Abstract
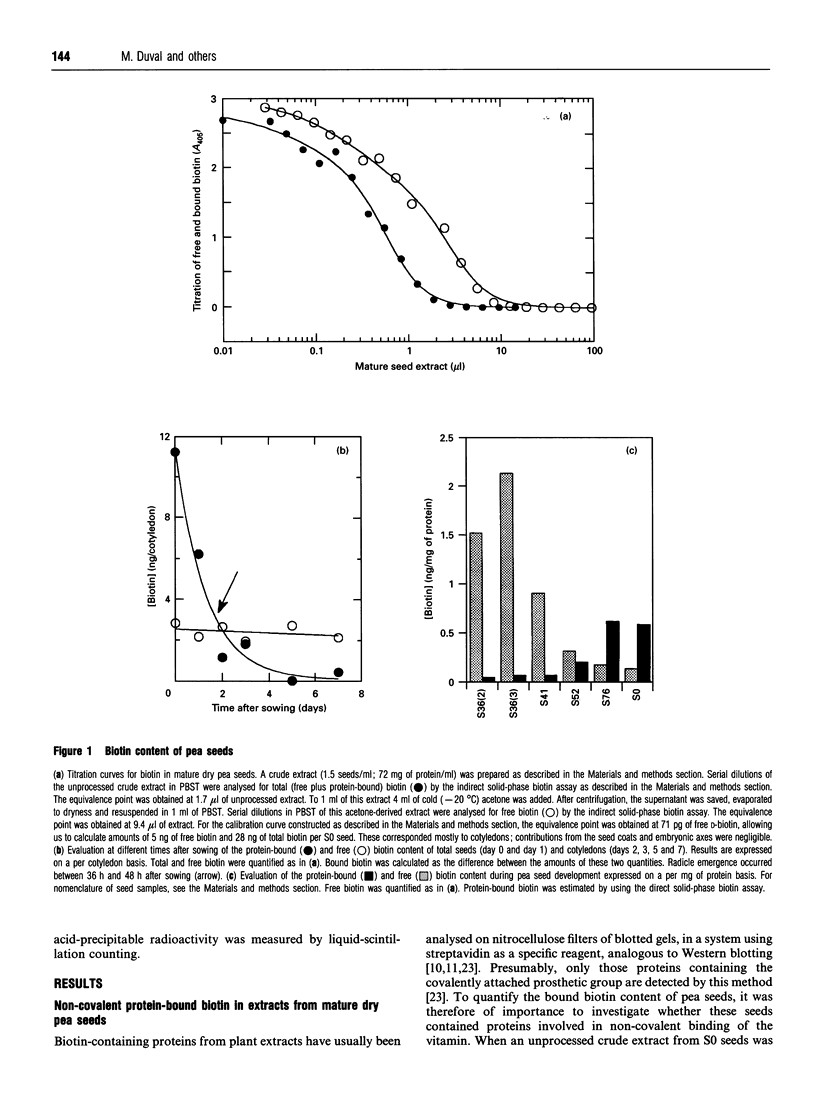
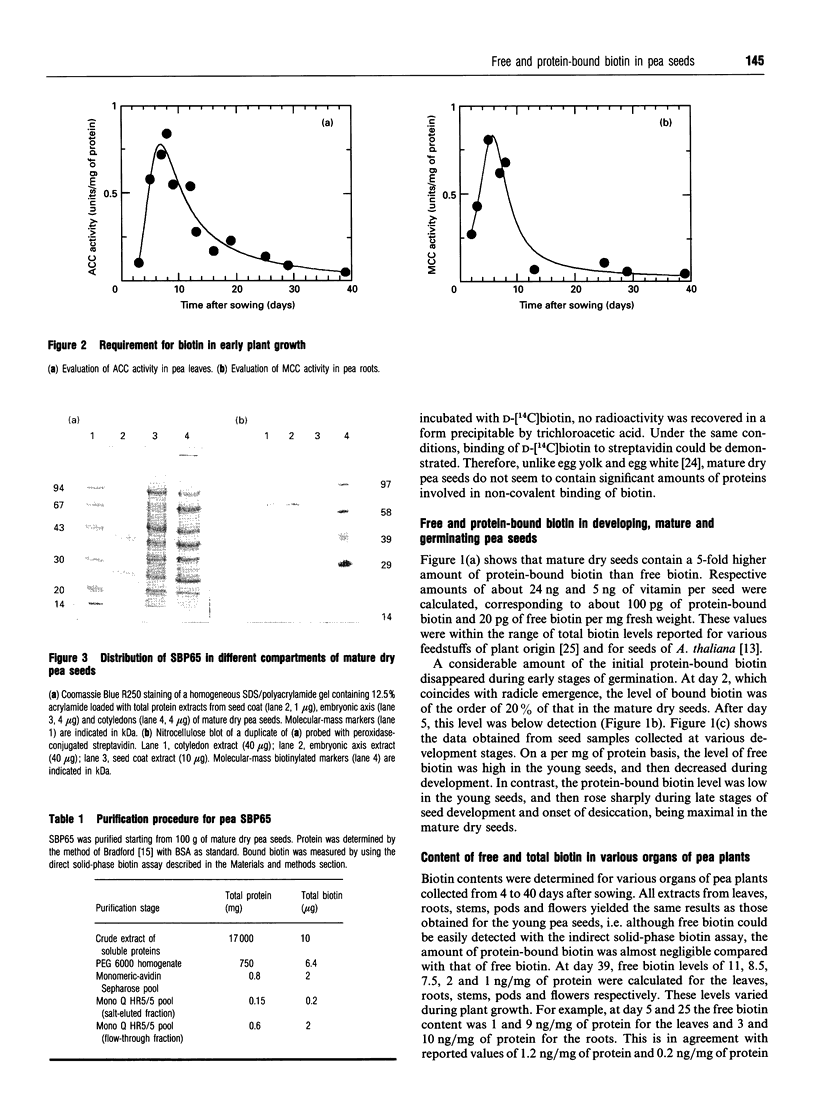
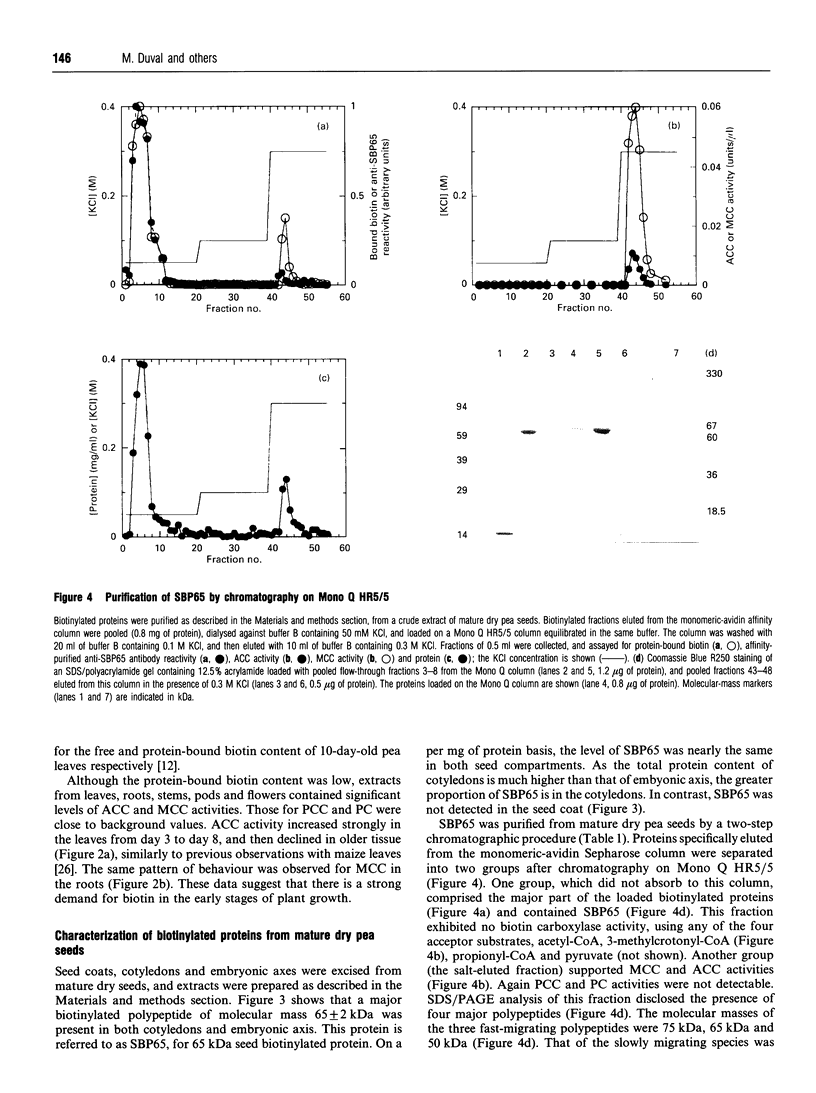
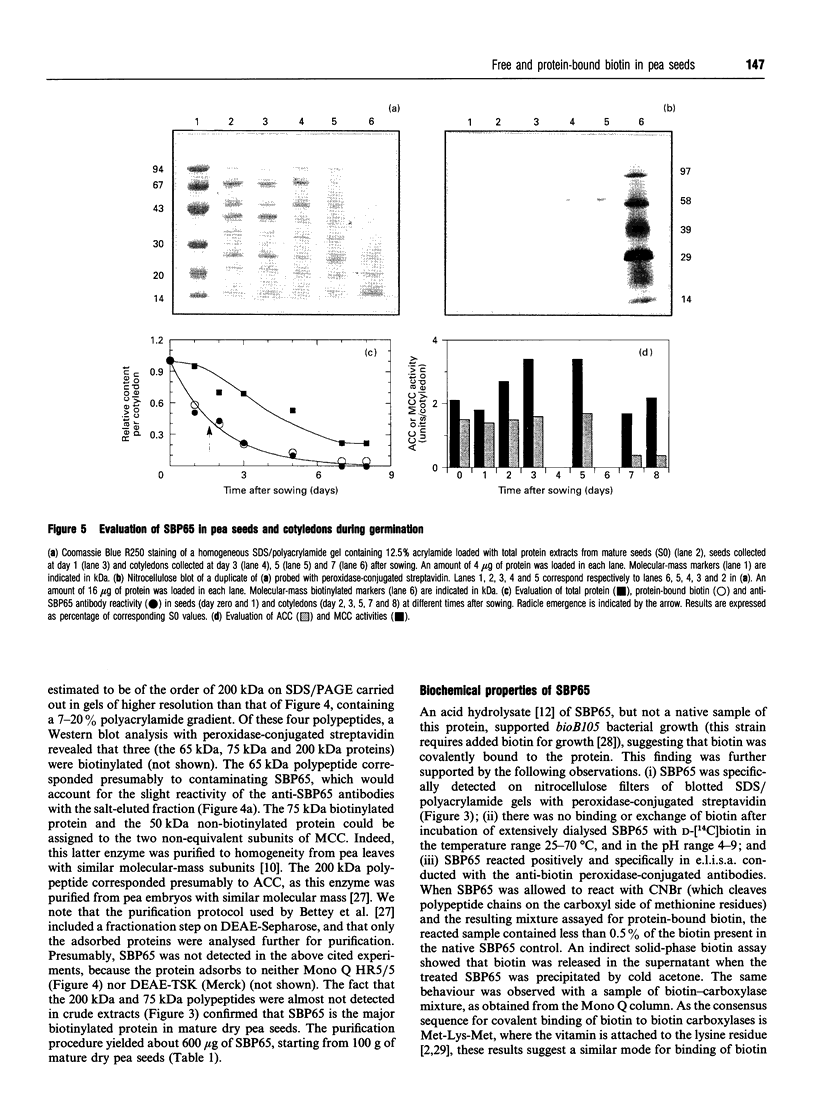
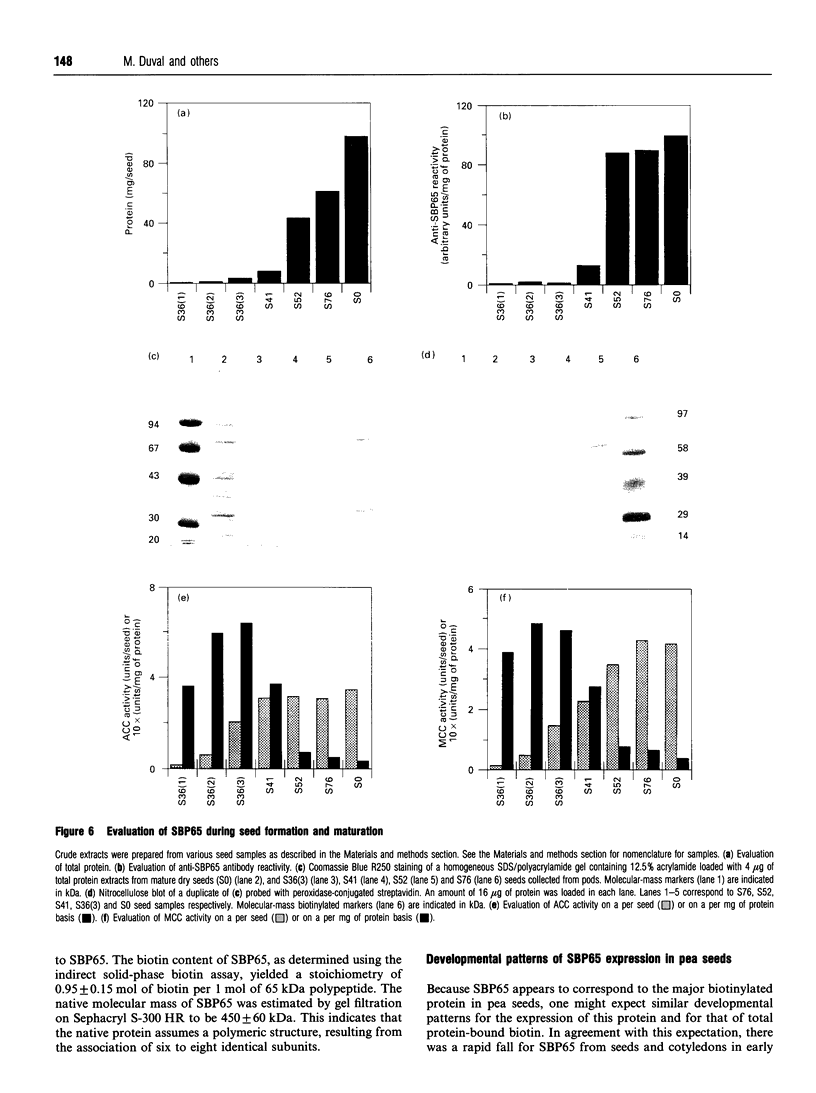
Mature dry pea seeds contain three major biotinylated proteins. Two of these of subunit molecular mass about 75 kDa and 200 kDa are associated with 3-methylcrotonyl-CoA carboxylase (EC 6.4.1.4) and acetyl-CoA carboxylase activities (EC 6.4.1.2) respectively. The third does not exhibit any of the biotin-dependent carboxylase activities found in higher organisms and represents the major part of the total protein-bound biotin in the seeds. This novel protein has been purified from a whole pea seed extract. Because in SDS/polyacrylamide gels the protein migrates with an apparent molecular mass of about 65 kDa, it is referred to as SBP65, for 65 kDa seed biotinylated protein. The molecular mass of native SBP65 is greater than 400 kDa, suggesting that the native protein assumes a polymeric structure, resulting from the association of six to eight identical subunits. The results of CNBr cleavage experiments suggest that biotin is covalently bound to the protein. The stoichiometry is 1 mol of biotin per 1 mol of 65 kDa polypeptide. The temporal and spatial pattern of expression of SBP65 is described. SBP65 is specifically expressed in the seeds, being absent from leaf, root, stem, pod and flower tissues of pea plants. The level of SBP65 increases dramatically during seed development. The protein is not detectable in very young seeds. Its accumulation pattern parallels that for storage proteins, being maximally expressed in the mature dry seeds. SBP65 disappears at a very high rate during seed germination. The level of free biotin has also been evaluated for various organs of pea plants. In all proliferating tissues examined (young developing seeds, leaf, root, stem, pod and flower tissues), free biotin is in excess of protein-bound biotin. Only in the mature dry seeds is protein-bound biotin (i.e. that bound to SBP65) in excess of free biotin. These temporal expression patterns, and the strict organ specificity for expression of SBP65, are discussed with regard to the possibility that in plants, as in mammals, biotin plays a specialized role in cell growth and differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Feel W., Chirala S. S., Wakil S. J. Cloning of the yeast FAS3 gene and primary structure of yeast acetyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4534–4538. doi: 10.1073/pnas.89.10.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alban C., Baldet P., Axiotis S., Douce R. Purification and Characterization of 3-Methylcrotonyl-Coenzyme A Carboxylase from Higher Plant Mitochondria. Plant Physiol. 1993 Jul;102(3):957–965. doi: 10.1104/pp.102.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldet P., Alban C., Axiotis S., Douce R. Localization of free and bound biotin in cells from green pea leaves. Arch Biochem Biophys. 1993 May 15;303(1):67–73. doi: 10.1006/abbi.1993.1256. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Ben-Hur H., Wilchek M. Colorimetric enzyme assays for avidin and biotin. Methods Enzymol. 1990;184:217–223. doi: 10.1016/0076-6879(90)84277-n. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burton J. D., Gronwald J. W., Somers D. A., Connelly J. A., Gengenbach B. G., Wyse D. L. Inhibition of plant acetyl-coenzyme A carboxylase by the herbicides sethoxydim and haloxyfop. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1039–1044. doi: 10.1016/s0006-291x(87)80236-x. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K., Chauhan J. Biotin. Vitam Horm. 1989;45:337–384. doi: 10.1016/s0083-6729(08)60398-2. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K., Chauhan J. Nonavidin biotin-binding proteins. Methods Enzymol. 1990;184:93–102. doi: 10.1016/0076-6879(90)84264-h. [DOI] [PubMed] [Google Scholar]
- Eisenberg M. A. Mode of action of alpha-dehydrobiotin, a biotin analogue. J Bacteriol. 1975 Jul;123(1):248–254. doi: 10.1128/jb.123.1.248-254.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Avidin and streptavidin. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- Henrikson K. P., Allen S. H., Maloy W. L. An avidin monomer affinity column for the purification of biotin-containing enzymes. Anal Biochem. 1979 Apr 15;94(2):366–370. doi: 10.1016/0003-2697(79)90374-9. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. The mechanism of biotin-dependent enzymes. Annu Rev Biochem. 1989;58:195–221. doi: 10.1146/annurev.bi.58.070189.001211. [DOI] [PubMed] [Google Scholar]
- Kohanski R. A., Lane M. D. Homogeneous functional insulin receptor from 3T3-L1 adipocytes. Purification using N alpha B1-(biotinyl-epsilon-aminocaproyl)insulin and avidin-sepharose. J Biol Chem. 1985 Apr 25;260(8):5014–5025. [PubMed] [Google Scholar]
- Kohanski R. A., Lane M. D. Monovalent avidin affinity columns. Methods Enzymol. 1990;184:194–200. doi: 10.1016/0076-6879(90)84274-k. [DOI] [PubMed] [Google Scholar]
- Kondo H., Shiratsuchi K., Yoshimoto T., Masuda T., Kitazono A., Tsuru D., Anai M., Sekiguchi M., Tanabe T. Acetyl-CoA carboxylase from Escherichia coli: gene organization and nucleotide sequence of the biotin carboxylase subunit. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9730–9733. doi: 10.1073/pnas.88.21.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. J., Rock C. O., Cronan J. E., Jr The dedB (usg) open reading frame of Escherichia coli encodes a subunit of acetyl-coenzyme A carboxylase. J Bacteriol. 1992 Sep;174(17):5755–5757. doi: 10.1128/jb.174.17.5755-5757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meslar H. W., Camper S. A., White H. B., 3rd Biotin-binding protein from egg yolk. A protein distinct from egg white avidin. J Biol Chem. 1978 Oct 10;253(19):6979–6982. [PubMed] [Google Scholar]
- Nikolau B. J., Wurtele E. S., Stumpf P. K. Use of streptavidin to detect biotin-containing proteins in plants. Anal Biochem. 1985 Sep;149(2):448–453. doi: 10.1016/0003-2697(85)90596-2. [DOI] [PubMed] [Google Scholar]
- Sakakibara H., Watanabe M., Hase T., Sugiyama T. Molecular cloning and characterization of complementary DNA encoding for ferredoxin-dependent glutamate synthase in maize leaf. J Biol Chem. 1991 Feb 5;266(4):2028–2035. [PubMed] [Google Scholar]
- Samols D., Thornton C. G., Murtif V. L., Kumar G. K., Haase F. C., Wood H. G. Evolutionary conservation among biotin enzymes. J Biol Chem. 1988 May 15;263(14):6461–6464. [PubMed] [Google Scholar]
- Scheiner J., De Ritter E. Biotin content of feedstuffs. J Agric Food Chem. 1975 Nov-Dec;23(6):1157–1162. doi: 10.1021/jf60202a039. [DOI] [PubMed] [Google Scholar]
- Schneider T., Dinkins R., Robinson K., Shellhammer J., Meinke D. W. An embryo-lethal mutant of Arabidopsis thaliana is a biotin auxotroph. Dev Biol. 1989 Jan;131(1):161–167. doi: 10.1016/s0012-1606(89)80047-8. [DOI] [PubMed] [Google Scholar]
- Shellhammer J., Meinke D. Arrested Embryos from the bio1 Auxotroph of Arabidopsis thaliana Contain Reduced Levels of Biotin. Plant Physiol. 1990 Jul;93(3):1162–1167. doi: 10.1104/pp.93.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D. A., Keith R. A., Egli M. A., Marshall L. C., Gengenbach B. G., Gronwald J. W., Wyse D. L. Expression of the Acc1 Gene-Encoded Acetyl-Coenzyme A Carboxylase in Developing Maize (Zea mays L.) Kernels. Plant Physiol. 1993 Mar;101(3):1097–1101. doi: 10.1104/pp.101.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtele E. S., Nikolau B. J. Differential Accumulation of Biotin Enzymes during Carrot Somatic Embryogenesis. Plant Physiol. 1992 Aug;99(4):1699–1703. doi: 10.1104/pp.99.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtele E. S., Nikolau B. J. Plants contain multiple biotin enzymes: discovery of 3-methylcrotonyl-CoA carboxylase, propionyl-CoA carboxylase and pyruvate carboxylase in the plant kingdom. Arch Biochem Biophys. 1990 Apr;278(1):179–186. doi: 10.1016/0003-9861(90)90246-u. [DOI] [PubMed] [Google Scholar]