Abstract
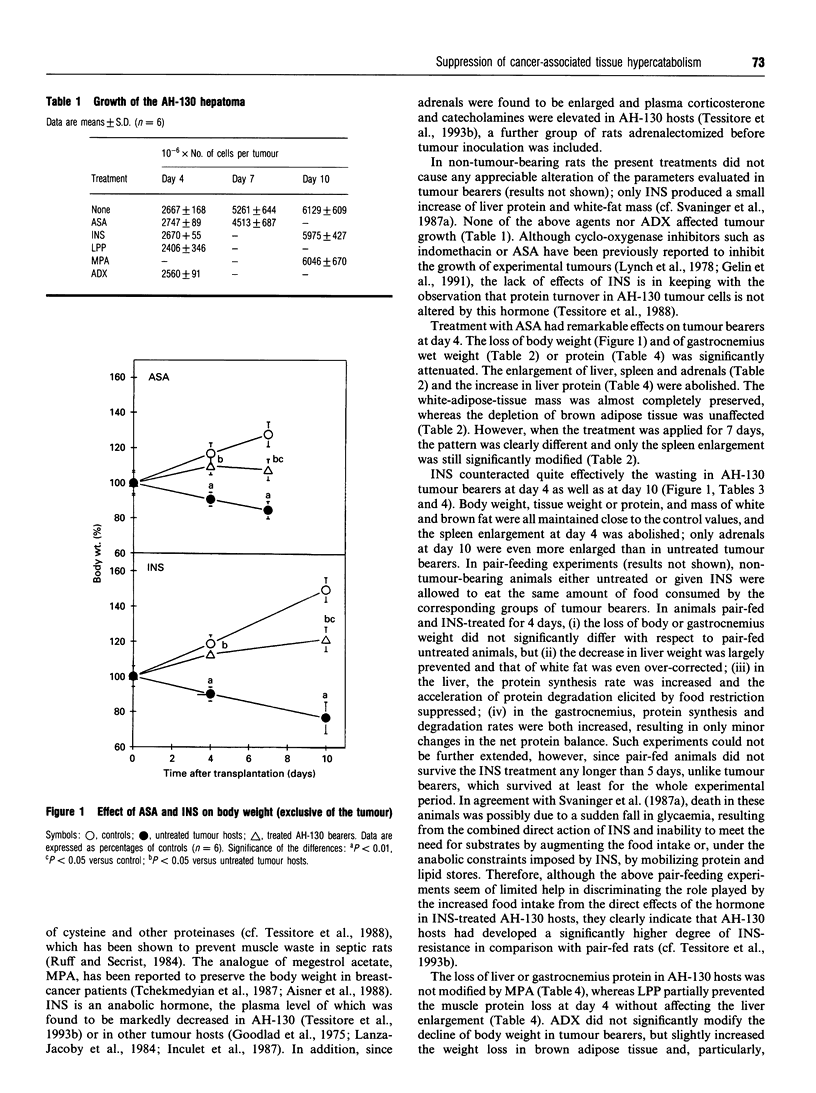
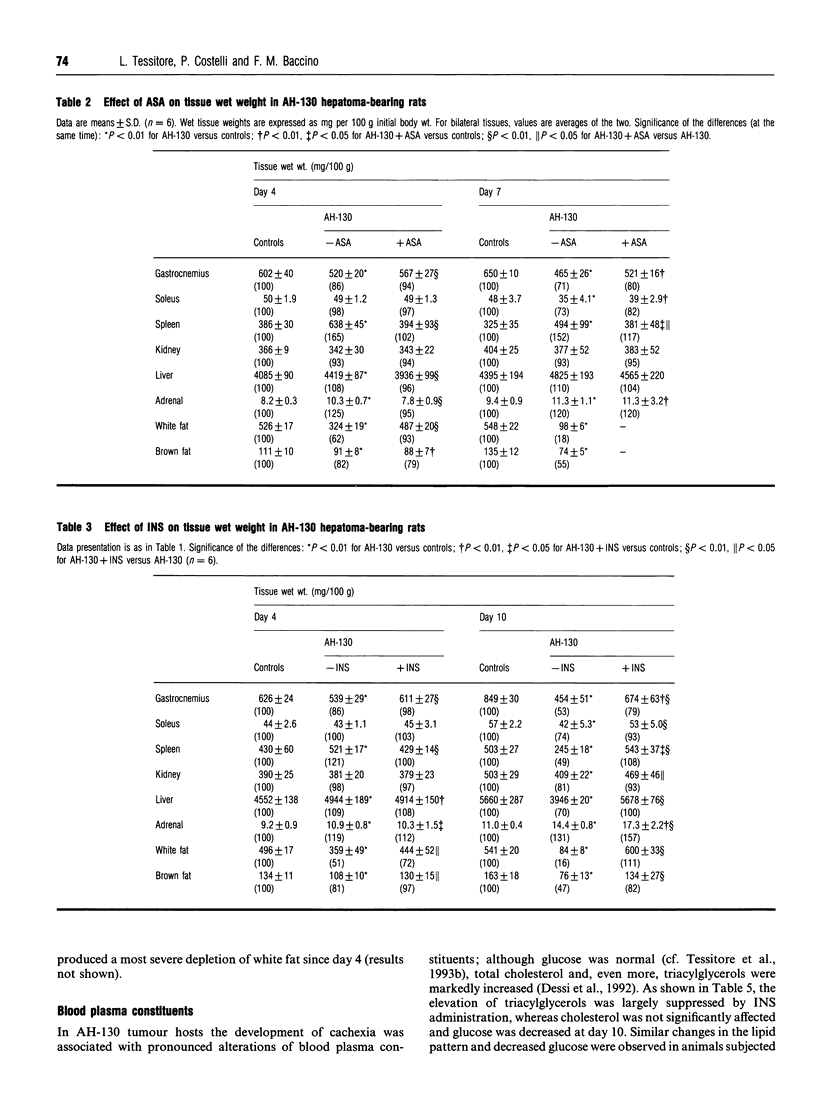
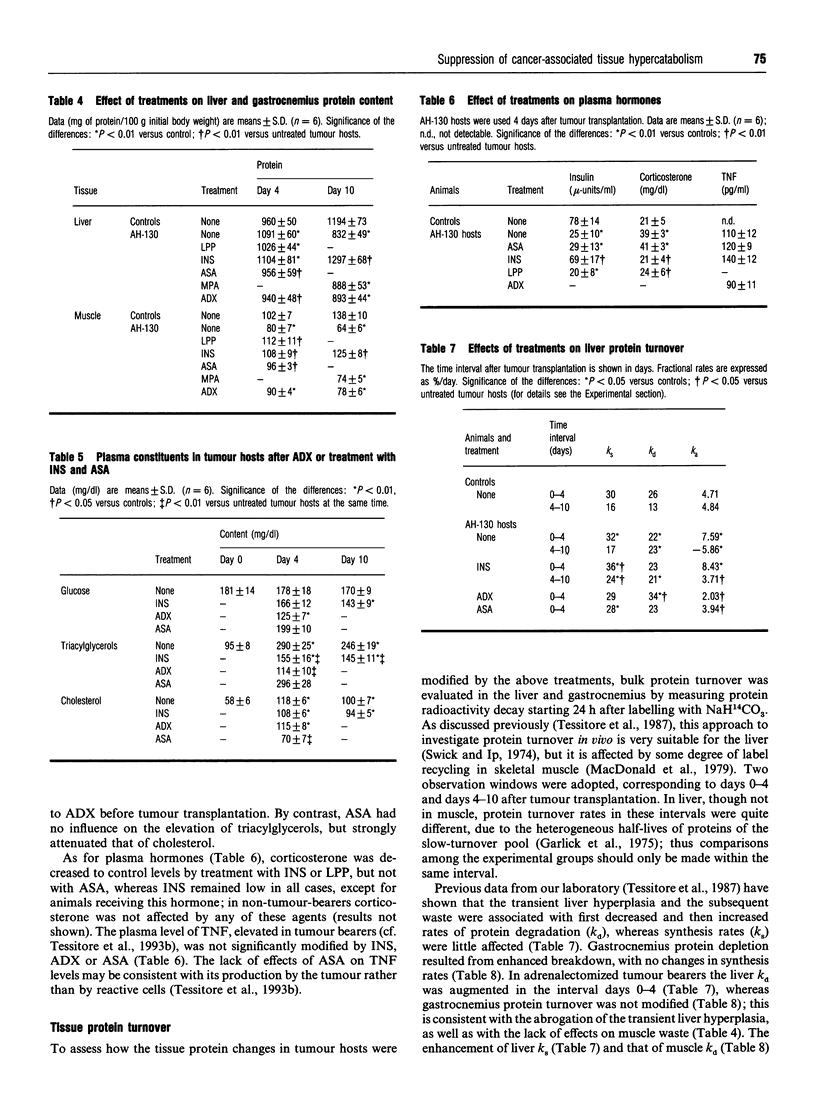
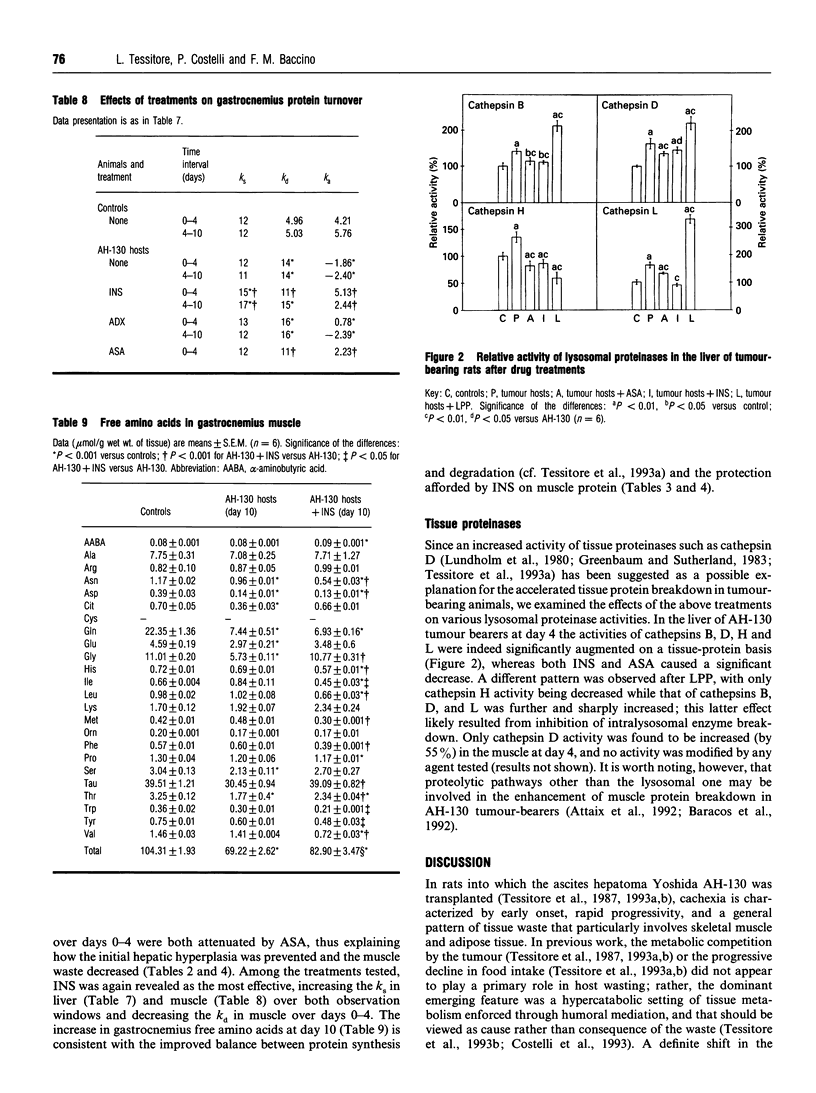
Marked loss of body weight and profound waste of both skeletal muscle and white adipose tissue occur in rats into which the ascites hepatoma Yoshida AH-130 has been transplanted, associated with marked perturbations in the hormonal homoeostasis and the presence of circulating tumour necrosis factor and high plasma levels of prostaglandin E2 [Tessitore, Costelli and Baccino (1993) Br. J. Cancer 67, 15-23]. On the basis of previous findings, the present study examined whether the development of cachexia in this model system could be significantly affected by adrenalectomy or by pharmacological treatments that may interfere with proximal or distal mediators of tissue hypercatabolism. In no instance was tumour growth modified. Medroxyprogesterone acetate, an anabolic-hormone-like drug, was completely ineffective. In adrenalectomized animals, although changes such as the elevation of plasma triacylglycerols and corticosterone were corrected, the general course of cachexia was not modified. A partial prevention of muscle waste was observed with acetylsalicylic acid, a non-steroidal anti-inflammatory drug, or with leupeptin, a proteinase inhibitor. Insulin afforded the most significant preservation of muscle protein and adipose-tissue mass, which were maintained close to control values even 10 days after transplantation. The effects of insulin on gastrocnemius muscle and liver protein content were exerted by slowing down protein turnover, mainly enhancing synthesis. Consistently, the total free amino acid concentration in the gastrocnemius of insulin-treated rats 10 days after tumour transplantation was close to that of controls. Although treatment with insulin decreased plasma corticosterone to normal values, it did not modify the circulating level of tumour necrosis factor. On the whole these data show that it seems possible to prevent, at least in part, the tissue waste that characterizes cancer cachexia by purely pharmacological means.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisner J., Tchekmedyian N. S., Tait N., Parnes H., Novak M. Studies of high-dose megestrol acetate: potential applications in cachexia. Semin Oncol. 1988 Apr;15(2 Suppl 1):68–75. [PubMed] [Google Scholar]
- Baccino F. M., Tessitore L., Cecchini G., Messina M., Zuretti M. F., Bonelli G., Gabriel L., Amenta J. S. Control of cell protein catabolism in rat liver. Effects of starvation and administration of cycloheximide. Biochem J. 1982 Aug 15;206(2):395–405. doi: 10.1042/bj2060395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron P. L., Lawrence W., Jr, Chan W. M., White F. K., Banks W. L., Jr Effects of parenteral nutrition on cell cycle kinetics of head and neck cancer. Arch Surg. 1986 Nov;121(11):1282–1286. doi: 10.1001/archsurg.121.11.1282. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S. A., Tisdale M. J. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989 May;59(5):677–681. doi: 10.1038/bjc.1989.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzetti F., Ammatuna M., Migliavacca S., Bonalumi M. G., Facchetti G., Pupa A., Terno G. Total parenteral nutrition prevents further nutritional deterioration in patients with cancer cachexia. Ann Surg. 1987 Feb;205(2):138–143. doi: 10.1097/00000658-198702000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. F. Total parenteral nutrition in the cancer patient. N Engl J Med. 1981 Aug 13;305(7):375–382. doi: 10.1056/NEJM198108133050705. [DOI] [PubMed] [Google Scholar]
- Cameron I. L. Effect of total parenteral nutrition on tumor-host responses in rats. Cancer Treat Rep. 1981;65 (Suppl 5):93–99. [PubMed] [Google Scholar]
- Chance W. T., Muggia-Sullam M., Chen M. H., Murphy R. F., Fischer J. E. Reversal of tumor-induced biochemical abnormalities by insulin treatment in rats. J Natl Cancer Inst. 1986 Aug;77(2):497–503. [PubMed] [Google Scholar]
- Chlebowski R. T. Critical evaluation of the role of nutritional support with chemotherapy. Cancer. 1985 Jan 1;55(1 Suppl):268–272. doi: 10.1002/1097-0142(19850101)55:1+<268::aid-cncr2820551311>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Costelli P., Carbó N., Tessitore L., Bagby G. J., Lopez-Soriano F. J., Argilés J. M., Baccino F. M. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993 Dec;92(6):2783–2789. doi: 10.1172/JCI116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWys W. Management of cancer cachexia. Semin Oncol. 1985 Dec;12(4):452–460. [PubMed] [Google Scholar]
- Dessí S., Batetta B., Anchisi C., Pani P., Costelli P., Tessitore L., Baccino F. M. Cholesterol metabolism during the growth of a rat ascites hepatoma (Yoshida AH-130). Br J Cancer. 1992 Nov;66(5):787–793. doi: 10.1038/bjc.1992.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Gelin J., Moldawer L. L., Lönnroth C., Sherry B., Chizzonite R., Lundholm K. Role of endogenous tumor necrosis factor alpha and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991 Jan 1;51(1):415–421. [PubMed] [Google Scholar]
- Goodlad G. A., Mitchell A. J., McPhail L., Clark C. M. Serum insulin and somatomedin levels in the tumour-bearing rat. Eur J Cancer. 1975 Oct;11(10):733–737. doi: 10.1016/0014-2964(75)90048-1. [DOI] [PubMed] [Google Scholar]
- Greenbaum L. M., Sutherland J. H. Host cathepsin D response to tumor in the normal and pepstatin-treated mouse. Cancer Res. 1983 Jun;43(6):2584–2587. [PubMed] [Google Scholar]
- Greenberg A. S., Nordan R. P., McIntosh J., Calvo J. C., Scow R. O., Jablons D. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 1992 Aug 1;52(15):4113–4116. [PubMed] [Google Scholar]
- Heslin M. J., Newman E., Wolf R. F., Pisters P. W., Brennan M. F. Effect of systemic hyperinsulinemia in cancer patients. Cancer Res. 1992 Jul 15;52(14):3845–3850. [PubMed] [Google Scholar]
- Hyltander A., Drott C., Körner U., Sandström R., Lundholm K. Elevated energy expenditure in cancer patients with solid tumours. Eur J Cancer. 1991;27(1):9–15. doi: 10.1016/0277-5379(91)90050-n. [DOI] [PubMed] [Google Scholar]
- Inculet R. I., Stein T. P., Peacock J. L., Leskiw M., Maher M., Gorschboth C. M., Norton J. A. Altered leucine metabolism in noncachectic sarcoma patients. Cancer Res. 1987 Sep 1;47(17):4746–4749. [PubMed] [Google Scholar]
- Kern K. A., Norton J. A. Cancer cachexia. JPEN J Parenter Enteral Nutr. 1988 May-Jun;12(3):286–298. doi: 10.1177/0148607188012003286. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Fiers W., Goldberg A. L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4273–4277. doi: 10.1073/pnas.84.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza-Jacoby S., Lansey S. C., Miller E. E., Cleary M. P. Sequential changes in the activities of lipoprotein lipase and lipogenic enzymes during tumor growth in rats. Cancer Res. 1984 Nov;44(11):5062–5067. [PubMed] [Google Scholar]
- Lowry S. F. Cancer cachexia revisited: old problems and new perspectives. Eur J Cancer. 1991;27(1):1–3. doi: 10.1016/0277-5379(91)90046-g. [DOI] [PubMed] [Google Scholar]
- Lundholm K., Bylund A. C., Holm J., Scherstén T. Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer. 1976 Jun;12(6):465–473. doi: 10.1016/0014-2964(76)90036-0. [DOI] [PubMed] [Google Scholar]
- Lundholm K., Ekman L., Karlberg I., Edström S., Scherstén T. Comparison of hepatic cathepsin D activity in response to tumor growth and to caloric restriction in mice. Cancer Res. 1980 May;40(5):1680–1685. [PubMed] [Google Scholar]
- Lynch N. R., Castes M., Astoin M., Salomon J. C. Mechanism of inhibition of tumour growth by aspirin and indomethacin. Br J Cancer. 1978 Oct;38(4):503–512. doi: 10.1038/bjc.1978.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. L., Augustine S. L., Burk T. L., Swick R. W. A comparison of methods for the measurement of protein turnover in vivo. Biochem J. 1979 Nov 15;184(2):473–476. doi: 10.1042/bj1840473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys P., Dijkmans R., Proost P., Van Damme J., Heremans H., Sobis H., Billiau A. Severe cachexia in mice inoculated with interferon-gamma-producing tumor cells. Int J Cancer. 1991 Aug 19;49(1):77–82. doi: 10.1002/ijc.2910490115. [DOI] [PubMed] [Google Scholar]
- Moley J. F., Morrison S. D., Gorschboth C. M., Norton J. A. Body composition changes in rats with experimental cancer cachexia: improvement with exogenous insulin. Cancer Res. 1988 May 15;48(10):2784–2787. [PubMed] [Google Scholar]
- Moley J. F., Morrison S. D., Norton J. A. Insulin reversal of cancer cachexia in rats. Cancer Res. 1985 Oct;45(10):4925–4931. [PubMed] [Google Scholar]
- Mori M., Yamaguchi K., Honda S., Nagasaki K., Ueda M., Abe O., Abe K. Cancer cachexia syndrome developed in nude mice bearing melanoma cells producing leukemia-inhibitory factor. Cancer Res. 1991 Dec 15;51(24):6656–6659. [PubMed] [Google Scholar]
- Morrison S. D. Feeding response of tumor-bearing rats to insulin and insulin withdrawal and the contribution of autonomous tumor drain to cachectic depletion. Cancer Res. 1982 Sep;42(9):3642–3647. [PubMed] [Google Scholar]
- Odedra B. R., Millward D. J. Effect of corticosterone treatment on muscle protein turnover in adrenalectomized rats and diabetic rats maintained on insulin. Biochem J. 1982 Jun 15;204(3):663–672. doi: 10.1042/bj2040663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock J. L., Gorschboth C. M., Norton J. A. Impact of insulin on doxorubicin-induced rat host toxicity and tumor regression. Cancer Res. 1987 Aug 15;47(16):4318–4322. [PubMed] [Google Scholar]
- Popp M. B., Kirkemo A. K., Morrison S. D., Brennan M. F. Tumor and host carcass changes during total parenteral nutrition in an anorectic rat-tumor system. Ann Surg. 1984 Feb;199(2):205–210. doi: 10.1097/00000658-198402000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp M. B., Morrison S. D., Brennan M. F. Total parenteral nutrition in a methylcholanthrene-induced rat sarcoma model. Cancer Treat Rep. 1981;65 (Suppl 5):137–143. [PubMed] [Google Scholar]
- Rofe A. M., Bourgeois C. S., Bais R., Conyers R. A. Metabolic response to insulin and glucose infusions in starved tumor-bearing rats. Cancer Res. 1989 Nov 1;49(21):5949–5953. [PubMed] [Google Scholar]
- Ruff R. L., Secrist D. Inhibitors of prostaglandin synthesis or cathepsin B prevent muscle wasting due to sepsis in the rat. J Clin Invest. 1984 May;73(5):1483–1486. doi: 10.1172/JCI111352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumley T. O., Jr, Copeland E. M., 3rd Intravenous hyperalimentation as nutritional support for the cancer patient--an update. J Surg Oncol. 1985 Nov;30(3):164–173. doi: 10.1002/jso.2930300309. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Kisner D., Haller D., Blecher M., Hamosh M. Cachexia of malignancy: potential role of insulin in nutritional management. Cancer. 1979 May;43(5 Suppl):2070–2076. doi: 10.1002/1097-0142(197905)43:5+<2070::aid-cncr2820430715>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Whole-body protein kinetics in patients with early and advanced gastrointestinal cancer: the response to glucose infusion and total parenteral nutrition. Surgery. 1988 Feb;103(2):148–155. [PubMed] [Google Scholar]
- Strelkov A. B., Fields A. L., Baracos V. E. Effects of systemic inhibition of prostaglandin production on protein metabolism in tumor-bearing rats. Am J Physiol. 1989 Aug;257(2 Pt 1):C261–C269. doi: 10.1152/ajpcell.1989.257.2.C261. [DOI] [PubMed] [Google Scholar]
- Sugden P. H., Fuller S. J. Regulation of protein turnover in skeletal and cardiac muscle. Biochem J. 1991 Jan 1;273(Pt 1):21–37. doi: 10.1042/bj2730021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaninger G., Drott C., Lundholm K. Role of insulin in development of cancer cachexia in nongrowing sarcoma-bearing mice: special reference to muscle wasting. J Natl Cancer Inst. 1987 May;78(5):943–950. [PubMed] [Google Scholar]
- Svaninger G., Gelin J., Lundholm K. Tumor-host wasting not explained by adrenal hyperfunction in tumor-bearing animals. J Natl Cancer Inst. 1987 Nov;79(5):1135–1141. [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]
- Tanaka Y., Tanaka T., Ishitsuka H. Antitumor activity of indomethacin in mice bearing advanced colon 26 carcinoma compared with those with early transplants. Cancer Res. 1989 Nov 1;49(21):5935–5939. [PubMed] [Google Scholar]
- Tchekmedyian N. S., Tait N., Moody M., Aisner J. High-dose megestrol acetate. A possible treatment for cachexia. JAMA. 1987 Mar 6;257(9):1195–1198. [PubMed] [Google Scholar]
- Tessitore L., Bonelli G., Baccino F. M. Early development of protein metabolic perturbations in the liver and skeletal muscle of tumour-bearing rats. A model system for cancer cachexia. Biochem J. 1987 Jan 1;241(1):153–159. doi: 10.1042/bj2410153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore L., Bonelli G., Cecchini G., Autelli R., Amenta J. S., Baccino F. M. Regulation of protein turnover versus growth state. Studies on the mechanism(s) of initiation of acidic vacuolar proteolysis in cells of stationary ascites hepatoma. Biochem J. 1988 Apr 15;251(2):483–490. doi: 10.1042/bj2510483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore L., Costelli P., Baccino F. M. Humoral mediation for cachexia in tumour-bearing rats. Br J Cancer. 1993 Jan;67(1):15–23. doi: 10.1038/bjc.1993.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore L., Costelli P., Bonetti G., Baccino F. M. Cancer cachexia, malnutrition, and tissue protein turnover in experimental animals. Arch Biochem Biophys. 1993 Oct;306(1):52–58. doi: 10.1006/abbi.1993.1479. [DOI] [PubMed] [Google Scholar]


