Abstract
Most acquired and inherited cardiomyopathies are characterized by regional left ventricular involvement and nonischemic myocardial scars, often with a disease-specific pattern. Irrespective of the etiology and pathophysiological mechanisms, myocardial disorders are invariably associated with cardiac fibrosis, which contributes to dysfunction and electrical instability. Accordingly, cardiac magnetic resonance plays a central role in the diagnostic work-up and prognostic risk stratification of cardiomyopathies, particularly with the increasing correlation between genetic background and specific disease phenotype. Starting from pattern and distribution of myocardial fibrosis at cardiac magnetic resonance, we provide a practical regional atlas of nonischemic myocardial scar to guide the diagnostic approach to nonischemic cardiomyopathies.
Key words: cardiomyopathies, disease-specific regional distribution, left ventricular scar, pattern of myocardial fibrosis
Central Illustration
Highlights
-
•
Presence of LV scars does not necessarily reflect coronary artery disease.
-
•
Regional LV scars can be seen in different myocardial disorders, often with typical distribution.
-
•
Clinical data combined with localization of LV scars is essential in raising etiological suspicion/diagnosis.
-
•
Cardiac magnetic resonance is the new gold standard for diagnosis and risk stratification of scars.
Up to 40% of patients referred to coronary angiography for chest pain, wall motion abnormalities, or evidence of structural heart disease in presence of cardiovascular risk factors have no evidence of significant coronary artery disease.1In a significant subset, cardiac magnetic resonance (CMR) is performed as part of the diagnostic work-up of several clinical scenarios including chest pain with normal coronary arteries, cardiomyopathies, or unexplained ventricular arrhythmias when a cardiomyopathy has not yet been suspected. The identification of a nonischemic left ventricular (LV) scar represents a significant challenge for cardiologists due to the need for differential diagnosis among a wide range of diseases, with major diagnostic, therapeutic, and prognostic implications for patients and their families. Myocardial fibrosis is a structural lesion resulting from several different damage mechanisms. Besides myocardial ischemia and necrosis, regional LV scars may be caused by direct or indirect damage affecting cardiomyocytes, the interstitium, and the microvasculature.2 Indeed, the recently issued European Society of Cardiology guidelines for the management of cardiomyopathies 3 emphasized the importance of scar assessment by CMR in defining the phenotype of cardiomyopathies and in guiding further diagnostic tests and therapeutic strategies. Of note, a new cardiomyopathy phenotype named “nondilated left ventricular cardiomyopathy,” defined as the presence of nonischemic LV scarring or fatty replacement regardless of the presence of global or regional wall motion abnormalities, or isolated global LV hypokinesia without scarring, has been introduced.
This paper reviews the pathogenesis of myocardial scar and provides an overview of value of location and distribution of LV scar patterns for diagnosis of cardiomyopathies, classified according to their distribution within the main coronary territories, ie, the 17 LV segments grouped into anterior, septal, apical, lateral, and inferior regions, and the well-known ring-like pattern remaining distinct (Central Illustration); moreover, a distribution of the most frequent genes involved per segment is proposed when the differential diagnosis focuses on inherited cardiomyopathies (Figure 1).
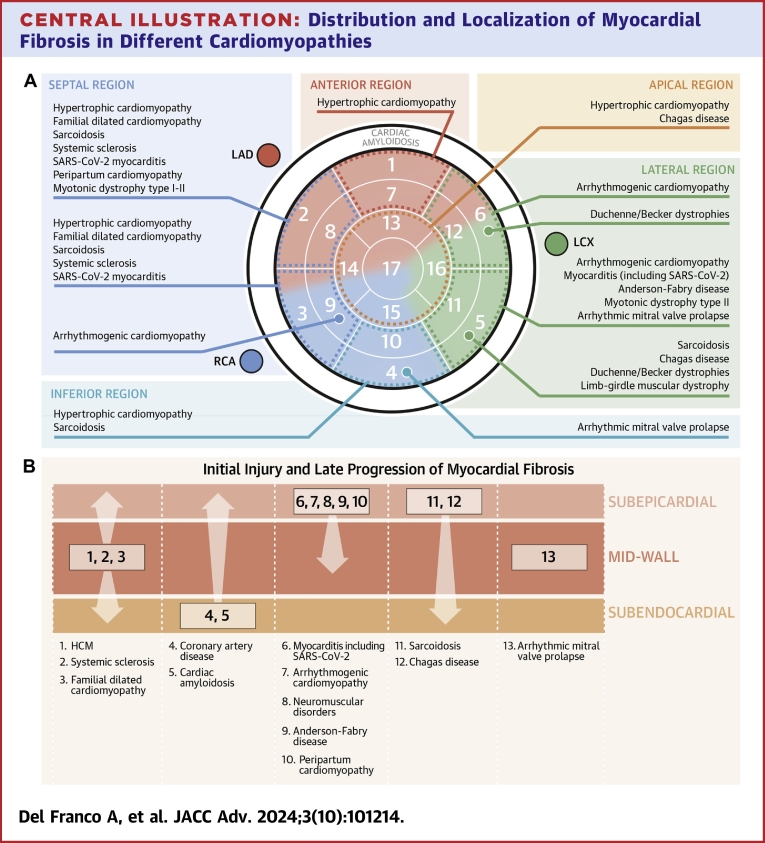
Central Illustration.
Distribution and Localization of Myocardial Fibrosis in Different Cardiomyopathies
(A) List of diseases follow a hierarchical downward prevalence sequence. Cardiac amyloidosis, which has no specific distribution, is a distinctive case. Left ventricle is divided into five regions: anterior, septal, apical, lateral, and inferior. The 17 segments are: basal anterior (1), basal anteroseptal (2), inferoseptal (3), basal inferior (4), basal inferolateral (5), basal anterolateral (6), mid anterior (7), mid anteroseptal (8), mid inferoseptal (9), mid inferior (10), mid inferolateral (11), mid anterolateral (12), apical anterior (13), apical septal (14), apical inferior (15), apical lateral (16), and apex (17). (B) Location of myocardial fibrosis from the layer that is initially involved and damage progression. HCM = hypertrophic cardiomyopathy; LAD = left anterior descending artery; LCX = left circumflex artery; RCA = right coronary artery.
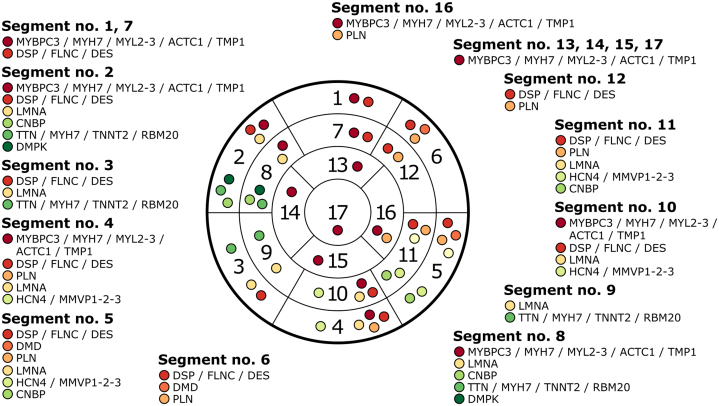
Figure 1.
Gene Map Displaying Distinct Expression Patterns for Each Left Ventricular Segments Affected by a Nonischemic Scar
ACTC1 = actin alpha cardiac muscle 1; CNBP = CCHC-type zinc finger nucleic acid binding protein; DES = desmin; DMD = dystrophin; DMPK = DM1 protein kinase; DSP = desmoplakin; FLNC = filamin C; HCN4 = hyperpolarization activated cyclic nucleotide gated potassium channel 4; LMNA = lamin A/C; MMVP1-2-3 = myxomatous mitral valve prolapse 1-2-3; MYBPC3 = myosin binding protein C3; MYH7 = myosin heavy chain 7; MYL2-3 = myosin regulatory light chain 2 to 3; PLN = phospholamban; RBM20 = RNA binding motif protein 20; TMP1 = tropomyosin 1; TNNI3 = cardiac troponin I3; TNNT2 = cardiac troponin T2; TTN = titin.
Pathogenesis of regional left ventricular scar
Myocardial fibrosis is characterized by an excessive deposition that may occur with two different modalities, reflecting different pathophysiological mechanisms: interstitial fibrosis, which reflects abnormal superactivation of the matrix and represents diffuse process; and replacement fibrosis following tissue damage, ie, the scar, which is typically regional.4
Replacement fibrosis is most frequently observed after an ischemic insult in patients with coronary artery disease but may also occur as a result of pressure or volume overload, genetic cardiomyopathies (eg, hypertrophic cardiomyopathy [HCM]), or inflammatory disease (eg, cardiac sarcoidosis, myocarditis).4 Genetic mechanisms and superimposed inflammation may often co-exist, as in the case of arrhythmogenic cardiomyopathy or Anderson-Fabry disease.
The distinction between replacement and interstitial fibrosis, however, is not clear-cut, as the two phenomena may overlap. LV scars represent the irreversible end-stage results of severe disease processes leading to cell death. Following a genetic, inflammatory, toxic, or metabolic damage mechanisms leading to myocardial cell death, the local tissue response includes a sequence of activation of inflammation, cell damage, and repair.5 As such, the histological and imaging features are qualitatively indistinguishable in LV scars, while the regional and transmural distribution may be specific for individual diseases.
Although histological analysis remains the gold standard for confirming the presence of myocardial fibrosis, several studies have demonstrated histological validation of CMR parameters for its assessment.6 A technique known as late gadolinium enhancement (LGE) uses the deposition of CMR contrast in the extracellular space, which is enlarged due to the loss of myocytes or deposition, to identify myocardial scar.4 In stable chronic conditions and in the absence of edema, LGE is considered an accurate proxy of scar evaluation in vivo and has entered routine clinical practice to aid in differential diagnoses and assess disease progression in cardiomyopathies.
Anterior Region
Scar in the anterior region of the LV typically affecting basal and mid segments is frequently observed in patients with HCM, the most prevalent genetic cardiovascular disorder,7 caused in 60% of cases by mutations in sarcomere protein genes (being myosin heavy chain and myosin binding protein 3 the most frequent).8 HCM is characterized by asymmetric LV wall hypertrophy, myocardial hypercontractility, diastolic dysfunction, and dynamic left ventricular outflow tract obstruction.9 Mitral valve morphological abnormalities are a key phenotypic manifestation of HCM; elongated mitral leaflets are a primary contributor to dynamic LV outflow tract obstruction (together with a small outflow tract dimension).10 Although electrocardiograms (ECGs) may appear normal in 4% to 6% of HCM adult patients, several patterns are HCM distinctive, such as pathological Q waves, giant symmetric negative T waves in the precordial leads in apical HCM, and pseudo-ST-elevation myocardial infarction (pseudo-STEMI) pattern with inverted T waves in DI and aVL.11 While generally considered a disease of the interventricular septum, anterior wall involvement is almost as constant, and the basal anterior segment is the most hypertrophied region.12 Fibrosis is detected as LGE by CMR in over 50% of patients and most frequently occurs at the mid-wall of the hypertrophied segments but may be transmural (Figure 2) and involve the right ventricular intersection sites with the LV (Table 1). LGE is inhomogeneous and asymmetrically distributed, preferentially involving the interventricular septal wall and anterior free wall at the basal- and mid-level but may expand to the apex.13 Further, fibrosis deposition might begin in the mid-wall and spread to the transmural distribution.14,15 Notably, patients with a positive genotype have more myocardial fibrosis than those who are genotype-negative. Extensive areas of replacement fibrosis (>15% of the whole LV) purport adverse prognostic significance in terms of risk of sudden cardiac death (SCD) and disease progression.14,15
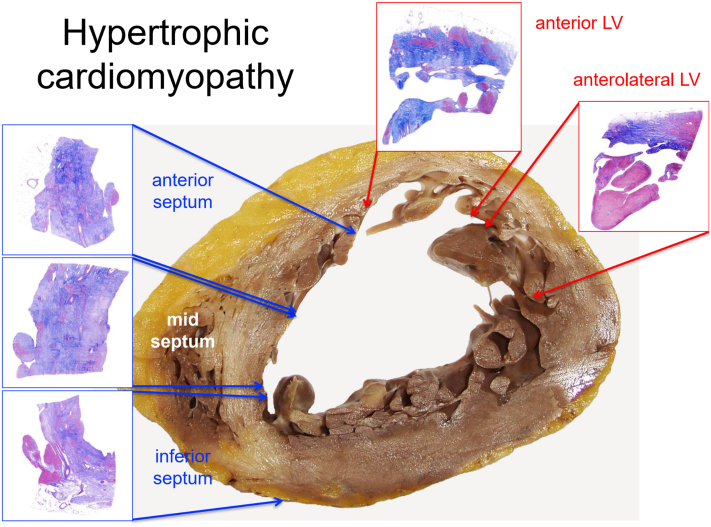
Figure 2.
Histological Findings in End-Stage Hypertrophic Cardiomyopathy
Transverse section at the mid-third of the native explanted heart of a 51-year-old male. Macroscopy and histology (scanned slides, Azan Mallory Trichrome) show transmural replacement fibrosis in the septum and anterolateral left ventricular wall. LV = left ventricle.
Table 1.
Left Ventricular Scars in the Anterior Region With Specific Disease Characteristics
| LV Scar | Distribution | Phenotype | Red Flags | Etiologies | Further Investigation | |
|---|---|---|---|---|---|---|
| Anterior Region | Mid-wall/Transmural | HCM | CLINICAL: |
|
Sarcomeric HCM | Genetic testing |
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
HCM = hypertrophic cardiomyopathy; LVH = left ventricular hypertrophy; SAM = systolic anterior motion; SCD = sudden cardiac death; STEMI = ST-segment elevation myocardial infarction.
Septal region
Septal fibrosis may result from a wide spectrum of conditions with heterogeneous etiologies and clinical manifestations including HCM,13 dilated cardiomyopathy (DCM), sarcoidosis, systemic sclerosis, and myotonic dystrophies (Table 2).16
Table 2.
Left Ventricular Scars in the Septal Region With Specific Disease Characteristics
| LV Scar | Distribution | Phenotype | Red Flags | Etiologies | Further Investigation | |
|---|---|---|---|---|---|---|
| Septal region | Mid-wall/transmural | NDLVC/DCM | CLINICAL: |
|
Familial DCM | Genetic testing |
| ECG: |
|
|||||
| ECHO: |
|
|||||
| RCM | CLINICAL: |
|
Systemic Sclerosis | Antinuclear antibody testing | ||
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
| HCM | CLINICAL: |
|
Sarcomeric HCM | Genetic testing | ||
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
| Subepicardial/mid-wall | NDLVC/DCM/ARVC | CLINICAL: |
|
Arrhythmogenic cardiomyopathy | Genetic testing | |
| ECG: |
|
|||||
|
||||||
| ECHO: |
|
|||||
| NDLVC | CLINICAL: |
|
Neuromuscular disorders | Genetic testing Muscle biopsya |
||
| ECG: |
|
|||||
| ECHO: |
|
|||||
| NDLVC/DCM | CLINICAL: |
|
Peripartum Cardiomyopathy | |||
| ECG: |
|
|||||
| ECHO: |
|
|||||
| NDLVC/DCM | CLINICAL: |
|
SARS-CoV-2 myocarditis | Molecular viral test | ||
| ECG: |
|
|||||
| ECHO: |
|
|||||
| Subepicardial/transmural | DCM/HCM | CLINICAL: |
|
Sarcoidosis | Thoracic FDG-PET imaging | |
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
|
||||||
ARVC = arrhythmogenic right ventricular cardiomyopathy; AV = atrioventricular; DCM = dilated cardiomyopathy; FDG-PET = fluorodeoxyglucose positron emission tomography; HCM = hypertrophic cardiomyopathy; GLS = global longitudinal strain; LBBB = left bundle branch block; LGE = late gadolinium enhancement; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; NDLVC = nondilated left ventricular cardiomyopathy; PH = pulmonary hypertension; RBBB = right bundle branch block; RCM = restrictive cardiomyopathy; RV = right ventricular; SAM = systolic anterior motion; SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2; SCD = sudden cardiac death; STEMI = ST-segment elevation myocardial infarction.
Indicated when genetic testing is negative and clinical suspicion remains high.
Septal involvement is a frequent finding also in patients with DCM phenotype,17 particularly in individuals with familial forms linked to titin, troponin T, myosin light chain 7, and lamin A/C gene mutations,18 the latter accounting for 10% of familial DCM. Cardiac involvement in laminopathies is characterized by a progressive form of DCM with remarkable electrical instability, often preceding structural abnormalities. Specifically, typical ECG abnormalities such as atrioventricular block, septal remodeling evidenced by Q waves in V1-V2, and fragmented QRS in at least two adjacent leads can be recognized.19 Global longitudinal strain and ejection fraction apart, early alterations in septal speckle tracking longitudinal strain are associated with increased mechanical dispersion, which in turn is associated with a greater burden of ventricular arrhythmia.20 Likewise, extensive mid-wall fibrosis in the septal region can be seen even when LV dilatation and/or dysfunction are absent (Figure 3).18,20 Not surprisingly, the presence of LGE represents a strong and independent predictor of major adverse arrhythmic cardiac events occurring on an intraventricular macroreentry basis.21,22
Figure 3.
Laminopathy
(A) A 12-lead electrocardiogram showing a slight intraventricular conduction disturbance and an occasional premature ventricular beat. (B) Ventricular tachycardia with basal septum exit demonstrated by right bundle morphology in V1 with positive concordance. (C) Two-dimensional echocardiography speckle tracking analysis highlighting the septal impairment. (D) Short-axis CMR image showing mid-wall and linear enhancement of the basal septum. CMR = cardiac magnetic resonance.
Of note, mid-wall septal LGE has been observed in patients with a previous acute myocarditis23 including severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection, and is associated with a worse outcome.
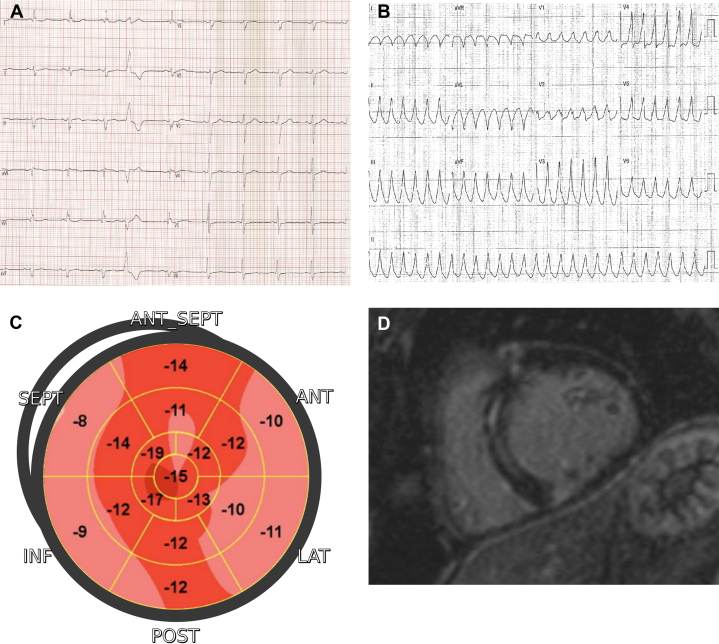
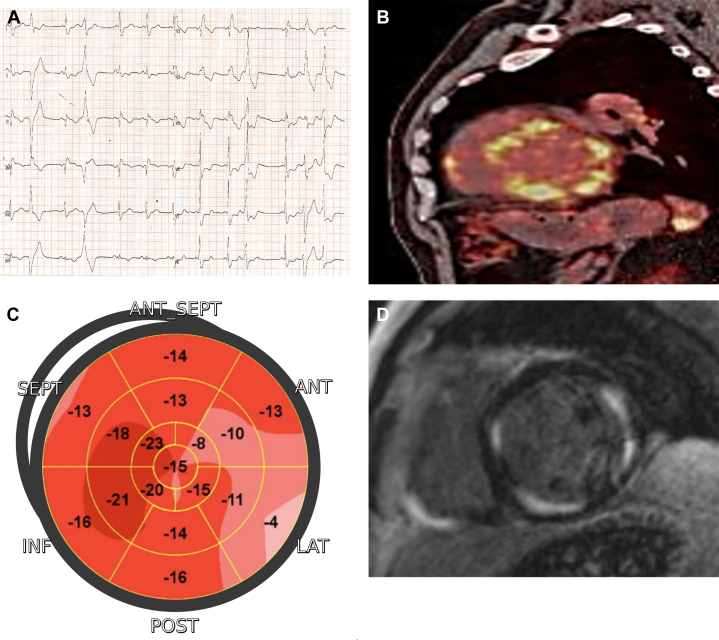
Cardiac sarcoidosis should be considered in young and middle-aged patients who present with unexplained ventricular tachycardia or high-degree atrioventricular block associated even with LV hypertrophy.24 Postmortem and imaging studies have demonstrated unrecognized myocardial involvement in up to 54% of patients with systemic sarcoidosis, with differences related to different imaging techniques and ethnicity.25 Common ECG abnormalities include PR prolongation, advanced atrioventricular blocks, and bundle branch blocks. Although echocardiography is not sensitive enough to identify mild or minor localized abnormalities, the most common observation is basal septal thinning with hyperechogenicity. Additional anomalies include abnormal wall motions, right ventricular dysfunction in absence of pulmonary hypertension, aneurysm formation, and LV wall thickening.26 Myocardial scar involvement usually presents as subepicardial to transmural fibrosis, mainly located in the interventricular septum and the inferior/inferolateral wall with a patchy distribution27 (Figures 4 and 5). Given that detection of LGE is unable to distinguish between acute and chronic processes, T1 mapping proved to be a more effective method of monitoring disease activity and response to immunosuppressive drugs than LGE alone.28
Figure 4.
Cardiac Sarcoidosis
(A) A 12-lead electrocardiogram showing an intraventricular conduction disturbance and polymorphic ventricular ectopic beats. (B) The 18-FDG myocardial PET demonstrating patchy/diffuse hypermetabolic activity. (C) Two-dimensional echocardiography speckle tracking analysis highlighting the impairment of the lateral wall. (D) Short-axis CMR image showing multifocal patchy enhancement in the inferoseptum and anterolateral and inferior LV walls. Notice how the enhancement correlates to segments with high metabolic activity. CMR = cardiac magnetic resonance; FDG = fluorodeoxyglucose; PET = positron emission tomography.
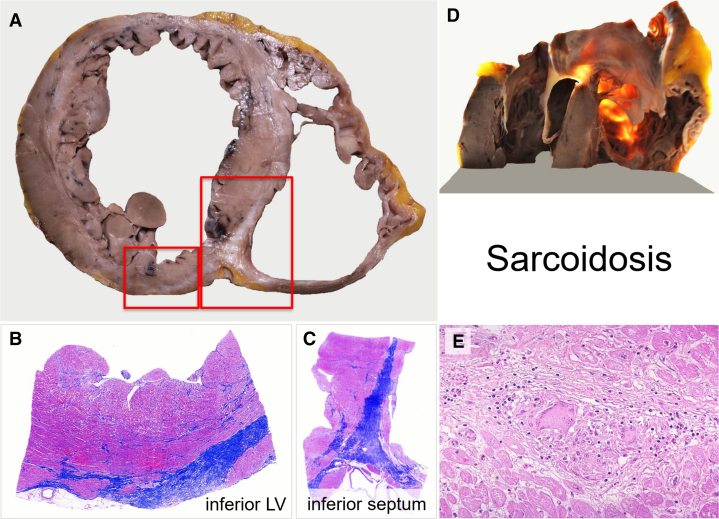
Figure 5.
Histological Findings in Cardiac Sarcoidosis
(A to C) Native explanted heart of a 35-year-old male showing marked biventricular dilatation and thinning of anterior/inferior ventricular walls and septum. Both macroscopic transverse and histologic sections show extensive epicardial-mid-wall replacement fibrosis in LV inferior wall and inferoseptum (scanned slides, Azan Mallory Trichrome). In D, transillumination highlights the ectasia of the right ventricular outflow with almost no myocardium. E shows a typical compact noncaseating granuloma with giant cells (hematoxylin-eosin, 400x). LV = left ventricle
In patients with systemic sclerosis, myocardial fibrosis is an early warning sign of cardiac involvement, may be seen in up to 80% of cases29 and is mainly detected in the septal region at the basal and mid-segments with a mid-wall linear or nodular patchy pattern.30 In this context, fibrosis is thought to result from ischemia-reperfusion damage, microvascular dysfunction, and myocardial inflammation.31 Despite preserved LV systolic function or only minor signs (like prolongation of QT interval/dispersion and increased LV filling pressure), an extensive quota of myocardial fibrosis has a detrimental impact on prognosis.32
Peripartum cardiomyopathy is a rare and potentially life-threatening condition. It is defined as the development of new-onset cardiomyopathy (LV ejection fraction <45% and without an identifiable cause of heart failure) during the peripartum episode, with an incidence ranging from 1/1,000 to 1/4,000 deliveries.33 Several studies reported different prevalence rates for chronic LGE in these patients, with recent data asserting a prevalence of about 10%.33 Its distribution with subepicardial or mid-wall pattern is usually linked to regional wall motion abnormalities and predominantly affects the anteroseptal and basal to midventricular regions. Detection of LGE at CMR could be often correlated with extensive disease involving both ventricles and a poor prognosis.34 Therefore, monitoring LGE is essential for the long-term management of peripartum cardiomyopathy and the prevention of detrimental consequences.
Apical region
Causes of nonischemic apical scarring include apical HCM and Chagas disease, both associated with a pattern that may be confined or preferentially involve the apical region (Table 3). In apical or mid-apical HCM, particularly when mid-ventricular obstruction is present, an extensive apical scar with transmural distribution may evolve into an apical aneurysm35 (Figure 6). The latter has important prognostic implications as a substrate for sustained ventricular arrhythmias.35
Table 3.
Left Ventricular Scars in the Apical Region With Specific Disease Characteristics
| LV Scar | Distribution | Phenotype | Red Flags | Etiologies | Further Investigation | |
|---|---|---|---|---|---|---|
| Apical region | Mid-wall/transmural | HCM | CLINICAL: |
|
Sarcomeric HCM | Genetic testing |
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
| Subepicardial/transmural | DCM | CLINICAL: |
|
Chagas Disease | Testing for parasite specific antibodies | |
| ECG: |
|
|||||
| ECHO: |
|
|||||
DCM = dilated cardiomyopathy; HCM = hypertrophic cardiomyopathy; LVH = left ventricular hypertrophy; SAM = systolic anterior motion; SCD = sudden cardiac death; STEMI = ST-segment elevation myocardial infarction.
Figure 6.
Apical Hypertrophic Cardiomyopathy
(A) A 12-lead electrocardiogram showing left ventricular hypertrophy and giant negative T-waves in precordial and inferolateral leads. (B) At 2D-echocardiography, the apical aneurysm becomes clearly evident in end-systolic apical 2-chamber view. (C) Speckle tracking analysis highlights the contractile impairment of the apex. (D) CMR image showing late gadolinium enhancement in the apical aneurysm. CMR = cardiac magnetic resonance.
Chagas disease is caused by the protozoan Trypanosoma Cruzi, affecting millions in endemic areas in South America and increasingly seen in the western world following migratory fluxes. The cardiac involvement is identified in 20% to 40% of patients who may present in the 2nd to 5th decade of life with DCM, conduction blocks and tachyarrhythmias, biventricular aneurysms, heart failure, thromboembolism, and SCD.36 While acute Chagas disease may show cardiac involvement in the form of a nonspecific myocarditis, chronic disease is characterized by diffuse mononuclear cell infiltration and granulomata, suggesting an exaggerated delayed immune response akin to that of tuberculosis in the lung.37 The upregulation of pro-inflammatory pathways modifies the expression of cardiac genes and proteins, causing hypertrophy, ventricular dilatation, and fibrosis that affect muscle and conduction system.38 Since Chagas disease typically affects the apical and inferolateral levels, LGE is commonly detected in these regions, with patterns ranging from subendocardial and mid-wall to subepicardial or transmural. As a result, wall motion abnormalities ranging from mild hypokinetic segments to dyskinetic ones reflect the degree of myocardial fibrosis. But a small level of LGE has also been identified in walls with normal function, supporting the utility of CMR as a screening test in individuals who have had a negative echocardiogram and even normal ECG. Finally, while extensive LGE has been associated with fatal arrhythmias, a relationship between its pattern of distribution and cardiovascular outcomes has not been clearly assessed.39
Lateral region
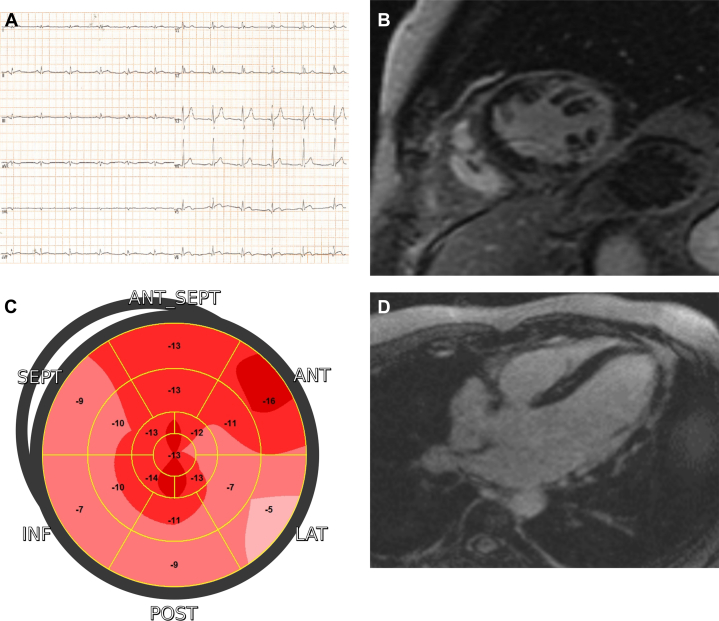
Myocarditis is one of the most common causes of scar in LV lateral wall, particularly in younger patients with acute coronary syndrome-like clinical presentation and normal coronary arteries (Figure 7, Table 4). Since no ECG or echocardiographic abnormalities are specific of myocarditis, CMR is required for a prompt diagnosis of myocardial inflammation.40 While the acute phase is characterized by myocardial edema, subepicardial/transmural LGE and thickening of involved segments, once the inflammatory process has healed, are often associated with a residual subepicardial scar. Inferolateral segments are usually involved, although localization in other segments does not rule out such diagnosis. Acute myocarditis may also evolve into inflammatory cardiomyopathy, even after a mild or clinically silent acute phase. In these cases, subepicardial scar is accompanied by persisting low-grade myocardial inflammation only detectable by T2 mapping CMR sequences or cardiac 18F-fluorodeoxyglucose positron emission tomography-computed tomography. In fact, in selected cases, endomyocardial biopsy can be indicated to guide further immunosuppressive or antiviral treatment.41
Figure 7.
Acute Myocarditis
(A) A 12-lead electrocardiogram showing inferolateral ST-segment elevation, mimicking myocardial infarction. (B) Short-axis T2-weighted CMR image showing myocardial edema in the lateral wall. (C) Two-dimensional echocardiography speckle tracking analysis highlighting reduced contractility in the same segments. (D) Short-axis CMR image showing matching fibrosis with an epi- and mid-wall distribution. CMR = cardiac magnetic resonance.
Table 4.
Left Ventricular Scars in the Lateral Region With Specific Disease Characteristics
| LV Scar | Distribution | Phenotype | Red Flags | Etiologies | Further Investigation | |
|---|---|---|---|---|---|---|
| Lateral region | Subepicardial/mid-wall | NDLVC/DCM/ARVC | CLINICAL: |
|
Arrhythmogenic cardiomyopathy | Genetic testing |
| ECG: |
|
|||||
|
||||||
| ECHO: |
|
|||||
| NDLVC/DCM | CLINICAL: |
|
Myocarditis (including SARS-CoV-2, see above) | Laboratory testsb Endomyocardial biopsyc |
||
| ECG: |
|
|||||
| ECHO: |
|
|||||
| NDLVC/DCM/HCM | CLINICAL: |
|
Neuromuscular disorders | Genetic testing Muscle biopsya |
||
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
| HCM | CLINICAL: |
|
Anderson-Fabry disease | Genetic testing for glycosphingolipid metabolism | ||
|
||||||
| ECG: |
|
|||||
|
||||||
|
||||||
| ECHO: |
|
|||||
| Subepicardial/transmural | DCM | CLINICAL: |
|
Chagas Disease | Testing for parasite specific antibodies | |
| ECG: |
|
|||||
| ECHO: |
|
|||||
| DCM/HCM | CLINICAL: |
|
Sarcoidosis | Thoracic FDG-PET imaging | ||
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
|
||||||
ARVC = arrhythmogenic right ventricular cardiomyopathy; AV = atrioventricular; DCM = dilated cardiomyopathy; ECG = electrocardiogram; FDG-PET = fluorodeoxyglucose positron emission tomography; HCM = hypertrophic cardiomyopathy; LGE = late gadolinium enhancement; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; NDLVC = nondilated left ventricular cardiomyopathy; PH = pulmonary hypertension; RBBB = right bundle branch block; RV = right ventricular; RVH = right ventricular hypertrophy; SAM = systolic anterior motion; SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2; SCD = sudden cardiac death; STEMI = ST-segment elevation myocardial infarction; TWI = T-wave inversion.
Indicated when genetic testing is negative and clinical suspicion remains high.
Including inflammatory and cardiac markers and rheumatologic screening.
In case of suspected fulminant myocarditis or acute myocarditis with acute heart failure, left ventricular dysfunction, and/or rhythm disorders; setting of immune checkpoint inhibitor therapy; acute myocarditis associated with peripheral eosinophilia.
SARS-CoV-2 infection can induce a specific type of myocarditis as a result of immune and hypercoagulability responses, with a chronic myocardial scar in up to 30% of patients who had been previously hospitalized 42 or 50% of patients who have been recovered.43 LV scars, in most cases associated with preserved biventricular systolic function, showed a unique distribution with a more involvement of the septal segments than non-SARS-CoV-2 myocarditis, beyond the basal or mid-cavity inferolateral segments.43,44 The involvement of various segments at the basal level allows the identification of a pattern named “reverse tako-tsubo” in speckle tracking.45 Moreover, it is likely that the septal involvement is the cause of LV dyssynchrony or arrhythmia.
In cases of recurrent or familial myocarditis, CMR can detect the progressive expansion of subepicardial LGE. In these patients, particularly when the clinical picture is characterized also by ventricular arrhythmias, an arrhythmogenic cardiomyopathy progressing through inflammatory hot phases should be suspected.46 Indeed, some forms of arrhythmogenic cardiomyopathy, while being caused by genetic variants affecting both desmosomal genes and others—psuch as desmoplakin, desmin, phospholamban and filamin C—may have a pathophysiology that heavily entails inflammatory pathways overlapping classic myocarditis.47,48 Arrhythmogenic cardiomyopathy affecting the left ventricle is a rare disorder with a prevalence of 1:2,000-1:5,000;48 although its true prevalence may be underestimated, it is increasing due to the widespread use of CMR. Electrical instability with ventricular arrhythmias is the most typical sign of the disease, which has been recognized as one of the leading causes of SCD in young athletes. At the onset and during its “hot phases,” arrhythmogenic cardiomyopathy may mimic acute myocarditis, presenting with episodes of chest pain, troponin release, and acute ECG modifications.49
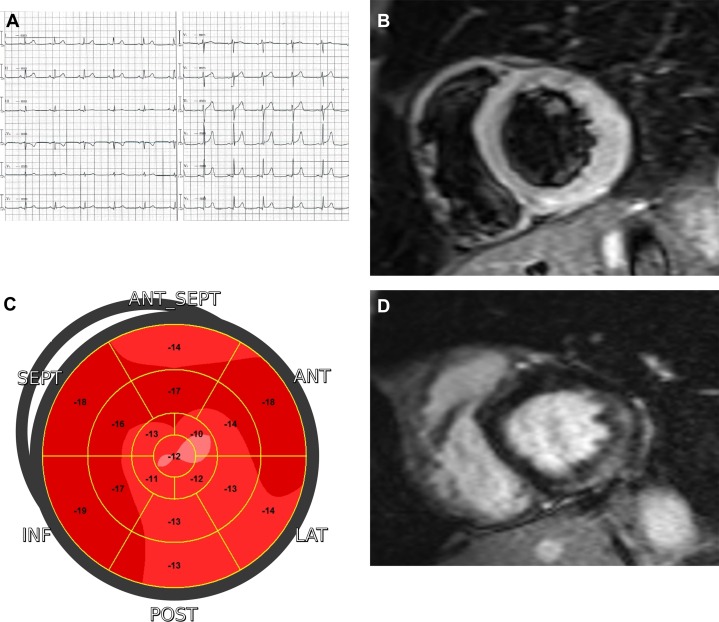
As disease progresses with a wavefront of myocardial loss and fibrofatty replacement from the epicardium to endocardium, LGE is localized at the epicardial level,50 localizing preferentially to the lateral wall (Figure 8 and 9). Although the endocardium is often spared, justifying the absence of wall motion abnormalities at echocardiography, transmural extension may occur in advanced cases. ECG abnormalities such as low-amplitude QRS complexes in the limb leads, fragmented QRS, and T-wave inversion or flattening in the lateral leads are suggestive findings. Additional features include ventricular arrhythmias with right bundle branch block morphology and superior axis not suppressed or elicited by exercise, normal or mildly depressed LV systolic function, and no or mild LV dilatation.48
Figure 8.
Desmoplakin-Related Arrhythmogenic Cardiomyopathy
(A) A 12-lead electrocardiogram showing low QRS voltages in limb leads and diphasic T-waves in the lateral leads. (B) Short-axis T2-weighted CMR image showing myocardial edema in the lateral wall. Short-axis (B) and 4-chamber (D) CMR images showing extensive late gadolinium enhancement as a stria proceeding from epicardium toward endocardium in the lateral wall with partial apical involvement. (C) Two-dimensional echocardiography speckle tracking analysis highlighting concordant regional impairment. CMR = cardiac magnetic resonance.
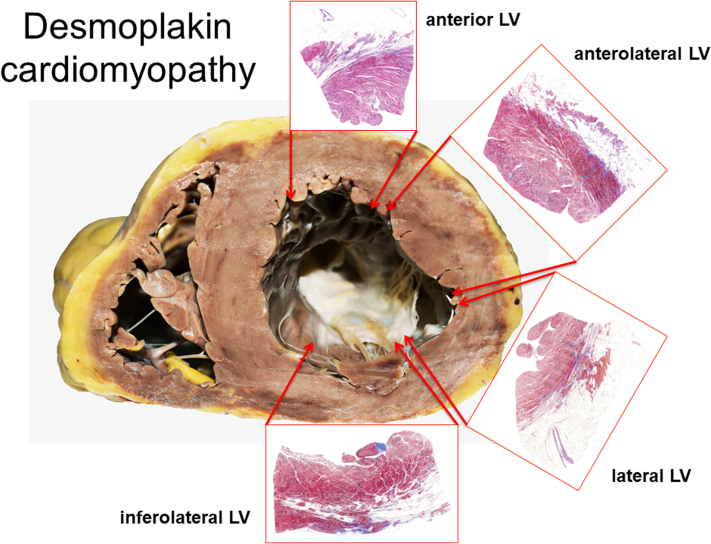
Figure 9.
Histological Findings in Desmoplakin-Related Arrhythmogenic Cardiomyopathy
Extensive/diffuse fibro-fatty replacement of the LV myocardium spreading from the epicardial toward the mid-wall layer in section from a 49-year-old man (scanned slides, Azan Mallory Trichrome). LV = left ventricle.
In patients with Anderson-Fabry disease, HCM associated with myocardial fibrosis involving the lateral region represents a classic feature.51,52 This lysosomal storage disease is marked by phenotypic variability that is both intra- and inter-family due to its multisystemic involvement, with the most affected organs being the neurological, cardiovascular, cochleovestibular, and cutaneous systems. Early signs of cardiac involvement in Anderson-Fabry disease include short PR intervals, alterations in tissue Doppler and speckle tracking imaging, and decreased native T1. Over time, these signs can progress to LV hypertrophy and fibrosis with corresponding changes in the ECG (chronotropic incompetence, bundle branch block), as well as changes in echocardiography (symmetric LV hypertrophy, right/biventricular hypertrophy, and valve thickening).53
Although LGE and inflammation appear mainly in the basal inferolateral wall, LV scars can occasionally be seen at the septal and/or apical regions; they usually have a mid-wall localization, with transmural lesions being observed in advanced stages.53 Although the disease has X-linked inheritance, female patients usually present cardiac involvement with lateral wall scar in some cases preceding the development of LV hypertrophy.53
Neuromuscular disorders should be considered as a possible alternative diagnosis, despite their rarity.54 Both in Duchenne and Becker muscular dystrophy, cardiac involvement is common and occurs even before the onset of other symptoms.55 Similarly, patients with limb girdle muscular dystrophy or myotonic dystrophies may experience severe cardiac symptoms and an increased risk of SCD.56 Cardiac dysfunction originates from an abnormal and unstable nucleotide repetition in the dystrophia myotonica protein kinase and cellular nucleic acid binding protein genes, which leads to a DCM phenotype with wall thinning and/or altered wall motions and LGE in the lateral wall, sometimes mistaken for the sequelae of myocarditis in patients with milder neurological manifestations.57 Furthermore, atrioventricular nodal or infra-nodal disease has been often documented. Myocardial fibrosis may indeed appear years before clinical signs of cardiac involvement or muscular weakness, both in carriers and patients.
Conversely, Friedreich's ataxia presents as a HCM phenotype with LGE involving the septum and inferolateral wall,56 progressing toward systolic impairment after the 4th decade.
Ring-like pattern
A unique scenario with LGE involving at least 3 consecutive segments in the short-axis view, resulting in a ring-like pattern usually with an epicardial and mid-wall distribution, has been recently reported.58,59 This pattern has been clinically associated with recurrent or familial myocarditis and a high burden of ventricular arrhythmias,47 but not with an impairment of LV ejection fraction. The latter suggests that this pattern, despite the extension and the distribution of the scar, may represent a major arrhythmogenic substrate without impairing LV function.60
Due to its association with arrhythmogenic cardiomyopathy, which affects the left ventricle,61,62 and the arrhythmogenic subtypes of DCM,63 the true incidence of this pattern is yet unclear. As a result, these individuals may exhibit a wide spectrum of phenotypic expression, from dilated to nondilated LV cardiomyopathy, and because of the increased risk of malignant arrhythmic events, they should always get appropriate therapeutic care and tailored decisions, particularly regarding primary prevention of SCD. Finally, further research is required to ascertain if a "cascade" CMR screening of the proband's family members is beneficial, even in absence of an arrhythmic profile.
Inferior region
Nonischemic myocardial fibrosis in this region may be a result of sarcoidosis27 or arrhythmogenic mitral valve prolapse (Table 5). The latter can be associated with mitral annular disjunction, the Pickelhaube sign—the high-velocity systolic signal with tissue Doppler imaging—and systolic curling.63 Although the genetic etiology of mitral valve prolapse remains largely unknown, some genes have been identified in familial aggregation, including hyperpolarization activated cyclic nucleotide-gated potassium channel 4. Inverted/biphasic T waves in the inferior leads are a common evidence, and premature ventricular complexes originate from the papillary muscle or mitral annular regions.64
Table 5.
Left Ventricular Scars in the Inferior Region With Specific Disease Characteristics
| LV Scar | Distribution | Phenotype | Red Flags | Etiologies | Further Investigation | |
|---|---|---|---|---|---|---|
| Inferior region | Mid-wall/transmural | HCM | CLINICAL: |
|
Sarcomeric HCM | Genetic testing |
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
| Mid-wall | NDLVC/DCM | CLINICAL: |
|
Arrhythmic mitral valve prolapse | Rhythm monitoring | |
| ECG: |
|
|||||
|
||||||
| ECHO: |
|
|||||
| Subepicardial/transmural | DCM/HCM | CLINICAL: |
|
Sarcoidosis | Thoracic FDG-PET imaging | |
| ECG: |
|
|||||
| ECHO: |
|
|||||
|
||||||
|
||||||
AV = atrioventricular; DCM = dilated cardiomyopathy; FDG-PET = fluorodeoxyglucose positron emission tomography; HCM = hypertrophic cardiomyopathy; LBBB = left bundle branch block; LVH = left ventricular hypertrophy; MAD = mitral annular disjunction; NDLVC = nondilated left ventricular cardiomyopathy; PH = pulmonary hypertension; PVC = premature ventricular complex; RBBB = right bundle branch block; RV = right ventricular; SAM = systolic anterior motion; SCD = sudden cardiac death; STEMI = ST fluorodeoxyglucose positron emission tomography elevation myocardial infarction.
Patients with arrhythmogenic mitral valve prolapse may typically present localized fibrosis at the inferior and inferolateral regions,63 and in some cases involving the base of the posteromedial papillary muscle. Mitral valve prolapse has an estimated annual risk of SCD ranging from 0.2% to 1.9%,64 higher in young women and in presence of bi-leaflet involvement. Although risk stratification is challenging, the presence of LGE together with complex ventricular ectopy, concomitant mitral annular disjunction, severe mitral regurgitation, and ECG repolarization abnormalities identifies the high-risk patients requiring further evaluation and closer monitoring.
Diffuse pattern
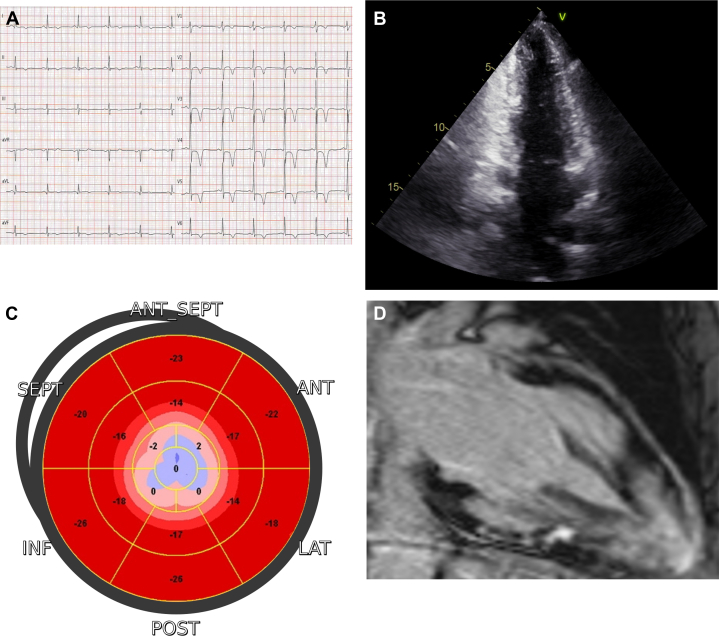
A distinct case that does not recognize any specific regionality is cardiac amyloidosis. Due to the deposition of different misfolded proteins in an extracellular space of the myocardium, cardiac amyloidosis usually results in heart failure, conduction system disease, and SCD. LGE proved to be a useful way to identify cardiac amyloidosis qualitatively and quantitatively and to provide prognostic information.65 In addition to echocardiographic abnormalities (LV hypertrophy, diastolic dysfunction, and pericardial effusion), a diffuse circumferential subendocardial LGE is present in at least one-third of patients 65 and represents a highly specific marker for the diagnosis of cardiac amyloidosis, with a specificity of nearly 95%. While subendocardial LGE is typical of patients with light-chain amyloidosis, the pattern of LGE in transthyretin-related cardiac amyloidosis is more extensive, with right ventricular involvement and a higher prevalence of transmural distribution.66 Moreover, given the potential challenges of LGE imaging due to the diffuse amyloid deposition throughout the heart, which may result in incorrect nulling, high values of native T1 and extracellular volume provide an additional accurate tool for diagnosis and quantitative measurement of disease burden.67
Right ventricle
The present review focuses primarily on LV injury, as qualitative and quantitative assessment by CMR of myocardial fibrosis in the right ventricle is challenging due to the thin-walled and trabeculated myocardium. Nevertheless, right ventricular involvement may be relevant in several cardiomyopathies. For instance, it is important to distinguish LGE confined to the right ventricular insertion sites, associated with a low risk of adverse events in patients with HCM 68 or DCM,69 from LGE extending to the right free wall, which is associated instead with a pronounced arrhythmic burden in cardiac sarcoidosis 70 or in myocarditis.71
Conclusions
Several causes other than ischemic heart disease may cause segmental myocardial fibrosis of the left ventricle with a disease-specific scar distribution that can guide differential diagnosis. Despite clinical and morphological variability of myocardial disease, the proposed atlas may serve as a reference to interpret CMR scar patterns in the context of clinical presentation, family history, and electrocardiographic and physical findings.
Funding support and author disclosures
Dr Olivotto was supported by the European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement number: 777204: “SILICOFCM - In Silico trials for drug tracing the effects of sarcomeric protein mutations leading to familial cardiomyopathy”; by the Italian Ministry of Health (Left ventricular hypertrophy in aortic valve disease and hypertrophic cardiomyopathy: genetic basis, biophysical correlates and viral therapy models” (RF-2013-02356787); and by the EnteCassa di Risparmio di Firenze (bando 2016) “juvenile sudden cardiac death: just know and treat.” The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Patel M.R., Peterson E.D., Dai D., et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng Z.H., Moffatt E., Kim A., et al. Sudden cardiac death and myocardial fibrosis, determined by autopsy, in persons with HIV. N Engl J Med. 2021;384:2306–2316. doi: 10.1056/NEJMoa1914279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbelo E., Protonotarios A., Gimeno J.R., et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44:3503–3626. doi: 10.1093/eurheartj/ehad194. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S., Ge Y., Singh A., Gräni C., Kwong R.Y. Multimodality imaging assessment of myocardial fibrosis. JACC Cardiovasc Imaging. 2021;14:2457–2469. doi: 10.1016/j.jcmg.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Thomas T.P., Grisanti L.A. The dynamic interplay between cardiac inflammation and fibrosis. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.529075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karamitsos T.D., Arvanitaki A., Karvounis H., Neubauer S., Ferreira V.M. Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc Imaging. 2020;13:1221–1234. doi: 10.1016/j.jcmg.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Elliott P., Andersson B., Arbustini E., et al. Classification of the cardiomyopathies: a position statement from the European society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 8.Poggesi C., Ho C.Y. Muscle dysfunction in hypertrophic cardiomyopathy: what is needed to move to translation? J Muscle Res Cell Motil. 2014;35:37–45. doi: 10.1007/s10974-014-9374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maron B.J. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. doi: 10.1056/NEJMra1710575. [DOI] [PubMed] [Google Scholar]
- 10.Maron M.S., Olivotto I., Harrigan C., et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation. 2011;124:40–47. doi: 10.1161/CIRCULATIONAHA.110.985812. [DOI] [PubMed] [Google Scholar]
- 11.Biagini E., Pazzi C., Olivotto I., et al. Usefulness of electrocardiographic patterns at presentation to predict long-term risk of cardiac death in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2016;118:432–439. doi: 10.1016/j.amjcard.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Maron B.J., Gottdiener J.S., Epstein S.E. Patterns and significance of distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy. A wide angle, two dimensional echocardiographic study of 125 patients. Am J Cardiol. 1981;48:418–428. doi: 10.1016/0002-9149(81)90068-0. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Zhao S., Yu S., et al. Patterns of replacement fibrosis in hypertrophic cardiomyopathy. Radiology. 2022;302:298–306. doi: 10.1148/radiol.2021210914. [DOI] [PubMed] [Google Scholar]
- 14.Galati G., Leone O., Pasquale F., et al. Histological and histometric characterization of myocardial fibrosis in end-stage hypertrophic cardiomyopathy: a clinical-pathological study of 30 explanted hearts. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003090. [DOI] [PubMed] [Google Scholar]
- 15.Chan R.H., Maron B.J., Olivotto I., et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 16.Chmielewski L., Bietenbeck M., Patrascu A., et al. Non-invasive evaluation of the relationship between electrical and structural cardiac abnormalities in patients with myotonic dystrophy type 1. Clin Res Cardiol. 2019;108:857–867. doi: 10.1007/s00392-019-01414-0. [DOI] [PubMed] [Google Scholar]
- 17.Halliday B.P., Baksi A.J., Gulati A., et al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging. 2019;12:1645–1655. doi: 10.1016/j.jcmg.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Augusto J.B., Eiros R., Nakou E., et al. Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: a comprehensive genotype-imaging phenotype study. Eur Heart J Cardiovasc Imaging. 2020;21:326–336. doi: 10.1093/ehjci/jez188. [DOI] [PubMed] [Google Scholar]
- 19.Ollila L., Nikus K., Holmström M., et al. Clinical disease presentation and ECG characteristics of LMNA mutation carriers. Open Heart. 2017;4 doi: 10.1136/openhrt-2016-000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasselberg N.E., Edvardsen T., Petri H., et al. Risk prediction of ventricular arrhythmias and myocardial function in Lamin A/C mutation positive subjects. Europace. 2014;16:563–571. doi: 10.1093/europace/eut291. [DOI] [PubMed] [Google Scholar]
- 21.Di Marco A., Anguera I., Schmitt M., et al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC Heart Fail. 2017;5:28–38. doi: 10.1016/j.jchf.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Guaricci A.I., Masci P.G., Muscogiuri G., et al. CarDiac magnEtic resonance for prophylactic implantable-cardioVerter defibrillAtor ThErapy in non-ischaemic dilated CardioMyopathy: an international registry. Europace. 2021;23:1072–1083. doi: 10.1093/europace/euaa401. [DOI] [PubMed] [Google Scholar]
- 23.Aquaro G.D., Perfetti M., Camastra G., et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol. 2017;70:1977–1987. doi: 10.1016/j.jacc.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 24.Freeman A.M., Curran-Everett D., Weinberger H.D., et al. Predictors of cardiac sarcoidosis using commonly available cardiac studies. Am J Cardiol. 2013;112:280–285. doi: 10.1016/j.amjcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Vignaux O., Dhote R., Duboc D., et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrast-enhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr. 2002;26:762–767. doi: 10.1097/00004728-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Lemay S., Massot M., Philippon F., et al. Ten questions cardiologists should Be able to answer about cardiac sarcoidosis: case-based approach and contemporary review. CJC Open. 2020;3:532–548. doi: 10.1016/j.cjco.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukushima K., Nagao M., Yamamoto A., et al. Discrepancy between significant fibrosis and active inflammation in patients with cardiac sarcoidosis: combined and image fusion analysis of cardiac magnetic resonance and 18F fluorodeoxyglucose positron emission tomography. Eur J Hybrid Imaging. 2019;3:9. doi: 10.1186/s41824-019-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greulich S., Kitterer D., Latus J., et al. Comprehensive cardiovascular magnetic resonance assessment in patients with sarcoidosis and preserved left ventricular ejection fraction. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005022. [DOI] [PubMed] [Google Scholar]
- 29.Bulkley B.H., Ridolfi R.L., Salyer W.R., Hutchins G.M. Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation. 1976;53:483–490. doi: 10.1161/01.cir.53.3.483. [DOI] [PubMed] [Google Scholar]
- 30.De Luca G., Bombace S., Monti L. Heart involvement in systemic sclerosis: the role of magnetic resonance imaging. Clin Rev Allergy Immunol. 2023;64:343–357. doi: 10.1007/s12016-022-08923-3. [DOI] [PubMed] [Google Scholar]
- 31.Pieroni M., De Santis M., Zizzo G., et al. Recognizing and treating myocarditis in recent-onset systemic sclerosis heart disease: potential utility of immunosuppressive therapy in cardiac damage progression. Semin Arthritis Rheum. 2014;43:526–535. doi: 10.1016/j.semarthrit.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Mavrogeni S.I., Schwitter J., Gargani L., et al. Cardiovascular magnetic resonance in systemic sclerosis: "Pearls and pitfalls". Semin Arthritis Rheum. 2017;47:79–85. doi: 10.1016/j.semarthrit.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Isaak A., Ayub T.H., Merz W.M., et al. Peripartum cardiomyopathy: diagnostic and prognostic value of cardiac magnetic resonance in the acute stage. Diagnostics. 2022;12:378. doi: 10.3390/diagnostics12020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haghikia A., Röntgen P., Vogel-Claussen J., et al. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: a cardiovascular magnetic resonance study. ESC Heart Fail. 2015;2:139–149. doi: 10.1002/ehf2.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D.Z.J., Montazeri M., Bataiosu R., et al. Clinical characteristics and prognostic importance of left ventricular apical aneurysms in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2022;15:1696–1711. doi: 10.1016/j.jcmg.2022.03.029. [DOI] [PubMed] [Google Scholar]
- 36.Vannucchi V., Tomberli B., Zammarchi L., et al. Chagas disease as a cause of heart failure and ventricular arrhythmias in patients long removed from endemic areas: an emerging problem in Europe. J Cardiovasc Med. 2015;16:817–823. doi: 10.2459/JCM.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 37.Milei J., Storino R., Fernandez Alonso G., Beigelman R., Vanzulli S., Ferrans V.J. Endomyocardial biopsies in chronic chagasic cardiomyopathy. Immunohistochemical and ultrastructural findings. Cardiology. 1992;80:424–437. doi: 10.1159/000175035. [DOI] [PubMed] [Google Scholar]
- 38.Cruz J.S., Machado F.S., Ropert C., Roman-Campos D. Molecular mechanisms of cardiac electromechanical remodeling during Chagas disease: role of TNF and TGF-β. Trends Cardiovasc Med. 2017;27:81–91. doi: 10.1016/j.tcm.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Senra T., Ianni B.M., Costa A.C.P., et al. Long-term prognostic value of myocardial fibrosis in patients with Chagas cardiomyopathy. J Am Coll Cardiol. 2018;72:2577–2587. doi: 10.1016/j.jacc.2018.08.2195. [DOI] [PubMed] [Google Scholar]
- 40.Leone O., Pieroni M., Rapezzi C., Olivotto I. The spectrum of myocarditis: from pathology to the clinics. Virchows Arch. 2019;475:279–301. doi: 10.1007/s00428-019-02615-8. [DOI] [PubMed] [Google Scholar]
- 41.Ammirati E., Buono A., Moroni F., et al. State-of-the-Art of endomyocardial biopsy on acute myocarditis and chronic inflammatory cardiomyopathy. Curr Cardiol Rep. 2022;24:597–609. doi: 10.1007/s11886-022-01680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yar A., Uusitalo V., Vaara S.M., et al. Cardiac magnetic resonance -detected myocardial injury is not associated with long-term symptoms in patients hospitalized due to COVID-19. PLoS One. 2023;18 doi: 10.1371/journal.pone.0282394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haberka M., Rajewska-Tabor J., Wojtowicz D., et al. A distinct septal pattern of late gadolinium enhancement specific for COVID-induced myocarditis: a multicenter cardiovascular magnetic resonance study. Kardiol Pol. 2023;81:463–471. doi: 10.33963/KP.a2023.0054. [DOI] [PubMed] [Google Scholar]
- 44.Kim J.Y., Han K., Suh Y.J. Prevalence of abnormal cardiovascular magnetic resonance findings in recovered patients from COVID-19: a systematic review and meta-analysis. J Cardiovasc Magn Reson. 2021;23:100. doi: 10.1186/s12968-021-00792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stöbe S., Richter S., Seige M., Stehr S., Laufs U., Hagendorff A. Echocardiographic characteristics of patients with SARS-CoV-2 infection. Clin Res Cardiol. 2020;109:1549–1566. doi: 10.1007/s00392-020-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ammirati E., Raimondi F., Piriou N., et al. Acute myocarditis associated with desmosomal gene variants. JACC Heart Fail. 2022;10:714–727. doi: 10.1016/j.jchf.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Miles C., Finocchiaro G., Papadakis M., et al. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation. 2019;139:1786–1797. doi: 10.1161/CIRCULATIONAHA.118.037230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basso C., Pilichou K., Bauce B., Corrado D., Thiene G. Diagnostic criteria, genetics, and molecular basis of arrhythmogenic cardiomyopathy. Heart Fail Clin. 2018;14:201–213. doi: 10.1016/j.hfc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Ammirati E., Frigerio M., Adler E.D., et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith E.D., Lakdawala N.K., Papoutsidakis N., et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nordin S., Kozor R., Medina-Menacho K., et al. Proposed stages of myocardial phenotype development in fabry disease. JACC Cardiovasc Imaging. 2019;12:1673–1683. doi: 10.1016/j.jcmg.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Pieroni M., Namdar M., Olivotto I., Desnick R.J. Anderson-Fabry disease management: role of the cardiologist. Eur Heart J. 2024;45:1395–1409. doi: 10.1093/eurheartj/ehae148. [DOI] [PubMed] [Google Scholar]
- 53.Pieroni M., Moon J.C., Arbustini E., et al. Cardiac involvement in fabry disease: JACC review topic of the week. J Am Coll Cardiol. 2021;77:922–936. doi: 10.1016/j.jacc.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 54.Finsterer J., Stöllberger C., Maeztu C. Sudden cardiac death in neuromuscular disorders. Int J Cardiol. 2016;203:508–515. doi: 10.1016/j.ijcard.2015.10.176. [DOI] [PubMed] [Google Scholar]
- 55.Menon S.C., Etheridge S.P., Liesemer K.N., et al. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatr Cardiol. 2014;35:1279–1285. doi: 10.1007/s00246-014-0929-z. [DOI] [PubMed] [Google Scholar]
- 56.Blaszczyk E., Gröschel J., Schulz-Menger J. Role of CMR imaging in diagnostics and evaluation of cardiac involvement in muscle dystrophies. Curr Heart Fail Rep. 2021;18:211–224. doi: 10.1007/s11897-021-00521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almogheer B., Antonopoulos A.S., Azzu A., et al. Diagnostic and prognostic value of cardiovascular magnetic resonance in neuromuscular cardiomyopathies. Pediatr Cardiol. 2022;43:27–38. doi: 10.1007/s00246-021-02686-y. [DOI] [PubMed] [Google Scholar]
- 58.Muser D., Santangeli P., Castro S.A., et al. Risk stratification of patients with apparently idiopathic premature ventricular contractions: a multicenter international CMR registry. JACC Clin Electrophysiol. 2020;6:722–735. doi: 10.1016/j.jacep.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 59.Muser D., Nucifora G., Muser D., et al. Prognostic value of nonischemic ringlike left ventricular scar in patients with apparently idiopathic nonsustained ventricular arrhythmias. Circulation. 2021;143:1359–1373. doi: 10.1161/CIRCULATIONAHA.120.047640. [DOI] [PubMed] [Google Scholar]
- 60.Chen W., Qian W., Zhang X., et al. Ring-like late gadolinium enhancement for predicting ventricular tachyarrhythmias in non-ischaemic dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2021;22:1130–1138. doi: 10.1093/ehjci/jeab117. [DOI] [PubMed] [Google Scholar]
- 61.Gigli M., Stolfo D., Graw S.L., et al. Phenotypic expression, natural history, and risk stratification of cardiomyopathy caused by filamin C truncating variants. Circulation. 2021;144:1600–1611. doi: 10.1161/CIRCULATIONAHA.121.053521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith E.D., Lakdawala N.K., Papoutsidakis N., Aubert G., Mazzanti A., McCanta A.C. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perazzolo Marra M., Basso C., De Lazzari M., et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basso C., Perazzolo Marra M., Rizzo S., et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 65.Fontana M., Pica S., Reant P., et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132:1570–1579. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dungu J.N., Valencia O., Pinney J.H., et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:133–142. doi: 10.1016/j.jcmg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Wang T.K.M., Brizneda M.V., Kwon D.H., et al. Reference ranges, diagnostic and prognostic utility of native T1 mapping and extracellular volume for cardiac amyloidosis: a meta-analysis. J Magn Reson Imag. 2021;53:1458–1468. doi: 10.1002/jmri.27459. [DOI] [PubMed] [Google Scholar]
- 68.Chan R.H., Maron B.J., Olivotto I., et al. Significance of late gadolinium enhancement at right ventricular attachment to ventricular septum in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2015;116:436–441. doi: 10.1016/j.amjcard.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 69.Claver E., Di Marco A., Brown P.F., et al. Prognostic impact of late gadolinium enhancement at the right ventricular insertion points in non-ischaemic dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2023;24:346–353. doi: 10.1093/ehjci/jeac109. [DOI] [PubMed] [Google Scholar]
- 70.Velangi P.S., Chen K.A., Kazmirczak F., et al. Right ventricular abnormalities on cardiovascular magnetic resonance imaging in patients with sarcoidosis. JACC Cardiovasc Imaging. 2020;13:1395–1405. doi: 10.1016/j.jcmg.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baritussio A., Cheng C.Y., Simeti G., et al. CMR predictors of favorable outcome in myocarditis: a single-center experience. J Clin Med. 2024;13:1229. doi: 10.3390/jcm13051229. [DOI] [PMC free article] [PubMed] [Google Scholar]