Abstract
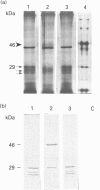
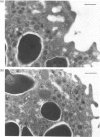
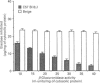
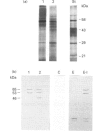
In this paper we report the serum antiprotease screening and the biochemical and functional characteristics of neutrophils in a variety of mouse strains with different susceptibilities for developing a protease-mediated injury. C57Bl/6J mice and their mutants tight-skin and pallid have a lower serum elastase inhibitory capacity (-30, -65 and -70% respectively) than other inbred strains (i.e. NMRI and Balb/c, which both have similar values). We demonstrate that these values are a consequence of a decreased concentration of the alpha 1-protease inhibitor for elastase [PI(E)], which is the major serum inhibitor of elastase and cathepsin G. In addition, neutrophil lysosomal dysfunctions characterized by abnormally high contents of elastase and cathepsin G, or defective lysosomal secretion are observed in tight-skin and pallid mice respectively. Another C57Bl/6J mutant with lysosomal abnormalities is the beige mouse. Negligible amounts of elastase and cathepsin G, as well as defective neutrophil degranulation, have been described previously in this strain. We found, however, discrete amounts of a latent form of neutrophil elastase that undergoes a spontaneous activation by a protease-dependent mechanism. We also report that neutrophil cathepsin G in this mouse is tightly bound to lysosomal membranes, but is released in near normal quantities during exocytosis. Cytosolic elastase and cathepsin G inhibitors, which were previously reported as being specific for the beige neutrophils, have also been detected in all the examined strains. Neutrophil functions, lysosomal enzyme content and serum antiprotease screening may represent key elements in the protease-antiprotease balance and may explain the different interstrain susceptibility to developing lesions in which an elastolytic activity has been implicated.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin G. Methods Enzymol. 1981;80(Pt 100):561–565. doi: 10.1016/s0076-6879(81)80044-4. [DOI] [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Boudier C., Holle C., Bieth J. G. Stimulation of the elastolytic activity of leukocyte elastase by leukocyte cathepsin G. J Biol Chem. 1981 Oct 25;256(20):10256–10258. [PubMed] [Google Scholar]
- Briscoe W. A., Kueppers F., Davis A. L., Bearn A. G. A case of inherited deficiency of serum alpha-antitrypsin associated with pulmonary emphysema. Am Rev Respir Dis. 1966 Oct;94(4):529–539. doi: 10.1164/arrd.1966.94.4.529. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- Davies M., Barrett A. J., Travis J., Sanders E., Coles G. A. The degradation of human glomerular basement membrane with purified lysosomal proteinases: evidence for the pathogenic role of the polymorphonuclear leucocyte in glomerulonephritis. Clin Sci Mol Med. 1978 Mar;54(3):233–240. doi: 10.1042/cs0540233. [DOI] [PubMed] [Google Scholar]
- Doherty J. B., Ashe B. M., Argenbright L. W., Barker P. L., Bonney R. J., Chandler G. O., Dahlgren M. E., Dorn C. P., Jr, Finke P. E., Firestone R. A. Cephalosporin antibiotics can be modified to inhibit human leukocyte elastase. Nature. 1986 Jul 10;322(6075):192–194. doi: 10.1038/322192a0. [DOI] [PubMed] [Google Scholar]
- Felleisen R., Klinkert M. Q. In vitro translation and processing of cathepsin B of Schistosoma mansoni. EMBO J. 1990 Feb;9(2):371–377. doi: 10.1002/j.1460-2075.1990.tb08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman W. H., Kato K., Anstiss C. L., Green S. Human serum beta-glucuronidase; its measurement and some of its properties. Clin Chim Acta. 1967 Mar;15(3):435–447. doi: 10.1016/0009-8981(67)90008-3. [DOI] [PubMed] [Google Scholar]
- Gardi C., Lungarella G. Isolation and partial characterization of a proteinase with elastolytic activity from mouse blood leukocytes. Biochem Int. 1988 Jan;16(1):185–191. [PubMed] [Google Scholar]
- Gardi C., Martorana P. A., de Santi M. M., van Even P., Calzoni P., Lungarella G. Different evolution of emphysema in two strains of mice with similar serum antielastase deficit. Ann N Y Acad Sci. 1991;624:329–330. doi: 10.1111/j.1749-6632.1991.tb17036.x. [DOI] [PubMed] [Google Scholar]
- Gardi C., Martorana P. A., de Santi M. M., van Even P., Lungarella G. A biochemical and morphological investigation of the early development of genetic emphysema in tight-skin mice. Exp Mol Pathol. 1989 Jun;50(3):398–410. doi: 10.1016/0014-4800(89)90048-8. [DOI] [PubMed] [Google Scholar]
- Gardi C., Martorana P. A., van Even P., de Santi M. M., Lungarella G. Serum antielastase deficiency in tight-skin mice with genetic emphysema. Exp Mol Pathol. 1990 Feb;52(1):46–53. doi: 10.1016/0014-4800(90)90057-k. [DOI] [PubMed] [Google Scholar]
- Groutas W. C. Inhibitors of leukocyte elastase and leukocyte cathepsin G. Agents for the treatment of emphysema and related ailments. Med Res Rev. 1987 Apr-Jun;7(2):227–241. doi: 10.1002/med.2610070205. [DOI] [PubMed] [Google Scholar]
- Haak R. A., Ingraham L. M., Baehner R. L., Boxer L. A. Membrane fluidity in human and mouse Chediak-Higashi leukocytes. J Clin Invest. 1979 Jul;64(1):138–144. doi: 10.1172/JCI109432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Iskandar S. S., Jennette J. C., Wilkman A. S., Becker R. L. Interstrain variations in nephritogenicity of heterologous protein in mice. Lab Invest. 1982 Mar;46(3):344–351. [PubMed] [Google Scholar]
- Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207–216. doi: 10.1146/annurev.me.36.020185.001231. [DOI] [PubMed] [Google Scholar]
- Jennette J. C., Tidwell R. R., Geratz J. D., Bing D. H., Falk R. J. Amelioration of immune complex-mediated glomerulonephritis by synthetic protease inhibitors. Am J Pathol. 1987 Jun;127(3):499–506. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Varani J., Oliver J., Ward P. A. Immunologic vasculitis in beige mice with deficiency of leukocytic neutral protease. J Immunol. 1979 May;122(5):1807–1811. [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Alpers C. E., Vissers M., Schulze M., Klebanoff S. J. The human neutrophil serine proteinases, elastase and cathepsin G, can mediate glomerular injury in vivo. J Exp Med. 1988 Sep 1;168(3):1169–1174. doi: 10.1084/jem.168.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger W., Hallström S., Redl H., Schlag G. Preliminary data on isolation of an elastase-like proteinase and its inhibitor from ovine neutrophil granulocytes. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):63–68. [PubMed] [Google Scholar]
- Keiser H., Greenwald R. A., Feinstein G., Janoff A. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. II. Degradation of isolated bovine nasal cartilage proteoglycan. J Clin Invest. 1976 Mar;57(3):625–632. doi: 10.1172/JCI108318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucey E. C., Stone P. J., Breuer R., Christensen T. G., Calore J. D., Catanese A., Franzblau C., Snider G. L. Effect of combined human neutrophil cathepsin G and elastase on induction of secretory cell metaplasia and emphysema in hamsters, with in vitro observations on elastolysis by these enzymes. Am Rev Respir Dis. 1985 Aug;132(2):362–366. doi: 10.1164/arrd.1985.132.2.362. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Lowrie C. T., Jordan H. W. Giant granules in leukocytes of the beige mouse. J Hered. 1967 Nov-Dec;58(6):299–300. doi: 10.1093/oxfordjournals.jhered.a107620. [DOI] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Martorana P. A., Brand T., Gardi C., van Even P., de Santi M. M., Calzoni P., Marcolongo P., Lungarella G. The pallid mouse. A model of genetic alpha 1-antitrypsin deficiency. Lab Invest. 1993 Feb;68(2):233–241. [PubMed] [Google Scholar]
- Martorana P. A., van Even P., Gardi C., Lungarella G. A 16-month study of the development of genetic emphysema in tight-skin mice. Am Rev Respir Dis. 1989 Jan;139(1):226–232. doi: 10.1164/ajrccm/139.1.226. [DOI] [PubMed] [Google Scholar]
- McGuire M. J., Lipsky P. E., Thiele D. L. Generation of active myeloid and lymphoid granule serine proteases requires processing by the granule thiol protease dipeptidyl peptidase I. J Biol Chem. 1993 Feb 5;268(4):2458–2467. [PubMed] [Google Scholar]
- Minnich M., Kueppers F., James H. Alpha-1-antitrypsin from mouse serum isolation and characterization. Comp Biochem Physiol B. 1984;78(2):413–419. doi: 10.1016/0305-0491(84)90051-8. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Nathoo S., Rasums A., Katz J., Ferguson W. S., Finlay T. H. Purification and properties of two different alpha 1-protease inhibitors from mouse plasma. Arch Biochem Biophys. 1982 Dec;219(2):306–315. doi: 10.1016/0003-9861(82)90161-8. [DOI] [PubMed] [Google Scholar]
- Novak E. K., Swank R. T. Lysosomal dysfunctions associated with mutations at mouse pigment genes. Genetics. 1979 May;92(1):189–204. doi: 10.1093/genetics/92.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J., Dubin A., Watorek W., Travis J. An elastase inhibitor from equine leukocyte cytosol belongs to the serpin superfamily. Further characterization and amino acid sequence of the reactive center. J Biol Chem. 1988 May 25;263(15):7364–7369. [PubMed] [Google Scholar]
- Powers J. C., Boone R., Carroll D. L., Gupton B. F., Kam C. M., Nishino N., Sakamoto M., Tuhy P. M. Reaction of azapeptides with human leukocyte elastase and porcine pancreatic elastase. New inhibitors and active site titrants. J Biol Chem. 1984 Apr 10;259(7):4288–4294. [PubMed] [Google Scholar]
- Rossi G. A., Hunninghake G. W., Gadek J. E., Szapiel S. V., Kawanami O., Ferrans V. J., Crystal R. G. Hereditary emphysema in the tight-skin mouse. Evaluation of pathogenesis. Am Rev Respir Dis. 1984 May;129(5):850–855. doi: 10.1164/arrd.1984.129.5.850. [DOI] [PubMed] [Google Scholar]
- Salvesen G., Enghild J. J. An unusual specificity in the activation of neutrophil serine proteinase zymogens. Biochemistry. 1990 Jun 5;29(22):5304–5308. doi: 10.1021/bi00474a013. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., Joosten L. A., van den Berg W. B., van de Putte L. B. Experimental arthritis in C57black/6 normal and beige (Chediak-Higashi) mice: in vivo and in vitro observations on cartilage degradation. Ann Rheum Dis. 1988 Nov;47(11):940–946. doi: 10.1136/ard.47.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijver G., Schalkwijk J., Robben J. C., Assmann K. J., Koene R. A. Antiglomerular basement membrane nephritis in beige mice. Deficiency of leukocytic neutral proteinases prevents the induction of albuminuria in the heterologous phase. J Exp Med. 1989 Apr 1;169(4):1435–1448. doi: 10.1084/jem.169.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J. Neutral proteinases from human inflammatory cells. A critical review of their role in extracellular matrix degradation. Clin Lab Med. 1983 Dec;3(4):645–666. [PubMed] [Google Scholar]
- Snider G. L., Lucey E. C., Stone P. J. Animal models of emphysema. Am Rev Respir Dis. 1986 Jan;133(1):149–169. doi: 10.1164/arrd.1986.133.1.149. [DOI] [PubMed] [Google Scholar]
- Stahl R. F., Fisher C. A., Kucich U., Weinbaum G., Warsaw D. S., Stenach N., O'Connor C., Addonizio V. P. Effects of simulated extracorporeal circulation on human leukocyte elastase release, superoxide generation, and procoagulant activity. J Thorac Cardiovasc Surg. 1991 Feb;101(2):230–239. [PubMed] [Google Scholar]
- Starcher B., James H. Evidence that genetic emphysema in tight-skin mice is not caused by neutrophil elastase. Am Rev Respir Dis. 1991 Jun;143(6):1365–1368. doi: 10.1164/ajrccm/143.6.1365. [DOI] [PubMed] [Google Scholar]
- Starcher B., Williams I. The beige mouse: role of neutrophil elastase in the development of pulmonary emphysema. Exp Lung Res. 1989 Sep;15(5):785–800. doi: 10.3109/01902148909062861. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J., Burleigh M. C. The degradation of articular collagen by neutrophil proteinases. Biochim Biophys Acta. 1977 Aug 11;483(2):386–397. doi: 10.1016/0005-2744(77)90066-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi K. H., Swank R. T. Inhibitors of elastase and cathepsin G in Chédiak-Higashi (beige) neutrophils. J Biol Chem. 1989 May 5;264(13):7431–7436. [PubMed] [Google Scholar]
- Takeuchi K., Wood H., Swank R. T. Lysosomal elastase and cathepsin G in beige mice. Neutrophils of beige (Chediak-Higashi) mice selectively lack lysosomal elastase and cathepsin G. J Exp Med. 1986 Mar 1;163(3):665–677. doi: 10.1084/jem.163.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Granelli-Piperno A., Griscelli C., Reich E. Specific protease deficiency in polymorphonuclear leukocytes of Chédiak-Higashi syndrome and beige mice. J Exp Med. 1978 Apr 1;147(4):1285–1290. doi: 10.1084/jem.147.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt S. M., Burgess A. W., Metcalf D. Isolation and surface labeling of murine polymorphonuclear neutrophils. J Cell Physiol. 1979 Jul;100(1):1–21. doi: 10.1002/jcp.1041000102. [DOI] [PubMed] [Google Scholar]
- de Santi M. M., Gardi C., Martorana P. A., van Even P., Lungarella G. Immunoelectron-microscopic demonstration of elastase in emphysematous lungs of tight-skin mice. Exp Mol Pathol. 1989 Aug;51(1):18–30. doi: 10.1016/0014-4800(89)90004-x. [DOI] [PubMed] [Google Scholar]
- von Fellenberg R., Kohler L., Grünig G., Pellegrini A. Comparison of neutrophil elastases and of neutrophil protease inhibitors in the horse and man. Am J Vet Res. 1985 Dec;46(12):2480–2484. [PubMed] [Google Scholar]