Abstract
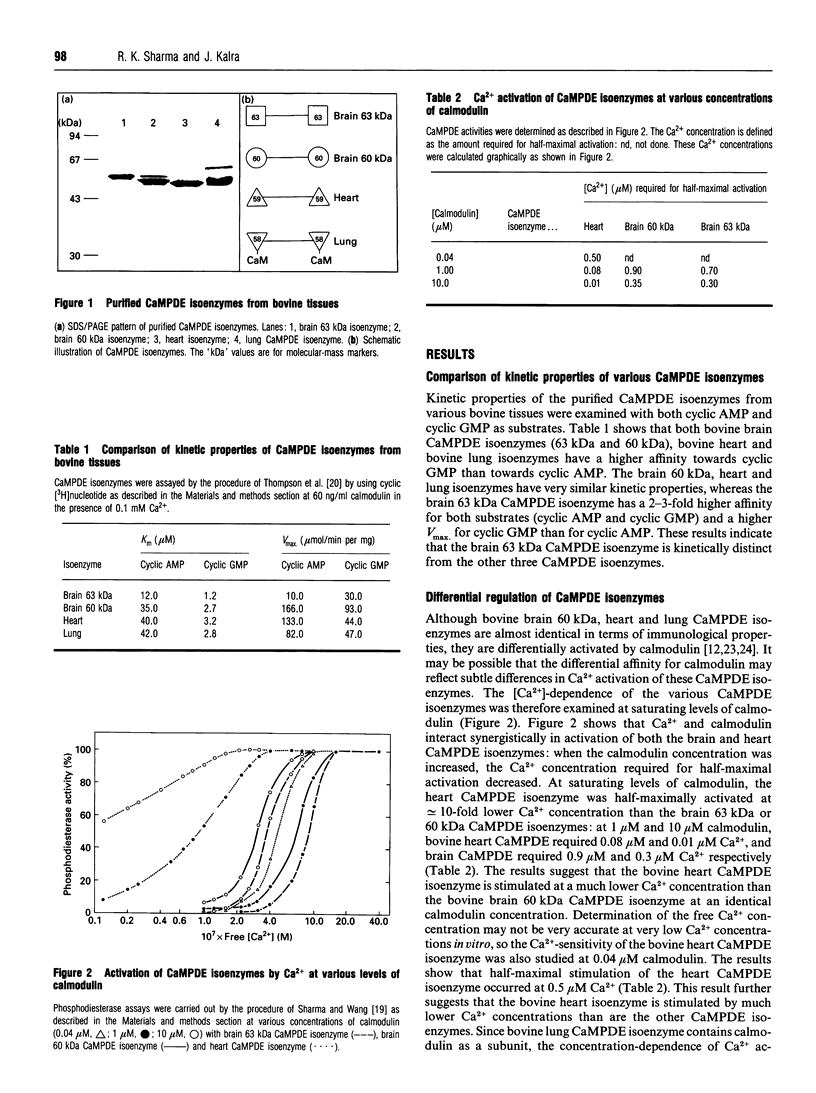
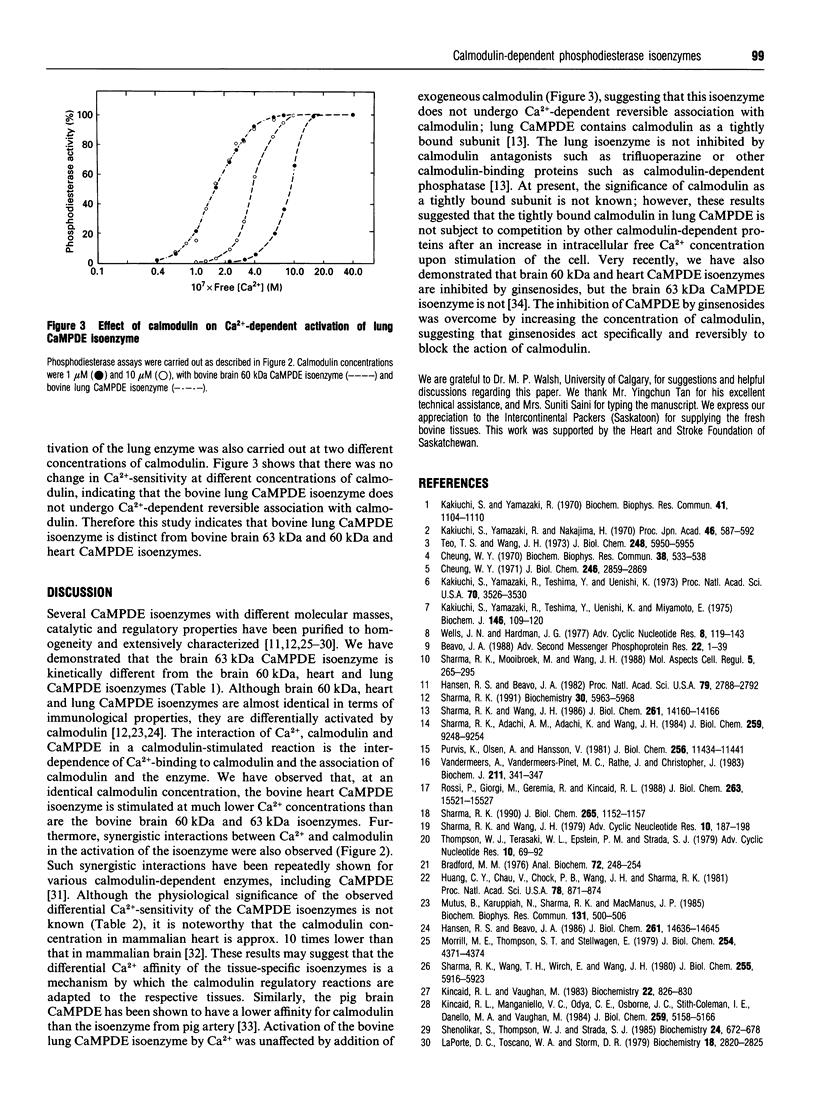
Calmodulin-dependent phosphodiesterase (CaMPDE) is one of the key enzymes involved in the complex interactions which occur between the cyclic-nucleotide and Ca2+ second-messenger systems. Calmodulin-dependent phosphodiesterase exists in different isoenzymic forms, which exhibit distinct molecular and/or catalytic properties. The kinetic properties suggest that the 63 kDa brain isoenzyme is distinct from the brain 60 kDa and heart and lung CaMPDE isoenzymes. The CaMPDE isoenzymes of 60 kDa from brain, heart and lung are regulated by calmodulin, but the affinities for calmodulin are different. At identical concentrations of calmodulin, the bovine heart CaMPDE isoenzyme is stimulated at a much lower Ca2+ concentration than the bovine brain or lung isoenzymes. The bovine lung CaMPDE isoenzyme contains calmodulin as a tightly bound subunit, so that a change in calmodulin concentration had no effect on the [Ca2+]-dependence of activation of this isoenzyme. These observations are consistent with the notion that differential regulation by calmodulin and Ca2+ is an important function of these isoenzymes, which provide fine-tuning mechanisms for calmodulin action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- Hansen R. S., Beavo J. A. Differential recognition of calmodulin-enzyme complexes by a conformation-specific anti-calmodulin monoclonal antibody. J Biol Chem. 1986 Nov 5;261(31):14636–14645. [PubMed] [Google Scholar]
- Hansen R. S., Beavo J. A. Purification of two calcium/calmodulin-dependent forms of cyclic nucleotide phosphodiesterase by using conformation-specific monoclonal antibody chromatography. Proc Natl Acad Sci U S A. 1982 May;79(9):2788–2792. doi: 10.1073/pnas.79.9.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Y., Chau V., Chock P. B., Wang J. H., Sharma R. K. Mechanism of activation of cyclic nucleotide phosphodiesterase: requirement of the binding of four Ca2+ to calmodulin for activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):871–874. doi: 10.1073/pnas.78.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R. Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3',5'-nucleotide phosphodiesterase (3). Biochem Biophys Res Commun. 1970 Dec 9;41(5):1104–1110. doi: 10.1016/0006-291x(70)90199-3. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y., Uenishi K., Miyamoto E. Multiple cyclic nucleotide phosphodiesterase activities from rat tissues and occurrence of a calcium-plus-magnesium-ion-dependent phosphodiesterase and its protein activator. Biochem J. 1975 Jan;146(1):109–120. doi: 10.1042/bj1460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y., Uenishi K. Regulation of nucleoside cyclic 3':5'-monophosphate phosphodiesterase activity from rat brain by a modulator and Ca2+. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3526–3530. doi: 10.1073/pnas.70.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C., Odya C. E., Osborne J. C., Jr, Stith-Coleman I. E., Danello M. A., Vaughan M. Purification and properties of calmodulin-stimulated phosphodiesterase from mammalian brain. J Biol Chem. 1984 Apr 25;259(8):5158–5166. [PubMed] [Google Scholar]
- Kincaid R. L., Vaughan M. Affinity chromatography of brain cyclic nucleotide phosphodiesterase using 3-(2-pyridyldithio)propionyl-substituted calmodulin linked to thiol-sepharose. Biochemistry. 1983 Feb 15;22(4):826–830. doi: 10.1021/bi00273a018. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Toscano W. A., Jr, Storm D. R. Cross-linking of iodine-125-labeled, calcium-dependent regulatory protein to the Ca2+-sensitive phosphodiesterase purified from bovine heart. Biochemistry. 1979 Jun 26;18(13):2820–2825. doi: 10.1021/bi00580a021. [DOI] [PubMed] [Google Scholar]
- Morrill M. E., Thompson S. T., Stellwagen E. Purification of a cyclic nucleotide phosphodiesterase from bovine brain using blue dextran-Sepharose chromatography. J Biol Chem. 1979 Jun 10;254(11):4371–4374. [PubMed] [Google Scholar]
- Mutus B., Karuppiah N., Sharma R. K., MacManus J. P. The differential stimulation of brain and heart cyclic-AMP phosphodiesterase by oncomodulin. Biochem Biophys Res Commun. 1985 Aug 30;131(1):500–506. doi: 10.1016/0006-291x(85)91830-3. [DOI] [PubMed] [Google Scholar]
- Olwin B. B., Keller C. H., Storm D. R. Calcium-dependent and calcium-independent affinities of calmodulin for calmodulin-binding proteins determined by fluorescence techniques. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:227–243. [PubMed] [Google Scholar]
- Rossi P., Giorgi M., Geremia R., Kincaid R. L. Testis-specific calmodulin-dependent phosphodiesterase. A distinct high affinity cAMP isoenzyme immunologically related to brain calmodulin-dependent cGMP phosphodiesterase. J Biol Chem. 1988 Oct 25;263(30):15521–15527. [PubMed] [Google Scholar]
- Sharma R. K., Adachi A. M., Adachi K., Wang J. H. Demonstration of bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase isozymes by monoclonal antibodies. J Biol Chem. 1984 Jul 25;259(14):9248–9254. [PubMed] [Google Scholar]
- Sharma R. K., Kalra J. Ginsenosides are potent and selective inhibitors of some calmodulin-dependent phosphodiesterase isozymes. Biochemistry. 1993 May 18;32(19):4975–4978. doi: 10.1021/bi00070a001. [DOI] [PubMed] [Google Scholar]
- Sharma R. K. Phosphorylation and characterization of bovine heart calmodulin-dependent phosphodiesterase. Biochemistry. 1991 Jun 18;30(24):5963–5968. doi: 10.1021/bi00238a021. [DOI] [PubMed] [Google Scholar]
- Sharma R. K. Purification and characterization of novel calmodulin-binding protein from cardiac muscle. J Biol Chem. 1990 Jan 15;265(2):1152–1157. [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Preparation and assay of the Ca2+--dependent modulator protein. Adv Cyclic Nucleotide Res. 1979;10:187–198. [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Purification and characterization of bovine lung calmodulin-dependent cyclic nucleotide phosphodiesterase. An enzyme containing calmodulin as a subunit. J Biol Chem. 1986 Oct 25;261(30):14160–14166. [PubMed] [Google Scholar]
- Shenolikar S., Thompson W. J., Strada S. J. Characterization of a Ca2+-calmodulin-stimulated cyclic GMP phosphodiesterase from bovine brain. Biochemistry. 1985 Jan 29;24(3):672–678. doi: 10.1021/bi00324a020. [DOI] [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- Vandermeers A., Vandermeers-Piret M. C., Rathe J., Christophe J. Purification and kinetic properties of two soluble forms of calmodulin-dependent cyclic nucleotide phosphodiesterase from rat pancreas. Biochem J. 1983 May 1;211(2):341–347. doi: 10.1042/bj2110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. N., Hardman J. G. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1977;8:119–143. [PubMed] [Google Scholar]


