Abstract
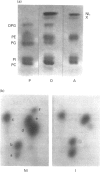
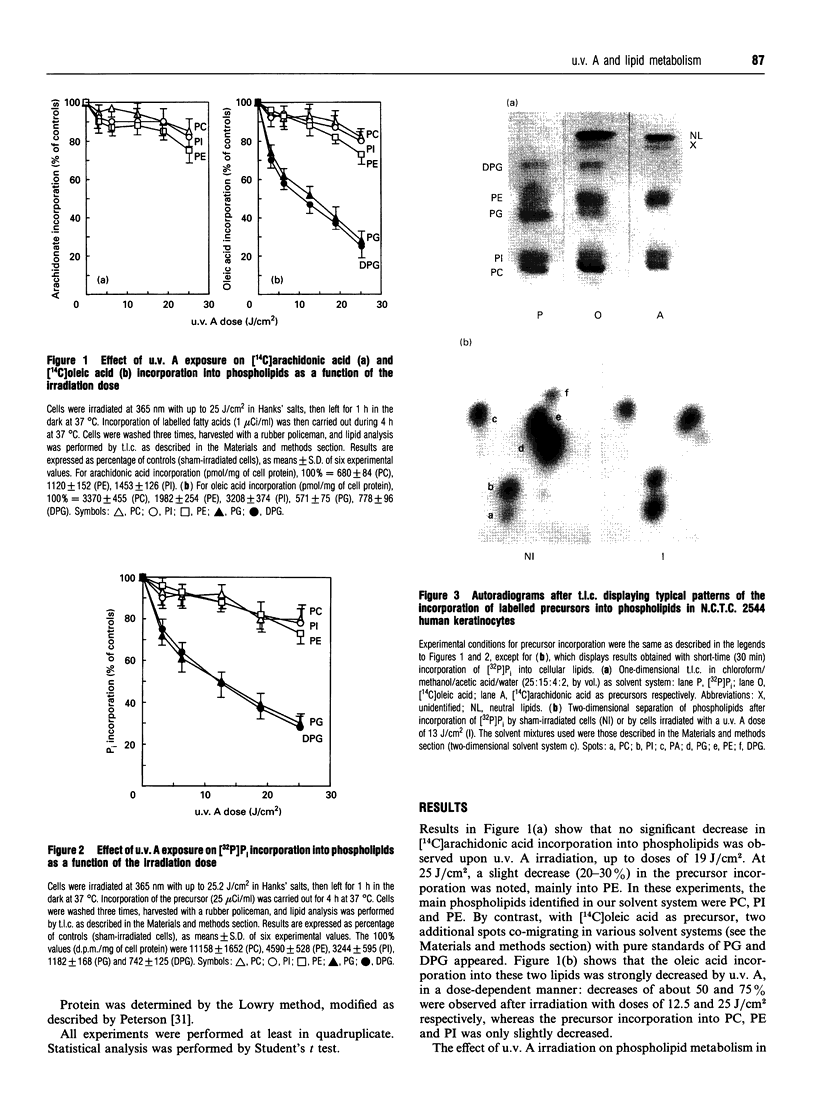
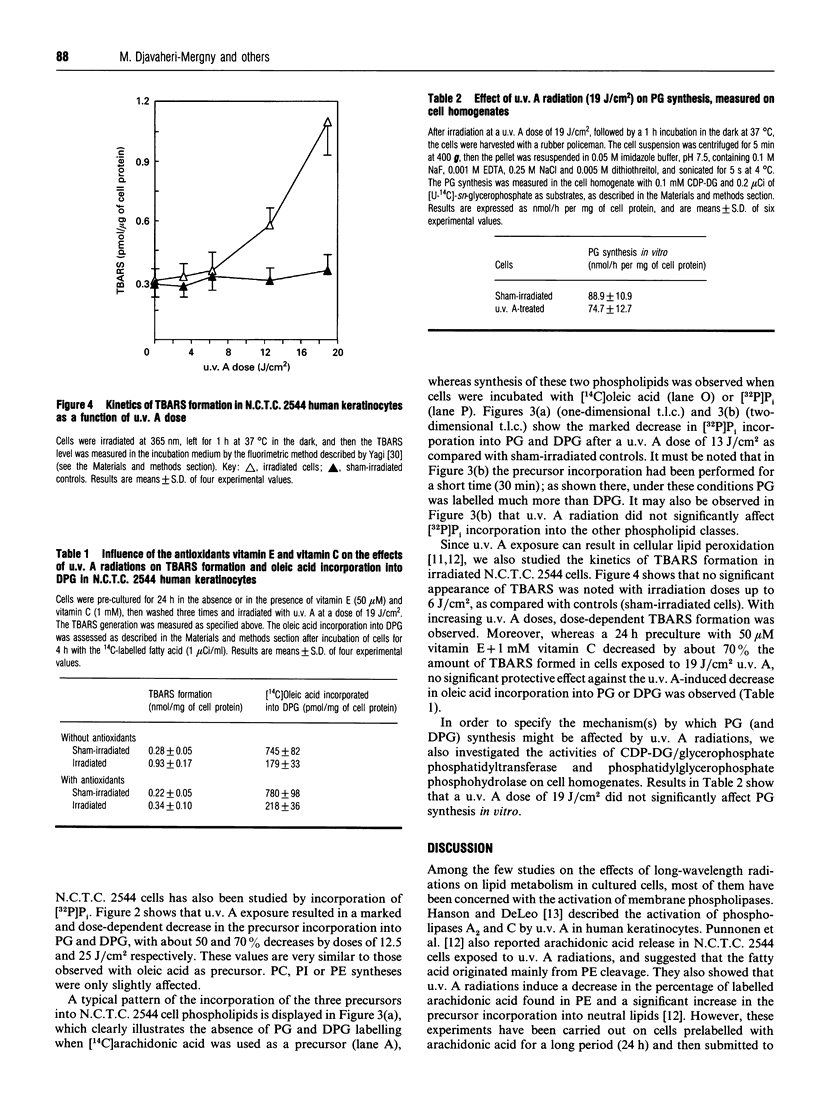
The effects of u.v. A radiations on phospholipid synthesis were studied in the N.C.T.C. 2544 human keratinocyte cell line, by using [14C]arachidonic acid, [14C]oleic acid or sodium [32P]orthophosphate as precursors. Cells were irradiated in Hanks' medium with 365 nm light at doses up to 19 J/cm2, and then phospholipid synthesis from the three precursors was studied. Under these conditions, only small alterations in the incorporation pattern of [14C]arachidonic into phospholipids [phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylinositol (PI)] were observed, for u.v. A irradiation doses up to 19 J/cm2. In contrast, with [14C]oleic acid as precursor, two additional spots were observed, which co-migrate with pure phosphatidylglycerol (PG) and diphosphatidylglycerol (DPG) standards. The incorporation of [14C]oleic acid into PG and DPG was decreased in a dose-dependent manner after u.v. A exposure, with about 50% and 75% decreases at 9.5 J/cm2 and 19 J/cm2 respectively. As for arachidonic acid incorporation, no significant differences in the synthesis of the major phospholipids (PC, PE, PI) were noted upon u.v. A exposure. The dramatic and selective decrease in PG and DPG syntheses was confirmed with [32P]orthophosphate as precursor. As DPG is a specific component of the mitochondrial inner membrane, it appears that one of the early kinds of damage induced by u.v. A irradiation could be the impairment of mitochondrial functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awasthi Y. C., Ruzicka F. J., Crane F. L. The relation between phospholipase action and release of NADH dehydrogenase from mitochondrial membrane. Biochim Biophys Acta. 1970 Apr 21;203(2):233–248. doi: 10.1016/0005-2736(70)90137-9. [DOI] [PubMed] [Google Scholar]
- Bergelson L. D., Dyatlovitskaya E. V., Torkhovskaya T. I., Sorokina I. B., Gorkova N. P. Phospholipid composition of membranes in the tumor cell. Biochim Biophys Acta. 1970 Jul 14;210(2):287–298. doi: 10.1016/0005-2760(70)90173-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bulos B., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 18. The masking of adenosine triphosphatase in submitochondrial particles and its reactivation by phospholipids. J Biol Chem. 1968 Jul 25;243(14):3901–3905. [PubMed] [Google Scholar]
- Davidson J. B., Stanacev N. Z. Biosynthesis of cardiolipin in mitochondria isolated from guinea pig liver. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1191–1199. doi: 10.1016/0006-291x(71)90032-5. [DOI] [PubMed] [Google Scholar]
- De Leo V. A., Hanson D., Weinstein I. B., Harber L. C. Ultraviolet radiation stimulates the release of arachidonic acid from mammalian cells in culture. Photochem Photobiol. 1985 Jan;41(1):51–56. doi: 10.1111/j.1751-1097.1985.tb03447.x. [DOI] [PubMed] [Google Scholar]
- Diffey B. L. Analysis of the risk of skin cancer from sunlight and solaria in subjects living in northern Europe. Photodermatol. 1987 Jun;4(3):118–126. [PubMed] [Google Scholar]
- FLEISCHER S., BRIERLEY G., KLOUWEN H., SLAUTTERBACK D. B. Studies of the electron transfer system. 47. The role of phospholipids in electron transfer. J Biol Chem. 1962 Oct;237:3264–3272. [PubMed] [Google Scholar]
- Freeman S. E., Gange R. W., Sutherland J. C., Sutherland B. M. Pyrimidine dimer formation in human skin. Photochem Photobiol. 1987 Aug;46(2):207–212. doi: 10.1111/j.1751-1097.1987.tb04758.x. [DOI] [PubMed] [Google Scholar]
- Getz G. S., Bartley W., Lurie D., Notton B. M. The phospholipids of various sheep organs, rat liver and of their subcellular fractions. Biochim Biophys Acta. 1968 Mar 4;152(2):325–339. doi: 10.1016/0005-2760(68)90040-4. [DOI] [PubMed] [Google Scholar]
- Gray G. M. Chromatography of lipids. II. The quantitative isolation of the minor (acidic) phospholipids and of phosphatidylethanolamine from the lipid extracts of mammalian tissues. Biochim Biophys Acta. 1967 Dec 5;144(3):519–524. doi: 10.1016/0005-2760(67)90040-9. [DOI] [PubMed] [Google Scholar]
- Hanson D. L., DeLeo V. A. Long wave ultraviolet radiation stimulates arachidonic acid release and cyclooxygenase activity in mammalian cells in culture. Photochem Photobiol. 1989 Apr;49(4):423–430. doi: 10.1111/j.1751-1097.1989.tb09190.x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Rapidly occurring DNA excision repair events determine the biological expression of u.v.-induced damage in human cells. Carcinogenesis. 1987 Sep;8(9):1251–1256. doi: 10.1093/carcin/8.9.1251. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Kligman A. M. The nature of photoaging: its prevention and repair. Photodermatol. 1986 Aug;3(4):215–227. [PubMed] [Google Scholar]
- Maroney A. C., Macara I. G. Phorbol ester-induced translocation of diacylglycerol kinase from the cytosol to the membrane in Swiss 3T3 fibroblasts. J Biol Chem. 1989 Feb 15;264(5):2537–2544. [PubMed] [Google Scholar]
- Matsui M. S., DeLeo V. A. Induction of protein kinase C activity by ultraviolet radiation. Carcinogenesis. 1990 Feb;11(2):229–234. doi: 10.1093/carcin/11.2.229. [DOI] [PubMed] [Google Scholar]
- Maziere C., Maziere J. C., Mora L., Polonovski J. Incorporation of exogenous fatty acids into phospholipids by cultured hamster fibroblasts. Effect of SV40 transformation. Biochim Biophys Acta. 1982 Sep 14;712(3):712–715. doi: 10.1016/0005-2760(82)90303-4. [DOI] [PubMed] [Google Scholar]
- Mazière C., Mazière J. C., Mora L., Auclair M., Polonovski J. Trifluoperazine increases fatty acid turnover in phospholipids in cultured human fibroblasts. Lipids. 1988 May;23(5):419–423. doi: 10.1007/BF02535513. [DOI] [PubMed] [Google Scholar]
- Mazière C., Mazière J. C., Mora L., Polonovski J. Changes in phospholipid polar head group turnover in SV40-transformed hamster fibroblasts. FEBS Lett. 1982 Mar 22;139(2):217–220. doi: 10.1016/0014-5793(82)80855-7. [DOI] [PubMed] [Google Scholar]
- Mazière C., Mazière J. C., Mora L., Polonovski J. Rapid analysis of cellular lipids without extraction. J Biochem Biophys Methods. 1987 Aug;14(5):267–272. doi: 10.1016/0165-022x(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Morlière P., Moysan A., Santus R., Hüppe G., Mazière J. C., Dubertret L. UVA-induced lipid peroxidation in cultured human fibroblasts. Biochim Biophys Acta. 1991 Jul 30;1084(3):261–268. doi: 10.1016/0005-2760(91)90068-s. [DOI] [PubMed] [Google Scholar]
- Nair V., Cooper C. S., Vietti D. E., Turner G. A. The chemistry of lipid peroxidation metabolites: crosslinking reactions of malondialdehyde. Lipids. 1986 Jan;21(1):6–10. doi: 10.1007/BF02534294. [DOI] [PubMed] [Google Scholar]
- PERRY V. P., SANFORD K. K., EVANS V. J., HYATT G. W., EARLE W. R. Establishment of clones of epithelial cells from human skin. J Natl Cancer Inst. 1957 May;18(5):709–717. [PubMed] [Google Scholar]
- Paltauf F., Schatz G. Promitochondria of anaerobicallly grown yeast. II. Lipid composition. Biochemistry. 1969 Jan;8(1):335–339. doi: 10.1021/bi00829a046. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Paddon H. B., Vance D. E. Phorbol esters stimulate phosphatidylcholine biosynthesis by translocation of CTP:phosphocholine cytidylyltransferase from cytosol to microsomes. Biochim Biophys Acta. 1984 Oct 4;795(3):447–451. doi: 10.1016/0005-2760(84)90171-1. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Punnonen K., Jansén C. T., Puntala A., Ahotupa M. Effects of in vitro UVA irradiation and PUVA treatment on membrane fatty acids and activities of antioxidant enzymes in human keratinocytes. J Invest Dermatol. 1991 Feb;96(2):255–259. doi: 10.1111/1523-1747.ep12462271. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Dawson R. M. Interactions of cytochrome c and [14C]. Biochem J. 1969 Oct;115(1):65–75. doi: 10.1042/bj1150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staberg B., Wulf H. C., Klemp P., Poulsen T., Brodthagen H. The carcinogenic effect of UVA irradiation. J Invest Dermatol. 1983 Dec;81(6):517–519. doi: 10.1111/1523-1747.ep12522855. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Keyse S. M. New trends in photobiology. The interaction of UVA radiation with cultured cells. J Photochem Photobiol B. 1990 Mar;4(4):349–361. doi: 10.1016/1011-1344(90)85014-n. [DOI] [PubMed] [Google Scholar]
- Urbach F. Potential effects of altered solar ultraviolet radiation on human skin cancer. Photochem Photobiol. 1989 Oct;50(4):507–513. doi: 10.1111/j.1751-1097.1989.tb05556.x. [DOI] [PubMed] [Google Scholar]
- Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]