Abstract
As a heterogeneous disease, breast cancer (BC) has been characterized by the uncontrolled proliferation of mammary epithelial cells. The tumor microenvironment (TME) also contains inflammatory cells, fibroblasts, the extracellular matrix (ECM), and soluble factors that all promote BC progression. In this sense, the macrophage migration inhibitory factor (MIF), a pleiotropic pro-inflammatory cytokine and an upstream regulator of the immune response, enhances breast tumorigenesis through escalating cancer cell proliferation, survival, angiogenesis, invasion, metastasis, and stemness, which then brings tumorigenic effects by activating key oncogenic signaling pathways and inducing immunosuppression. Against this background, this review was to summarize the current understanding of the MIF pathogenic mechanisms in cancer, particularly BC, and address the central role of this immunoregulatory cytokine in signaling pathways and breast tumorigenesis. Furthermore, different inhibitors, such as small molecules as well as antibodies (Abs) or small interfering RNA (siRNA) and their anti-tumor effects in BC studies were examined. Small molecules and other therapy target MIF. Considering MIF as a promising therapeutic target, further clinical evaluation of MIF-targeted agents in patients with BC was warranted.
Keywords: Macrophage migration inhibitory factor, MIF, breast cancer, small molecules, extracellular matrix
Introduction
Breast cancer (BC) has been acknowledged as the most common type of cancer and the second leading cause of cancer-related death in women worldwide. In 2020, about 2.3 new cases of BC (11.7% of cancers) were diagnosed, and an estimated 684 996 deaths also occurred globally. 1 As a heterogeneous disease, BC consists of multiple distinct subtypes that are at variance in terms of their morphological features, clinical presentations, and responses to therapy. 2 Breast cancer subtypes are usually divided into four categories based on the immunohistochemical expression of hormone receptors (HRs), estrogen receptor (ER+), progesterone receptor (PR+), and human epidermal growth factor receptor (HER2+): luminal A or HR+/HER2– (HR-positive/HER2-negative) luminal B or HR+/HER2+ (HR-positive/HER2-positive), HER2 positive or HR–/HER2+ (HR-negative/HER2-positive), and triple negative BC (TNBC) or HR–/HER2– (HR-negative/HER2-negative). 3
The luminal subtypes of cancer, accounting for 70% of breast tumors, express HRs for estrogen and/or progesterone, and generally have the best prognosis. Contrarily, both HER2-positive and BL or TNBC do not express the estrogen receptor (ER), progesterone receptor (PR), and HER2. They are also associated with poor clinical outcomes due to their high rates of recurrence and distant metastasis. 2 The BC development and progression are driven by the acquisition of genetic and epigenetic alterations in mammary epithelial cells (ECs). However, accumulating evidence over the past decade has established a crucial role for the tumor microenvironment (TME), containing stromal cells (SCs), including fibroblasts, adipocytes, vascular cells (VCs), and immune cells that surround the malignant epithelial ones and modulate BC pathogenesis. 4 Through paracrine signaling mediated by direct cell–cell contact and secreted factors, the stroma further stimulates cancer cell proliferation, survival, invasion, metastasis, stemness, and drug resistance. 5 So far, much focus has been on tumor-associated macrophages (TAMs), derived from circulating monocytes recruited to the tumor site. TAMs facilitate an immunosuppressive and pro-angiogenic microenvironment that fuels BC growth and spread. 6 In this study, there was an attempt to understand the complex interactions between cancer cells and the TME in BC. Accordingly, an important protein, called the macrophage migration inhibitory factor (MIF), along with its structure and role in cancer was investigated. Following the detailed discussion about its various inhibitors, there is great hope that a path to new therapeutic opportunities will be revealed.
Role of TME in BC
As the mammary stroma undergoes marked remodeling during the BC development, it is characterized by some changes in extracellular matrix (ECM) components, fibroblasts, immune cells, and angiogenesis. 7 Cancer-associated fibroblasts (CAFs) are also known as activated fibroblasts that promote tumorigenesis through the secretion of growth factors (GFs), cytokines, chemokines, and ECM-degrading proteases. 8 In this respect, dense collagen fibers restrict drug penetration, thereby reducing therapeutic efficacy. 9 Of note, the immune cells in the breast TME consist of T and B cells, natural killer (NK) cells, neutrophils, myeloid-derived suppressor cells (MDSCs), as well as dendritic cells (DCs) and TAMs. 10 Whereas cytotoxic CD8+ T and NK cells have anti-tumor functions, regulatory T cells (Tregs), MDSCs, M2-polarized TAMs, and CD4+ T helper 2 (Th2) cells mediate immunosuppression, which allows cancer cells to evade immune destruction. 11 Macrophages are highly plastic innate immune cells that can change their functional phenotypes in response to microenvironmental signals and exhibit highly polarized states between M1 and M2. 12 M1 or classically activated macrophages have pro-inflammatory properties and anti-tumor activities by migrating to inflamed tissues, targeting pathogens by producing reactive oxygen species (ROS), and having high antigen expression potential. For this reason, anti-tumor macrophages are commonly called M1 macrophages, which stimulate cytotoxic T lymphocytes (CTLs) to activate adaptive immune responses. In contrast, M2 or activated macrophages secrete anti-inflammatory cytokines to induce immune tolerance and Tregs and Th2 T cell subsets lacking cytotoxic function. M2 macrophages as pro-tumors in cancer facilitate tissue repair functions, stimulate angiogenesis with vascular endothelial growth factor (VEGF) and promote tissue growth with transforming growth factor beta (TGF-β). In nature, there is a wide variety of macrophage phenotypes, but for simplicity, TAMs are described as either M1-like (anti-tumor) or M2-like (pro-tumor). 13 In BC, TAMs preferentially polarize toward the M2 phenotype due to cytokines, such as interleukin-4 (IL-4), IL-10, and TGF-β secreted by malignant and SCs. 6 Through the production of ECM components, enzymes, GFs, and chemokines, TAMs then stimulate the BC initiation, progression, and metastasis. 14 Angiogenesis or the growth of new blood vessels correspondingly accelerates tumor expansion and provides routes for cancer dissemination. 15 Therefore, the complex interplay between BC cells and the surrounding microenvironment has emerged as an important driver of the disease pathogenesis. Therapeutic strategies targeting the tumor milieu in addition to the malignant ECs may thus improve patient outcomes.
Migration Inhibitory Factor
The MIF, known as the glycosylation-inhibiting factor (GIF), L-dopachrome isomerase, or phenylpyruvate tautomerase (PPT), is a pleiotropic protein with the properties of an inflammatory cytokine, a chaperone, an enzyme, and an upstream regulator of the host immune response.16,17 It antagonizes the anti-inflammatory effects of glucocorticoids (GCs) to sustain the immune cell survival and pro-inflammatory cytokine production. 18 Moreover, the MIF is ubiquitously expressed in multiple cell types, including the immune cells (namely, monocytes, macrophages, T and B cells, and neutrophils), endothelial cells, ECs, fibroblasts, adipocytes, pituitary cells, and various cancer cells. 19 As an important component of the TME, the MIF further enhances the progression of numerous solid and hematological malignancies via diverse mechanisms.20,21 The elevated expression of the MIF has been accordingly detected in BC tissue and serum compared with normal controls.22,23 Higher MIF levels have been additionally associated with lymph node metastasis, advanced tumor stage, distant metastasis, poorer overall and disease-free survival, and increased mortality.23,24 Notably, the MIF upregulation is more prominent in TNBC and HER2+ subtypes, indicating enhanced invasion and metastatic potentials. 25 Given its pathogenic effects, the MIF represents a promising therapeutic target in BC. Against this background, this review aims to summarize the current understanding of how MIF can promote BC progression through multiple mechanisms. The preclinical efficacy of targeting the MIF was also discussed.
Structure and genetic qualities
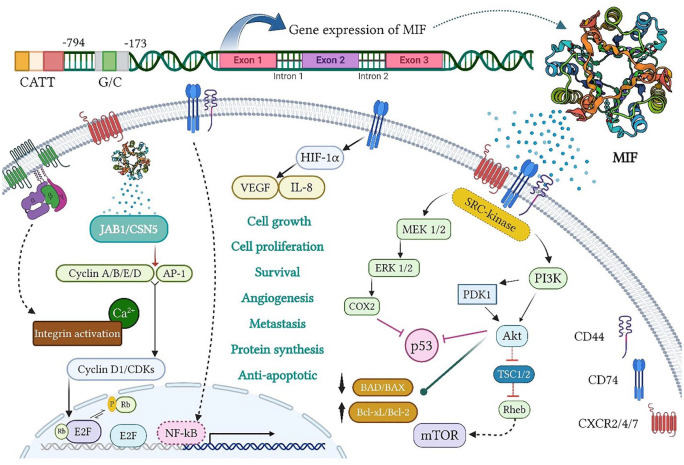
The MIF is encoded by a single gene of less than 1 kb, located on chromosome 22q11.2, consisting of three exons of 66, 107, and 172 bp, separated by two introns of 94 and 188 bp. Besides, two MIF promoter polymorphisms (namely, CATT5-8 and G/C) are at –794 and –173 positions, respectively (see Figure 1). 26 It has about 90% interspecies homology between rodents and mammals, resulting in a 0.6 kb messenger RNA (mRNA) transcript in both species. The MIF also encodes an evolutionarily conserved protein with the molecular weight of 12.5 kDa, containing 115 amino acids.27,28 In the 1990s, using techniques such as nuclear magnetic resonance (NMR) spectroscopy, size exclusion chromatography (SEC), dynamic light scattering (DLS), analytical ultracentrifugation (AUC), and cross-linking, mammalian MIF was identified as a monomer, a dimer, a trimer, or a mixture of oligomers.27,29,30 The NMR outcomes also established considerable conformational flexibility in the C-terminus in the MIF, whereas the N-terminus was relatively rigid.29,31 The crystal structures of the MIF in humans, mice, and other species have further revealed that it can crystallize as a trimer with a prominent solvent-permeable channel.21,32,33 In this line, Philo et al 34 detected the MIF trimer in solution, and even found an inactive MIF monomer. Therefore, the MIF oligomerization could be related to its function and the intrinsic activity of the MIF tautomerase, which was dependent on a hydrophobic pocket between adjacent monomeric subunits in a functional trimer. 35 This activity required the N-terminal proline of the MIF as a catalytic base, and other important residues, including conserved Lys-32, Ile-64, Tyr-95, and Asn-97. 36
Figure 1.
MIF gene expression and biological activity. The MIF transcription from its gene on chromosome 22q11.2 includes three exons, separated by two introns, under two MIF promoters (namely, CATT5 8 and G/C), and located at 794 and 173 positions, respectively. The MIF signaling is also generated by interactions with CD74 and CD44 as well as CXCR2/4/7 on the cell surface. First, it stimulates the activation of extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) and phosphatidyl 4,5-bisphosphate 3-kinase/protein kinase B (PI3K/AKT) pathways. The activation of ERK/cyclooxygenase-2 (COX2) and PI3K/AKT also inhibits p53-dependent apoptosis and promotes tumor cell proliferation. On the contrary, the PI3K/AKT and mammalian target of rapamycin (mTOR) activation causes the inactivation of pro-apoptotic proteins, BAD and BAX, and the expression of anti-apoptotic proteins, Bcl-xL and Bcl-2, which increase the survival and invasion of cancer cells. During hypoxia, the MIF binding to CD74 contributes to the activation and stabilization of hypoxia-inducible factor-1 subunit alpha (HIF1α), which then enhances the expression of angiogenic GFs, including vascular endothelial growth factor (VEGF) and IL-8, thereby promoting angiogenesis. Moreover, extracellular MIF binding to G protein-coupled chemokine receptors (GPCRs), namely, CXCR2, CXCR4, and CXCR7, individually or in a CD74-dependent manner, triggers the activation of integrin and its subsequent pathways, which play a key role in cancer cell invasion. Intracellularly, the MIF functionally interacts with cytosolic Jab1/CSN5, leading to the retention of the Skp1-Cullin1 F-box (SCF) complex in an activated form as well as the induction of cyclin activity and/or c-Jun/AP-1 phosphorylation. Meanwhile, the CD74/CD44 receptor complex releases the intracellular domain (ICD) of CD74 and translocates into the nucleus, thereby boosting nuclear factor kappa B (NF-κB) activation. MIF indicates migration inhibitory factor.
Biological activity
Unlike most cytokines, the MIF is intracellularly stored in vesicle-like structures and released in response to a number of stimuli, including lipopolysaccharide (LPS), tumor necrosis factor alpha (TNF-α), hypoxia, hydrogen peroxide (H2O2), thrombin, and angiotensin II, and is induced but not suppressed by GCs. Most of the studies have shown that the MIF tautomerase activity affects CD74 receptor binding and downstream signaling activities.27,37 According to Pantouris et al, 38 creating mutations and exploiting small molecules to target the active site of tautomerase interfere with CD74 binding. As the biological functions of the MIF are autocrine and paracrine, the induction of signaling pathways requires the recruitment of CD44 or CXCR in addition to the CD74 receptor (a central hub in the MIF signaling). The possible complexes between these receptors include CD74/CD44, CD74/CXCR2, CD74/CXCR4, and CD74/CXCR4/CXCR7. 37 The binding of the MIF to CD74 thus initiates the activation of the Src family kinases, or the internalization and subsequent transmembrane-regulated proteolysis of CD74. These downstream signaling pathways may depend on CD44 as a co-receptor or co-factor because the deletion of CD44 abrogates the effect of the MIF signaling in CD74-expressing cells. As a final point, this pathway interacts with transcription factors (TFs), NF-κB, and runt-related TF (RUNX), and regulates the anti-apoptotic gene, B-cell lymphoma-extra large (Bcl-xL), thereby improving cell survival. On the contrary, CD44 intramembrane domain is of importance in the MIF signaling by targeting the SH2/SH3 domains of the non-receptor tyrosine kinases of the Src family, and binding the MIF to the CD74/CD44, which causes fast and transient autophosphorylation of tyrosine-416 of Src. Then, the downstream pathways of PI3K and AKT are phosphorylated and the corresponding signaling pathways are activated (see Figure 1). It also phosphorylates ERK1/2 in a pKa-dependent and protein kinase C (PKC)-independent manner.37,39 Moreover, binding to CD74/CXCR2 and CD74/CXCR4 complexes leads to the activation of ERK1/2, AKT pathways, and downstream events, such as calcium influx and integrin activation through the Gi alpha subunit of the G protein.37,40 However, CXCR7 does not interact with any type of G proteins and exerts its effect through β-arrestin-2, which triggers the long-term activation of ERK1/2 and c-Jun N-terminal kinase 3 (JNK3).37,41 As evidenced, the MIF activates the PI3K-AKT pathway only through CXCR7. 42 The MIF also physically interacts with various intracellular proteins, such as the c-Jun activation domain-binding protein 1/COP9 signalosome subunit 5 (JAB1/CSN5), as an important subunit of the photomorphogenic 9 signalosome complex (COP9-CSN), also acts as a negative regulator of the SCF complex. As well, the formation of the JAB1/CSN5 and the MIF complex results in the dendylation of the SCF complex and the loss of its ubiquitination, which then stabilizes other intracellular proteins, such as c-JUN, c-Myn, WEE1, p21, p27, or β-catenin. It further induced the activation of cyclin and Ap-1 proteins and affects the cell cycle progression. 37
In this respect, Hanahan and Weinberg 43 described 10 biological properties acquired by cancer cells that could be effective in their formation, growth, and invasion. Studies have further shown that the MIF is involved in a number of cancer hallmarks with its pleiotropic functions, as follows:
1. Avoidance of growth suppressors: Among the most important tumor suppressor proteins that prevent uncontrolled cell growth and division are p53 and Rb. The MIF has been demonstrated to antagonize these proteins (p53 and Rb) directly or indirectly. For example, the MIF-deficient mice exposed to carcinogens developed smaller tumors than wild-type mice. 44 The MIF further antagonizes the p53 actions and enhances the interaction of mouse double minute 2 homolog (MDM2) with p53 after physically binding to p53, and thus increases its degradation. Second, the MIF may indirectly impact p53 by expressing COX-2. 45
2. Apoptosis resistance: The MIF delays apoptosis by affecting some proteins. As an example, the PI3K/AKT activation (namely, the MIF signaling downstream of CD74) decreases the expression of the pro-apoptotic genes, BAD and BAX. 46 It has been also observed that cancer cells with silenced MIF experience augmented cytochrome C release, downregulation of Bcl-2 and Bcl-xL proteins, and increased BAD and BAX and p53 suppressor proteins, which generally promote apoptosis. On the contrary, the activation of NF-κB by the MIF has been suggested to control the dynamics and stability of mitochondria. 47
3. Induction of angiogenesis: There is a positive correlation between the MIF and VEGF, as reported in various types of cancer. In fact, the MIF and VEGF have been highly present in patients with liver cancer compared with controls, both in serum and cancer tissues. 48 In addition to the direct regulation of VEGF and angiogenesis, the MIF is indirectly involved in angiogenesis through the HIF because it is a major TF that is effective in the expression of genes, eg, VEGF, under hypoxic conditions. 49
4. Invasion and metastasis: Invasive cancers mean being separated from the primary site, entering and exiting the bloodstream, and adapting to the new environment to create metastasis. It has been reported that the MIF at high levels in tumors make them prone to migration and metastasis. For example, nuclear receptor subfamily 3 group c member 2 (NR3C2) in the MIF is a tumor suppressor gene that reduces the growth, migration, and invasion of cancer cells. In addition, the MIF escalates the epithelial-to-mesenchymal transition (EMT) of cancer cells, and thus cells with high levels of MIF decrease the E-cadherin expression, which maintains the integrity of the epithelial structure and multiplies N-cadherin, vimentin, and Zeb1, which promote migration.50,51
5. Regulation of cellular energy: The potentiation of the Warburg effect and the MIF increases lactate production through the regulation of lactate dehydrogenase A (LDHA) by HIF-1α. 52
6. Maintenance of proliferative signaling: Various studies have so far shown that the MIF knockout or knockdown has a direct effect on cell proliferation. To give an example, the MIF loss in a colorectal cancer cell line results in a significant drop in proliferation, which probably involves the AKT/GSK-3β signaling pathway, as its phosphorylation is impaired by the MIF depletion. 53
7. Avoidance of the immune system destruction: The MIF suppresses the immune system and impairs its capacity in the TME. For example, blocking the interaction between the MIF and CD74 in a metastatic melanoma model has reduced immunosuppression in various immune cells and more M1 (with anti-tumor phenotype), CD4+ T cells, CD8+ T cells, and NK ones have been observed in the treated group compared with the controls, ultimately decreasing the number of metastatic foci in the former group. Moreover, the MIF knockout mice in a colon carcinoma model have shown smaller tumors than the wild-type group, due to the boosted expression of IL-2 by the MIF, mainly utilized by Tregs, which lead to their proliferation. 54
8. Tumor-inducing inflammation: There is a mutual relationship between inflammation and cancer. The MIF is one type of pro-inflammatory cytokines and the main regulator of inflammation that directly and indirectly promotes the expression of other inflammatory molecules, such as IL-2, IL-6, IL-8, TNF, interferon gamma (IFN-γ), and IL. Under these conditions, tumor-stimulating molecules, such as GFs, pro-angiogenic cytokines, and enzymes that can promote invasion, such as colony-stimulating factor 1 (CSF1), epidermal growth factor (EGF), VEGF, and matrix metalloproteinase 9 (MMP-9) are increased.55,56
Pathogenic Mechanisms of MIF in BC
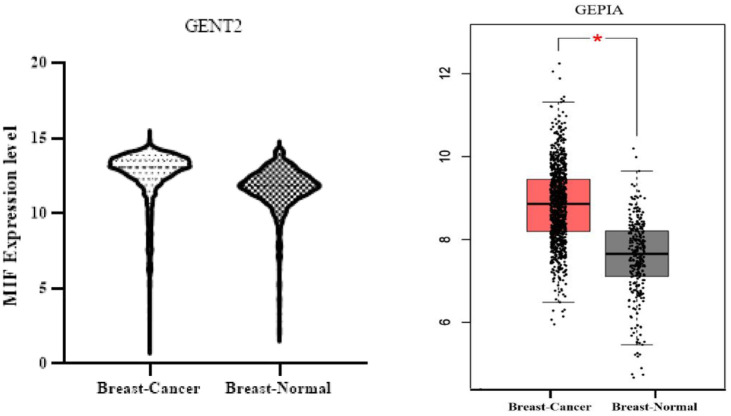
The level of aberrant MIF protein expression in cancer, as compared with a healthy sample, and its prognostic significance is particularly relevant. This had been investigated in the study by Charan et al, 57 measuring the level of MIF expression in human TNBC samples using tissue microarray (TMA) and immunohistochemistry (IHC), and then a sharp rise in the MIF expression had been detected in patients. Higher MIF expression had been also found to be substantially associated with worse overall survival in the cases with TNBC. 57 Richard et al, 58 using IHC for the MIF in breast tissues, had further observed a significant increase in the MIF expression in carcinomas compared with non-tumor samples. Moreover, they had applied enzyme-linked immunosorbent assay (ELISA) to compare the levels of MIF in the serum of patients, indicating that the MIF concentration in those with BC had been almost four times higher than the amount recorded in the healthy people. 58 In the study by Lin et al, 59 the samples from 560 patients with BC had been similarly analyzed via DNA sequencing. The results had also established that cases with three specific genotypes, namely, C/G, C/C, and C/G-C/C of the rs755622 MIF variant (as the most common type) were more likely to develop BC. 59 This had been additionally recorded in other works,60,61 implying the leading role of the MIF in the BC development and progression and its association with the survival of patients and response to chemotherapy drugs. Moreover, the MIF expression had been evaluated using the GENT2 and GEPIA databases, and the findings had found that the expression of this protein had been higher in patients with BC compared with the normal samples (see Figure 2).
Figure 2.
Overexpression of MIF using GENT2 and GEPIA databases. From the GENT2 database with the address http://gent2.appex.kr/gent2/, the level of MIF gene expression in 5574 cancer samples and 475 normal samples was obtained and the related graph was drawn using GraphPad Prism program. Then, using the GEPIA database with the address http://gepia.cancer-pku.cn/detail.php?gene=mif and the expression DIY section, the MIF gene expression level was obtained based on Box Plots by comparing 1085 cancer samples and 291 normal samples. Both databases showed increased MIF gene expression. MIF indicates migration inhibitory factor.
MIF promotes BC cell proliferation and survival
In 2020, a study by Charan et al demonstrated that MIF overexpression in TNBC enhances growth and metastasis. Taken together, the results of this study indicated that the use of small molecular weight MIF inhibitors could be a promising strategy to inhibit TNBC progression and metastasis. Thus, MIF plays a key role in BC tumor growth, as observed in various human BC cell lines and syngeneic breast tumor models. 57 The MIF further stimulates the growth and proliferation of BC cells via the activation of the ERK1/2 and AKT signaling pathways. 46 The ERK1/2 and AKT are thus among key intermediates that control cell cycle progression, metabolism, survival, angiogenesis, and motility. The MIF-induced ERK1/2 and AKT phosphorylation can also be abolished by CD74 neutralizing antibodies (Abs) in MDA-MB-231 TNBC cells. 46 In this line, Verjans et al 25 had concluded that the recombinant MIF could stimulate the proliferation of non-invasive BC cell lines, MDA-MB-468 (as an infiltrating adenocarcinoma) and ZR-75-1 (the infiltrating ductal carcinoma), as well as highly aggressive MDA-MB-231 cells (namely, invasive ductal carcinoma). Lue et al 46 in their study on different BC cell lines, including Michigan Cancer Foundation-7 (MCF-7; as an infiltrating ductal adenocarcinoma), MDA-MB-468 (the infiltrating ductal adenocarcinoma), and ZR-75-1 (ie, infiltrating ductal carcinoma) had shown that the MIF/CD74 interaction could activate the PI3K/AKT signaling pathway and promote cell survival. 46 On the contrary, the activation of the CXCL8 pathway involving CXCR1/2 ligands (ie, CXCL8, CXCL1, and CXCL2) could enhance BC cell survival and chemoresistance. 62 The MIF could further protect MCF-7 cells against apoptosis, induced by oxidative stress or chemotherapeutic drugs through the upregulation of the inhibitor of apoptosis proteins (IAPs). 63 Besides, Wu et al 64 had argued that the MIF knockdown could increase the chemosensitivity of BC cells to doxorubicin and cause autophagic cell death. 64 Hence, the MIF could promote BC cell growth, inhibit apoptosis, and mediate chemoresistance.
MIF induces angiogenesis in breast tumors
As evidenced, sustained angiogenesis is critical for BC progression, invasion, and metastasis. The MIF accordingly upregulates the production of pro-angiogenic factors, VEGF, and IL-8, in BC cells through HIF-1α and NF-κB signaling. In their in vitro and in vivo study using MCF-7 cells, Oda et al 65 had accordingly revealed that the MIF could activate HIF-1α under hypoxic conditions through a p53-dependent manner. Culture supernatants from the MIF-overexpressing MDA-MB-231 cells could further induce endothelial cell migration and tube formation, which might be blocked by neutralizing the VEGF and IL-8 Abs. 23 In co-culture models, BC cells could further stimulate VEGF secretion by macrophages in an MIF-dependent manner to promote angiogenesis. 19 Clinical data have correspondingly shown positive correlations between the MIF, CD74, VEGF, and microvessel density in invasive breast carcinomas. Elevated serum MIF has been further associated with increased serum VEGF in node-positive BC patients. Compared with the MIF-low tumors, the MIF-high ones have demonstrated greater VEGF expression, vessel count, and hemoglobin saturation index by cryosectioning and imaging. This close relationship in BC cell lines has revealed that the MIF depletion is associated with reduced activation of the ERK/MAPK signaling pathway, which is required for VEGF-C expression. 66 The angiopoietin (Ang)-Tie2 system has also been introduced as another regulator of tumor angiogenesis. 67 Ang-2 cooperates with VEGF to destabilize vessel structure and primes the vasculature to respond to angiogenic stimuli. In breast tumors, the MIF upregulates the Ang-2 expression in TAMs to enhance angiogenesis. 68 As a whole, these findings indicated that the MIF could promote breast tumor vascularization by increasing pro-angiogenic factors in cancer and SCs.
MIF stimulates BC metastasis
The metastatic cascade is a complex, multi-step process that enables cancer cell dissemination from the primary tumor to distant organs. Each step, namely, local invasion, intravasation, survival in circulation, extravasation, and colonization, is thus facilitated by the MIF. 69 Fersching et al, 60 examining biomarkers related to apoptosis in the serum of BC patients undergoing neoadjuvant chemotherapy had accordingly reported a positive correlation between the MIF and intercellular adhesion molecule 1 (ICAM-1). 60 Lv et al 70 had further observed that the increased MIF expression in BC cells was directly correlated with the expression of Snail, Vimentin, and Twist, as well as the activation of MMP-2, thereby the knockdown of MIF could decrease the expression of these proteins. Furthermore, the activation of HMGB1/TLR4/NF-κB axis through the MIF could induce lung metastasis in BC because the MIF overexpression could promote the migration of BC cells by increasing the TLR4 expression. 70 As mentioned earlier, there was a high correlation between the MIF and IL-8. In the study by George et al, 71 two estrogen receptor-positive and -negative BC cell lines, T47D and MDA-MB-231, had been accordingly investigated wherein the IL-8 treatment had augmented the proliferation, migration, and invasion of the T47D and MDA-MB-231 cells. In vivo studies by Charan et al 57 had further established that the MIF inhibition in orthotopic breast carcinoma mouse models had significantly inhibited TNBC growth and lung metastasis in a dose-dependent manner. 57 In the 4T1 mouse model of BC, the MIF had also amplified tumor growth and metastasis by increasing the prevalence of a highly immunosuppressive subpopulation of MDSC within the tumor. 72 As noted, the MIF was a potent endogenous mediator of COX-2 expression, and Majumder et al 73 had shown that indomethacin, a COX-1/COX-2 inhibitor, had inhibited cell proliferation and migration using the highly metastatic C3L5 mouse BC model. In general, MIF could facilitate several stages of BC metastatic cascade, so its inhibition could reduce BC metastasis, as a vital factor in the disease progression and mortality rate.
Targeting MIF in BC Treatment
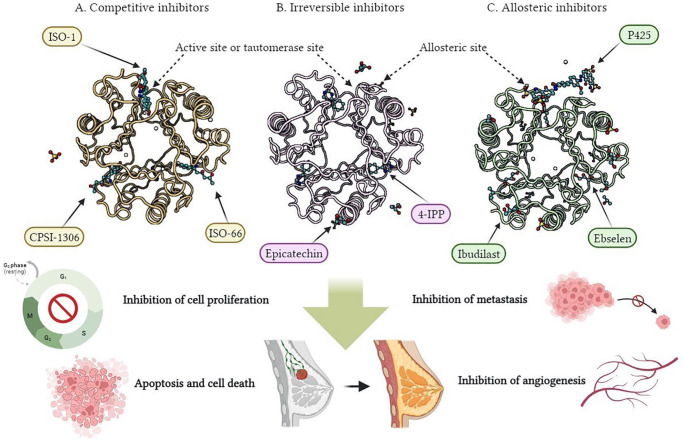
The biological activities of the MIF have led to its recognition as a significant therapeutic target, but the development of small molecule inhibitors has intensified due to the obvious limitations of protein (Ab) and nucleic acid (small interfering RNA [siRNA])-based strategies, such as high cost and inconvenience of application. 26 Small molecules also cause the inactivation of the MIF tautomerase through some mechanisms, 26 namely, (1) binding to the active site (namely, competitive inhibitors), (2) covalent modification of active site residues (namely, irreversible inhibitors), and (3) allosteric inhibition (see Figure 3). Therefore, the inhibition of the MIF enzymatic active site has been studied as a strategy for the production of the MIF inhibitors. Active site binding and covalent attachment of small molecules to Pro1 have been the most widely used approaches to date. Pro1 is a proline residue. Pro1 is the first residue of MIF and plays a crucial role in its function. This Pro1 residue and its interactions within MIF are significant for the activation of CD74, a cell surface receptor. 37
Figure 3.
Three-dimensional (3D) structure of the MIF trimer (Protein Data Bank entry, https://www.rcsb.org/, selected for display: 1CA7) and the binding site of various classes of small molecule inhibitors of the MIF tautomerase, including (A) competitive inhibitors (namely, ISO-1, ISO-66, and CPSI-1306), (B) irreversible inhibitors (ie, 4-IPP and epicatechin), and (C) allosteric inhibitors (that is, ibudilast, ebselen, and P425). They can be therapeutic strategies to inhibit the MIF signaling and play a significant role in improving BC with effects, such as apoptosis and cell death, cell proliferation inhibition, angiogenesis, and metastasis.
Binding to active site
Competitive MIF inhibitors that directly bind to the active site include the isoxazoline class, and the best MIF inhibitor investigated in this class is S,R-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1). This inhibitor has shown significant interactions with Lys-32, Ile-64, and Asn-97 in the MIF structure. The MIF inhibition by ISO-1 is also a key tool in elucidating the complex biological role of this protein in cancer and inflammation, resulting in reduced cell migration, proliferation, and invasion in several human cancer cell lines, such as DU145, A549, LN229, HS683, and LN-18 and in a number of in vivo studies in mice, and improving survival in endotoxemia, colitis, melanoma, prostate, and colorectal cancers.74,75 For example, Cotzomi-Ortega et al, 76 knowing that one of the consequences of blocking the autophagic pathway was the increase in ROS due to the accumulation of damaged mitochondria, had found that the MIF secretion in the BC cell line 66cl4 was attributable to the higher production of ROS caused by the inhibition of autophagy. Secreted MIF could also have important autocrine effects on the promotion of malignancy, as cell death in 66cl4 cells could be dramatically augmented upon treatment with ISO-1. Most importantly for promoting malignancy, it could add to the efficiency of migration in 67NR and 4T1 cell lines, indicating the paracrine effect of cytokine secretion in promoting malignancy, and this elevated migration could be prevented by ISO-1. 76 Despite these favorable results regarding ISO-1, in vivo clinical use of this compound could be hindered owing to inappropriate stability and toxicity. Therefore, compounds from the same class of inhibitors, including ISO-66, CPSI-2705, and CPSI-1306, were introduced, and CPSI-1306 compound received much more attention thanks to its longer half-life in vivo. 74 In two studies in 2020 and 2022, Charan et al investigated the effects of the MIF reduction on TNBC. In the first study, the administration of a small synthetic MIF inhibitor, CPSI-1306, diminished the proliferation and survival of BC cells in vitro. CPSI-1306 also induced apoptosis by enhancing ROS, mitochondria membrane potential alteration, cytochrome C release, and activation of caspases. In addition, CPSI-1306 could inhibit cell survival and apoptosis signaling molecules, including the AKT, PDK, and RAF expression. In vivo, the mice treated with this inhibitor had undergone smaller tumors, which could be associated with the reduced expression of Ki67 in cancer cells. 57 The second study had correspondingly established that treatment with CPSI-1306 and MIF reduction in human TNBC grafts was connected with the lower infiltration of MDSCs in the tumor and the spleen. In addition, treatment with CPSI-1306 could increase the infiltration of CD8+ T cells and decrease granulocyte colony-stimulating factor (GCSF), granulocyte-macrophage colony-stimulating factor (GMCSF), IL-2, and IL-4 compared with the control group. 77 Das et al 78 had also investigated the effect of CPSI-1306 on BC, and found that CPSI-1306 significantly induced apoptosis and reduced the viability of metastatic BC (MBC)-related MDA-MB 468 and MDA-MB 231 cells in a dose- and time-dependent manner. CPSI-1306 further dwindled the mitochondrial membrane potential and induced apoptosis by increasing apoptogenic signals, including apoptosis-inducing factor (AIF) and cytochrome-C. In the mouse model of preclinical mammary tumor MVT-1, CPSI-1306 had significantly reduced tumor growth and metastasis to the lungs and caused a fall in the number of Ki67-positive proliferative cells and CD31-positive blood vessels in the tumor. 78
Covalent modification of active site residues
Irreversible inhibitors typically bind to the terminal nucleophilic proline of the MIF and include 2-oxo-4-phenyl-3-butanoate, phenylpyrimidines, acetaminophen analogs, and epicatechin. The 4-Iodo-6-phenylpyrimidine (4-IPP) as a potent inhibitor with an IC50 less than 10 times that of ISO-1 by irreversible binding to the Pro1 residue of the MIF accordingly has appropriate anticancer activities on the lung and head and neck cancer cells. 74 Das et al 79 had accordingly shown that the increase of MIF in the culture medium of the MDA-MB-231 cell line had caused resistance to chemotherapy agents. By examining the MIF expression in BC tissues, it had been observed that the amount of this protein had elevated in chemotherapy-resistant tissues compared with the non-resistant ones. Anti-apoptotic proteins, Bcl2 and Bcl-xl, had been further induced by the MIF through reducing BAX and BAD, and the MIF had been able to redouble the level of phosphorylated AKT in 231 cell line. Then, using 4-IPP inhibitor, it had been observed that the induction of chemical resistance in the target cell line had been eliminated, and the anti-apoptotic effect of MIF had been reversed and the phosphorylation of AKT had been inhibited. 79 Moreover, the natural product inhibitor of MIF, sulforaphane (SFN), had decreased the MIF expression in a mouse model of BC, using 4T1 cell line, which had significantly reduced the induction of monocytic MDSCs. 72 Considering epicatechin as the main polyphenolic compound of cocoa beans, one study had concluded that 4-O-methyl-epicatechin and 3-O-methyl-epicatechin could have favorable anti-proliferative functions in MCF-7 BC cells compared with other derivatives. 80 There are also studies of phenylpyrimidines with MIF-independent effects as radiosensitizers targeting p53 and Nrf2 81 and adjuvant chemotherapy to inhibit ABC transporter-mediated multidrug resistance (MDR), 82 which have received good results in inhibiting BC. Also, MIF-independent effects of SFN on BC through histone deacetylases involved in chromatin remodeling, increase in sulfate-related metabolites and glutathione-related metabolites, gene expression and Nrf2 antioxidant signaling and targeting the RAF/MEK/ERK signaling pathway are well evident.83-85 This is also the case for epicatechin and through the increase of death receptor (DR4/DR5), ROS production, upregulation of pro-apoptotic proteins and metastasis-related genes, Cdh1, Mtss1, Pten, Bmrs, Fat1, and Smad4, showed anticancer activity in human and mouse BC cells.86,87
Allosteric inhibition
Allosteric inhibitors have been of particular interest due to the pleiotropic nature of the MIF, as they have the potential to bind to critical regions while maintaining the integrity of other receptor binding sites. Given the essential motifs and regions for inhibitory interactions with the MIF, allosteric modulation may thus become a more precise and desirable way to design targeted therapies. Demonstrated in a study in 2010, ibudilast as an anti-inflammatory drug, mainly used to treat bronchial asthma, allergic conjunctivitis, and cerebrovascular disorders (CVDs), and also known as AV411 (3-isobutyryl-2-isopropylpyrazolo-[1,5-a] pyridine) and its analog AV1013, the MIF allosteric inhibitors, had reduced the MIF-mediated chemotaxis. The X-ray crystal structures have further shown that ibudilast binds to an allosteric site adjacent to the tautomerase active site and makes a conformational change in Tyr-36, which then alters the dimensions of the tautomerase active site.26,75 In a phase 1b/2a study evaluating the combination of ibudilast and temozolomide (TMZ) in patients with glioblastoma, with MIF inhibition by daily ibudilast and monthly cycles of TMZ with immune checkpoint blocking properties, The 6-month progression-free survival (PFS-6) rate was acceptable and had a good efficacy. 88
Ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one), an anti-inflammatory drug, has also developed a novel mechanism for targeting protein–protein interactions as it covalently modifies Cys-80 and inhibits enzyme activity by disrupting the MIF trimer structure. Considering that the MIF trimer is very stable, the monomer is very unstable and tends to aggregate rapidly when detached from it. This drug reduces GC overriding and AKT phosphorylation. 75 In a 2017 study, the MCF-7 cell line had been utilized as a valuable BC model to evaluate the anti-proliferative properties of ebselen, proving that the compound in combination with γ radiation (6 Gy) could modulate gene expression and inflammatory cytokine response, and then induce apoptosis and anti-proliferative effects. 89 Ebselen oxide, and some derivatives, were also found to exert an efficient allosteric inhibition of overexpressed HER2, as well as mutated and truncated oncogenic forms of HER2, which are resistant to current therapies and significantly blocked HER2+ breast tumor progression. 90
Another MIF allosteric inhibitor, named P425 (6′-[(3,3-dimethoxy[1,1′-biphenyl]-4,4′-diyl)bis(azo)]bis[4-amino-5hydroxy-1,3-naphthalene disulfonic acid] tetrasodium salt), was identified in a study in 2012. 91 The P425, as a member of the family of large azo and sulfone organic acids, could form a cap on the active site and engage in hydrogen (H) bonds with Lys-32, Asn-109, and Asn-110 on the surface of the two-trimer MIF protein. Other names for this inhibitor were Pontamine Sky Blue or Chicago Sky Blue. It could significantly inhibit the MIF activity for the tautomerization of 4-hydroxyphenylpyruvate and block the interaction between the MIF and its receptor, CD74. The P425 could further attenuate GC elevation, secretion of MMPs, and inhibition of p53-mediated apoptosis induced by the MIF. Although p425 has shown significant effects on the MIF suppression, no study has been so far conducted on the use of this inhibitor in BC, so it is appropriate to see whether the promising biological activity of this inhibitor is maintained in this condition in vivo.26,75 Two other allosteric inhibitors also include epoxyazadiradione and spirohexenolide. Epoxyazadiradione forms H bonds with Asn-105 and Asn-102 of a monomer and covers it against Tyr-99 of an adjacent monomer, which inhibits the MIF tautomerase activity, iNOS induction, NF-κB translocation, and macrophage chemotaxis. Conformational changes are also large enough to affect the distal active site and inhibit tautomerase activity very interestingly, as the X-ray crystal structure of epoxyazadiradione-MIF has confirmed this interaction. 92 Two studies in 2018 and 2021 comparably investigated the effect of epoxyazadiradione on cell viability, mitochondrial potential, cell migration, apoptosis, and protein expression in cell culture models of TNBC and ER+ BC cells, and reported that epoxyazadiradione could inhibit PI3K/Akt-dependent mitochondrial depolarization, induce apoptosis, and reduce cell migration, angiogenesis, and breast tumor growth.93,94 Of note, spirohexenolide A does not inhibit the MIF tautomerase activity, but its large size probably prevents binding to the active site and inhibits the MIF-mediated AKT phosphorylation. 95
Monoclonal Abs
The use of monoclonal Abs against the MIF or its receptor, CD74, has been to date of interest in cancer therapy. 74 Anti-MIF monoclonal Abs, BAXG03, BAXB01, and BAXM159, have accordingly shown good effects in prostate and colon cancers. For example, in a phase-I clinical trial, imalumab (BAX69), an MIF inhibitor, had been applied to treat patients with advanced solid tumors, and then the tolerable dose and the biologically active dose of this substance had been determined. 96 Moreover, the Abs against the CD74 receptor, such as milatuzumab, had brought significant anti-tumor effects in mice, and phase I and I/II clinical trials in patients with B-cell lymphoma had been associated with satisfactory results.97,98 In a study, anti-MIF and anti-CD74 Abs, which could disrupt the MIF/CD74 interactions, had strongly blocked the proliferation of BC cells (namely, MDA-MB-231 and MDA-MB-468) induced by autocrine or exogenous MIF. 25
Supplementary inhibitors of MIF
Other alternative ways to neutralize the biological activity of MIF are now being investigated, eg, HSP90 increases in cancer cells and prevents the MIF degradation. Accordingly, the MIF degradation amplifies and causes favorable anticancer activities when HSP90 is inhibited in cancer cell lines. 74 Pharmacological HSP90 inhibitor 17-(alkylamino)-17-(demethoxygeldanamycin) (17AAG) or siRNA-mediated knockdown of HSP90 also reduces the MIF protein levels and cell proliferation, and induces the apoptosis of human cancer cells. In an ErbB2 transgenic model of HER2-positive BC, systemic treatment with the HSP90 inhibitor 17AAG had diminished the MIF expression and blocked the growth of the MIF-expressing tumors. 99 Another study had further suggested that the HER2/Erb2 overexpression in BC had stabilized many tumor-promoting HSP90 clients, such as the MIF, AKT, and heat shock factor 1 (HSF-1) as an oncogenic master GF. Accordingly, HER2 inhibition could suppress HSP90 activation with subsequent MIF destabilization. 100 Ganetespib, a small molecule inhibitor of HSP90, had been also used in patients with MBC in a phase II clinical trial. Ganetespib had been accordingly well tolerated, and the results had indicated strong inhibitory effects on HSP90-dependent oncoproteins associated with BC pathogenesis. 101 Resveratrol (3, 4′, 5-trihydroxy-trans-stilbene), a natural phytoalexin found in grapes, could further inhibit cell growth, induce apoptosis, and enhance chemotherapy in various types of cancer. It could also decrease cell proliferation and colony formation and increase senescence and apoptosis in triple negative BC cells (MDA-MB-231), which are resistant and sensitive to the common cancer drug, paclitaxel. 102 In the study by Fujita et al, 103 the anti-proliferative effect of Aza-resveratrol derivatives (3, 4′, 5-trihydroxy-trans-aza-acetylbene) had been investigated in MCF-7 BC cell line and the MIF had been evaluated as a main target protein in MCF-7 cell lysates. The findings had revealed that Aza-resveratrol and its derivatives were strong inhibitors of MIF tautomerase activity, thereby exerting their anticancer effects in this way. 103 Other MIF inhibitors had been also considered, such as vitamin E (binding to the active site and reducing the production of pro-inflammatory cytokines), thyroxine (located in the hydrophobic pocket of the MIF and lowering inflammatory effects), non-metastatic protein 23 H1 (Nm23H1; binding Cys145 of NM23H1 and Cys60 of MIF and decreasing p53 suppression), anti-rheumatic drug iguratimod (the MIF inhibition and synergy with GCs). 74
Small interfering RNA
A practical and precise method to achieve protein knockdown is siRNA. Therefore, the MIF can be a suitable target for this method because it is overexpressed in many solid tumors and is associated with a poor prognosis. In the study by Simpson et al, 72 the shRNA knockdown of the MIF in invasive and MBC 4T1 cell lines had thus reduced primary tumor growth and significantly minimized the number of lung micrometastases in Balb/c mice, which could be caused by the drop in the highly immunosuppressive subpopulation of MDSCs and affect some aspects of the immune system. In a study to clarify the role of MIF in cell survival and migration, the MIF-specific siRNA (si-MIF) had been utilized in MDA-MB-468 and MDA-MB-231 TNBC cells, wherein the MIF loss in these cells had significantly increased apoptosis and decreased colony formation, migration, and wound healing capacity. 57 Zhang et al 104 had additionally utilized a glucan-based cationic nanoparticle to deliver siRNA against the MIF in mice inoculated with 4T1 breast tumors, and reported that the MIF knockdown had downgraded immunosuppression and elevated the number of infiltrating CD8+ T cells in tumors treated with siMIF-NP, resulting from the decreased level of CD206 (macrophage marker M2) and the increase in the level of MHCII (important for antigen presentation to CD8+ T cells) of TAMs. In addition, investigating the function of the MIF siRNA in the tumorigenesis of MCF-7 cells in a xenograft mouse model had established that the MIF siRNA could significantly suppress tumor growth in nude mice. 64 The mentioned studies had supported the targeting of MIF using different inhibitors, such as small molecule, along with neutralizing Abs or siRNA knockdown, as summarized in Table 1.
Table 1.
Studies on the use of various MIF inhibitors in BC.
| Author(s) | MIF inhibitors | Target/Binding mode | In vitro model | In vivo model | Main findings | Country |
|---|---|---|---|---|---|---|
| Cotzomi-Ortega et al 76 | ISO-1 | Tautomerase site/competitive | 66cl4, 67NR or 4T1 cell lines | — | Cell death, prevention of malignancy promotion | Mexico |
| Charan et al 57 | CPSI-1306 | Tautomerase site/competitive | MDA-MB-468, MDA-MB-231, and MVT-1 | Female FVB, C57BL/6, and NOD/SCID/IL-2 gamma (NSG) mice | Growth inhibition and apoptosis induction in TNBC cells. Activation of pro-apoptotic proteins and suppression of cell survival markers. Inhibition of tumor growth along with progression and metastasis in a mouse model |
United States |
| Charan et al 77 | CPSI-1306 | Tautomerase site/competitive | — | A xenograft and MIF knockout mouse model | Diminished infiltration of MDSCs and increased infiltration of CD8+ T cells in tumor and spleen. Reduction of GCSF, GMCSF, IL-2 and IL-4, cytokines, and chemokines |
United States |
| Das et al 78 | CPSI-1306 | Tautomerase site/competitive | MDA-MB 468 and MDA-MB 231 | MVT-1 orthotopic mouse model | Decreased AKT, apoptosis, and low cell viability. Reduction in tumor growth and lung metastasis |
United States |
| Das et al 79 | 4-IPP | Terminal nucleophilic proline/irreversible | MDA-MB-231 | — | Apoptosis, reduced induction of chemoresistance, and AKT phosphorylation | India |
| Simpson et al 72 | SFN | Terminal nucleophilic proline/irreversible | — | A female Balb/c 4T1 mouse model | Decreased MIF expression and reduced induction of monocytic MDSCs | United States |
| Delgado et al 80 | Epicatechin | Terminal nucleophilic proline/irreversible | MCF-7 | — | Anti-proliferative activity | Spain and Portugal |
| Thabet and Moustafa 89 | Ebselen | Cys-80/allosteric | MCF-7 | — | Decrease in VEGF level and proliferative capacity. Lower expression of NF-κB, TNF-α, IL-2, INF-γ, and TGF-β. Increased expression of granzyme-B, TRAIL, and IL-10 genes |
Egypt |
| Kumar et al 93 | Epoxyazadiradione | Asn-105 and Asn-102/allosteric | MDA-MB-231 and MCF-7 | An orthotopic NOD/SCID mice model | Apoptosis induction PI3K/AKT inhibition and cell survival. Inhibition of migration and angiogenesis through factors such as COX-2, OPN, VEGF, and MMP-9. Reduction of breast tumor growth and angiogenesis in a mouse model |
India and United States |
| Lakshmi et al 94 | Epoxyazadiradione | Asn-105 and Asn-102/allosteric | MDA-MB-231 | — | Arrest in G2/M by downregulation of cyclin A2/cdk2 and apoptosis. Decreased NF-κB, EGFR, MMP-9, and fibronectin. Inhibition of migration and colony formation. Interference in cellular metabolism |
India |
| Verjans et al 25 | Anti-MIF and anti-CD74 Abs | MIF/CD74 disruption | MDA-MB-231 and MDA-MB-468 | — | Prevention of cell proliferation | Germany |
| Schulz et al 99 | 17AAG | HSP90 inhibitor | — | ErbB2 transgenic model of HER2-positive BC | Reduction of MIF and tumor growth | Germany |
| Jhaveri et al 101 | Ganetespib | HSP90 inhibitor | — | Patients with MBC in a phase II clinical trial | Potent inhibitory effects on oncoproteins associated with BC pathogenesis | United States |
| Fujita et al 103 | Aza-resveratrol | Tautomerase activity of MIF | MCF-7 | — | Anti-proliferative effects | Japan |
| Simpson et al 72 | Anti-MIF shRNA | Disruption of protein synthesis | 4T1 | A balb/c mouse model | Reduction in primary tumor growth and low number of lung micrometastases. Drop in MDSCs |
United States |
| Charan et al 57 | MIF-specific siRNA (si-MIF) | Disruption of protein synthesis | MDA-MB-468 and MDA-MB-231 | — | Increased apoptosis. Decline in colony forming ability, migration, and wound healing capacity |
United States |
| Zhang et al 104 | Glucan-based cationic nanoparticle containing siRNA against MIF (siMIF-NP) | Disruption of protein synthesis | MDA-MB-231 and 4T1 | A 4T1 mouse model | Lower cell proliferation and higher apoptosis. Diminished immunosuppression and elevated number of infiltrating CD8+ T cells in treated tumors |
United States |
| Wu et al 64 | MIF-specific siRNA (si-MIF) | Disruption of protein synthesis | MCF-7 | A xenograft mouse model with MCF-7 | Decreased cell proliferation and survival. Autophagy induction Suppression of tumor growth in nude mice |
United States |
Abbreviations: GCSF, granulocyte colony-stimulating factor; GMCSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; MDSC, myeloid-derived suppressor cell; MIF, migration inhibitory factor; MMP, matrix metalloproteinase; TNBC, triple-negative breast cancer; VEGF, vascular endothelial growth factor.
Limitations
The MIF is a pleiotropic chemokine with multiple effects on cancer cells, TME, and systemic effects that increase proliferation, migration, and inhibition of autophagy, apoptosis, immune modulation, and angiogenesis, metabolic disorders, and metastasis.24,28
A key weakness in existing reports is the use of recombinant MIF protein, which may have reduced biological activity, and endotoxin contamination, which can induce artificial activity. Therefore, protein purification should be done properly and with complete reports on purification methods and endotoxin levels. 27
Small molecules that target MIF tautomerase activity competitively or allosterically, have been key in the development of MIF as a drug target, but this enzyme site, which is the best starting point for screening these inhibitors, should not be solely targeted. Consider other binding sites that can affect MIF interactions in vivo (disrupting MIF/CD74 interactions), either by steric interference or by inducing conformational changes in the rest of the protein. This is because it is now widely accepted that disruption of enzyme activity alone is not sufficient to completely abrogate the biological effects of MIF. On the contrary, it is difficult to measure the oxidation and reduction activity of MIF, as well as reliable and agreed assays in laboratory conditions for the biological activity of MIF. 27 Nevertheless, many questions about the cellular mechanisms of MIF within the primary or metastatic tumor remain unresolved, but considering its suitability as a therapeutic option, experimental studies on MIF inhibitors seem promising and may lead to longer patient survival and better prognosis for BC.24,26
Conclusions and Future Directions
The TME has emerged as a key factor in the pathogenesis of BC. The pro-inflammatory cytokine, MIF, which is part of this environment, accordingly increases breast tumorigenesis by augmenting cancer cell proliferation, survival, angiogenesis, invasion, metastasis, and stemness. Studies have thus far established that the MIF expression in BC tissues is much higher than that in the normal ones, and this overexpression indicates a poor prognosis in patients with BC. Research on targeting the MIF using various inhibitors, such as small molecules, Abs, or siRNA have further shown good results, such as a reduction in breast tumor growth and metastasis. Current therapeutic strategies to target the MIF are also mainly focused on the development of small inhibitors of its tautomerase or biological activities, as discussed in detail earlier. Due to the upstream role of MIF in coordinating TME and tumorigenesis in BC, the use of these inhibitors with effects, such as apoptosis and cell death, cell proliferation inhibition, angiogenesis, and metastasis can be taken into account as an appropriate therapeutic strategy to inhibit the MIF signaling. In view of this, experimental studies on the MIF inhibitors seem to be promising and lead to longer patient survival and better prognosis in BC. Therefore, the MIF is a valuable therapeutic target in BC, and further evaluation of the MIF-based combination regimens, eg, combining a potent MIF inhibitor with any of the promising chemotherapy and immunotherapy options in clinical trials, can be beneficial for these patients with limited treatment options.
Acknowledgments
The authors would like to sincerely thank the staffs of the Research Center for Molecular Medicine of Hamadan University of Medical Sciences for their cooperation.
Footnotes
ORCID iD: Ali Shojaeian  https://orcid.org/0000-0002-1166-385X
https://orcid.org/0000-0002-1166-385X
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Ali Khezrian: Investigation; Methodology; Writing – original draft.
Ali Shojaeian: Conceptualization; Investigation; Methodology; Project administration; Supervision; Validation; Writing – original draft; Writing – review & editing.
Armin Khaghani Boroujeni: Investigation; Validation; Writing – original draft.
Razieh Amini: Supervision; Validation; Writing – original draft.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and material: Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. [DOI] [PubMed] [Google Scholar]
- 2. Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929-2943. [PMC free article] [PubMed] [Google Scholar]
- 3. Orrantia-Borunda E, Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. Subtypes of breast cancer. In: Mayrovitz HN, ed. Breast Cancer. Brisbane, QLD, Australia: Exon Publications; 2022:31-42. [PubMed] [Google Scholar]
- 4. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582-598. [DOI] [PubMed] [Google Scholar]
- 9. Nguyen TV, Sleiman M, Moriarty T, Herrick WG, Peyton SR. Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials. 2014;35:5749-5759. [DOI] [PubMed] [Google Scholar]
- 10. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185-S198. [DOI] [PubMed] [Google Scholar]
- 12. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643-675. [DOI] [PubMed] [Google Scholar]
- 13. Mehta AK, Kadel S, Townsend MG, Oliwa M, Guerriero JL. Macrophage biology and mechanisms of immune suppression in breast cancer. Front Immunol. 2021;12:643771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qian B-Z, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature Med. 2011;17:1359-1370. [DOI] [PubMed] [Google Scholar]
- 16. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song S, Xiao Z, Dekker FJ, Poelarends GJ, Melgert BN. Macrophage migration inhibitory factor family proteins are multitasking cytokines in tissue injury. Cell Mol Life Sci. 2022;79:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol Endocrinol. 2007;21:1267-1280. [DOI] [PubMed] [Google Scholar]
- 19. Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer: macrophage migration inhibitory factor (MIF)—the potential missing link. QJM. 2010;103:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Savaskan NE, Fingerle-Rowson G, Buchfelder M, Eyüpoglu IY. Brain miffed by macrophage migration inhibitory factor. Int J Cell Biol. 2012;2012:139573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suzuki M, Sugimoto H, Nakagawa A, Tanaka I, Nishihira J, Sakai M. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat Struct Biol. 1996;3:259-266. [DOI] [PubMed] [Google Scholar]
- 22. Bando H, Matsumoto G, Bando M, et al. Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn J Cancer Res. 2002;93:389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu X, Wang B, Ye C, et al. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008;261:147-157. [DOI] [PubMed] [Google Scholar]
- 24. Richard V, Kindt N, Saussez S. Macrophage migration inhibitory factor involvement in breast cancer. Int J Oncol. 2015;47:1627-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verjans E, Noetzel E, Bektas N, et al. Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer. BMC Cancer. 2009;9:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu L, Li Y, Sun H, et al. Current developments of macrophage migration inhibitory factor (MIF) inhibitors. Drug Discov Today. 2013;18:592-600. [DOI] [PubMed] [Google Scholar]
- 27. Bloom J, Sun S, Al-Abed Y. MIF, a controversial cytokine: a review of structural features, challenges, and opportunities for drug development. Expert Opin Ther Targets. 2016;20:1463-1475. [DOI] [PubMed] [Google Scholar]
- 28. Guda MR, Rashid MA, Asuthkar S, et al. Pleiotropic role of macrophage migration inhibitory factor in cancer. Am J Cancer Res. 2019;9:2760-2773. [PMC free article] [PubMed] [Google Scholar]
- 29. Mühlhahn P, Bernhagen J, Czisch M, et al. NMR characterization of structure, backbone dynamics, and glutathione binding of the human macrophage migration inhibitory factor (MIF). Protein Sci. 1996;5:2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nishihira J, Kuriyama T, Sakai M, Nishi S, Ohki S-y, Hikichi K. The structure and physicochemical properties of rat liver macrophage migration inhibitory factor. Biochim Biophys Acta. 1995;1247:159-162. [DOI] [PubMed] [Google Scholar]
- 31. Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 1994;33:14144-14155. [DOI] [PubMed] [Google Scholar]
- 32. Buchko GW, Abendroth J, Robinson H, et al. Crystal structure of a macrophage migration inhibitory factor from Giardia lamblia. J Struct Funct Genomics. 2013;14:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun HW, Bernhagen J, Bucala R, Lolis E. Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1996;93:5191-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Philo JS, Yang T-H, LaBarre M. Re-examining the oligomerization state of macrophage migration inhibitory factor (MIF) in solution. Biophys Chem. 2004;108:77-87. [DOI] [PubMed] [Google Scholar]
- 35. Bendrat K, Al-Abed Y, Callaway DJ, et al. Biochemical and mutational investigations of the enzymatic activity of macrophage migration inhibitory factor. Biochemistry. 1997;36:15356-15362. [DOI] [PubMed] [Google Scholar]
- 36. Kleemann R, Rorsman H, Rosengren E, Mischke R, Mai NT, Bernhagen J. Dissection of the enzymatic and immunologic functions of macrophage migration inhibitory factor: full immunologic activity of N-terminally truncated mutants. Eur J Biochem. 2000;267:7183-7193. [DOI] [PubMed] [Google Scholar]
- 37. Jankauskas SS, Wong DWL, Bucala R, Djudjaj S, Boor P. Evolving complexity of MIF signaling. Cell Signal. 2019;57:76-88. [DOI] [PubMed] [Google Scholar]
- 38. Pantouris G, Syed MA, Fan C, et al. An analysis of MIF structural features that control functional activation of CD74. Chem Biol. 2015;22:1197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gore Y, Starlets D, Maharshak N, et al. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784-2792. [DOI] [PubMed] [Google Scholar]
- 40. Lue H, Dewor M, Leng L, Bucala R, Bernhagen J. Activation of the JNK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on CXCR4 and CD74. Cell Signal. 2011;23:135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rajagopal S, Kim J, Ahn S, et al. β-arrestin-but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci USA. 2010;107:628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chatterjee M, Borst O, Walker B, et al. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ Res. 2014;115:939-949. [DOI] [PubMed] [Google Scholar]
- 43. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [DOI] [PubMed] [Google Scholar]
- 44. Feroz W, Sheikh AMA. Exploring the multiple roles of guardian of the genome: P53. Egypt J Med Hum Genet. 2020;21:1-23. [Google Scholar]
- 45. Xin D, Rendon BE, Zhao M, Winner M, McGhee Coleman A, Mitchell RA. The MIF homologue D-dopachrome tautomerase promotes COX-2 expression through β-catenin–dependent and–independent mechanisms. Mol Cancer Res. 2010;8:1601-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lue H, Thiele M, Franz J, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046-5059. [DOI] [PubMed] [Google Scholar]
- 47. De R, Sarkar S, Mazumder S, et al. Macrophage migration inhibitory factor regulates mitochondrial dynamics and cell growth of human cancer cell lines through CD74–NF-κB signaling. J Biol Chem. 2018;293:19740-19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang D, Luo L, Chen W, et al. Significance of the vascular endothelial growth factor and the macrophage migration inhibitory factor in the progression of hepatocellular carcinoma. Oncol Rep. 2014;31:1199-1204. [DOI] [PubMed] [Google Scholar]
- 49. Zis O, Zhang S, Dorovini-Zis K, Wang L, Song W. Hypoxia signaling regulates macrophage migration inhibitory factor (MIF) expression in stroke. Mol Neurobiol. 2015;51:155-167. [DOI] [PubMed] [Google Scholar]
- 50. Lai X, Li Q, Wu F, et al. Epithelial-mesenchymal transition and metabolic switching in cancer: lessons from somatic cell reprogramming. Front Cell Dev Biol. 2020;8:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang S, He P, Wang J, et al. A novel MIF signaling pathway drives the malignant character of pancreatic cancer by targeting NR3C2. Cancer Res. 2016;76:3838-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li J, Zhang J, Xie F, Peng J, Wu X. Macrophage migration inhibitory factor promotes Warburg effect via activation of the NF‑κB/HIF‑1α pathway in lung cancer. Int J Mol Med. 2018;41:1062-1068. [DOI] [PubMed] [Google Scholar]
- 53. Huang G, Ma L, Shen L, et al. MIF/SCL3A2 depletion inhibits the proliferation and metastasis of colorectal cancer cells via the AKT/GSK-3β pathway and cell iron death. J Cell Mol Med. 2022;26:3410-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mora Barthelmess R, Stijlemans B, Van Ginderachter JA. Hallmarks of cancer affected by the MIF cytokine family. Cancers. 2023;15:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Günther S, Fagone P, Jalce G, Atanasov AG, Guignabert C, Nicoletti F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: from pathogenic factors to therapeutic targets. Drug Discov Today. 2019;24:428-439. [DOI] [PubMed] [Google Scholar]
- 56. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Charan M, Das S, Mishra S, et al. Macrophage migration inhibitory factor inhibition as a novel therapeutic approach against triple-negative breast cancer. Cell Death Dis. 2020;11:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Richard V, Kindt N, Decaestecker C, et al. Involvement of macrophage migration inhibitory factor and its receptor (CD74) in human breast cancer. Oncol Rep. 2014;32:523-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin S, Wang M, Liu X, et al. Association of genetic polymorphisms in MIF with breast cancer risk in Chinese women. Clin Exp Med. 2017;17:395-401. [DOI] [PubMed] [Google Scholar]
- 60. Fersching DM, Nagel D, Siegele B, et al. Apoptosis-related biomarkers sFAS, MIF, ICAM-1 and PAI-1 in serum of breast cancer patients undergoing neoadjuvant chemotherapy. Anticancer Res. 2012;32:2047-2058. [PubMed] [Google Scholar]
- 61. Martinez LM, Vallone VB, Labovsky V, et al. Changes in the peripheral blood and bone marrow from untreated advanced breast cancer patients that are associated with the establishment of bone metastases. Clin Exp Metastasis. 2014;31:213-232. [DOI] [PubMed] [Google Scholar]
- 62. Singh JK, Simões BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15:210-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park S-J, Ahmad F, Philp A, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu MY, Fu J, Xu J, O’Malley BW, Wu RC. Steroid receptor coactivator 3 regulates autophagy in breast cancer cells through macrophage migration inhibitory factor. Cell Res. 2012;22:1003-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oda S, Oda T, Nishi K, et al. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS ONE. 2008;3:e2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang J, Zhang G, Yang S, et al. Macrophage migration inhibitory factor regulating the expression of VEGF-C through MAPK signal pathways in breast cancer MCF-7 cell. World J Surg Oncol. 2016;14:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nat Rev Mol Cell Biol. 2009;10:165-177. [DOI] [PubMed] [Google Scholar]
- 68. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-727. [DOI] [PubMed] [Google Scholar]
- 69. Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679-695. [DOI] [PubMed] [Google Scholar]
- 70. Lv W, Chen N, Lin Y, et al. Macrophage migration inhibitory factor promotes breast cancer metastasis via activation of HMGB1/TLR4/NF kappa B axis. Cancer Lett. 2016;375:245-255. [DOI] [PubMed] [Google Scholar]
- 71. George AL, Bednarczyk R, Rajoria S, et al. CXCL8 is a secretory inflammatory stimulus of the activated TME that modulates breast cancer phenotype. Cancer Res. 2013;73:1432. [Google Scholar]
- 72. Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. 2012;189:5533-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Majumder M, Xin X, Liu L, Girish GV, Lala PK. Prostaglandin E2 receptor EP 4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci. 2014;105:1142-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mangano K, Mazzon E, Basile MS, et al. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget. 2018;9:17951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Trivedi-Parmar V, Jorgensen WL. Advances and insights for small molecule inhibition of macrophage migration inhibitory factor. J Med Chem. 2018;61:8104-8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cotzomi-Ortega I, Rosas-Cruz A, Ramírez-Ramírez D, et al. Autophagy inhibition induces the secretion of macrophage migration inhibitory factor (MIF) with autocrine and paracrine effects on the promotion of malignancy in breast cancer. Biology. 2020;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Charan M, Das S, Mishra S, Varikuti S, Satoskar AR, Ganju RK. Abstract P4-04-12: macrophage migration inhibitory factor regulates triple-negative breast cancer progression by enhancing the recruitment of immune suppressive cells. Cancer Res. 2022;82:P4-04-12. [Google Scholar]
- 78. Das S, Chatterjee N, Mishra S, et al. CPSI-1306: a novel macrophage migration inhibitory factor inhibitor against aggressive breast cancer. Cancer Res. 2018;78:5870. [Google Scholar]
- 79. Das S, Kundu M, Parekh A, Bharadwaj D, Mandal M. Cancer stem cell induces chemoresistance in breast cancer via macrophage migration inhibitory factor mediated activation of AKT pathway. Cancer Res. 2019;79:4676. [Google Scholar]
- 80. Delgado L, Fernandes I, González-Manzano S, de Freitas V, Mateus N, Santos-Buelga C. Anti-proliferative effects of quercetin and catechin metabolites. Food Funct. 2014;5:797-803. [DOI] [PubMed] [Google Scholar]
- 81. Jung SY, Nam KY, Park JI, et al. Radiosensitizing effect of novel phenylpyrimidine derivatives on human lung cancer cells via cell cycle perturbation. J Pharmacol Exp Ther. 2019;370:514-527. [DOI] [PubMed] [Google Scholar]
- 82. Silbermann K, Li J, Namasivayam V, Stefan SM, Wiese M. Rational drug design of 6-substituted 4-anilino-2-phenylpyrimidines for exploration of novel ABCG2 binding site. Eur J Med Chem. 2021;212:113045. [DOI] [PubMed] [Google Scholar]
- 83. Cao S, Hu S, Jiang P, Zhang Z, Li L, Wu Q. Effects of sulforaphane on breast cancer based on metabolome and microbiome. Food Sci Nutr. 2023;11:2277-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jabbarzadeh Kaboli P, Afzalipour Khoshkbejari M, Mohammadi M, et al. Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer–contradictory effects and future perspectives. Biomed Pharmacother. 2020;121:109635. [DOI] [PubMed] [Google Scholar]
- 85. Zhang Y, Lu Q, Li N, Xu M, Miyamoto T, Liu J. Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. NPJ Breast Cancer. 2022;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pereyra-Vergara F, Olivares-Corichi IM, Perez-Ruiz AG, Luna-Arias JP, García-Sánchez JR. Apoptosis induced by (−)-epicatechin in human breast cancer cells is mediated by reactive oxygen species. Molecules. 2020;25:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pérez-Durán J, Luna A, Portilla A, et al. (−)-Epicatechin inhibits metastatic-associated proliferation, migration, and invasion of murine breast cancer cells in vitro. Molecules. 2023;28:6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Youssef G, Lathia J, Lee EQ, et al. Phase 1b/2a study evaluating the combination of MN-166 (ibudilast) and temozolomide in patients with newly diagnosed and recurrent glioblastoma (GBM). Am Soc Clin Oncol. 2024;42:1. [Google Scholar]
- 89. Thabet NM, Moustafa EM. Synergistic effect of Ebselen and gamma radiation on breast cancer cells. Int J Radiat Biol. 2017;93:784-792. [DOI] [PubMed] [Google Scholar]
- 90. Blasquez L, Bouzinba-Segard H, Bourdoulous S, Faure C. Ebselen oxide and derivatives are new allosteric HER2 inhibitors for HER2-positive cancers. Mol Oncol. 2023;17:1981-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bai F, Asojo OA, Cirillo P, et al. A novel allosteric inhibitor of macrophage migration inhibitory factor (MIF). J Biol Chem. 2012;287:30653-30663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alam A, Haldar S, Thulasiram HV, et al. Novel anti-inflammatory activity of epoxyazadiradione against macrophage migration inhibitory factor: inhibition of tautomerase and proinflammatory activities of macrophage migration inhibitory factor. J Biol Chem. 2012;287:24844-24861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kumar D, Haldar S, Gorain M, et al. Epoxyazadiradione suppresses breast tumor growth through mitochondrial depolarization and caspase-dependent apoptosis by targeting PI3K/Akt pathway. BMC Cancer. 2018;18:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lakshmi S, Renjitha J, B Sasidhar S, Priya S. Epoxyazadiradione induced apoptosis/anoikis in triple-negative breast cancer cells, MDA-MB-231, by modulating diverse cellular effects. J Biochem Mol Toxicol. 2021;35:1-17. [DOI] [PubMed] [Google Scholar]
- 95. Yu W-L, Jones BD, Kang M, Hammons JC, La Clair JJ, Burkart MD. Spirohexenolide A targets human macrophage migration inhibitory factor (hMIF). J Nat Prod. 2013;76:817-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mahalingam D, Patel MR, Sachdev JC, et al. Phase I study of imalumab (BAX69), a fully human recombinant antioxidized macrophage migration inhibitory factor antibody in advanced solid tumours. Br J Clin Pharmacol. 2020;86:1836-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Haran M, Mirkin V, Braester A, et al. A phase I-II clinical trial of the anti-CD74 monoclonal antibody milatuzumab in frail patients with refractory chronic lymphocytic leukaemia: a patient based approach. Br J Haematol. 2017;182:125-128. [DOI] [PubMed] [Google Scholar]
- 98. Martin P, Furman RR, Rutherford S, et al. Phase I study of the anti-CD74 monoclonal antibody milatuzumab (hLL1) in patients with previously treated B-cell lymphomas. Leuk Lymphoma. 2015;56:3065-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schulz R, Marchenko ND, Holembowski L, et al. Inhibiting the HSP90 chaperone destabilizes macrophage migration inhibitory factor and thereby inhibits breast tumor progression. J Exp Med. 2012;209:275-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schulz R, Streller F, Scheel AH, et al. HER2/ErbB2 activates HSF1 and thereby controls HSP90 clients including MIF in HER2-overexpressing breast cancer. Cell Death Dis. 2014;5:e980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jhaveri K, Chandarlapaty S, Lake D, et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer. 2014;14:154-160. [DOI] [PubMed] [Google Scholar]
- 102. Sprouse AA, Herbert BS. Resveratrol augments paclitaxel treatment in MDA-MB-231 and paclitaxel-resistant MDA-MB-231 breast cancer cells. Anticancer Res. 2014;34:5363-5374. [PubMed] [Google Scholar]
- 103. Fujita Y, Islam R, Sakai K, et al. Aza-derivatives of resveratrol are potent macrophage migration inhibitory factor inhibitors. Invest New Drugs. 2012;30:1878-1886. [DOI] [PubMed] [Google Scholar]
- 104. Zhang M, Yan L, Kim JA. Modulating mammary tumor growth, metastasis and immunosuppression by siRNA-induced MIF reduction in tumor microenvironment. Cancer Gene Ther. 2015;22:463-474. [DOI] [PubMed] [Google Scholar]