Abstract
Background
Potassium‐sparing diuretics, which block the epithelial sodium channel (ENaC), are widely prescribed for hypertension as a second‐line drug in patients taking other diuretics (e.g. thiazide diuretics) and much less commonly prescribed as monotherapy. Therefore, it is essential to determine the effects of ENaC blockers on blood pressure (BP), heart rate and withdrawals due to adverse effects (WDAEs) when given as a first‐line or second‐line therapy.
Objectives
To quantify the dose‐related reduction in systolic blood pressure (SBP) and diastolic blood pressure (DBP) of ENaC blocker therapy as a first‐line or second‐line drug in patients with primary hypertension.
Search methods
We searched CENTRAL (The Cochrane Library 2012), MEDLINE (1950 to August 2012), EMBASE (1980 to August 2012) and reference lists of articles.
Selection criteria
Double‐blind, randomized, controlled trials in patients with primary hypertension that evaluate, for a duration of 3 to 12 weeks, the BP lowering efficacy of: 1) fixed‐dose monotherapy with an ENaC blocker compared with placebo; or 2) an ENaC blocker in combination with another class of anti‐hypertensive drugs compared with the respective monotherapy (without an ENaC blocker).
Data collection and analysis
Two authors independently assessed the risk of bias and extracted data. Study authors were contacted for additional information. WDAE information was also collected from the trials.
Main results
No trials evaluating the BP lowering efficacy of ENaC blockers as monotherapy in patients with primary hypertension were identified. Only 6 trials evaluated the BP lowering efficacy of low doses of amiloride and triamterene as a second drug in 496 participants with a baseline BP of 151/102 mm Hg. The additional BP reduction caused by the ENaC blocker as a second drug was estimated by comparing the difference in BP reduction between the combination and monotherapy groups. The addition of low doses of amiloride and triamterene in these trials did not reduce BP. An estimate of the dose‐related BP lowering efficacy for ENaC blockers was not possible because of a lack of trial data at higher doses.
Authors' conclusions
ENaC blockers do not have a statistically or clinically significant BP lowering effect at low doses but trials at higher doses are not available. The review did not provide a good estimate of the incidence of harms associated with ENaC blockers.
Keywords: Humans; Amiloride; Amiloride/administration & dosage; Antihypertensive Agents; Antihypertensive Agents/administration & dosage; Blood Pressure; Blood Pressure/drug effects; Diuretics; Diuretics/administration & dosage; Dose‐Response Relationship, Drug; Drug Therapy, Combination; Drug Therapy, Combination/methods; Hypertension; Hypertension/drug therapy; Randomized Controlled Trials as Topic; Sodium Channel Blockers; Sodium Channel Blockers/administration & dosage; Triamterene; Triamterene/administration & dosage
Plain language summary
The blood pressure lowering effect of ENaC blockers is not known
Potassium‐sparing diuretics, which block the epithelial sodium channel (also called ENaC blockers), are a class of drugs commonly prescribed to prevent loss of potassium but also might help to lower elevated blood pressure. This class includes drugs such as amiloride (Midamor, Amilzide) and triamterene (Dyrenium, Dyazide). We asked how much this class of drugs lowers blood pressure, when used alone or when used as the second drug to treat hypertension. The available scientific literature was searched to find all the trials that had assessed this question. No trials were found studying the blood pressure lowering ability of ENaC blockers when used alone. We found 6 trials studying the blood pressure lowering ability of amiloride and triamterene, when added as a second drug, in 496 participants. All 6 trials studied the ENaC blockers at low doses and there was no blood pressure lowering effect. Trials studying these drugs at higher doses are needed in order to determine if they lower blood pressure. The harms associated with ENaC blockers could not be estimated in this review because of the low doses studied and the short duration of the trials.
Background
Potassium‐sparing diuretics, which block the epithelial sodium channel (ENaC), are indicated in combination with other diuretics to decrease the loss of potassium in the kidney. They may decrease blood pressure by inducing mild natriuresis and plasma volume reduction. The magnitude of the blood pressure reduction associated with this class of drugs is not known.
A systematic review of the dose‐related blood pressure lowering efficacy of ENaC blockers as either first‐line or second‐line therapy has not been previously performed. The information derived from this review will facilitate future reviews of head‐to‐head comparisons with other drug classes and assist clinicians in choosing optimal doses of ENaC blockers.
Objectives
Primary objective:
To quantify the dose‐related systolic and/or diastolic blood pressure lowering efficacy of ENaC blockers, as monotherapy or when added as a second drug, in patients with primary hypertension.
Secondary objectives:
To determine the effects of ENaC blockers, as monotherapy or when added as a second drug, on:
variability of blood pressure
pulse pressure
heart rate
withdrawals due to adverse effects
Methods
Criteria for considering studies for this review
Types of studies
Included studies were randomized controlled trials (RCTs) and their design must have met the following criteria:
double‐blind
washout period of at least 2 weeks prior to randomization
random allocation to ENaC blocker group(s) and parallel placebo group
duration of follow‐up of at least three weeks
office blood pressure measurements at baseline (following washout) and at one or more time points between 3 and 12 weeks post‐treatment
Types of participants
Participants with a baseline office systolic blood pressure of at least 140 mmHg and/or a diastolic blood pressure of at least 90 mmHg were included.
Participants were not restricted by age, gender, baseline risk or any other co‐morbid conditions.
Patients with creatinine levels greater than 1.5 times the normal level were excluded.
Participants who were taking medications that affect blood pressure other than the study medications were excluded.
Types of interventions
Monotherapy trials
ENaC blocker as monotherapy, including amiloride and triamterene
parallel placebo arm
Trials in which titration to a higher dose was based on blood pressure response were not eligible if the titration occurred before 3 weeks of treatment because dose‐response relationships cannot be analyzed if patients within each randomized group are taking different doses. However, trials in which a response‐dependent titration took place during or after the 3 to 12 week interval were eligible if pre‐titration data were given. For forced titration trials, data at each dose level were extracted, provided this dose was given for a 3 to 12 week period.
Combination therapy trials
-
ENaC blocker in combination with another antihypertensive drug class, including:
angiotensin‐converting enzyme (ACE) inhibitors
angiotensin receptor blockers
beta blockers
calcium channel blockers
centrally‐acting drugs (but limited to guanabenz, rilmenidine, clonidine, moxonidine, methyldopa and guanfacine).
diuretics
renin inhibitors
The addition of a potassium‐sparing diuretic must have been the only difference between the combination and monotherapy groups. In the case of fixed‐dose combination, the pill should be identical in appearance and taste to the individual components. In other cases where drugs are administered separately in the combination group, the monotherapy group should receive a matching placebo (i.e. double‐dummy design).
All dosages and combinations of these drugs were considered. Trials in which titration to a higher dose was based on blood pressure response were excluded. For forced titration trials, data from the lowest dose given within 3 to 12 weeks period were extracted.
Types of outcome measures
Primary:
Monotherapy and combination therapy trials
Change from baseline of trough and/or peak systolic and diastolic blood pressure at 3 to 12 weeks. If blood pressure measurements were available at more than one time within the accepted window, the weighted means of blood pressures taken in the 3 to 12 week range were used.
Secondary:
Monotherapy trials
Standard deviation of the change in blood pressure compared with placebo.
Change in standard deviation of blood pressure compared with placebo.
Change in pulse pressure compared with placebo.
Change in heart rate compared with placebo.
Number of patient withdrawals due to adverse effects compared with placebo.
Combination therapy trials
Standard deviation of the change in blood pressure with combination therapy compared with monotherapy.
Change in standard deviation of blood pressure with combination therapy compared with monotherapy.
Change in pulse pressure with combination therapy compared with monotherapy.
Change in heart rate with combination therapy compared with monotherapy.
Number of patient withdrawals due to adverse effects with combination therapy compared with monotherapy.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched for primary studies:
Cochrane Central Register of Controlled Trials (CENTRAL),The Cochrane Library 2012
MEDLINE (1950 to August 2012)
EMBASE (1980 to August 2012)
Electronic databases were searched using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (Higgins 2009) with selected MeSH terms and free text terms relating to potassium‐sparing diuretics and hypertension. No language restrictions were used. The MEDLINE search strategy was translated into the other databases using the appropriate controlled vocabulary as applicable.
Full search strategies for all databases are included in the Appendices (Appendix 1; Appendix 2; Appendix 3).
Searching other resources
Previously published meta‐analyses on the dose‐response of potassium‐sparing diuretics, as well as narrative reviews, were used to help identify references to trials.
Data collection and analysis
Selection of studies
The databases listed above were searched and potentially relevant citations were identified. The initial screen of these abstracts excluded articles whose titles and/or abstracts were clearly irrelevant. The full text of remaining articles was then retrieved (and translated into English where required) to assess whether the trials met the prespecified inclusion criteria. The bibliographies of pertinent articles, reviews and texts were searched for additional citations. Two independent reviewers (BSH and JJW) assessed the eligibility of the trials using a standardized trial inclusion form. A third reviewer (JMHC) resolved discrepancies.
Data extraction and management
Data from included studies were extracted by one reviewer (BSH or JMHC) using standardized data extraction forms and checked by a second reviewer (JMHC or BSH). If data were presented numerically (in tables or text) and graphically (in figures), the numeric data were preferred because of possible measurement error when estimating from graphs. All numeric calculations and extractions from graphs or figures were confirmed by a second reviewer. Any discrepancies were resolved by consensus.
The position of the patient during blood pressure measurement may affect the blood pressure lowering effect. However, in order not to lose valuable data, if only one position was reported, data from that position were extracted. When blood pressure measurement data were available in more than one position, data were extracted in accordance with the following order of preference: 1) sitting; 2) standing; and 3) supine.
Assessment of risk of bias in included studies
Two reviewers (BSH and JMHC) independently assessed the risk of bias in included studies using the Cochrane Collaboration’s recommended tool, which is a domain‐based critical evaluation of the following domains: sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reporting; and other sources of bias (Higgins 2009). Assessments of risk of bias are provided in the ‘Risk of bias’ table for each study.
Dealing with missing data
If there were multiple reports from the same study, the duplicate publications were scanned for additional data. If necessary, investigators were contacted (by email, letter and/or fax) to obtain the missing information.
In the case of missing values for standard deviation of the change in blood pressure or heart rate, the standard deviation was imputed based on the information in the same trial or from other trials using the same dose. The following hierarchy (listed from high to low preference) was used to impute standard deviation values:
Pooled standard deviation calculated either from the t statistic corresponding to an exact p‐value reported or from the 95% confidence interval of the mean difference between treatment group and placebo.
Standard deviation of change in blood pressure/heart rate from a different position than that of the blood pressure data/heart rate used.
Standard deviation of blood pressure/heart rate at the end of treatment.
Standard deviation of blood pressure/heart rate at the end of treatment measured from a different position than that of the blood pressure/heart rate data used.
Standard deviation of blood pressure/heart rate at baseline (except if this measure was used for entry criteria).
Weighted mean standard deviation of change in blood pressure/heart rate from other trials using the same class of drug (at any dose).
Assessment of heterogeneity
If there was significant statistical heterogeneity (P‐value <0.10) associated with an effect estimate, a random effects model was applied. This model provides a more conservative statistical comparison of the difference between intervention and control because a confidence interval around the effect estimate is wider than a confidence interval around a fixed effect estimate. If a statistically significant difference was still present using the random effects model, the fixed effect pooled estimate and 95% CI was reported because of the tendency of smaller trials, which are more susceptible to publication bias, to be overweighted with a random effects analysis.
Assessment of reporting biases
No language restrictions were applied.
Data synthesis
Data were processed in accordance with the Cochrane Handbook 2009 for Systematic Reviews of Interventions (Higgins 2009). Data synthesis and analyses were done using Review Manager 5.0 software.
Blood pressure and heart rate (continuous outcomes) were expressed as the mean (±SD) change from baseline to follow‐up. Otherwise continuous outcomes were pooled as weighted mean difference (WMD). Withdrawals due to adverse effects (dichotomous outcome) for each comparison were expressed as relative risks with 95%confidence intervals (CI). If there was a statistically significant relative risk difference, the associated number needed to treat/harm was also calculated.
Direct and indirect comparisons
Where possible, direct and indirect comparisons of effect sizes between doses were performed for each ENaC blocker. In the direct method, only trials that randomized participants to different doses were included in the analysis. In the indirect method, an "adjusted indirect comparison" and the associated standard error were calculated using the method described by Bucher 1997 and Song 2003. A P‐value <0.05 was considered statistically significant for all comparisons.
Subgroup analysis and investigation of heterogeneity
Where possible, subgroup analyses were used to examine the results for specific categories of participants. Possible subgroup analyses included:
Race: black, white, other.
Age: adults (18‐69 years), older people (70 years and older).
Baseline severity of hypertension: mild, moderate, severe.
The robustness of the results was tested using several sensitivity analyses, including:
Trials that are industry‐sponsored versus non‐industry sponsored.
Trials that assess drug as primary drug of investigation versus trials that assess drug as comparator.
Trials with blood pressure data measured in the sitting position versus other measurement positions.
Trials with published standard deviations of blood pressure change versus imputed standard deviations.
Heterogeneity amongst included studies were explored qualitatively (by comparing the characteristics of included studies) and quantitatively (using the chi‐squared test of heterogeneity and I2 statistic). Where appropriate, data from each study were pooled using a fixed effect model, except where substantial heterogeneity exists. The funnel plot was used to look for small study bias.
Results
Description of studies
See also Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The original study flow diagram is included in Figure 1.
1.
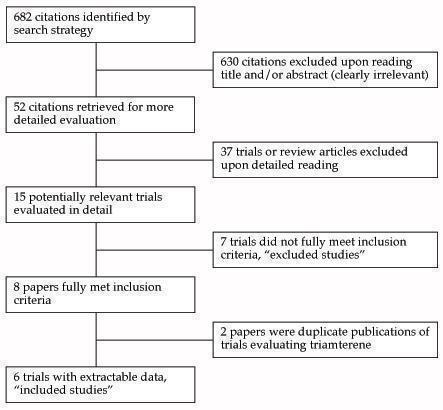
QUOROM flow diagram
The 2012 update search strategy identified 1233 citations, of which only 6 (0.9%) studies met the inclusion criteria and had extractable data to evaluate the dose‐related blood pressure lowering efficacy of amiloride (4 studies) and triamterene (2 studies), when added as a second antihypertensive drug to hydrochlorothiazide (HCTZ) and chlorthalidone, respectively (Figure 2). No studies were identified that evaluated amiloride or triamterene as monotherapy in patients with primary hypertension. Each included study is summarized in the Characteristics of included studies table.
2.
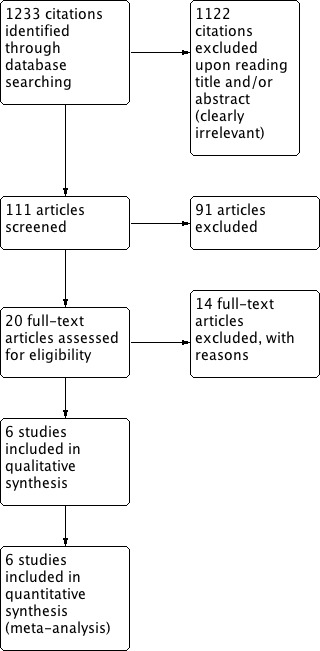
Study flow diagram.
Included studies
All 6 studies evaluating the antihypertensive efficacy of ENaC blockers when added as a second drug using office blood pressure measurements were published during the 1980s. No further studies have been published since 1987. All 6 included studies were published in English. Only one included study (Myers 1987) reported the source of funding (manufacturer of amiloride, Merck Frosst) while the remaining 5 did not report the source of funding. One duplicate publication of each of the 2 triamterene studies (Webb 1984 I; Webb 1984 II) were identified.
Baseline characteristics of the 6 included studies are provided in Table 1. A total of 496 participants with a weighted mean age of 57.1 years and a baseline BP of 151.2/102.4 mmHg were treated at a fixed dose for a weighted mean duration of 7.7 weeks. Only 2 trials evaluating the BP lowering efficacy of amiloride reported the measurements of BP were taken at trough levels (Andersson 1984; Salmela 1986).
1. Overview of the 6 studies investigating ENaC blockers when added as a second drug.
| ENaC blocker | Dose range (mg/day) | Number of studies | ENaC patients (n) | Placebo patients (n) | Mean duration (weeks) | Mean age (years) | Baseline BP (mm Hg) |
| Amiloride | 2.5 ‐ 5 | 4 | 112 | 114 | 5.0 | 65.7 | 156.8/103.2 |
| Triamterene | 50 | 2 | 132 | 138 | 10.0 | 50.0 | 146.5/101.7 |
Imputation of missing variance data
None of the included trials reported the standard deviation of the change in blood pressure. Therefore, we imputed these values according to imputation hierarchy described in the Methods section for all 6 studies. Of these studies, 3 were imputed using endpoint SD and 3 were imputed using the weighted mean estimates of SD of SBP and DBP change from trials meeting the inclusion criteria of a Cochrane systematic review of the blood pressure lowering efficacy of diuretics as second‐line therapy for primary hypertension (Chen 2009). In this review, the weighted mean SD of SBP and DBP change values for the combination group were 13.2 (SD 1.5) mmHg and 8.1 (SD 0.8) mmHg, respectively. For the monotherapy group, the weighted mean SD of SBP and DBP change values were 13.6 (SD 1.8) mmHg and 8.3 (SD 1.1) mmHg, respectively.
Excluded studies
Seven studies were excluded because they did not meet the prespecified inclusion criteria and the reasons for exclusion are listed in the Characteristics of excluded studies table. The three main reasons for exclusion were that crossover studies did not report pre‐crossover BP data and that pre‐titration BP data were not reported in trials in which dose titration was based on blood pressure response. An additional study met the inclusion criteria but did not have extractable BP data (Siegel 1992).
Risk of bias in included studies
Allocation
All the trial publications simply reported that the trial was “randomized” but did not provide any details about the method of randomization. Given the fact that many investigators use the term “randomized” when it is not justified, such vague reporting is insufficient to determine whether or not the allocation sequence was properly randomized and adequately concealed. Authors should report their methods of sequence generation and allocation concealment clearly.
Blinding
Four trials described the blinding method as using “identical” capsules. Two trial publications simply reported that the trial was “double‐blind” but did not provide any details about the blinding methods. The success of blinding in patients or investigators was not assessed in any of the included trials.
Incomplete outcome data
It is unlikely that attrition bias would have had an impact on the systematic review since most of the randomized patients in each trial completed the double‐blind treatment period.
Selective reporting
This would not affect the blood pressure measurements as these were the primary outcome of most of these trials. There is a potential for selective reporting bias for heart rate since only 2 of the included trials reported this outcome.
Other potential sources of bias
Selection Bias
Another potential source of bias in this review is patient selection bias. One of the exclusion criteria reported in 2 trials was participants with a known intolerance or hypersensitivity to ENaC blockers (Myers 1987; Webb 1984 I). This suggests that investigators have knowledge of each participant’s prior experience with this drug class and thus may select for patients who have been found to tolerate treatment with ENaC blockers. It was not possible, however, to prove selection bias since none of the included trials described details about patient recruitment.
Publication Bias
Although trials must have been completed and provided to the regulators in order for the drug to be approved as monotherapy and as second‐line therapy in patients with primary hypertension, only 6 trials evaluating ENaC blockers as second‐line antihypertensive therapy met the inclusion criteria for our review. Only two trials were identified that evaluated ENaC blockers as monotherapy compared with placebo (both failed to meet other inclusion criteria for this review). Furthermore, many of the doses that have been approved by regulators have little or no published trial evidence to support their use. For instance, the highest approved dose amiloride, 20mg, appeared only in three non‐randomized German studies performed in the 1970s and 1980s (Baumann 1976; ;Haimerl 1985; Vetter 1973) .
Another source of bias that is likely to have a significant impact on this review is the selective publication of trials with positive results. The most common way to investigate whether or not a review is subject to publication bias is to examine for funnel plot asymmetry as smaller studies with null results remained unpublished. However, due to the small number of trials included in this review, funnel plots could not be generated to adequately assess whether publication bias is likely.
The results of this review underscore the need for all studies, regardless of the findings, to be published and accessible for secondary analysis. In order to improve transparency in research and knowledge sharing, the World Health Organization (WHO) and other regulatory bodies have set standards for trial registration and reporting and are urging research institutions and companies to register all medical studies that test treatments on humans.
Effects of interventions
Dose‐ranging BP lowering efficacy of amiloride when added as a second drug
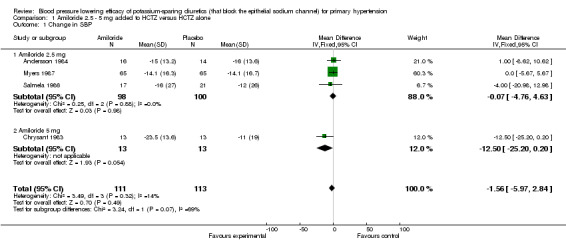
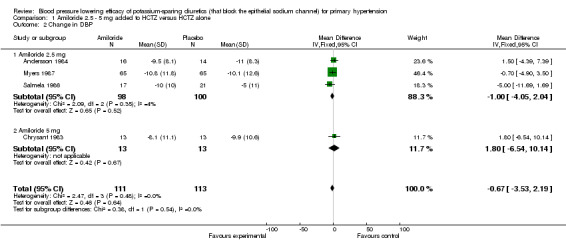
Three of the included trials (Andersson 1984; Myers 1987; Salmela 1986) evaluated amiloride 2.5 mg/day and only 1 trial (Chrysant 1983) evaluated amiloride 5 mg/day ‐ the manufacturer's recommended starting dose ‐ when added to HCTZ 25 mg/day as compared to HCTZ 25 mg/day alone. There were insufficient data to demonstrate a statistically significant difference between the combination of amiloride 2.5‐5 mg/day plus HCTZ versus HCTZ alone in lowering SBP and DBP. No trials evaluating amiloride above 5 mg/day and up to the manufacturer's maximum recommended daily dose of 20 mg/day were identified so a dose‐response relationship with amiloride could not be determined. The available data do not suggest any blood pressure lowering effect with amiloride in low doses.
Dose‐ranging BP lowering efficacy of triamterene when added as a second drug
Only 2 trials (Webb 1984 I; Webb 1984 II) evaluated the BP lowering efficacy of triamterene 50 mg/day (below the manufacturer's recommended dosage range) when added to chlorthalidone versus chlorthalidone alone. Based on the limited available data, the addition of triamterene 50 mg did not demonstrate a reduction in BP. Although triamterene is recommended up to a daily dose of 300 mg/day, no trials studied triamterene at doses higher than 50 mg/day. Whether doses higher than 50 mg/day have a blood pressure lowering effect cannot be determined.
Summary of the blood pressure lowering efficacy of ENaC blockers as a second drug
To estimate the effect of ENaC blockers at a dose of half the recommended starting dose, we combined the data for amiloride 2.5 mg/day with the data for triamterene 50 mg/day. This demonstrated no effect on systolic BP, ‐0.03 [95% CI ‐2.90, 2.83] mmHg and no effect on diastolic BP, ‐0.22 [95% CI ‐2.01, 1.57] mmHg (analysis not shown). Due to lack of data, an estimate of the effect of higher doses or whether there was a dose response effect could not be determined.
Pulse pressure
Pulse Pressure (PP) was not reported as an outcome in any of the included studies. Therefore, the value of change in PP was calculated by subtracting DBP change from SBP change for each treatment arm in the trial. Both SBP and DBP data were provided in all 6 included studies assessing amiloride and triamterene. This analysis did not suggest any effect of ENaC blockers on pulse pressure.
Blood pressure variability
The variability of blood pressure at both baseline and endpoint was reported in 3 of the included amiloride trials (Chrysant 1983; Myers 1987; Salmela 1986) and none of the triamterene trials. The limited data did not suggest any effect of ENaC blockers on blood pressure variability.
Heart rate
Only 2 of the included trials provided heart rate data (Chrysant 1983; Myers 1987) and these did not suggest any effect on heart rate.
Withdrawals due to adverse effects
An analysis of withdrawals due to adverse effects during 3 to 12 weeks of treatment with ENaC blockers was reported in 5 of the included trials. The overall estimate showed no statistically significant effect on ENaC blockers on this outcome, RR 0.53 [95% CI 0.19, 1.51].
Discussion
Summary of main results
Due to the very limited number of published studies, there is insufficient evidence for the ENaC blockers to estimate the dose‐response on blood pressure or the secondary outcomes of this review. No placebo‐controlled studies were identified that evaluated the dose‐related BP lowering efficacy of ENaC blocker monotherapy. Six trials were identified by our comprehensive search that assessed the effect of addition of ENaC blockers, amiloride and triamterene to other antihypertensive drugs. Of the 4 amiloride trials, 3 assessed a dose (2.5 mg) at half the recommended starting dose and only 1 small trial assessed amiloride at the recommended starting dose of 5 mg. Only 2 trials evaluated triamterene (50 mg) at half the manufacturer's recommended dose.
The 6 trials that met the pre‐specified inclusion criteria had a mean duration of 7.7 weeks and reported data on 496 participants (244 treated with ENaC blockers and 252 treated with placebo) with a weighted mean age of 57 years, weighted mean baseline blood pressure of 151/102 mmHg and a mean pulse pressure of 49 mmHg.
What is the magnitude of the effect of ENaC blockers on BP?
Given the limited data available, it is not possible to determine whether these drugs have any BP lowering effect over the recommended dose range, both as first‐line and second‐line drugs. The 5 trials that assessed doses of amiloride and triamterene at half the recommended starting dose showed no reduction in BP and ruled out a BP lowering effect of >3/2 mmHg. This suggests that these drugs do not have a blood pressure lowering effect but more trials at higher doses are needed. Other classes of drugs that have a significant effect on blood pressure did have a significant BP lowering effect at doses half the recommended starting dose (Heran 2008a, Heran 2008b, Musini 2008, Chen 2009). Complete reporting of all the ENaC blocker trials that have been completed are needed.
What is the effect of ENaC blockers on BP variability?
The variability of blood pressure at both baseline and endpoint was reported in only 3 of the included trials and this did not suggest any effect; however, this is clearly not sufficient data to answer this question.
Is there evidence of a dose‐response relationship for heart rate?
There is a possibility of selective reporting bias of resting heart rate since only 2 trials reported data for this outcome. Based on these 2 trials, ENaC blockers do not appear to affect heart rate; however, the data are insufficient.
Is there evidence of a dose‐response relationship for withdrawals due to adverse effects?
There were not enough data to determine a dose‐related effect of individual ENaC blockers on WDAE. The available data demonstrate that ENaC blockers as second‐line therapy did not affect WDAE compared with monotherapy. However, these studies evaluated low doses of amiloride and triamterene that were ineffective in lowering BP. Also, due to a lack of available trial data at higher doses, the short‐term effect of these drugs on WDAE is not known. Furthermore, there is a high risk of patient selection bias as participants with a known intolerance or hypersensitivity to ENaC blockers were excluded from 2 of the trials.
Short‐term trials are not the best type of trial to assess adverse effects and longer RCTs and other types of data can assist, such as non‐randomized trials or post‐marketing surveillance studies. Nevertheless, there is no justification for not reporting all withdrawals due to adverse effects in all completed trials.
Limitations of the review
Given that ENaC blockers are commonly prescribed as adjunctive therapy in patients receiving diuretics for primary hypertension, the lack of published RCT evidence of the dose‐ranging BP lowering efficacy for this class of drugs is unacceptable. It is clear that many trials assessing the efficacy of ENaC blockers have not been published. We know that because many of the doses that have been approved by regulators could not be included in this review. For example, there were no trials assessing triamterene in the manufacturer's recommended dose range. Also, only 1 small trial contributes efficacy data for the initial recommended dose of 5 mg of amiloride. The highest approved dose of amiloride, 20mg, was only utilized in a non‐randomized fashion in two before‐and‐after studies. From this, we know that trials must have been completed and provided to the regulators for the other doses of both drugs.
Authors' conclusions
Implications for practice.
Specific findings of this review
No placebo‐controlled studies were identified that evaluated the dose‐related BP lowering efficacy of ENaC blockers as monotherapy.
The review provides very limited data on the dose‐related blood pressure lowering efficacy of 2 different ENaC blockers, amiloride and triamterene, when added as a second drug. In these trials, adding a low dose of amiloride or triamterene did not significantly reduce BP further as compared to monotherapy.
Amiloride or triamterene did not appear to affect blood pressure variability, pulse pressure, or heart rate; however, available data are insufficient.
Low doses of amiloride or triamterene, when added as a second drug, did not change WDAE as compared to monotherapy.
Implications of these findings
The major limitation of this review is that the available published trials most likely do not represent all the trials that have been completed. The available data suggest that doses 0.5 times the recommended starting dose do not lower blood pressure. This review does not provide physicians with information about the blood pressure lowering effects of higher doses of ENaC blockers in patients with elevated blood pressure.
This review did not provide any evidence of an increase in withdrawals due to adverse effects. However, this finding is severely limited by the short duration of the included trials and a high risk of patient selection bias. Therefore, this systematic review is not a good measure of the incidence of adverse effects with this class of drugs.
Implications for research.
It is evident that for amiloride and triamterene, trials reporting blood pressure lowering data on doses recommended for use are not published. It should be mandatory that all clinical trials be registered and the results of these trials be published or otherwise made available in full detail.
Full dose‐response data for doses within the recommended and beyond the recommended dose range are needed to properly appreciate the dose‐response relationship for amiloride and triamterene on blood pressure.
Trials should measure and report blood pressure data for peak effects as well as trough effects.
All trials should report both systolic and diastolic BP, heart rate, standard deviation of BP and heart rate data, as well as all withdrawals due to adverse effects and serious adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 17 October 2012 | New citation required but conclusions have not changed | no new trials found, conclusions remain unchanged |
| 1 August 2012 | New search has been performed | searches re‐run, review updated |
Acknowledgements
The authors would like to acknowledge the assistance provided by the Cochrane Hypertension Group and in particular Mr. Stephen Adams who retrieved the papers for this review.
Tha authors would like to acknowledge the assistance provided by Mr. Ciprian Jauca who translated the original text from German articles and screened them against the eligibility criteria.
Appendices
Appendix 1. MEDLINE search strategy
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
exp double blind method/
(double adj blind$).ti,ab.
(double adj mask$).ti,ab.
or/1‐11
(animals not (human and animals)).sh.
12 not 13
exp hypertension/
hypertens$.ti,ab.
exp blood pressure/
(blood adj pressure).ti,ab.
or/15‐18
exp sodium channel blockers/
((epithelial ADJ (Na or sodium) ADJ channel ADJ2 (block$ or inhibit$)).ti,ab
(ENaC ADJ (block$ or inhibit$)).ti,ab
((K or potassium) ADJ sparing ADJ diuretic$).ti,ab
amiloride.ti,ab.
exp amiloride/
triamterene.ti,ab.
exp triamterene/
MK 870.ti,ab.
or/20‐28
exp placebos/
placebo$.ti,ab.
or/30‐31
14 and 19 and 29 and 32
Appendix 2. EMBASE search strategy
random$.mp.
factorial$.mp.
crossover$.mp.
cross over$.mp.
cross‐over$.mp.
placebo$.mp.
(doubl$ adj blind$).mp.
(singl$ adj blind$).mp.
assign$.mp.
allocat$.mp.
volunteer$.mp.
or/1‐11
Crossover Procedure/
Double Blind Procedure/
Randomized Controlled Trial/
Single Blind Procedure/
or/13‐16
12 or 17
exp hypertension/
hypertens$.ti,ab.
exp blood pressure/
blood pressure.ti,ab.
or/19‐22
(epithelial adj (Na or sodium) adj channel adj2 (block$ or inhibit$)).ti,ab.
(ENaC adj (block$ or inhibit$)).ti,ab.
((K or potassium) adj sparing adj diuretic$).ti,ab.
exp amiloride/
amiloride.ti,ab.
exp triamterene/
triamterene.ti,ab.
MK 870.ti,ab.
or/24‐31
exp placebos/
placebo$.ti,ab.
or/33‐34
18 and 23 and 32 and 35
Appendix 3. CENTRAL search strategy
((doubl*) NEXT (blind* or mask*)):ti,ab
((epithelial NEXT (Na or sodium) NEXT channel) NEXT/2 (block* or inhibit*)):ti,ab
(ENaC NEXT (block* or inhibit*)):ti,ab
((K or potassium) NEXT sparing NEXT diuretic*):ti,ab
amiloride:ti,ab
triamterene:ti,ab
(MK870 or MK 870):ti,ab
#2 or #3 or #4 or #5 or #6 or #7
hypertens*:ti,ab
(blood NEXT pressure*):ti,ab
#9 or #10
placebo*:ti,ab
#1 and #8 and #11 and #12
Data and analyses
Comparison 1. Amiloride 2.5 ‐ 5 mg added to HCTZ versus HCTZ alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in SBP | 4 | 224 | Mean Difference (IV, Fixed, 95% CI) | ‐1.56 [‐5.97, 2.84] |
| 1.1 Amiloride 2.5 mg | 3 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐4.76, 4.63] |
| 1.2 Amiloride 5 mg | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐12.5 [‐25.20, 0.20] |
| 2 Change in DBP | 4 | 224 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐3.53, 2.19] |
| 2.1 Amiloride 2.5 mg | 3 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐4.05, 2.04] |
| 2.2 Amiloride 5 mg | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐6.54, 10.14] |
| 3 Total withdrawals due to adverse effects | 3 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.08, 18.20] |
1.1. Analysis.
Comparison 1 Amiloride 2.5 ‐ 5 mg added to HCTZ versus HCTZ alone, Outcome 1 Change in SBP.
1.2. Analysis.
Comparison 1 Amiloride 2.5 ‐ 5 mg added to HCTZ versus HCTZ alone, Outcome 2 Change in DBP.
1.3. Analysis.
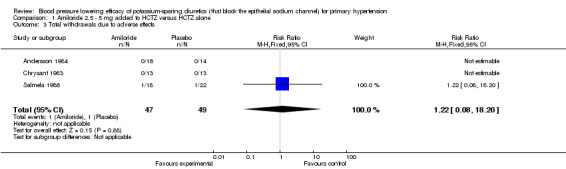
Comparison 1 Amiloride 2.5 ‐ 5 mg added to HCTZ versus HCTZ alone, Outcome 3 Total withdrawals due to adverse effects.
Comparison 2. Triamterene 50 mg added to chlorthalidone versus chlorthalidone alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in SBP | 2 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐3.63, 3.61] |
| 2 Change in DBP | 2 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.01, 2.41] |
| 3 Total withdrawals due to adverse effects | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.15, 1.45] |
2.1. Analysis.
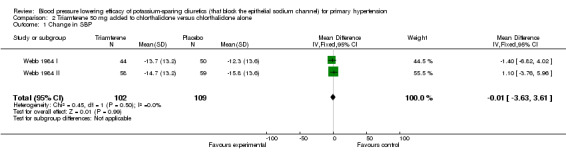
Comparison 2 Triamterene 50 mg added to chlorthalidone versus chlorthalidone alone, Outcome 1 Change in SBP.
2.2. Analysis.
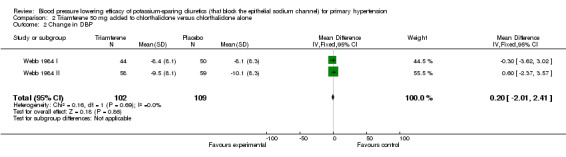
Comparison 2 Triamterene 50 mg added to chlorthalidone versus chlorthalidone alone, Outcome 2 Change in DBP.
2.3. Analysis.
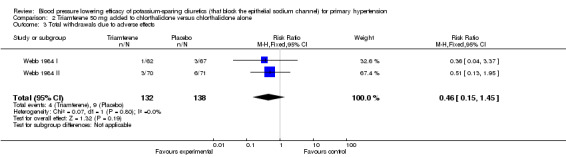
Comparison 2 Triamterene 50 mg added to chlorthalidone versus chlorthalidone alone, Outcome 3 Total withdrawals due to adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersson 1984.
| Methods | 2‐4 week washout period; 4‐week placebo period; inclusion criteria=supine DBP 100‐115 mm Hg at 3 outpatient visits at 2‐week intervals during placebo period; 12‐week double‐blind treatment, consisting of 6‐week treatment at initial fixed dose, non‐responders (defined as diastolic BP ≥95 mm Hg) had dosage of both drugs doubled after 6 weeks | |
| Participants | All patients: n=30 (14 males, 16 females); mean age=51 (range 31‐65) years Amiloride 2.5mg/HCTZ 25mg: n=16; baseline standing SBP=161 mm Hg, DBP=112 mm Hg; baseline supine SBP=165 mm Hg, DBP=107 mm Hg HCTZ 25 mg: n=14; baseline standing SBP=168 mm Hg, DBP=113 mm Hg; baseline supine SBP=172 mm Hg, DBP=108 mm Hg |
|
| Interventions |
Combination: Amiloride 2.5 mg (A 2.5)/HCTZ 25 mg once daily; patients received A 2.5/HCTZ 25 for 6 weeks; at week 6, dose was increased to A 5/HCTZ 50 once daily if target BP not achieved Monotherapy: HCTZ 25 mg once daily; patients received 25 mg for 6 weeks; at week 6, dose was increased to 50 mg once daily if target BP not achieved |
|
| Outcomes | Trough standing SBP/DBP using mercury sphygmomanometer Trough supine SBP/DBP using mercury sphygmomanometer WDAE |
|
| Notes | Used only week 6 BP data; BP change and SD of change not reported; week 6 BP reported, week 6 SD not reported; baseline BP reported, baseline SD not reported; imputed overall trial mean SD of change for SBP and DBP; week 6 BP data from Table 1, p. 198 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no dropouts." |
| Selective reporting (reporting bias) | Low risk | BP lowering efficacy was primary outcome. Safety/tolerability reported. |
| Other bias | Unclear risk | Funding source not reported. |
Chrysant 1983.
| Methods | 6‐week open‐label placebo period; inclusion criteria=DBP 100‐120 mm Hg; 12‐week double‐blind treatment, consisting of 4‐week treatment at initial fixed dose; at week 4, timolol once daily was added if supine DBP >90 mm Hg | |
| Participants | All patients: n=26 males Amiloride 2.5mg/HCTZ 25mg: n=13; mean age=53(7.2) years; baseline sitting SBP=157(18.0) mm Hg, DBP=107(7.2) mm Hg; baseline supine SBP=150(18.0) mm Hg, DBP=102(7.2) mm Hg, HR=77(7.2) bpm HCTZ 25 mg: n=13; mean age=49(10.8) years; baseline sitting SBP=154(21.6) mm Hg, DBP=107(7.2) mm Hg; baseline supine SBP=153(18.0) mm Hg, DBP=105(7.2) mm Hg, HR=83(18.0) bpm |
|
| Interventions |
Combination: Amiloride 2.5 mg/HCTZ 25 mg once daily Monotherapy: HCTZ 25 mg once daily |
|
| Outcomes | Sitting SBP/DBP using mercury sphygmomanometer Supine SBP/DBP using mercury sphygmomanometer Supine HR WDAE |
|
| Notes | Used only week 4 BP data; BP change and SD of change not reported, week 4 BP and SEM reported; baseline BP and SEM reported; calculated SD of change from N and SEM of change; imputed week 4 SBP/DBP SD for SD of change; time of post‐dose BP measurement not reported; BP data from Figure 1b, p. 149; HR data from Figure 2, p. 151 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "However, most of the observed side effects were not serious enough to require discontinuation of treatment, with the exception of one patient, who had to be taken off timolol..." Comment: No patients withdrew prematurely during first 4 weeks of double‐blind treatment. |
| Selective reporting (reporting bias) | Low risk | BP lowering efficacy was primary outcome. Safety/tolerability reported. |
| Other bias | Unclear risk | Funding source not reported. |
Myers 1987.
| Methods | 4‐week placebo period; inclusion criteria=supine and standing DBP 95‐114 mm Hg; 12‐week double‐blind treatment, consisting of 4‐week treatment at initial fixed dose, non‐responders (defined as supine DBP ≥90 mm Hg) had dosage of both drugs doubled after 4 weeks | |
| Participants | All patients: n=130 (45 males, 85 females); 116 white, 10 oriental, 4 black; mean age=71.7(4.6) years Amiloride 2.5mg/HCTZ 25mg: n=65 (21 males, 44 females); 56 white, 6 oriental, 3 black; mean age=72.1(5.6) years; baseline sitting SBP=150.3(14.1) mm Hg, DBP=99.4(3.2) mm Hg, HR=76.5(8.8) bpm; baseline standing SBP=150.6(13.6) mm Hg, DBP=100.5(5.8) mm Hg, HR=80.1(9.0) bpm HCTZ 25 mg; n=65 (24 males, 41 females); 60 white, 4 oriental, 1 black; mean age=71.2(4.8) years; baseline sitting SBP=150.0(13.2) mm Hg, DBP=100.6(3.0) mm Hg, HR=76.0(9.1) bpm; baseline standing SBP=149.5(14.1) mm Hg, DBP=101.8(5.4) mm Hg, HR=77.5(8.8) bpm |
|
| Interventions |
Combination: Amiloride 2.5 mg/HCTZ 25 mg once daily Monotherapy: HCTZ 25 mg once daily |
|
| Outcomes | Standing SBP/DBP using mercury sphygmomanometer Supine SBP/DBP using mercury sphygmomanometer Supine HR |
|
| Notes | Used only week 4 BP data; BP change and SD of change not reported; week 4 BP and SD reported; baseline BP and SD reported; imputed week 4 SBP/DBP SD for SD of change; time of post‐dose BP measurement not reported; week 4 BP data from Figures 1 and 2, pp. 1027‐8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...with the tablets being identical to each other and placebo in both appearance and taste." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HR and safety/tolerability data at week 4 not reported. Method of analysis (intention‐to‐treat or per protocol) not reported. Unclear how losses to follow up and dropouts were dealt with. |
| Selective reporting (reporting bias) | Low risk | BP lowering efficacy was primary outcome. |
| Other bias | High risk | Funding source is manufacturer of amiloride (Merck Frosst Canada Inc). |
Salmela 1986.
| Methods | 4‐week placebo baseline period; inclusion criteria=supine DBP 95‐115 mm Hg; 16‐week double‐blind treatment, consisting of 8‐week treatment at initial fixed dose, non‐responders (defined as supine DBP >90 mm Hg) had dosage of both drugs doubled after 8 weeks | |
| Participants | All patients: n=40 (12 males, 28 females); mean age=66.7 (range 55‐80) years Amiloride 2.5mg/HCTZ 25mg: n=18 (4 males, 14 females); mean age=66.9(7.8) years; baseline standing SBP=178(28) mm Hg, DBP=106(8) mm Hg; baseline supine SBP=178(28) mm Hg, DBP=101(7) mm Hg HCTZ 25 mg: n=22; mean age=66.7(7.4) years; baseline standing SBP=170(31) mm Hg, DBP=103(12) mm Hg; baseline supine SBP=177(23) mm Hg, DBP=101(6) mm Hg |
|
| Interventions |
Combination: Amiloride 2.5 mg/HCTZ 25 mg once daily Monotherapy: HCTZ 25 mg once daily taken in the morning |
|
| Outcomes | Trough standing SBP/DBP using mercury sphygmomanometer Trough supine SBP/DBP using mercury sphygmomanometer WDAE |
|
| Notes | Used only week 8 BP data; BP change and SD of change not reported, week 8 BP and SD reported; baseline BP and SD reported; imputed week 8 SBP/DBP SD for SD of change; week 8 BP data from Table 2, p. 89; HR data from Figure 2, p. 151 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The tablets were identical in appearance." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "In the HCT group...one patient had a generalized rash with fever and thrombocytosis 2 weeks after starting the therapy. This led to her drop out. In the HCT group, the other cases of drop outs were: 1 case of hypercalcaemia and 1 patient was unavailable for follow up due to a gall bladder operation. In the HCT+A group, generalised rash was observed in 1 patient during the first 2 weeks of therapy. This led her to drop out." Method of analysis (intention‐to‐treat or per protocol) not reported. Unclear how losses to follow up and dropouts were dealt with. |
| Selective reporting (reporting bias) | Low risk | BP lowering efficacy was primary outcome. Safety/tolerability reported. |
| Other bias | High risk | Quote: "The criteria for exclusion from the study were...previously demonstrated adverse reactions or hypersensitivity to HCT (hydrochlorothiazide) and/or A (amilorde)." Comment: Patient selection bias. Funding source not reported. |
Webb 1984 I.
| Methods | 4‐week single‐blind placebo run‐in period; inclusion criteria=standing DBP 95‐114 mm Hg; 10‐week double‐blind treatment, followed by 6‐week partial crossover phase and 2‐week placebo phase | |
| Participants | All patients: n=129 (86 males, 43 females); mean age=50 (range 21‐66) years Triamterene 50 mg/chlorthalidone 25 mg: n=62 (41 males, 21 females); mean age=50 years; n=53 with baseline BP data; baseline standing SBP=150.6 mm Hg, DBP=103.4 mm Hg; baseline supine SBP=150.0 mm Hg, DBP=99.0 mm Hg Chlorthalidone 25 mg: n=67 (45 males, 22 females); mean age=50 years; n=59 with baseline BP data; baseline standing SBP=146.7 mm Hg, DBP=102.1 mm Hg; baseline supine SBP=146.2 mm Hg, DBP=97.0 mm Hg |
|
| Interventions |
Combination: Triamterene 50 mg/chlorthalidone 25 mg once daily Monotherapy: Chlorthalidone 25 mg once daily taken before breakfast |
|
| Outcomes | Standing SBP/DBP using mercury sphygmomanometer Supine SBP/DBP using mercury sphygmomanometer WDAE |
|
| Notes | Duplicate publication = Hort 1991; BP change and SD of change not reported; weeks 4, 6, 8, 10 BP reported, weeks 4, 6, 8, 10 SD not reported; baseline BP reported, baseline SD not reported; imputed overall trial mean SD of change for SBP and DBP; calculated weighted mean BP during weeks 4‐10; time of post‐dose BP measurement not reported; BP data from Table 3, p. 136 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote [from Webb 1984 I]: "Study medications were administered as white tablets containing 25 mg of chlorthalidone or 25 mg of chlorthalidone and 50 mg of triamterene. Identical matching placebo tablets were dispensed to maintain the double‐blind nature of the study. All doses consisted of two tablets, one of active medication, the other of placebo..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote 1 [from Webb 1984 I]: "Evaluable blood pressure data were obtained from a maximum of fifty‐nine of the sixty‐five patients who started on chlorthalidone 25 mg/day as Group A and fifty‐three of the fifty‐eight who started on the combination as Group B." Quote 1 [from Hort 1991]: "Of these 129 patients, 6 were excluded from the efficacy evaluation, 1 in the combination group because of chest and abdominal pains not related to therapy and who was withdrawn, 1 in each group because concomitant antihypertensive therapy was given, 1 in the chlorthalidone alone group and 2 in the combination group (Other patients in Table II) because their blood pressure was below the entry criteria. The 5 patients who remained in the study were included in the tolerability analysis. The 10 weeks of the double‐blind phase of the study, up to the partial crossover, were completed by 50 (75%) of the 67 patients taking chlorthalidone alone and by 44 (71%) of the 62 receiving the combination. The remaining 34 patients did not return for assessment." Quote 2 [from Hort 1991]: "Mean end‐point analysis, for which the values at each patient's last visit of active treatment were examined, used two sample t‐tests." |
| Selective reporting (reporting bias) | High risk | Quote [from Hort 1991]: "Mean changes in pulse rate were neither clinically nor statistically significant in either group." Comment: Quantitative HR data not reported. |
| Other bias | High risk | Quote [from Hort 1991]: "Exclusion criteria were...intolerance to chlorthalidone, triamterene or to a sulphonamide‐derived drug..." Comment: Patient selection bias. Funding source not reported. |
Webb 1984 II.
| Methods | 4‐week single‐blind placebo run‐in period; inclusion criteria=standing DBP 95‐114 mm Hg; 10‐week double‐blind treatment, followed by 6‐week partial crossover phase and 2‐week placebo phase | |
| Participants | All patients: n=141 (70 males, 71 females); 119 white, 20 black, 2 other; mean age=50 (range 20‐68) years Triamterene 50 mg/chlorthalidone 50 mg: n=70 (34 males, 36 females); mean age=50 years; n=66 with baseline BP data; baseline standing SBP=145.1 mm Hg, DBP=100.8 mm Hg; baseline supine SBP=147.0 mm Hg, DBP=97.4 mm Hg Chlorthalidone 50 mg: n=71 (36 males, 35 females); mean age=50 years; n=66 with baseline BP data; baseline standing SBP=144.2 mm Hg, DBP=100.8 mm Hg; baseline supine SBP=144.7 mm Hg, DBP=96.4 mm Hg |
|
| Interventions |
Combination: Triamterene 50 mg/chlorthalidone 50 mg once daily Monotherapy: Chlorthalidone 50 mg once daily taken before breakfast |
|
| Outcomes | Standing SBP/DBP using mercury sphygmomanometer Supine SBP/DBP using mercury sphygmomanometer WDAE |
|
| Notes | BP change and SD of change not reported; weeks 4, 6, 8, 10 BP reported, weeks 4, 6, 8, 10 SD not reported; baseline BP reported, baseline SD not reported; imputed overall trial mean SD of change for SBP and DBP; calculated weighted mean BP during weeks 4‐10; time of post‐dose BP measurement not reported; BP data from Table 3, p. 142 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Study medications were administered as white tablets containing 50 mg of chlorthalidone or 50 mg of chlorthalidone and 50 mg of triamterene. Identical matching placebo tablets were dispensed as required to maintain the double‐blind nature of the study. All doses consisted of two tablets, one of active medication, the other of placebo..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Evaluable blood pressure data were obtained from a maximum of sixty‐seven of the seventy‐one patients who started on chlorthalidone 50 mg/day as Group A, and sixty‐six of the seventy patients who started on the combination as Group B provided evaluable blood pressure data for Part I." |
| Selective reporting (reporting bias) | High risk | Quantitative HR data not reported. |
| Other bias | Unclear risk | Funding source not reported. |
BP = blood pressure; SBP = systolic blood pressure; DBP = diastolic blood pressure; PP = pulse pressure; HR = heart rate; bpm = beats per minute; WDAE = withdrawals due to adverse effects; SD = standard deviation; SE = standard error; 95% CI = 95% confidence interval; HCTZ = hydrochlorothiazide
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersen 1985 | Crossover trial with no pre‐crossover data reported for first 3 weeks of treatment (amiloride 5/HCTZ 50 mg/day vs. HCTZ 50 mg/day plus potassium chloride 26 mmol). Potassium chloride may have antihypertensive effect, so control arm is likely inappropriate. |
| Baroni 1985 | Crossover trial with no pre‐crossover data reported. |
| Baumann 1976 | Before and after study ‐ no randomization, no blinding, no control group. |
| Haimerl 1985 | Before and after study ‐ no randomization, no blinding, no control group. |
| Hintzen 1973 | No washout period was described. BP follow‐up period of less than 3 weeks. |
| Koskelainen 1985 | Crossover trial with no pre‐crossover data reported for first 12 weeks of treatment (amiloride 5/HCTZ 50 mg/day vs. HCTZ 50 mg/day). |
| Larochelle 1985 | Dose titrated to BP response after 2 weeks of double‐blind treatment. |
| Lumme 1986 | Dose titrated to BP response after 4 weeks of double‐blind treatment. Pre‐titration BP data not reported. |
| Perola 1985 | Crossover trial with no pre‐crossover data reported for first 4 weeks of treatment (triamterene 50/furosemide 40 mg/day vs. furosemide 40 mg/day). |
| Salako 1973 | No blinding protocol was reported. No placebo control ‐ potassium chloride may have an antihypertensive effect, so control arm was likely inappropriate. |
| Siegel 1992 | Quantitative BP data not reported. |
| Simpson 1961 | Majority of study participants had comorbid hyperuricemia , which m ay indicate and exacerbate underlying renal dysfunction . Creatinine clearance was not reported. S econdary causes of hypertension were not thoughly ruled out. |
| Spiekerman 1966 | No post‐washout baseline BP data reported. |
| Vetter 1973 | Before and after study ‐ no randomization, no blinding, no control group. |
Differences between protocol and review
There are no differences between the protocol and the published review.
Contributions of authors
Dr. Balraj S. Heran designed the search strategy, undertook the search, screened the search results, collected data for the review, screened the retrieved papers against the eligibility criteria, appraised the risk of bias of the included studies, extracted the data from the included studies, entered data into RevMan, analyzed and interpreted the data, and wrote the first draft of the review.
Jenny M.H. Chen extracted data and appraised the risk of bias of all included studies.
Josh J. Wang screened the search results and the retrieved papers against the eligibility criteria.
Dr. James M. Wright conceived and designed the review, assisted with the analysis and interpretation of data, as well as provided a clinical perspective.
Sources of support
Internal sources
Departments of Anesthesiology, Pharmacology & Therapeutics and Medicine, Faculty of Medicine, University of British Columbia, Canada.
External sources
British Columbia Ministry of Health Grant to the Therapeutics Initiative, Canada.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andersson 1984 {published data only}
- Andersson P O, Andersen H H, Hagman A, Henning R. Potassium sparing by amiloride during thiazide therapy in hypertension. Clinical Pharmacology and Therapeutics 1984;36(2):197‐200. [DOI] [PubMed] [Google Scholar]
Chrysant 1983 {published data only}
- Chrysant S G, Brown J L, Hagstrom D. Antihypertensive and metabolic effects of hydrochlorothiazide, amiloride‐hydrochlorothiazide, and timolol. Journal of Clinical Pharmacology 1983;23(4):147‐54. [DOI] [PubMed] [Google Scholar]
Myers 1987 {published data only}
- Myers M G. Hydrochlorothiazide with or without amiloride for hypertension in the elderly. A dose‐titration study. Archives of Internal Medicine 1987;147(6):1026‐30. [PubMed] [Google Scholar]
Salmela 1986 {published data only}
- Salmela P I, Juustila H, Kinnunen O, Koistinen P. Comparison of low doses of hydrochlorothiazide plus amiloride and hydrochlorothiazide alone in hypertension in elderly patients. Annals of Clinical Research 1986;18(2):88‐92. [PubMed] [Google Scholar]
Webb 1984 I {published data only}
- Hort J F, Wilkins H M. Changes in blood pressure, serum potassium and electrolytes with a combination of triamterene and a low dose of chlorthalidone. Current Medical Research & Opinion 1991;12(7):430‐40. [DOI] [PubMed] [Google Scholar]
- Webb E L, Godfrey J C, Gertel A. The efficacy of a potassium‐sparing combination of chlorthalidone and triamterene in the control of mild and moderate hypertension. I. Journal of International Medical Research 1984;12(3):133‐9. [DOI] [PubMed] [Google Scholar]
Webb 1984 II {published data only}
- Spiers D R, Wade R C. Double‐blind parallel study of a combination of chlorthalidone 50 mg and triamterene 50 mg in patients with mild and moderate hypertension. Current medical research and opinion 1996;13(7):409‐15. [DOI] [PubMed] [Google Scholar]
- Webb E L, Godfrey J C, Gertel A. The efficacy of a potassium‐sparing combination of chlorthalidone and triamterene in the control of mild and moderate hypertension. II. Journal of International Medical Research 1984;12(3):140‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersen 1985 {published data only}
- Andersen B, Snorrason S P, Ragnarsson J, Hardarson T. Hydrochlorothiazide and potassium chloride in comparison with hydrochlorothiazide and amiloride in the treatment of mild hypertension. Acta Medica Scandinavica 1985;218(5):449‐54. [DOI] [PubMed] [Google Scholar]
Baroni 1985 {published data only}
- Baroni G, Lessi D R R P, Branco P P. Anti‐hypertensive and biochemical effects. Hydrochlorthiazide, long acting furosemide and combination amiloride/hydrochlorthiazide in the treatment of hypertension. [Portuguese]. Revista Brasileira de Medicina 1985;42(10):352‐7. [Google Scholar]
Baumann 1976 {published data only}
- Baumann VJC, Braun HD. Potassium‐sparing effect on hypertension and congestive heart failure with Moduretik [Kaliumsparende Saluretikabehandlung des Hochdruckes und der feuzden Herzdekompensation mit Moduretik]. Zeitschrift fur Allgermeinmedizin 1976;18:971‐5. [PubMed] [Google Scholar]
Haimerl 1985 {published data only}
- Haimerl VF, Lehmann K, Kopcke W. Treatment of Essential Hypertension with a K‐Sparing Diuretic Combination. Results of a German Multicenter Study with Moduretic. Fortschr Med 1985;103:812‐16. [PubMed] [Google Scholar]
Hintzen 1973 {published data only}
- Hintzen AHJ. Some Aspects of the use of Moduretic in the Treatment of Patients with Mild Hypertension . Acta Cardiologica 1973 ; 28 :331‐339. [PubMed] [Google Scholar]
Koskelainen 1985 {published data only}
- Koskelainen J, Turpeinen T, Lehto H. Metabolic effects of hydrochlorothiazide and hydrochlorothiazide‐amiloride and trichlormethiazide‐triamterene combinations. Current Therapeutic Research ‐ Clinical and Experimental 1985;37(3):554‐65. [Google Scholar]
Larochelle 1985 {published data only}
- Larochelle P, Logan A G. Hydrochlorothiazide‐amiloride versus hydrochlorothiazide alone for essential hypertension: Effects on blood pressure and serum potassium level. Canadian Medical Association Journal 1985;132(7):801‐5. [PMC free article] [PubMed] [Google Scholar]
Lumme 1986 {published data only}
- Lumme J A, Jounela A J. Cardiac arrhythmias in hypertensive outpatients on various diuretics. Correlation between incidence and serum potassium and magnesium levels. Annals of Clinical Research 1986;18(4):186‐90. [PubMed] [Google Scholar]
Perola 1985 {published data only}
- Perola P, Lehto H, Lammintausta R, Viikari J. Metabolic effects of furosemide and the combination of furosemide and triamterene. Current Therapeutic Research ‐ Clinical and Experimental 1985;37(3):545‐53. [Google Scholar]
Salako 1973 {published data only}
- Salako LA, Falase AO. A Comparison of Moduretic and Hydrochlorothiazide in the Treatment of Hypertension in African Patients . East African Medical Journal 1973 ; 50 ( 1 ):38‐48. [PubMed] [Google Scholar]
Siegel 1992 {published data only}
- Siegel D, Hulley S B, Black D M, Cheitlin M D, Sebastian A, Seeley D G, et al. Diuretics, serum and intracellular electrolyte levels, and ventricular arrhythmias in hypertensive men. JAMA 1992;267(8):1083‐9. [PubMed] [Google Scholar]
- Siegel D, Saliba P, Haffner S. Glucose and insulin levels during diuretic therapy in hypertensive men. Hypertension 1994;23(6 Pt 1):688‐94. [DOI] [PubMed] [Google Scholar]
Simpson 1961 {published data only}
- Simpson FO, Waal HJ. Clinical Trial of Triamterene in Hypertensive Patients . New Zealand Medical Journal 1961 ; 63 :199‐203. [PubMed] [Google Scholar]
Spiekerman 1966 {published data only}
- Spiekerman RE, Berge KG, Thurber DL, Gedge SW, McGuckin WF. Potassium‐Sparing Effects of Triamterene in the Treatment of Hypertension . Circulation 1966 ; 34 :524‐531. [DOI] [PubMed] [Google Scholar]
Vetter 1973 {published data only}
- Vetter G. Clinical trial of a new potassium‐sparing diuretic for hypertension [Klinische Prufun eines neuen kaliumsparenden Diuretikums zur Hochdruckbehandlung]. Pharmakotherapie in Kurze 1973;115(49):2249‐50. [PubMed] [Google Scholar]
Additional references
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter D. The results of direct and indirect treatment comparisons in meta‐analysis of randomised controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Chen 2009
- Chen JMH, Heran BS, Wright JM. Blood pressure lowering efficacy of diuretics as second‐line therapy for primary hypertension. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007187.pub2] [DOI] [PubMed] [Google Scholar]
Heran 2008a
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008b
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.. [Google Scholar]
Musini 2008
- Musini VM, Fortin PM, Bassett K, Wright JM. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007066.pub2] [DOI] [PubMed] [Google Scholar]
Song 2003
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. British Medical Journal 2003;326:472‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]


