medicine Huangqi type formulations for nephrotic syndrome. Cochrane Database of Systematic Reviews 2, Issue 2008. [DOI: 10.1002/14651858.CD006335.pub2] [DOI] [PubMed] [Google Scholar]


