Abstract
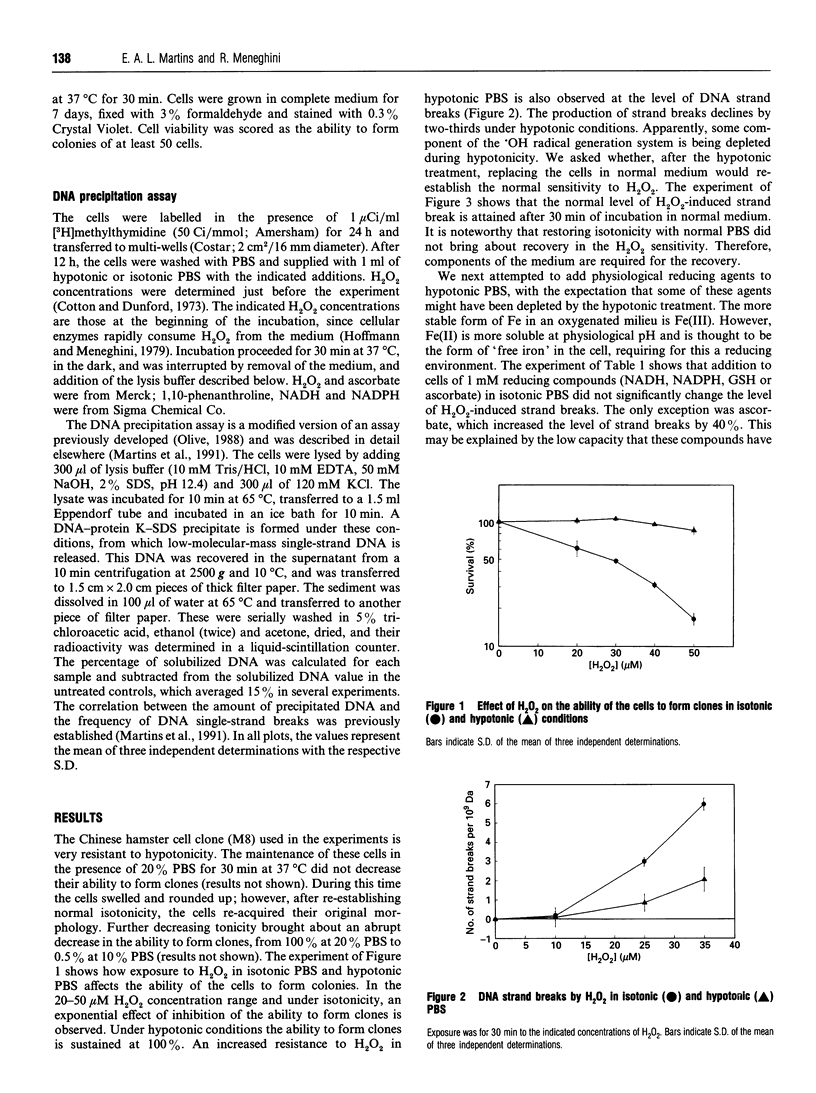
Chinese hamster fibroblasts (line V79) withstand well exposure for 30 min to hypotonic medium, corresponding to 25% physiological phosphate-buffered saline (PBS). Under these conditions, the cells become resistant to two effects of H2O2: DNA damage and inhibition of cell clone formation. The normal sensitivity to the DNA-damaging action of H2O2 is restored if, after exposure to hypotonic PBS, the cells are incubated in isotonic cell-culture medium. However, restoration of sensitivity is not observed on incubation in isotonic PBS. The normal sensitivity to H2O2 is also restored if one of the following reducing agents is added to hypotonic PBS: ascorbate, NADH and NADPH, in this order of decreasing efficiency. The recovery of sensitivity to H2O2 by ascorbate is completely inhibited by 1,10-phenanthroline, indicating that ascorbate is mediating the reduction of Fe(III). The decrease in the sensitivity to the DNA-damaging action of H2O2 is not a peculiarity of hypotonic PBS, since it appears to be caused by hypo-osmolarity in general: it is also observed in culture medium of 25% the isotonic concentration, and in 0.07 M sucrose. One explanation for this phenomenon is that hypotonic stress leads to a depletion of reducing species, in particular ascorbate. Under these conditions Fe(II) tends to be oxidized to Fe(III) and the Fenton chemistry is mitigated. However, other possibilities are that hypotonicity brings about structural modifications in the chromatin, rendering it less accessible to H2O2, or that it attenuates the Ca(2+)-activation of endonuclease, induced by oxidative stress.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calderaro M., Martins E. A., Meneghini R. Oxidative stress by menadione affects cellular copper and iron homeostasis. Mol Cell Biochem. 1993 Sep 8;126(1):17–23. doi: 10.1007/BF01772204. [DOI] [PubMed] [Google Scholar]
- Cantoni O., Fiorani M., Cattabeni F., Bellomo G. DNA breakage caused by hydrogen peroxide produced during the metabolism of 2-methyl-1,4-naphthoquinone (menadione) does not contribute to the cytotoxic action of the quinone. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S220–S222. doi: 10.1016/0006-2952(91)90415-2. [DOI] [PubMed] [Google Scholar]
- Cantoni O., Sestili P., Cattabeni F., Bellomo G., Pou S., Cohen M., Cerutti P. Calcium chelator Quin 2 prevents hydrogen-peroxide-induced DNA breakage and cytotoxicity. Eur J Biochem. 1989 Jun 15;182(2):209–212. doi: 10.1111/j.1432-1033.1989.tb14819.x. [DOI] [PubMed] [Google Scholar]
- Dancis A., Roman D. G., Anderson G. J., Hinnebusch A. G., Klausner R. D. Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake, and transcriptional control by iron. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3869–3873. doi: 10.1073/pnas.89.9.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M., Nackerdien Z., Chao B. C., Gajewski E., Rao G. Chemical nature of in vivo DNA base damage in hydrogen peroxide-treated mammalian cells. Arch Biochem Biophys. 1991 Mar;285(2):388–390. doi: 10.1016/0003-9861(91)90378-v. [DOI] [PubMed] [Google Scholar]
- Eide D., Davis-Kaplan S., Jordan I., Sipe D., Kaplan J. Regulation of iron uptake in Saccharomyces cerevisiae. The ferrireductase and Fe(II) transporter are regulated independently. J Biol Chem. 1992 Oct 15;267(29):20774–20781. [PubMed] [Google Scholar]
- Eisenstein R. S., Garcia-Mayol D., Pettingell W., Munro H. N. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Aruoma O. I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991 Apr 9;281(1-2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992 Jul 27;307(1):108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. E., Meneghini R. Action of hydrogen peroxide on human fibroblast in culture. Photochem Photobiol. 1979 Jul;30(1):151–155. doi: 10.1111/j.1751-1097.1979.tb07128.x. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Jonas S. K., Riley P. A., Willson R. L. Hydrogen peroxide cytotoxicity. Low-temperature enhancement by ascorbate or reduced lipoate. Biochem J. 1989 Dec 15;264(3):651–655. doi: 10.1042/bj2640651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Kühn L. C. mRNA-protein interactions regulate critical pathways in cellular iron metabolism. Br J Haematol. 1991 Sep;79(1):1–5. doi: 10.1111/j.1365-2141.1991.tb07998.x. [DOI] [PubMed] [Google Scholar]
- Martins E. A., Chubatsu L. S., Meneghini R. Role of antioxidants in protecting cellular DNA from damage by oxidative stress. Mutat Res. 1991 Sep-Oct;250(1-2):95–101. doi: 10.1016/0027-5107(91)90166-l. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Mello-Filho A. C., Meneghini R. Iron is the intracellular metal involved in the production of DNA damage by oxygen radicals. Mutat Res. 1991 Nov;251(1):109–113. doi: 10.1016/0027-5107(91)90220-i. [DOI] [PubMed] [Google Scholar]
- Menghini R. Genotoxicity of active oxygen species in mammalian cells. Mutat Res. 1988 May;195(3):215–230. [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive P. L. DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen. 1988;11(4):487–495. doi: 10.1002/em.2850110409. [DOI] [PubMed] [Google Scholar]
- Raaphorst G. P., Law P., Kruuv J. Water content and spin lattice relaxation times of cultured mammalian cells subjected to various salt, sucrose, or DMSO solutions. Physiol Chem Phys. 1978;10(2):177–191. [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Richardson D. R., Baker E. Intermediate steps in cellular iron uptake from transferrin. Detection of a cytoplasmic pool of iron, free of transferrin. J Biol Chem. 1992 Oct 25;267(30):21384–21389. [PubMed] [Google Scholar]
- Rothman R. J., Serroni A., Farber J. L. Cellular pool of transient ferric iron, chelatable by deferoxamine and distinct from ferritin, that is involved in oxidative cell injury. Mol Pharmacol. 1992 Oct;42(4):703–710. [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Sugiyama M., Tsuzuki K., Ogura R. Effect of ascorbic acid on DNA damage, cytotoxicity, glutathione reductase, and formation of paramagnetic chromium in Chinese hamster V-79 cells treated with sodium chromate(VI). J Biol Chem. 1991 Feb 25;266(6):3383–3386. [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. The role of transferrin in the mechanism of cellular iron uptake. Biochem J. 1990 Oct 1;271(1):1–9. doi: 10.1042/bj2710001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. F., Blakely W. F., Joner E. I. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985 Sep;103(3):383–392. [PubMed] [Google Scholar]
- Ward J. F., Evans J. W., Limoli C. L., Calabro-Jones P. M. Radiation and hydrogen peroxide induced free radical damage to DNA. Br J Cancer Suppl. 1987 Jun;8:105–112. [PMC free article] [PubMed] [Google Scholar]
- Watkins J. A., Altazan J. D., Elder P., Li C. Y., Nunez M. T., Cui X. X., Glass J. Kinetic characterization of reductant dependent processes of iron mobilization from endocytic vesicles. Biochemistry. 1992 Jun 30;31(25):5820–5830. doi: 10.1021/bi00140a018. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem J. 1979 Aug 15;182(2):625–628. doi: 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Superoxide as an intracellular radical sink. Free Radic Biol Med. 1993 Jan;14(1):85–90. doi: 10.1016/0891-5849(93)90512-s. [DOI] [PubMed] [Google Scholar]
- de Mello Filho A. C., Meneghini R. Protection of mammalian cells by o-phenanthroline from lethal and DNA-damaging effects produced by active oxygen species. Biochim Biophys Acta. 1985 Oct 30;847(1):82–89. doi: 10.1016/0167-4889(85)90156-9. [DOI] [PubMed] [Google Scholar]


