Abstract
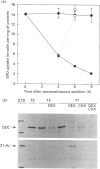
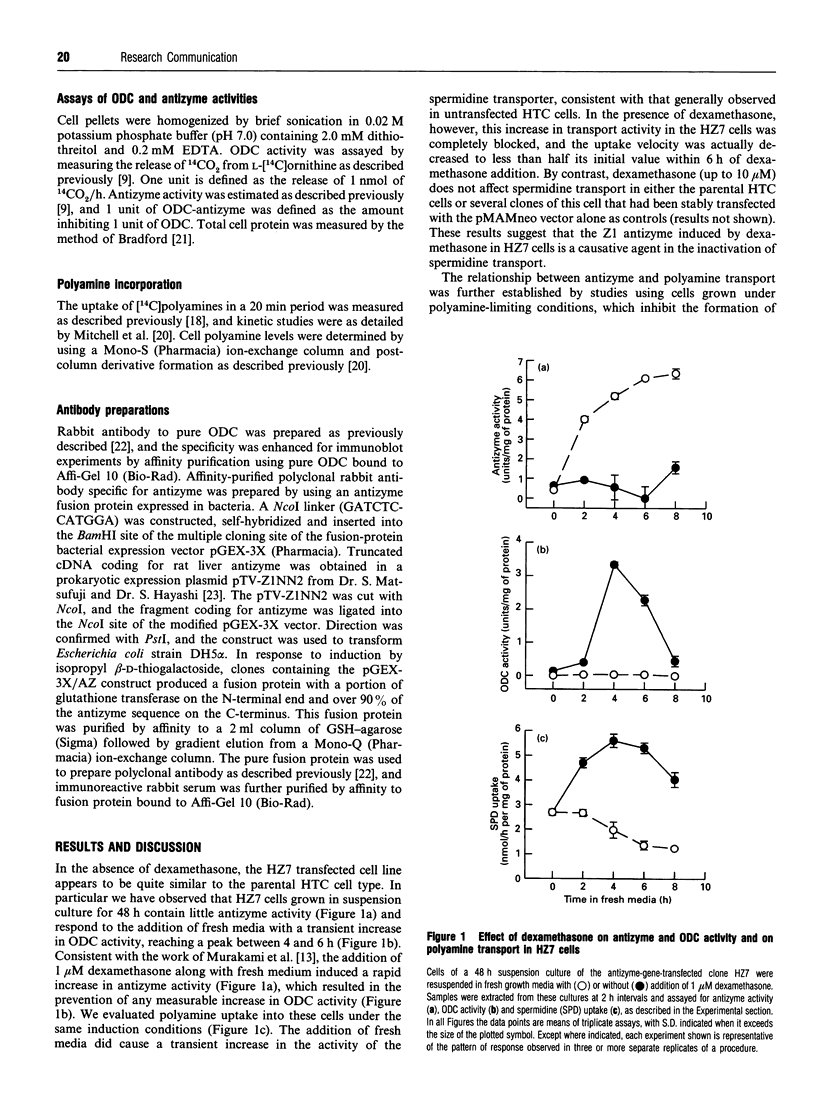
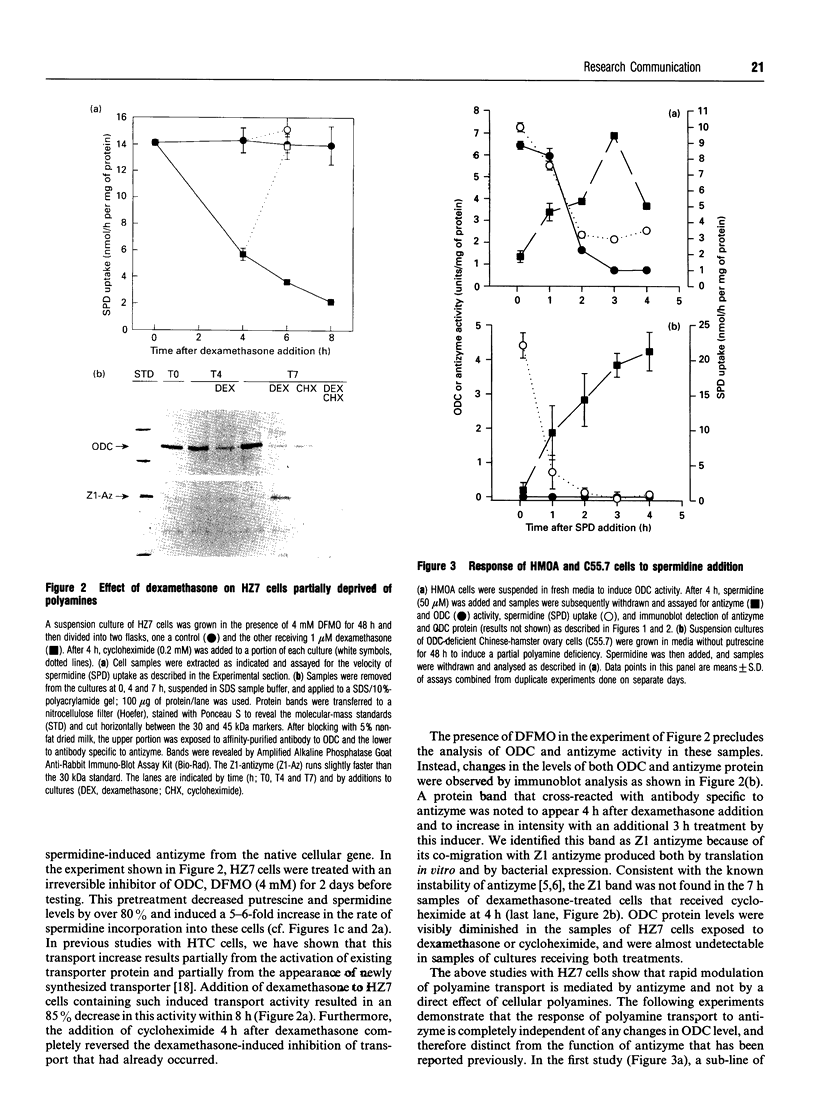
Antizyme, a spermidine-induced protein that binds and stimulates ornithine decarboxylase degradation, is now shown also to mediate the rapid feedback inhibition of polyamine uptake into mammalian cells. Using a cell line (HZ7) transfected with truncated antizyme cDNA, and mutant ornithine decarboxylase cell lines, we demonstrate that this newly discovered action of antizyme is distinct from its role in modulating polyamine biosynthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L., Seppänen P., Jänne J. Intracellular putrescine and spermidine deprivation induces increased uptake of the natural polyamines and methylglyoxal bis(guanylhydrazone). Biochem J. 1980 Dec 15;192(3):941–945. doi: 10.1042/bj1920941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovich Z., Kahana C. Involvement of the 20S proteasome in the degradation of ornithine decarboxylase. Eur J Biochem. 1993 Apr 1;213(1):205–210. doi: 10.1111/j.1432-1033.1993.tb17749.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byers T. L., Pegg A. E. Regulation of polyamine transport in Chinese hamster ovary cells. J Cell Physiol. 1990 Jun;143(3):460–467. doi: 10.1002/jcp.1041430309. [DOI] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Kyriakidis D., Fong W. F., Canellakis E. S. Ornithine decarboxylase antizyme is a normal component of uninduced H-35 cells and rat liver. Eur J Biochem. 1977 Dec;81(3):545–550. doi: 10.1111/j.1432-1033.1977.tb11980.x. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y., Hoshino K., Igarashi K. Characterization of the inducible polyamine transporter in bovine lymphocytes. Eur J Biochem. 1988 Sep 15;176(2):409–414. doi: 10.1111/j.1432-1033.1988.tb14297.x. [DOI] [PubMed] [Google Scholar]
- Khan N. A., Quemener V., Moulinoux J. P. Polyamine membrane transport regulation. Cell Biol Int Rep. 1991 Jan;15(1):9–24. doi: 10.1016/0309-1651(91)90078-w. [DOI] [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Purification and some properties of a protein inhibitor (antizyme) of ornithine decarboxylase from rat liver. J Biol Chem. 1984 Aug 25;259(16):10036–10040. [PubMed] [Google Scholar]
- Li X., Coffino P. Degradation of ornithine decarboxylase: exposure of the C-terminal target by a polyamine-inducible inhibitory protein. Mol Cell Biol. 1993 Apr;13(4):2377–2383. doi: 10.1128/mcb.13.4.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Coffino P. Regulated degradation of ornithine decarboxylase requires interaction with the polyamine-inducible protein antizyme. Mol Cell Biol. 1992 Aug;12(8):3556–3562. doi: 10.1128/mcb.12.8.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Tardif C. Initial characterization of a HTC cell variant partially resistant to the anti-proliferative effect of ornithine decarboxylase inhibitors. Exp Cell Res. 1978 Sep;115(2):387–393. doi: 10.1016/0014-4827(78)90292-6. [DOI] [PubMed] [Google Scholar]
- Matsufuji S., Miyazaki Y., Kanamoto R., Kameji T., Murakami Y., Baby T. G., Fujita K., Ohno T., Hayashi S. Analyses of ornithine decarboxylase antizyme mRNA with a cDNA cloned from rat liver. J Biochem. 1990 Sep;108(3):365–371. doi: 10.1093/oxfordjournals.jbchem.a123207. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Chen H. J. Conformational changes in ornithine decarboxylase enable recognition by antizyme. Biochim Biophys Acta. 1990 Jan 19;1037(1):115–121. doi: 10.1016/0167-4838(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Diveley R. R., Jr, Bareyal-Leyser A. Feedback repression of polyamine uptake into mammalian cells requires active protein synthesis. Biochem Biophys Res Commun. 1992 Jul 15;186(1):81–88. doi: 10.1016/s0006-291x(05)80778-8. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Diveley R. R., Jr, Bareyal-Leyser A., Mitchell J. L. Abnormal accumulation and toxicity of polyamines in a difluoromethylornithine-resistant HTC cell variant. Biochim Biophys Acta. 1992 Aug 12;1136(2):136–142. doi: 10.1016/0167-4889(92)90248-a. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Hoff J. A., Bareyal-Leyser A. Stable ornithine decarboxylase in a rat hepatoma cell line selected for resistance to alpha-difluoromethylornithine. Arch Biochem Biophys. 1991 Oct;290(1):143–152. doi: 10.1016/0003-9861(91)90600-n. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Matsufuji S., Murakami Y., Hayashi S. Single amino-acid replacement is responsible for the stabilization of ornithine decarboxylase in HMOA cells. Eur J Biochem. 1993 Jun 15;214(3):837–844. doi: 10.1111/j.1432-1033.1993.tb17987.x. [DOI] [PubMed] [Google Scholar]
- Morris D. R. A new perspective on ornithine decarboxylase regulation: prevention of polyamine toxicity is the overriding theme. J Cell Biochem. 1991 Jun;46(2):102–105. doi: 10.1002/jcb.240460203. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Hayashi S. Role of antizyme in degradation of ornithine decarboxylase in HTC cells. Biochem J. 1985 Mar 15;226(3):893–896. doi: 10.1042/bj2260893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Matsufuji S., Kameji T., Hayashi S., Igarashi K., Tamura T., Tanaka K., Ichihara A. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature. 1992 Dec 10;360(6404):597–599. doi: 10.1038/360597a0. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Matsufuji S., Miyazaki Y., Hayashi S. Destabilization of ornithine decarboxylase by transfected antizyme gene expression in hepatoma tissue culture cells. J Biol Chem. 1992 Jul 5;267(19):13138–13141. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz R. B., Steglich C., Scheffler I. E. Molecular and genetic characterization of an ornithine decarboxylase-deficient Chinese hamster cell line. J Biol Chem. 1990 May 25;265(15):8880–8886. [PubMed] [Google Scholar]
- Pohjanpelto P. Cycloheximide elicits in human fibroblasts a response characteristic for initiation of cell proliferation. Exp Cell Res. 1976 Oct 1;102(1):138–142. doi: 10.1016/0014-4827(76)90308-6. [DOI] [PubMed] [Google Scholar]
- Rinehart C. A., Jr, Chen K. Y. Characterization of the polyamine transport system in mouse neuroblastoma cells. Effects of sodium and system A amino acids. J Biol Chem. 1984 Apr 25;259(8):4750–4756. [PubMed] [Google Scholar]
- Seiler N., Dezeure F. Polyamine transport in mammalian cells. Int J Biochem. 1990;22(3):211–218. doi: 10.1016/0020-711x(90)90332-w. [DOI] [PubMed] [Google Scholar]
- Steglich C., Grens A., Scheffler I. E. Chinese hamster cells deficient in ornithine decarboxylase activity: reversion by gene amplification and by azacytidine treatment. Somat Cell Mol Genet. 1985 Jan;11(1):11–23. doi: 10.1007/BF01534730. [DOI] [PubMed] [Google Scholar]