Abstract
Background
Blood transfusion services play a very key role in modern health care service delivery. About 118.5 million blood donations were collected globally in 2022. However, about 1.6 million units of blood are destroyed annually due to transfusion-transmissible infections (TTIs). There is a very high risk of TTIs through donated blood to recipients if safe transfusion practices are not observed. This study determined the prevalence and factors associated with TTIs among blood donors in Arua regional blood bank, Uganda.
Methods
This study was a retrospective cross-sectional design that involved a review of a random sample of 1370 blood donors registered between January 1st, 2018 and December 31st, 2019 at Arua regional blood bank, Uganda. Descriptive statistics were used to describe the characteristics of the blood donors. The binary logistic regression was used to determine the factors associated with TTIs.
Results
The majority of the blood donors were male (80.1%), and the median donor age was 23 years (IQR = 8 years). The overall prevalence of TTIs was found to be 13.8% (95%CI: 12.0-15.6%), with specific prevalences of 1.9% for HIV, 4.1% for HBV, 6.6% for HCV and 2.8% for treponema pallidum. Male sex (AOR = 2.10, 95%CI: 1.32–3.36, p-value = 0.002) and lapsed donor type compared to new donor type (AOR = 0.34, 95%CI: 0.13–0.87, p-value = 0.025) were found to be associated with TTIs.
Conclusion
The prevalence of TTIs among blood donors of West Nile region, Uganda was found to be significantly high, which implies a high burden of TTIs in the general population. Hence, there is need to implement a more stringent donor screening process to ensure selection of risk-free donors, with extra emphasis on male and new blood donors. Additionally, sensitization of blood donors on risky behaviors and self-deferral will reduce the risk of donating infected blood to the recipients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09838-4.
Keywords: Blood donor, Voluntary donor, Transfusion-transmissible infections (TTIs), Seropositive blood donor
Background
Blood transfusion services play a very key role in the modern health care service delivery [1]. About 118.5 million blood donations were collected globally in 2022, and over a half of these were collected in the low and middle-income countries that host 80% of the world population [2]. In Uganda, there were 300,000 units of blood collected in 2021/2022 financial year [3].
Blood transfusion is indicated among the corrections of severe anaemia due to various aetiologies like; severe malaria, cancers, sickle cell anaemia and other conditions, injuries that are associated with active haemorrhage and surgeries to improve treatment outcome [4]. Nonetheless, one has to weigh the risks and benefits of blood transfusion against those of anaemia [4]. The greatest challenge of blood transfusion is the risk associated with transfusion-transmissible infections (TTIs) that majorly include; Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), Hepatitis B Virus (HBV) and Treponema pallidum [2]. In the general population, infections like HIV might not be screened for, and even with massive efforts, only 84% of estimated cases know their status [5]. For this reason, people who are carrying infections in the latent phase may not be motivated to go for blood tests, since they are asymptomatic. As a result, about 1.6 million blood units are destroyed annually due to TTIs [6], which in turn reduces the overall blood supply to the healthcare facilities [2, 7, 8].
Globally, there are approximately 58 million individuals chronically infected with HCV, more than 296 million with HBV, and 39 million with HIV [9]. The prevalence of TTIs among blood donors globally as reported by WHO varies from 0.005 to 6.02% for HBV, 0.002–1.67% for HCV, and 0.001–1.60% for HIV [2]. In Sub-Saharan Africa, the overall prevalence of TTIs was reported in Ethiopia at 4.1% [10], Eritrea at 3.6% [11], Malawi at 10.7% [12], Eastern Democratic Republic of Congo (D.R.C) at 14.7% [13], Western Kenya at 9.4% [14], Northern Tanzania at 10.1% [15], whereas in Southwestern Uganda, the burden of TTIs was estimated at 5.67% [16].
As a reduction and prevention strategy towards TTI transmission, WHO recommends a mandatory screening for at least HIV, HBV, HCV and Treponema pallidum by blood banks [2]. In spite of blood banks’ testing for common infections, there are still dangers of other untested infections that include yellow fever virus, leishmaniasis, malaria etc. [17]. In the West Nile region of Uganda, the prevalence of TTIs and its associated factors are still unknown. Moreover, the inadequacy of data on TTIs burden has serious implications on efforts toward reduction of new infections by asymptomatic seropositive blood donors in the communities. Therefore, the importance of documenting this problem cannot be overemphasized. Meanwhile, globally, there are several factors known to be associated with TTIs such as donor age [18–20], sex [19–21], being employed [22], being a commercial donor [21], level of education [23] and donor type (Repeat or new donor) [11, 23]. This study, therefore, aimed to determine the prevalence and factors associated with transfusion-transmissible infections among blood donors in Arua regional blood bank, Uganda.
Methods
Study design and setting
This study was purely quantitative, and employed a retrospective cross-sectional design to review records of blood donors registered between January 1st, 2018 and December 31st, 2019 in Arua regional blood bank. The study inclusion criteria was ‘all blood donors registered between the period of January 1st, 2018 and December 31st, 2019 at Arua Regional Blood Bank’ whereas the exclusion criteria was ‘all blood donors missing information on key variables such as sex, age, rhesus group, blood group and sero results’. The sample size to determine the prevalence of TTIs was calculated using the Kish Leslie formula for a single proportion [24]. Where: Zα/2 = 1.96 was the standard z normal value corresponding to the default confidence interval of 95%, which was used to derive sufficient statistical power for the study; P = 3.7% was the prevalence of TTIs among blood donors [11]; d = 0.01 was the tolerable sampling error since prevalence is less than 10%. Hence, study sample size of 1,370 blood donors. A total population of 5,343 blood donors were included into sampling frame, and then systematic sampling technique was used to select the blood donors; whereby the initial participant was selected using simple random sampling, and then a constant sampling interval of 4 (i.e. 5,343 blood donor population divided by 1,370 sample size) was employed to reach the study sample size of 1,370 blood donors. The data collection involved the use of a data abstraction form to collect socio-demographic data (such as: donor identification number, age, sex, birth district and availability of telephone contact) and clinical data (such as: donor type, frequency of donation, donation site, blood group, rhesus factor, donor TTIs status and notification status). Research Assistants were recruited and trained prior to the commencement of data collection.
The study was carried out in Arua Regional Blood Bank which is located in Arua Regional Referral Hospital in the North-Western part of Uganda, approximately 480 km North of Kampala Capital City [25]. The Regional Blood Bank serves 12 Districts and 1 City of West Nile, namely: Adjumani, Arua, Koboko, Madi-Okollo, Maracha, Moyo, Nebbi, Obongi, Pakwach, Terego, Yumbe, Zombo and Arua City. The West Nile region shares boarders with Democratic Republic of Congo in the West and South Sudan in the North [26]. The population of West-Nile is estimated at 3.9 million people, with about 500,000 households [27]. Arua Regional Blood Bank collects averagely 80 blood units daily, and approximately 10 units of donations are made at the Bank (static post) and about 70 units collected by two field teams daily [25]. The type of blood donors registered by the blood bank is 100% voluntary non-remunerated blood donors (VNRBD), majority are regular donors and about 80% are students [25]. The collected blood is utilized mainly by Arua Regional Referral Hospital, 9 general Hospitals and a number of Health Centre IVs in the region. At the time of donation, all blood donors go through screening sets of questions about previous illnesses and medical conditions and physical examination for blood donation eligibility according to the blood donation criteria. The inclusion criteria for blood donors are; having body weight > 45 kg, no history of high risk sexual behavior and practice, blood transfusion, jaundice, HIV, hepatitis, Treponema pallidum, surgery and hypertension, and current fever [25].
Serological analysis
Routine screening by the blood bank includes: anti-HIV, Treponema pallidum, hepatitis B surface antigen and anti-hepatitis C using the Abbott ARCHITECT i2000 SR analyzer (manufactured by Flextronics, Singapore) for first line testing. However, for the second line testing, confirmation is done using Enzyme Linked Immunosorbent Assay (ELISA) which uses the semi-automated IRE 96 Reader & IW 96 Washer (manufactured by SFRI Medical Diagnostics, France). Alternatively, the Treponema Pallidum Haeagglutination (TPHA) (manufactured by Abbott, USA) test is used for detection of syphilitic infection. The blood unit was considered sero-positive, when both the first and second test results were positive. The Arua regional blood bank subscribed to the European Society for External Quality Assessment (ESFEQA) to ensure compliance with international standards. Additionally, all tests were done according to the manufacture’s guidelines.
Data management and analysis
All collected data were entered into Microsoft excel and exported to STATA version 17.0 for statistical analysis. Participants’ characteristics were described using proportions for categorical variables whereas median and interquartile range (IQR) were used to summarize continuous variables (such as age). Proportions were also used to determine the prevalence of the TTIs among the blood donors. A Venn-diagram was used to describe the sero-positivity concordance of the different TTIs.
Binary logistic regression was used to determine the factors (socio-demographic and clinical) associated with TTIs. The outcome variable, Overall TTIs, was defined as a blood donor having a sero-positive result on any of the four TTIs (HIV, HCV, HBV and Treponema pallidum), and hence coded 1 for sero-positive otherwise 0. At bivariate analysis, variables with p-values less than 0.2, and variables cited important in the literature were considered for multivariate analysis. Interaction terms were assessed for statistical significance using the chunk test, and confounding was assessed by comparing adjusted and unadjusted models using a 10% cut-off. The odds ratios were used as the measure of association, and variables with p-values less than 0.05 (in the final model) were considered statistically significant. The final model was also tested using the Pearson chi-square goodness-of-fit test, and we obtained a p-value > 0.05 that suggested that the model was adequately fitted.
Results
Description of the blood donors
The study involved 1,370 randomly selected blood donors registered between January 1st, 2018 and December 31st, 2019 at Arua regional blood bank, Uganda. The majority of the donors (80.1%) were male. The minimum and maximum donor age was 16 and 59 years respectively, with a median age of 23 years (interquartile range (IQR) = 8 years). Many of the donors (44.2%) hailed from Nebbi district, and more than three quarters (78.8%) did not have telephone contacts. Further, more than a half (57.5%) were repeat donors. All the donors were VNRBD and the majority (97.8%) donated from the out-reach or mobile donation points. Also, the majority of the donors were of blood group O (40.8%), and Rhesus group positive (98.0%) (Table 1).
Table 1.
Socio-demographic and clinical characteristics of 1370 blood donors included in the study, Arua regional blood bank, January 1st, 2018 to December 31st, 2019
| Variable | Category | Frequency | % |
|---|---|---|---|
| Sex | Male | 1097 | 80.1 |
| Female | 273 | 19.9 | |
| Age (cont) | (min = 16yrs; max = 59yrs; median = 23yrs; IQR = 8yrs) | ||
| Birth district | Arua | 479 | 34.9 |
| Nebbi | 605 | 44.2 | |
| Other | 286 | 20.9 | |
| Contact available | No | 1079 | 78.8 |
| Yes | 291 | 21.2 | |
| Donor type | New | 491 | 35.9 |
| Repeat | 788 | 57.5 | |
| Lapsed | 91 | 6.6 | |
| Donor category | VNRBD | 1370 | 100.0 |
| Site | Blood bank | 30 | 2.2 |
| Mobile | 1340 | 97.8 | |
| Blood group | A | 496 | 36.2 |
| AB | 74 | 5.4 | |
| B | 241 | 17.6 | |
| O | 559 | 40.8 | |
| Rhesus group | Pos | 1342 | 98.0 |
| Neg | 28 | 2.0 |
Cont-continuous variable; IQR- Interquartile range; min- minimum; max- maximum; Pos- Positive; Neg- Negative; other-Adjumani, Koboko, Madi-Okollo, Maracha, Moyo, Obongi, Pakwach, Terego, Yumbe & Zombo); VNRBD- Voluntary Non-Remunerated Blood Donors
Prevalence of TTIs
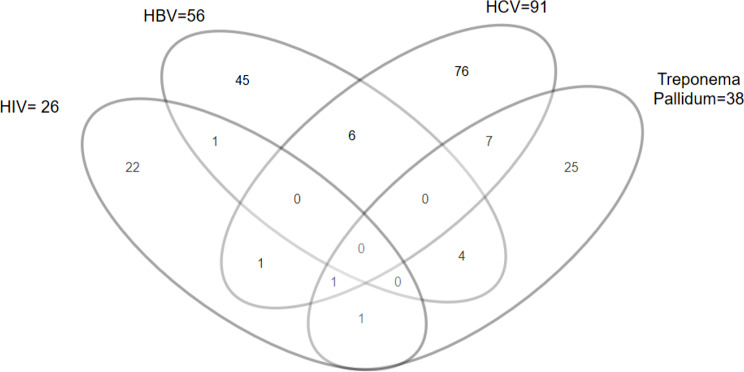
The TTIs considered in this study were: HIV, HBV, HCV and Treponema pallidum. The prevalence of HIV was 1.9% (95% confidence interval (CI): 1.3–2.8), HBV was 4.1% (95%CI: 3.1–5.3), HCV was 6.6% (95% CI: 5.4–8.1) and Treponema pallidum was 2.8% (95% CI: 2.0-3.8). Consequently, the overall prevalence of the TTIs was 13.8% (95% CI: 12.0- 15.6) (Table 2). Meanwhile, there were 21 (11.1%) donors with sero-positive concordance on at least 2 TTIs, and the majority [7] of these had HCV and Treponema pallidum sero-positive concordance. Only 1 donor was sero-positive on 3 TTIs i.e. HIV, HCV and Treponema pallidum, and none was sero-positive on all the 4 TTIs (Fig. 1).
Table 2.
Prevalence of HIV, HBV, HCV and Treponema pallidum among 1370 blood donors in Arua Regional Blood Bank, January 1st, 2018 to December 31st, 2019
| Variable | Category | Number | Prevalence (%) | 95% CI |
|---|---|---|---|---|
| HIV | Pos | 26 | 1.9 | 1.3–2.8 |
| Neg | 1344 | |||
| HBV | Pos | 56 | 4.1 | 3.1–5.3 |
| Neg | 1314 | |||
| HCV | Pos | 91 | 6.6 | 5.4–8.1 |
| Neg | 1279 | |||
| Treponema pallidum | Pos | 38 | 2.8 | 2.0- 3.8 |
| Neg | 1332 | |||
| Overall TTIs | Pos | 189 | 13.8 | 12.0- 15.6 |
| Neg | 1181 |
CI = Confidence Interval ; Pos- Positive ; Neg- Negative
Fig. 1.
Venn diagram showing sero-positivity concordance among 1370 blood donors in Arua regional blood bank, between January 1st, 2018 and December 31st, 2019
Factors associated with TTIs
At bivariate analysis, we included social demographic factors (age, sex, birth district and contact available) and clinical factors (donor type, site, blood group and rhesus group). Only being male and having lapsed compared to being a new donor were statistically significant. At multivariate level, the adjusted regression model also suggested that variables: being male (AOR = 2.10, 95%CI: 1.32–3.36, p = 0.002) and being lapsed compared to new donor (AOR = 0.34, 95%CI: 0.13–0.87, p = 0.025) were significantly associated with TTIs (Table 3).
Table 3.
Binary logistic regression model to determine factors associated with transfusion-transmissible infections, Arua Regional Blood Bank, Uganda, January 1st, 2018 to December 31st, 2019
| Bivariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | COR | 95%CI | P-value | AOR | 95%CI | P-value |
| Sex | Female | 1 | 1 | ||||
| Male | 2.05 | 1.28–3.26 | 0.003 | 2.10 | 1.32–3.36 | 0.002 | |
| Age (cont) | 1.00 | 0.98–1.02 | 0.732 | ||||
| Birth district | Arua | 1 | |||||
| Nebbi | 0.92 | 0.65–1.30 | 0.643 | ||||
| Other | 1.01 | 0.66–1.54 | 0.957 | ||||
| Contact available | No | 1 | |||||
| Yes | 0.82 | 0.55–1.21 | 0.325 | ||||
| Donor type | New | 1 | 1 | ||||
| Repeat | 1.04 | 0.76–1.44 | 0.789 | 1.01 | 0.73–1.40 | 0.929 | |
| Lapsed | 0.35 | 0.14–0.91 | 0.031 | 0.34 | 0.13–0.87 | 0.025 | |
| Site | Blood bank | 1 | |||||
| Mobile | 2.27 | 0.54–9.61 | 0.265 | ||||
| Blood group | A | 1 | 1 | ||||
| AB | 1.77 | 0.96–3.25 | 0.068 | 1.81 | 0.98–3.36 | 0.059 | |
| B | 0.98 | 0.62–1.54 | 0.932 | 1.00 | 0.64–1.58 | 0.993 | |
| O | 0.98 | 0.68–1.39 | 0.898 | 0.95 | 0.66–1.35 | 0.763 | |
| Rhesus group | Pos | 1 | 1 | ||||
| Neg | 1.04 | 0.36–3.04 | 0.939 | 1.10 | 0.37–3.23 | 0.862 | |
COR- Crude Odds Ratio; AOR- Adjusted Odds Ratio; 95% CI- 95% Confidence Interval; cont- Continous variable; Pos- Positive ; Neg- Negative
Discussion
Blood transfusion is one of the integral parts of life-saving procedures of modern medicine despite the very high risk of transmitting infectious diseases such as HIV, HBV, HCV, treponema pallidum and malaria. Our study sought to determine the prevalence and factors associated with transfusion-transmissible infections among blood donors in Arua regional blood bank, Uganda. The overall prevalence of TTIs revealed by this study was 13.8%, which is higher than what is reported in several studies in the region [10, 11, 14, 16, 28]. This high prevalence could lead to a reduction in pool of donors, especially for those that shall be permanently referred to chronic care. The high prevalence of TTIs also means an increased burden of destruction of infected blood, and hence increasing the overall operational costs of the blood bank. Studies with similar rates were reported in Kenya (14.1%) [29] and Ethiopia (12.4%) [30]. This could perhaps be due to similarities in the risky sexual behaviors of these populations and the blood donor selection criteria. In contrast, lower overall prevalence has been reported in Southwestern Uganda (5.67%), but also in Western Province of Rwanda (2.1%), Eritrea (3.6%), Ethiopia (4.1%), Western Kenya (9.4%), Northern Tanzania (10.1%) and Malawi (10.7%) [10–12, 14–16, 31]. Meanwhile, a higher prevalence was reported in Eastern D.R.C (14.7%) [13]. These variations in the TTI prevalence could be due to the differences in health care systems in these settings as compared to the West Nile region of Uganda. As such, regions with lower TTI prevalence could be having a healthier donor population, in respect to these TTIs, compared to the West Nile region of Uganda and vice versa.
Specifically, the prevalence of HCV was the highest with 6.6% followed by HBV at 4.1%, Treponema pallidum at 2.8% and HIV at 1.9%. Comparatively, a study conducted among a similar donor community in the southwestern part of Uganda got lower prevalence on all the TTIs with HCV at 2.22%, HBV at 1.87%, HIV at 1.03% and Treponema pallidum at 0.34% [16]. The discrepancies might be due to the differences in population risks or effectiveness and stringency in procedures of donor screening. However, our findings are similar on the prevalence of HCV being higher than that of HIV and HBV among the Ugandan donor community, although, this is contrary to what is reported in many other studies in Sub-Saharan Africa [10, 11, 13, 14]. This can also be attributed to the fact that, a lot more attention has been given to HIV and HBV, with less being done to treat and prevent HCV transmission in Ugandan communities. Hence, there is need to scale up interventions to prevent HCV transmission. Further, the prevalence of HIV remained comparatively the same but our study showed higher burden of treponema pallidum among donors in West Nile region than in the South Western region of Uganda.
Our regression results suggested that factors: male sex and lapsed donor category were significantly associated with TTIs. Male donors were more than twice as likely to have TTIs as the female donors. Perhaps this could be attributed to the risky sexual behaviors among the males compared to the females in the community. This finding was consistent with studies conducted in Honduras [32] and Kenya [29]. However, other studies conducted in Eritrea [11] reported male donors to be less likely to have TTIs. Meanwhile, the lapsed donors were 66% less likely to have TTIs compared to the new donors. This could be due to the fact that the lapsed donors have been previously screened, counselled and registered into the blood bank database, and hence less likely to have TTIs. On the contrary, other studies have reported the repeat donors to be associated with a higher prevalence of TTIs compared to the new donors [11, 20]. Our study was limited by the fact that it was a retrospective design and hence we were not able to obtain sufficient data to assess for additional potential confounders like occupation, marital status and educational level, as these were not captured at the time of registration of blood donors. Additionally, this study may also be limited by the blood donor eligibility criteria or/and blood unit screening tests that may not be the same in all blood banks (especially outside Uganda), and hence limiting the applicability of our results. Meanwhile, the major study strength was having sufficient statistical power owing to the large sample size (n = 1,370 donors).
Conclusion
The overall prevalence of 13.8% is high, which indicates, approximately, 1 in every 7 blood donors have at least one of the TTIs, which implies a high burden of TTIs in the general population and infectivity potential of the viruses and bacteria. Consequently, reviewing the current protocols in order to encourage pre-donation counselling and testing to enable seropositive donors to be deferred and referred for further management promptly. Factors, sex and donor category were significantly associated with TTIs. Hence, there is need to implement a more stringent donor screening process to ensure selection of risk-free donors, with extra emphasis on male and new blood donors. Our findings also justify the use of pathogen reduction technology (PRT) to treat infected blood products with pathogen-inactivating agent in order to ensure safety of blood, and maintain the blood supply that is needed at the health facilities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We want to appreciate the research assistants for their efforts during data collection and management of Arua Regional Blood Bank for supporting the study.
Abbreviations
- AOR
Adjusted Odds Ratio
- CEU
Clinical Epidemiology Unit
- CI
Confidence Interval
- COR
Crude Odds Ratio
- HBV
Hepatitis B Virus
- HCV
Hepatitis C Virus
- HIV
Human Immunodeficiency Virus
- IQR
Interquartile Range
- MoH
Ministry of Health
- PRT
Pathogen Reduction Technology
- RD
Replacement Donors
- RPR
Rapid Plasma Reagin
- SOMREC
School of Medicine Research and Ethical Committee
- TPHA
Test and Treponema Pallidum Hemagglutination
- TTIs
Transfusion Transmissible Infections
- VNRBD
Voluntary Non-Remunerated Blood Donors
- WHO
World Health Organization
Author contributions
NC and HK formulated the research protocol. DO and NC extracted data. DO and NC participated in data cleaning, analysis and interpretation. DO and NC wrote the initial draft of the manuscript. DO, NC, HK, JFZ, RA, TO and FA critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The ethical approval to conduct this study was obtained from School of Medicine Research and Ethical Committee (SOMREC), College of Health Sciences, Makerere University (#REC REF: 2020-057). Permission to review the blood donor files was sought from the Arua District Local Government and Head of Arua Regional Blood Bank, Uganda. We applied for waiver of consent since this study involved review of data, and there was no chance of interfacing with the study participants. The study protocol and conduct adhered to the principles in the Declaration of Helsinki and good clinical practice (GCP).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prasad S, Bai K. Seropositivity of HIV, Hepatitis B and C, and syphilis among blood donors: a retrospective study. Asian J Transfus Sci. 2014;8(1):66–7. 10.4103/0973-6247.126705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Blood safety and availability [Internet]. 2023 [cited 2024 Feb 10]. https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability
- 3.New Vision. New Vision. 2023 [cited 2024 Feb 10]. How UBTS can collect 450,000 units of blood annually. https://www.newvision.co.ug/articledetails/NV_153784
- 4.Yaddanapudi S, Yaddanapudi LN. Indications for blood and blood product transfusion. Indian J Anaesth. 2014;58(5):538. 10.4103/0019-5049.144648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. UNAIDS data 2019 [Internet]. UNAIDS. 2019. https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf
- 6.World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. World health organization; 2014. [PubMed]
- 7.Riley W, Love K, Saxon M, Tobian A, Bloch EM, Kasirye R, et al. A model for estimating the Burden of Disease of Transfusion-transmitted infection. Int J Public Health. 2024;69:1607165. 10.3389/ijph.2024.1607165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanagasabai U, Trends, and Gaps in National Blood Transfusion Services. — 14 Sub-Saharan African Countries, 2014–2016. MMWR Morb Mortal Wkly Rep [Internet]. 2018 [cited 2024 Aug 26];67. https://www.cdc.gov/mmwr/volumes/67/wr/mm6750a4.htm [DOI] [PMC free article] [PubMed]
- 9.World Health Organization. Fact sheets [Internet]. 2024 [cited 2024 Feb 17]. https://www.who.int/news-room/fact-sheets
- 10.Abdella S, Berheto TM, Tolera G, Belete W, Deressa T, Feleke A, et al. Sero-prevalence of transfusion transmittable infections: HIV, Hepatitis B, C and Treponema pallidum and associated factors among blood donors in Ethiopia: a retrospective study. PLoS ONE. 2020;15(10):e0241086. 10.1371/journal.pone.0241086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siraj N, Achila OO, Issac J, Menghisteab E, Hailemariam M, Hagos S, et al. Seroprevalence of transfusion-transmissible infections among blood donors at National Blood Transfusion Service, Eritrea: a seven-year retrospective study. BMC Infect Dis. 2018;18(1):1–9. 10.1186/s12879-018-3174-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singogo E, Chagomerana M, Van Ryn C, M’bwana R, Likaka A, M’baya B, et al. Prevalence and incidence of transfusion-transmissible infections among blood donors in Malawi: a population-level study. Transfus Med. 2023;33(6):483–96. 10.1111/tme.13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilumbulumbu MK, Ketha JK, Tshimanga VK, Bunduki GK, Valimungighe MM, Kitamwivirirwa TK et al. High prevalence of transfusion-transmissible infections among Volunteer Blood donors in Rural Area of Eastern Democratic Republic of the Congo (D.R.C). Archives Curr Res Int. 2018;1–8.
- 14.Onyango CG, Ogonda L, Guyah B, Okoth P, Shiluli C, Humwa F, et al. Seroprevalence and determinants of transfusion transmissible infections among voluntary blood donors in Homabay, Kisumu and Siaya counties in western Kenya. BMC Res Notes. 2018;11(1):171. 10.1186/s13104-018-3276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mremi A, Yahaya JJ, Nyindo M, Mollel E. Transfusion-transmitted infections and associated risk factors at the Northern Zone Blood Transfusion Center in Tanzania: a study of blood donors between 2017 and 2019. PLoS ONE. 2021;16(3):e0249061. 10.1371/journal.pone.0249061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apecu RO, Mulogo EM, Bagenda F, Byamungu A, Ii YB, Bazira J, et al. Seroprevalence of human immunodeficiency virus (HIV), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV) and Syphilis among Voluntary Blood donors in Rural Southwestern Uganda: a retrospective study. International Journal of TROPICAL DISEASE & Health; 2017. pp. 1–13.
- 17.Diseases and Organisms | Blood Safety | CDC [Internet]. 2022 [cited 2023 Jul 16]. https://www.cdc.gov/bloodsafety/bbp/diseases-organisms.html
- 18.Xie DD, Li J, Chen JT, Eyi UM, Matesa RA, Obono MMO, et al. Seroprevalence of Human Immunodeficiency Virus, Hepatitis B Virus, Hepatitis C Virus, and Treponema pallidum infections among blood donors on Bioko Island, Equatorial Guinea. PLoS ONE. 2015;10(10):e0139947. 10.1371/journal.pone.0139947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teklemariam Z, Mitiku H, Weldegebreal F. Seroprevalence and trends of transfusion transmitted infections at Harar blood bank in Harari regional state, Eastern Ethiopia: eight years retrospective study. BMC Hematol. 2018;18(1):24. 10.1186/s12878-018-0115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed Y, Bekele A. Seroprevalence of transfusion transmitted infection among blood donors at Jijiga blood bank, Eastern Ethiopia: retrospective 4 years study. BMC Res Notes. 2016;9(1):129. 10.1186/s13104-016-1925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okoroiwu HU, Okafor IM, Asemota EA, Okpokam DC. Seroprevalence of transfusion-transmissible infections (HBV, HCV, Syphilis and HIV) among prospective blood donors in a tertiary health care facility in Calabar, Nigeria; an eleven years evaluation. BMC Public Health. 2018;18(1):645. 10.1186/s12889-018-5555-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biadgo B, Shiferaw E, Woldu B, Alene KA, Melku M. Transfusion-transmissible viral infections among blood donors at the North Gondar district blood bank, northwest Ethiopia: a three year retrospective study. PLoS ONE. 2017;12(7):e0180416. 10.1371/journal.pone.0180416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pessoni LL, de Aquino ÉC, de Alcântara KC. Prevalence and trends in transfusion-transmissible infections among blood donors in Brazil from 2010 to 2016. Hematol, Transfus Cell Ther. 2019;41:310–5. [DOI] [PMC free article] [PubMed]
- 24.Kish L. Survey Sampling. New York: John Wiley and Sons, Inc; 1965. [Google Scholar]
- 25.UBTS, UBTS-Home [Internet]. 2023 [cited 2024 Feb 10]. https://www.ubts.go.ug/
- 26.Wikipedia. Arua Regional Referral Hospital. In: Wikipedia [Internet]. 2020 [cited 2024 Feb 10]. https://en.wikipedia.org/w/index.php?title=Arua_Regional_Referral_Hospital&oldid=985688489
- 27.UBOS. National-Population-and-Housing-Census-2024-Dissemination-of-Preliminary-Results [Internet]. Plot 9 Colville Street, Kampala Uganda: Uganda Bureau of Statistics; 2024 Jun. https://www.ubos.org/wp-content/uploads/publications/National-Population-and-Housing-Census-2024-Dissemination-of-Preliminary-Results.pdf
- 28.Mohamed Z, Kim JU, Magesa A, Kasubi M, Feldman SF, Chevaliez S, et al. High prevalence and poor linkage to care of transfusion-transmitted infections among blood donors in Dar‐es‐Salaam, Tanzania. J Viral Hepatitis. 2019;26(6):750–6. 10.1111/jvh.13073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartonjo G, Oundo J, Ng’ang’a Z. Prevalence and associated risk factors of transfusion transmissible infections among blood donors at Regional Blood Transfusion Center Nakuru and Tenwek Mission Hospital, Kenya. The Pan African Medical Journal [Internet]. 2019 Sep 16 [cited 2023 Jul 22];34(31). https://www.panafrican-med-journal.com/content/article/34/31/full [DOI] [PMC free article] [PubMed]
- 30.Heyredin I, Mengistie B, Weldegebreal F. Sero-prevalence of transfusion-transmittable infections and associated factors among blood donors in Eastern Ethiopia: an institutional-based cross-sectional study. SAGE Open Med. 2019;7:2050312119834468. 10.1177/2050312119834468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nsekuye O, Omolo J, El-Khatib Z, Matsiko E, Uwayo HD, Ndicunguye F et al. Risk factors associated with transfusion transmissible infections among blood donors at Karongi Regional Centre for Blood Transfusion-Western Province of Rwanda. Journal of Interventional Epidemiology and Public Health [Internet]. 2024 Jun 21 [cited 2024 Aug 24];7(2). https://www.afenet-journal.net/content/series/7/3/2/full/
- 32.Hernández-Arriaga G, Ruglas K, Alas-Pineda C, Chinchilla-López C, Arriaga-Mendoza G, Bejarano-Cáceres S, et al. Prevalence of infectious diseases and its associated factors among the blood donors of the Honduran Red Cross – Northern region between 2014 and 2016. PLoS ONE. 2018;13(11):e0207338. 10.1371/journal.pone.0207338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.