Abstract
Background
Diabetes mellitus (DM) patients without coronary artery disease (CAD) have a higher all-cause mortality rate than patients with neither DM nor CAD. We examined cause-specific death of DM patients with and without CAD.
Methods
We conducted a cohort study of all patients who underwent CAG in Western Denmark between 2003 and 2016. Using Danish health registries, patients were followed for a maximum of 10 years and stratified according to their DM and CAD status. Outcomes included all-cause-, cancer-, circulatory-, and endocrinologic death. Ten-year cumulative risks were computed as well as adjusted and unadjusted hazard ratios (aHR and HR).
Results
A total of 132,432 patients (28,524 deaths, median follow-up of 6.2 years) were included. Compared to patients with neither DM nor CAD, DM patients without CAD had a higher 10-year risk of all-cause death (27.9% versus 19.7%, aHR 1.43 [95% CI 1.35–1.52]), cancer death (7.2% versus 5.4%, aHR 1.29 [95% CI 1.15–1.46]), circulatory death (9.1% versus 6.9%, aHR 1.35 [95% CI 1.22–1.49]), and endocrinologic death (3.9% versus 0.3%, aHR 14.02 [95% CI 10.95–17.95]). Among endocrinologic deaths, 87% were due to classical complications of DM, such as diabetic nephropathy and ketoacidosis, in DM patients without CAD.
Conclusion
Diabetes patients without CAD exhibit a higher risk of all-cause mortality, driven primarily by elevated rates of cancer, circulatory, and endocrinologic deaths, particularly related to diabetic microvascular complications.
Keywords: diabetes, coronary artery disease, death
Introduction
Patients with diabetes mellitus (DM) have a significantly greater risk of developing cardiovascular disease (CVD) compared to patients without DM.1 CVD death rates are estimated to be 1.7 times higher among DM patients compared to those without DM. DM is associated with several risk factors for CVD development including hyperglycemia, obesity, hypertension, chronic kidney disease, and dyslipidaemia while still remaining an independent CVD risk factor.2,3 Efficient management of these modifiable risk factors, as well as smoking, exercise, and diet, improves cardiovascular outcomes.4–6
A considerable number of patients with DM have no or only mild coronary artery disease (CAD) when examined by coronary angiography (CAG).7,8 We and others7,8 have previously shown that DM patients without angiographic CAD have a risk of myocardial infarction (MI) that is similar to patients with neither DM nor CAD and even comparable to an age-matched general population.9 However, despite the low risk of MI, DM patients without CAD remain at increased risk of all-cause death.9 Furthermore, DM patients without CAD are at increased risk of atherosclerotic cardiovascular diseases other than MI, ie ischemic stroke and peripheral artery disease, as well as other cardiac diseases, such as non-ischemic heart failure and atrial fibrillation.8–13 Thus, there is a need to explore specific causes of death among DM patients without CAD to potentially unveil areas for future research and potential for prevention. In the current study, we sought to investigate the causes of death that may contribute to excess mortality among DM patients without CAD.
Methods
Data Sources
A registry-based cohort study was conducted using data from the Western Denmark Heart Registry; a clinical database that contains information on all cardiac procedures performed in Western Denmark since 1999.14 The Western Denmark Heart Registry contains both patient data and procedural characteristics, including diagnostic, invasive, and surgical cardiovascular procedures. As such, the database also includes a detailed description of more than 240,000 CAGs, describing the presence and extent of CAD in patients with and without DM.14 In addition to the Western Denmark Heart Registry, several other databases were used; The Danish Civil Registration System assigns at the time of birth or immigration a unique 10-digit number to every Danish resident. This personal identifier (the civil registration number) can be used in every registry in Denmark, which allows for valid linkage of individual patient data across all healthcare registries in Denmark. The Danish Civil Registration System also monitors the occurrence of death or emigration from Denmark.15,16 The Danish National Patient Registry has collected data on all inpatient hospital admissions and discharges since 1977 and out-patient contacts since 1995. The registry includes dates of admission and discharge, as well as discharge diagnoses according to the International Classification of Diseases 10th revision (ICD-10).17 We also used the Danish National Prescription Registry containing information on every prescription redeemed at any Danish pharmacy since 1994.18 Finally, the Danish Register of Causes of Death was used. It has kept track of all deaths occurring in Denmark since 1970. As of 1994, causes of death have been registered according to ICD-10 diagnosis codes set by the World Health Organization (WHO). Diagnosis codes are provided by death certificates, which are filled out by medical doctors, who list causes of death as 1) immediate cause of death, 2) contributory causes, and 3) the underlying cause of death, defined as a condition or disease that triggered the process that resulted in death.19
Patient Selection
This study included all patients with a CAG registered in the Western Denmark Heart Registry between January 1, 2003, to December 31, 2016. If a patient had more than one CAG examination during the study period, the first CAG was used as the index procedure. Furthermore, patients were stratified according to their CAD and DM status.
CAD status was obtained from the Western Denmark Heart Registry14 and defined as angiographic lumen narrowing (≥50%) in at least one coronary artery or as diffuse CAD with non-obstructive stenosis (1–49%) in two or more coronary arteries. The absence of stenosis in all coronary arteries or mild angiographic lumen narrowing (<50%) in a single coronary artery was defined as “no CAD”.
DM was defined as either (1) receiving insulin (with or without supplemental oral glucose-lowering agents), oral glucose-lowering medicine alone, or non-pharmacological dietary treatment for DM at the time of CAG as recorded in the Western Denmark Heart Registry, and/or (2) having a DM diagnosis recorded in the Danish National Patient Registry before index CAG, and/or (3) redeeming one or more prescription(s) for DM medication within 6 months before CAG, as recorded in the Danish National Prescription Registry.20 Type 1 DM was defined as being in treatment with insulin without any previous redemption of prescription for non-insulin glucose lowering drugs. The remaining DM patients were classified as type 2 DM, ie treatment with non-insulin glucose lowering drugs ± insulin or non-pharmacological dietary treatment for diabetes.
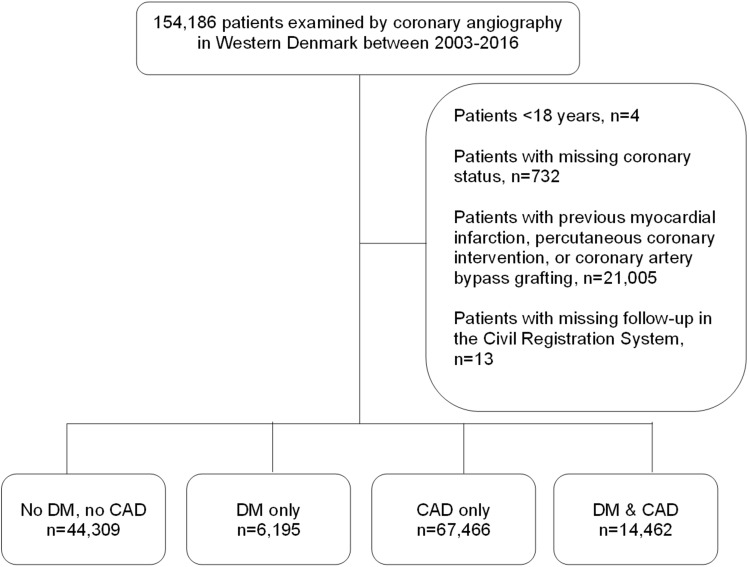
The following patients were excluded: patients <18 years, patients with missing CAD status, patients with previous MI or coronary revascularization, patients with invalid personal identifiers in the Danish Civil Registration System, and/or emigration before index CAG (Figure 1).
Figure 1.
Patient selection. CAD: coronary artery disease; DM: diabetes mellitus.
Medication
The Danish National Prescription Registry was used to collect treatment records according to the Anatomical Therapeutic Chemical (ATC) codes (Table S1). Medical treatment was defined as redemption of one or more prescriptions within 6 months before index CAG.
Comorbidity
Comorbidities were assessed by inpatient and outpatient primary and secondary discharge diagnoses from the Danish National Patient Registry before start of follow-up (Table S2). DM-related microvascular complications were defined as a composite of hospital-diagnosed retinopathy, nephropathy, and peripheral neuropathy. Additionally, the Western Denmark Heart Registry was used to collect data on smoking, body mass index, and hypertension.
Outcomes
Outcomes were categorized into all-cause death, as well as cause-specific death, including, but not limited to, cancer-related death, circulatory death, and endocrinological death.21 We also examined death due to DM-related complications, including diabetes-related nephropathy and ketoacidosis. The Danish Register of Causes of Death was used to identify cause-specific death by using the underlying cause of death obtained from death certificates based on ICD-10 codes (Table S3). Knowing that deaths due to infectious diseases are underestimated when using only the underlying cause of death, we performed sensitivity analysis of this outcome to also include the contributing causes of death.22 All-cause death was based on patients’ vital status (dead, alive, or emigrated) obtained from the Danish Civil Registration System.
Statistical Analyses
Follow-up began on the date of index CAG and continued until an outcome, emigration, or end of follow-up (31 December 2017), whichever came first. Follow-up was restricted to maximum 10 years. Events that occurred beyond 10 years were censored. As a result, 10-year cumulative incidence proportion (CIP) curves were computed as well as hazard ratios (HRs). Using Cox proportional hazard regression adjusted and unadjusted hazard ratios (aHR and HR) were calculated for all-cause death and cause-specific death in each patient group. All outcomes met the proportional hazards assumption as verified by the log–log plots. CIP curves were computed accounting for the competing risk of dying from other causes in each outcome. In Cox regression analyses, HRs were adjusted for sex and age. Patients with neither DM nor CAD were used as reference. Furthermore, we also estimated the distribution of causes of death stratified by DM and CAD status.
We performed a sensitivity analysis in which we would censor follow-up if patients changed DM and/or CAD status after CAG, as well as a sensitivity analysis in which we additionally adjusted for hypertension, statin treatment, smoking, body mass index, and microvascular disease.
In subgroup analyses, DM patients without CAD were stratified according to the presence or absence of DM-related microvascular complications (ie, retinopathy, nephropathy, and peripheral neuropathy) at index CAG. Patients without microvascular disease were used as reference. Among patients without CAD, we further stratified DM patients by type of diabetes using patients with DM as reference. In sensitivity analysis, we estimated sub-distribution HRs of cause-specific death accounting for the competing risk of other causes of death using the Fine-Gray model.23
The abstract of this paper was presented at the European Society of Cardiology Congress 2022 as a moderated poster presentation with interim findings.24
Ethical Considerations
This study was approved by the Danish Data Protection Agency (record no. 1–16-02-193-18), and the requirement for informed consent was waived by the legal office at the Region of Central Denmark, Viborg, Denmark (record no 14–45-70-24-22).
Results
Following index CAG, a total of 132,432 patients were included in the study. Among these, 44,309 (33.5%) had neither DM nor CAD, 6,195 (4.7%) had DM only, 67,466 (50.9%) had CAD only, and 14,462 (10.9%) had both DM and CAD (Figure 1). The median follow-up was 6.2 years (IQR 3.8–10.0 years).
Baseline Characteristics
Baseline characteristics are presented in Table 1. Patients with CAD were older and more likely to be male and active smokers compared to patients without CAD, regardless of DM status. Furthermore, CAD patients were also more likely to be admitted urgently, have PAD, and receive treatment with statins, beta-blockers, aspirin, and adenosine diphosphate inhibitors. DM patients were more likely to have hypertension, previous ischemic stroke, microvascular disease (ie, hospital-diagnosed retinopathy, nephropathy, and/or peripheral neuropathy) and be obese or overweight compared to patients without DM, irrespective of CAD status. They were also more often treated with antihypertensive drugs. DM patients without CAD were more often diagnosed with heart failure, atrial fibrillation/flutter, and chronic obstructive pulmonary disease compared to patients with neither DM nor CAD.
Table 1.
Baseline Characteristics
| No DM, no CAD (n=44,309) |
DM only (n=6,195) | CAD only (n=67,466) |
DM and CAD (n=14,462) |
|
|---|---|---|---|---|
| Male, % | 22,166 (50.0) | 3216 (51.9) | 46,892 (69.5) | 10,044 (69.5) |
| Age, median (IQR) | 61 years (52–70) | 62 years (54–70) | 67 years (58–75) | 68 years (60–74) |
| Current smoking, %a | 9510 (21.5) | 1186 (19.1) | 20,859 (30.9) | 3611 (25.0) |
| Body mass index, median (IQR) | 26 kg/m2 (23–29) | 30 kg/m2 (26–35) | 26 kg/m2 (24–29) | 28 kg/m2 (25–32) |
| Weight (IQR) | 78 kg (67–90) | 88 kg (75–103) | 80 kg (70–90) | 85 kg (74–97) |
| eGFR, mean (SD) | 79.7 (20.1) | 77.2 (24.4) | 75.7 (20.3) | 71.9 (24.5) |
| Comorbidity | ||||
| Hypertension, % | 21,404 (48.3) | 4807 (77.6) | 36,367 (53.9) | 11,508 (79.6) |
| Heart failure, % | 6748 (15.2) | 1233 (19.9) | 11,296 (19.9) | 3398 (23.5) |
| Ischemic stroke, % | 670 (1.5) | 225 (3.6) | 1563 (2.3) | 704 (4.9) |
| Hemorrhagic stroke, % | 89 (0.2) | 15 (0.2) | 151 (0.2) | 49 (0.3) |
| Peripheral artery disease, % | 1015 (2.3) | 235 (3.8) | 3654 (5.4) | 1631 (11.3) |
| Atrial fibrillation/flutter, % | 6991 (15.8) | 1156 (18.7) | 8192 (12.1) | 2199 (15.2) |
| Chronic pulmonary disease, % | 3714 (8.4) | 687 (11.1) | 4472 (6.6) | 1248 (8.6) |
| Nephropathy, % | 59 (0.1) | 432 (7.0) | 90 (0.3) | 1162 (8.0) |
| Peripheral neuropathy, % | 96 (0.2) | 329 (5.3) | 173 (0.3) | 944 (6.5) |
| Retinopathy, % | 33 (0.1) | 905 (14.6) | 40 (0.1) | 2428 (16.8) |
| Medical treatment | ||||
| Statin, % | 18,921 (42.7) | 4485 (72.4) | 56,999 (84.5) | 12,517 (86.6) |
| Beta-blocker, % | 21,906 (49.4) | 3392 (54.8) | 47,860 (70.9) | 10,051 (69.5) |
| ACE inhibitor, % | 12,456 (28.1) | 2872 (46.4) | 23,885 (35.4) | 7466 (51.6) |
| ARB, % | 6234 (14.1) | 1821 (29.4) | 10,411 (15.4) | 4275 (29.6) |
| Calcium channel blocker, % | 10,796 (24.4) | 2185 (35.3) | 19,148 (28.4) | 5891 (40.7) |
| Thiazide, % | 7008 (15.8) | 1285 (20.7) | 10,844 (16.1) | 2962 (20.5) |
| Aspirin, % | 22,660 (51.1) | 3847 (62.1) | 56,219 (83.3) | 11,943 (82.6) |
| ADP receptor inhibitor, % | 2839 (6.4) | 414 (6.7) | 38,536 (57.1) | 6990 (48.3) |
| VKA, % | 5947 (13.4) | 972 (15.7) | 5996 (8.9) | 1685 (11.7) |
| DOAC, % | 1091 (2.5) | 196 (3.2) | 1351 (2.0) | 364 (2.5) |
| Insulin | 0 (0.0%) | 1752 (28.3%) | 0 (0.0%) | 4700 (32.5%) |
| Non-insulin | 0 (0.0%) | 3923 (63.3%) | 0 (0.0%) | 9287 (64.2%) |
| SGLT-2 inhibitors | 0 (0.0%) | 197 (3.2%) | 0 (0.0%) | 460 (3.2%) |
| GLP-1 analogues | 0 (0.0%) | 247 (4.0%) | 0 (0.0%) | 499 (3.5%) |
| Procedural priority | ||||
| Elective, % | 30,162 (68.1) | 4537 (73.2) | 30,148 (44.7) | 7576 (52.4) |
| Subacute, % | 10,384 (23.4) | 1282 (20.7) | 18,514 (27.4) | 4151 (28.7) |
| Acute, % | 3763 (8.5) | 376 (6.1) | 18,803 (27.9) | 2735 (18.9) |
| Procedural indication | ||||
| STEMI | 1967 (4.4) | 179 (2.9) | 16,649 (24.7) | 2235 (15.5) |
| NSTEMI/UAP | 5230 (11.8) | 673 (10.9) | 15,352 (22.8) | 3392 (23.5) |
| Stable angina pectoris | 17856 (40.3) | 2698 (43.6) | 22,662 (33.6) | 5513 (38.1) |
| Other | 19256 (43.5) | 2645 (42.7) | 12,803 (19.0) | 3322 (23.0) |
| Coronary artery disease | ||||
| 0 VD | 44309 (100) | 6196 (100) | 0 (0) | 0 (0) |
| 1 VD | 0 (0) | 0 (0) | 29,398 (43.6) | 4863 (33.6) |
| 2 VD | 0 (0) | 0 (0) | 14,852 (22.0) | 3246 (22.4) |
| 3 VD | 0 (0) | 0 (0) | 12,615 (18.7) | 3797 (26.3) |
| Diffuse VD | 0 (0) | 0 (0) | 10,601 (15.7) | 2556 (17.7) |
Abbreviations: ACE, angiotensin-converting enzyme; ADP: Adenosine diphosphate; ARB: angiotensin-II receptor blocker; CAD: coronary artery disease; DM: diabetes mellitus; DOAC: Direct-Acting Oral Anticoagulants; eGFR: estimated glomerular filtration rate; GLP-1: Glucagon-Like Peptide-1; IQR: interquartile range; NSTEMI: Non-ST-segment–elevation; SD: standard deviation; STEMI: ST-segment–elevation myocardial infarction; SGLT-2: Sodium-Glucose Cotransporter-2; UAP: Unstable angina pectoris; VD: vessel disease; VKA: vitamin K antagonist. a13% missing values for smoking.
Outcomes
During the study period, 28,524 patients died. A total of 6,943 patients died of cancer, 12,365 died of circulatory causes, and 1,120 died of endocrine disorders. The remaining 8,096 deaths were caused by the other cause-specific outcomes (Table 2).
Table 2.
Ten-Year Risk of All-Cause Death and Cause-Specific Death by DM and CAD
| Patients | Events | 10-year CIP* (95% CI) | Unadjusted HR (95% CI) | Adjusted† HR (95% CI) | |
|---|---|---|---|---|---|
| All-cause death | |||||
| No DM or CAD | 44,309 | 6877 | 19.7% (19.3–20.1) | ref | ref |
| DM only | 6,195 | 1291 | 27.9% (26.5–29.3) | 1.46 (1.37–1.55) | 1.43 (1.35–1.52) |
| CAD only | 67,466 | 15,611 | 30.4% (29.9–30.8) | 1.64 (1.59–1.69) | 1.22 (1.19–1.26) |
| DM & CAD | 14,462 | 4745 | 43.3% (42.3–44.4) | 2.59 (2.50–2.69) | 1.95 (1.76–2.02) |
| Infection | |||||
| No DM or CAD | 44,309 | 245 | 0.7% (0.6–0.8) | ref | ref |
| DM only | 6,195 | 40 | 0.9% (0.6–1.2) | 1.29 (0.92–1.80) | 1.27 (0.91–1.78) |
| CAD only | 67,466 | 578 | 1.2% (1.1–1.3) | 1.72 (1.48–2.00) | 1.17 (1.00–1.36) |
| DM & CAD | 14,462 | 153 | 1.5% (1.3–1.8) | 2.41 (1.97–2.95) | 1.68 (1.37–2.06) |
| Cancer death | |||||
| No DM or CAD | 44,309 | 1887 | 5.4% (5.2–5.7) | ref | ref |
| DM only | 6,195 | 319 | 7.2% (6.4–8.0) | 1.33 (1.18–1.49) | 1.29 (1.15–1.46) |
| CAD only | 67,466 | 3897 | 7.8% (7.6–8.1) | 1.51 (1.43–1.59) | 1.14 (1.08–1.21) |
| DM & CAD | 14,462 | 840 | 7.9% (7.3–8.4) | 1.72 (1.58–1.86) | 1.30 (1.19–1.41) |
| Hematologic, non cancer death | |||||
| No DM or CAD | 44,309 | 52 | 0.1% (0.1–0.2) | ref | ref |
| DM only | 6,195 | 3 | 0.1% (0.0–0.2) | 0.45 (0.14–1.44) | 0.44 (0.14–1.42) |
| CAD only | 67,466 | 77 | 0.2% (0.1–0.2) | 1.08 (0.76–1.53) | 0.80 (0.56–1.16) |
| DM & CAD | 14,462 | 21 | 0.2% (0.1–0.3) | 1.54 (0.93–2.56) | 1.17 (0.70–1.96) |
| Endocrinologic death | |||||
| No DM or CAD | 44,309 | 98 | 0.3% (0.2–0.4) | ref | ref |
| DM only | 6,195 | 175 | 3.9% (3.4–4.6) | 14.08 (11.00–18.03) | 14.02 (10.95–17.95) |
| CAD only | 67,466 | 145 | 0.3% (0.3–0.4) | 1.08 (0.84–1.40) | 0.89 (0.69–1.15) |
| DM & CAD | 14,462 | 702 | 6.6% (6.1–7.2) | 27.68 (22.40–34.21) | 23.08 (18.62–28.61) |
| Endocrinologic death coded as diabetes-related | |||||
| No DM or CAD | 44,309 | 26 | 0.1% (0.1–0.1) | ref | ref |
| DM only | 6,195 | 155 | 3.6% (3.0–4.2) | 47.17 (31.14–71.47) | 46.15 (30.46–69.92) |
| CAD only | 67,466 | 74 | 0.2% (0.2–0.2) | 2.08 (1.33–3.26) | 1.61 (1.03–2.52) |
| DM & CAD | 14,462 | 674 | 6.4% (5.9–7.0) | 100.84 (68.15–149.22) | 77.82 (52.47–115.42) |
| Psychiatric death | |||||
| No DM or CAD | 44,309 | 183 | 0.6% (0.5–0.7) | ref | ref |
| DM only | 6,195 | 15 | 0.5% (0.3–0.7) | 0.67 (0.40–1.14) | 0.68 (0.40–1.15) |
| CAD only | 67,466 | 319 | 0.8% (0.3–0.8) | 1.31 (1.09–1.57) | 0.98 (0.81–1.18) |
| DM & CAD | 14,462 | 73 | 0.9% (0.7–1.1) | 1.66 (1.26–2.18) | 1.29 (0.98–1.70) |
| Neurologic death | |||||
| No DM or CAD | 44,309 | 155 | 0.5% (0.04–0.6) | ref | ref |
| DM only | 6,195 | 27 | 0.6% (0.4–0.9) | 1.49 (0.93–2.11) | 1.38 (0.92–2.08) |
| CAD only | 67,466 | 254 | 0.6% (0.5–0.7) | 1.21 (0.99–1.48) | 0.86 (0.70–1.06) |
| DM & CAD | 14,462 | 54 | 0.5% (0.4–0.7) | 1.39 (1.02–1.90) | 1.01 (0.74–1.38) |
| Circulatory death | |||||
| No DM or CAD | 44,309 | 2472 | 6.9% (6.7–7.2) | ref | ref |
| DM only | 6,195 | 444 | 9.1% (8.2–9.9) | 1.38 (1.24–1.52) | 1.35 (1.22–1.49) |
| CAD only | 67,466 | 7310 | 13.5% (13.2–13.8) | 2.10 (2.01–2.20) | 1.53 (1.46–1.60) |
| DM & CAD | 14,462 | 2139 | 18.8% (18.0–19.5) | 3.14 (2.97–3.33) | 2.30 (2.17–2.44) |
| Respiratory death | |||||
| No DM or CAD | 44,309 | 846 | 2.5% (2.3–2.6) | ref | ref |
| DM only | 6,195 | 115 | 2.5% (2.1–3.0) | 1.06 (0.88–1.29) | 1.05 (0.86–1.27) |
| CAD only | 67,466 | 1220 | 2.5% (2.3–2.6) | 1.05 (0.96–1.15) | 0.81 (0.74–0.88) |
| DM & CAD | 14,462 | 277 | 2.6% (2.3–2.9) | 1.26 (1.10–1.44) | 0.97 (0.85–1.11) |
| Digestive system | |||||
| No DM or CAD | 44,309 | 270 | 0.8% (0.7–0.9) | ref | ref |
| DM only | 6,195 | 45 | 1.0% (0.7–1.3) | 1.29 (0.94–1.77) | 1.28 (0.94–1.76) |
| CAD only | 67,466 | 531 | 1.0% (0.9–1.1) | 1.42 (1.23–1.64) | 1.16 (1.00–1.35) |
| DM & CAD | 14,462 | 156 | 1.4% (1.2–1.6) | 2.17 (1.78–2.65) | 1.80 (1.47–2.20) |
| Dermatologic | |||||
| No DM or CAD | 44,309 | 3 | 0.0% (0.0–0.0) | ref | ref |
| DM only | 6,195 | 1 | 0.0% (0.0–0.2) | 2.59 (0.27–24.91) | 2.58 (0.27–24.80) |
| CAD only | 67,466 | 10 | 0.0% (0.0–0.0) | 2.40 (0.66–8.74) | 1.78 (0.47–6.67) |
| DM & CAD | 14,462 | 4 | 0.0% (0.0–0.1) | 5.00 (1.12–22.40) | 3.80 (0.82–17.48) |
| Musculoskeletal/connective tissue | |||||
| No DM or CAD | 44,309 | 60 | 0.2% (0.1–0.2) | ref | ref |
| DM only | 6,195 | 5 | 0.0% (0.0–0.2) | 0.65 (0.26–1.61) | 0.65 (0.26–1.62) |
| CAD only | 67,466 | 83 | 0.2% (0.1–0.2) | 1.00 (0.72–1.39) | 1.00 (0.71–1.41) |
| DM & CAD | 14,462 | 16 | 0.1% (0.1–0.2) | 1.00 (0.58–1.74) | 1.02 (0.58–1.78) |
| Genito-urinary | |||||
| No DM or CAD | 44,309 | 128 | 0.3% (0.3–0.4) | ref | ref |
| DM only | 6,195 | 23 | 0.5% (0.3–0.8) | 1.40 (0.90–2.19) | 1.38 (0.88–2.15) |
| CAD only | 67,466 | 303 | 0.6% (0.5–0.7) | 1.72 (1.40–2.11) | 1.22 (0.98–1.50) |
| DM & CAD | 14,462 | 63 | 0.6% (0.4–0.7) | 1.87 (1.38–2.53) | 1.34 (0.99–1.83) |
| Obstetric/perinatal | |||||
| No DM or CAD | 44,309 | 1 | - | ref | ref |
| DM only | 6,195 | 0 | - | - | - |
| CAD only | 67,466 | 1 | - | - | - |
| DM & CAD | 14,462 | 0 | - | - | - |
| Accidental | |||||
| No DM or CAD | 44,309 | 156 | 0.5% (0.4–0.5) | ref | ref |
| DM only | 6,195 | 22 | 0.5% (0.3–0.7) | 1.10 (0.71–1.73) | 1.10 (0.70–1.72) |
| CAD only | 67,466 | 260 | 0.5% (0.5–0.6) | 1.21 (0.99–1.48) | 0.92 (0.75–1.13) |
| DM & CAD | 14,462 | 71 | 0.7% (0.5–0.9) | 1.74 (1.31–2.30) | 1.35 (1.01–1.80) |
| Suicide | |||||
| No DM or CAD | 44,309 | 64 | 0.2% (0.1–0.2) | ref | ref |
| DM only | 6,195 | 8 | 0.1% (0.1–0.3) | 0.97 (0.47–2.03) | 0.97 (0.46–2.01) |
| CAD only | 67,466 | 84 | 0.2% (0.1–0.2) | 0.95 (0.69–1.32) | 0.80 (0.57–1.12) |
| DM & CAD | 14,462 | 12 | 0.1% (0.1–0.2) | 0.71 (0.38–1.32) | 0.61 (0.33–1.13) |
| Congenital/symptoms | |||||
| No DM or CAD | 44,309 | 219 | 0.6% (0.5–0.7) | ref | ref |
| DM only | 6,195 | 41 | 0.9% (0.6–1.2) | 1.46 (1.05–2.04) | 1.45 (1.04–2.02) |
| CAD only | 67,466 | 458 | 0.9% (0.8–1.0) | 1.52 (1.29–1.78) | 1.12 (0.95–1.33) |
| DM & CAD | 14,462 | 128 | 1.2% (1.0–1.4) | 2.23 (1.79–2.77) | 1.68 (1.35–2.10) |
| Missing | |||||
| No DM or CAD | 44,309 | 38 | 0.1% (0.1–0.2) | ref | ref |
| DM only | 6,195 | 8 | 0.2% (0.1–0.3) | 1.66 (0.77–3.55) | 1.64 (0.77–3.52) |
| CAD only | 67,466 | 81 | 0.2% (0.1–0.2) | 1.56 (1.06–2.29) | 1.41 (0.95–2.10) |
| DM & CAD | 14,462 | 36 | 0.3% (0.2–0.5) | 3.68 (2.33–5.80) | 3.56 (2.11–5.34) |
Notes: CAD indicates coronary artery disease; CI: confidence interval; CIP: cumulative incidence proportion; DM: diabetes mellitus; HR: hazard ratio. * Accounting for the competing risk of other causes of death. † Adjusted for sex and age.
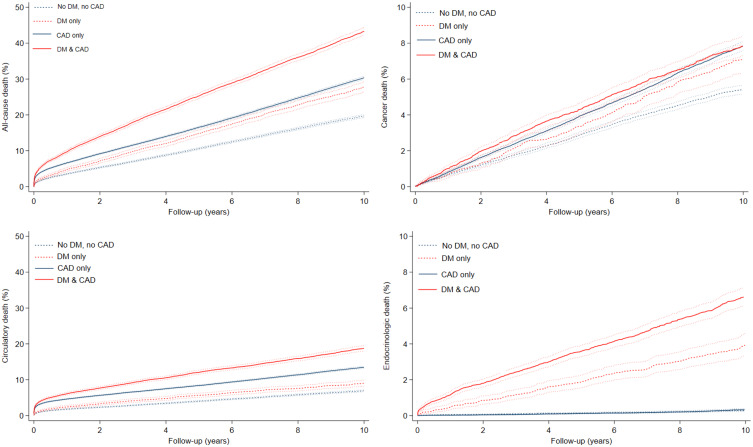
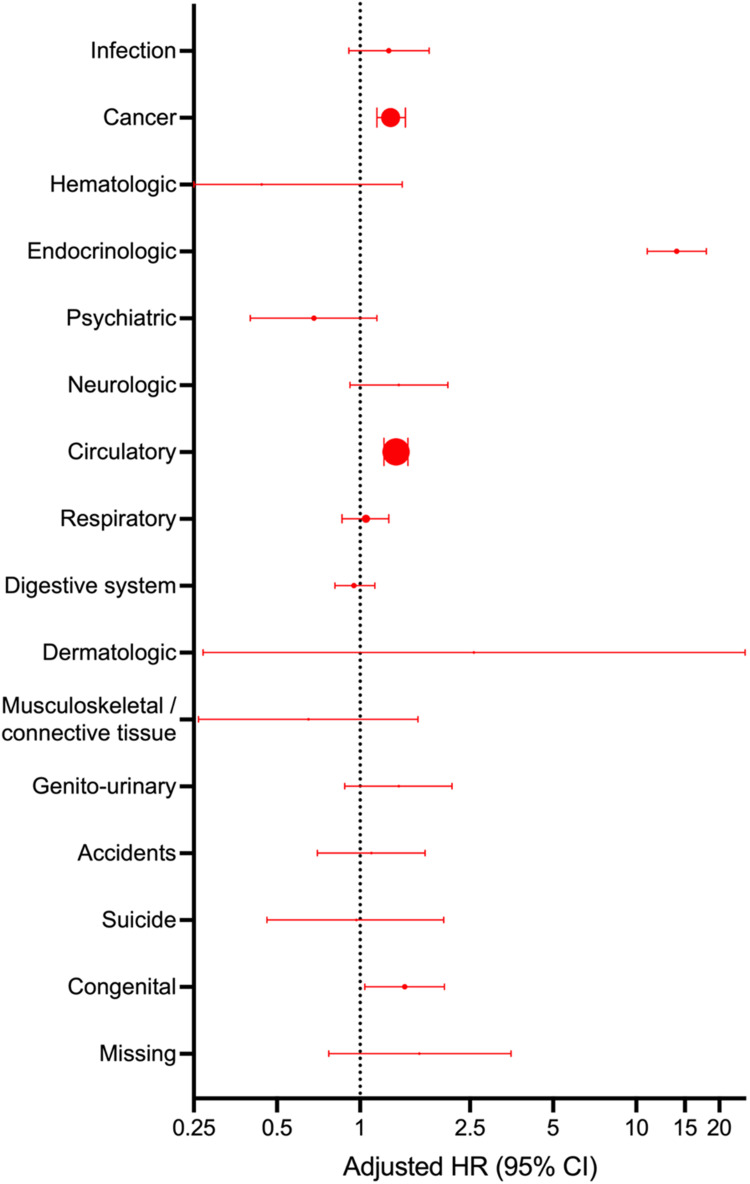
DM patients without CAD had a higher risk of all-cause death (10-year risk difference 8.2% [95% CI 6.7–9.7]), cancer death (10-year risk difference 1.8% [95% CI 1.0%–2.6%]), circulatory death (10-year risk difference 2.2% [95% CI 1.3%–3.1%]) and most notably, endocrinologic death (10-year risk difference 3.6% [95% CI 3.0%–4.2%]) compared to patients with neither DM nor CAD (Table 2, Figure 2). When comparing DM patients without CAD to patients with neither DM nor CAD, the relative risk of the remaining outcomes was imprecise and had very broad confidence intervals. Additionally, the effect sizes of these cause-specific deaths constituted only a small portion of all-cause death (Figure 3).
Figure 2.
Cumulative incidences rate curves of all-cause death, cancer death, circulatory death, and endocrinologic death in patients with and without diabetes mellitus (DM), stratified by the presence or absence of coronary artery disease (CAD).
Figure 3.
Hazard ratio of cause-specific death in diabetes patients without coronary artery disease compared with patients with neither diabetes nor coronary artery disease. HR indicates hazard ratio. Size of dot indicates the weight of the cause-specific death compared to all-cause death.
DM patients without CAD had similar risk of death due to infectious diseases (0.9% versus 0.7%, aHR 1.29.
[95% CI 0.92–1.80], Table 2) compared to patients with neither DM nor CAD when only considering the underlying causes of death. However, when including contributing causes of death, we found that the 10-year risk of death due to infections increased to 5.0% among patients with DM only and to 3.4% among patients with neither DM nor CAD (10-year risk difference 1.6%, aHR 1.54 [95% CI 1.34–1.78]).
Patients with both DM and CAD had the highest 10-year cumulative incidence of death among all the aforementioned outcomes (all-cause death: 43.3% [95% CI 42.3%–44.4%], circulatory death: 18.8%.
[95% CI 18.0%–19.5%], endocrinologic death: 6.4% [95% CI 6.1%–7.2%]) except for cancer death, where patients with both DM and CAD had a similar cumulative incidence to those with CAD only (7.9% versus 7.8%, Table 2, Figure 2).
Endocrinologic Death
During follow-up, 7.9% (n = 3,508) of patients with neither DM nor CAD at the time of CAG were subsequently diagnosed with DM. When further investigating the composition of endocrinologic death among DM patients without CAD and patients with neither DM nor CAD, we found that deaths caused by classical DM-related complications were much more common among DM patients without CAD than patients with neither DM nor CAD (3.6% versus 0.1%, aHR 46.15 [95% CI 30.46–69.92], Table 2). Endocrinologic death accounted for 13.6% of all deaths among DM patients without CAD (Table 3). Among these, 88.6% were due to DM-related complications, which include diabetes-related nephropathy and ketoacidosis (Table 4).
Table 3.
Distribution of Causes of Death by DM and CAD
| No DM, no CAD (n=44,309) |
DM only (n=6,195) | CAD only (n=67,466) |
DM and CAD (n=14,462) |
|
|---|---|---|---|---|
| All-cause death | 6877 (100%) | 1291 (100%) | 15,611 (100%) | 4745 (100%) |
| Infection | 245 (3.6%) | 40 (3.1%) | 578 (3.7%) | 153 (3.2%) |
| Cancer | 1887 (27.4%) | 319 (24.7%) | 3897 (25.0%) | 840 (17.7%) |
| Hematologic, non-cancerous | 52 (0.8%) | 3 (0.2%) | 77 (0.5%) | 21 (0.4%) |
| Endocrinologic | 98 (1.4%) | 175 (13.6%) | 145 (0.9%) | 702 (14.8%) |
| Psychiatric | 183 (2.7%) | 15 (1.2%) | 319 (2.0%) | 73 (1.5%) |
| Neurologic | 155 (2.3%) | 27 (2.1%) | 254 (1.6%) | 54 (1.1%) |
| Circulatory | 2472 (35.9%) | 444 (34.4%) | 7310 (46.8%) | 2139 (45.1%) |
| Respiratory | 846 (12.3%) | 115 (8.9%) | 1220 (7.8%) | 277 (5.8%) |
| Digestive system | 270 (3.9%) | 45 (3.5%) | 531 (3.4%) | 156 (3.3%) |
| Skin | 3 (0.0%) | 1 (0.1%) | 10 (0.1%) | 4 (0.4%) |
| Musculoskeletal/connective tissue | 60 (0.9%) | 5 (0.4%) | 83 (0.5%) | 16 (0.3%) |
| Genito-urinary | 128 (1.9%) | 23 (1.8%) | 303 (1.9%) | 63 (1.3%) |
| Obstetric/perinatal | 1 (0.0%) | 0 (0.0%) | 1 (0.0%) | 0 (0.0%) |
| Accidents | 156 (2.3%) | 22 (1.7%) | 260 (1.7%) | 71 (1.5%) |
| Suicide | 64 (0.9%) | 8 (0.6%) | 84 (0.5%) | 12 (0.3%) |
| Congenital/symptoms | 219 (3.2%) | 41 (3.2%) | 458 (2.9%) | 128 (2.7%) |
| Missing | 38 (0.6%) | 8 (0.6%) | 81 (0.5%) | 36 (0.8%) |
Notes: CAD indicates coronary artery disease; DM: diabetes mellitus.
Table 4.
Most Frequent Causes of Death Within Cancer-Related, Circulatory, and Endocrinologic Death
| No DM, no CAD | DM only | |
|---|---|---|
| Cancer death | 1887 (100%) | 319 (100%) |
| Gastro-intestinal cancer | 489 (25.9%) | 99 (31.0%) |
| Lung cancer | 502 (26.6%) | 81 (25.3%) |
| Female reproductive organ cancer | 193 (10.2%) | 39 (12.2%) |
| Urinary cancer incl. prostate | 215 (11.4%) | 33 (10.3%) |
| Endocrinologic death | 98 (100%) | 175 (100%) |
| Diabetes-related | 26 (26.5%) | 155 (88.6%) |
| Obesity | 11 (11.2%) | 9 (5.1%) |
| Cystic fibrosis | 50 (11.2%) | 11 (6.2%) |
| Circulatory death | 2,472 (100%) | 444 (100%) |
| Ischemic heart disease | 561 (22.7%) | 112 (25.2%) |
| Non-ischemic cardiomyopathy | 437 (17.7%) | 86 (19.4%) |
| Valve disease | 479 (19.4%) | 81 (18.2%) |
| Stroke | 334 (13.5%) | 55 (12.4%) |
Notes: CAD indicates coronary artery disease; DM: diabetes mellitus.
Subgroup and Sensitivity Analyses
Censoring follow-up if patients changed DM or CAD status during follow-up did not impact the results (Table S4). When adjusting for additional cardiovascular risk factor, the risk of all-cause death, cancer-related and circulatory death associated with DM and CAD increased compared to the age and sex adjusted model (Table S5). When stratifying DM patients without CAD based on DM-related microvascular complications at inclusion, we found that the presence of microvascular disease increased the risk of all-cause death and endocrinologic death (Table S6). We found that 8.9% (n = 554) of DM patients without CAD had type 1 DM. Furthermore, patients with type 1 diabetes had higher mortality than patients with type 2 DM, particularly driven by higher rates of endocrinologic death (Table S7). Accounting for the competing risk of other causes of death in the Fine-Gray regression analysis did not noticeably change the risk associated with DM in the three most common causes of death, when compared to patients with neither DM nor CAD (Table S8).
Discussion
We have previously shown that DM patients without CAD have a reduced risk of MI and cardiac death while being at increased risk of all-cause mortality compared to patients with neither DM nor CAD. The aim of the current study was to examine reasons for this discrepancy by investigating cause-specific deaths among patients with DM stratified by CAD status. Despite the absence of CAD, DM was associated with >8% higher absolute 10-year mortality when compared to patients with neither DM nor CAD. Excess mortality was partially driven by increased risks of circulatory and cancer-related deaths. However, endocrinologic death was the largest contributor of excess mortality, particularly among patients with type 1 DM, with a 10-year risk difference of 3.6% compared to patients with neither DM nor CAD, driven by DM-related complications such as diabetic nephropathy and ketoacidosis.
An important finding is that a major part of the excess mortality in DM patients without CAD is driven by DM-related microvascular disease. Chronic hyperglycemia negatively affects the microvasculature of several organs through endothelial damage, oxidative stress, and sorbitol production. This may lead to reduced kidney function, impaired vision, and reduced peripheral sensitivity.25 DM duration and poor glycaemic control are among the most important risk factors for DM-related microvascular disease, while hypertension, dyslipidemia, alcohol consumption, and smoking26 are other key contributing risk factors.26,27 A randomized controlled trial of 11,140 patients found that DM duration was independently associated with adverse microvascular events, defined as new or worsening retinopathy or nephropathy (HR 1.28 [95% CI 1.23–1.33]), as well as death (HR 1.15 [95% CI 1.10–1.20]) in patients with type 2 DM. The youngest age group (55–64 years) with the longest DM duration (>10 years) had the greatest risk of microvascular events.28 However, these patients had an elevated risk of CAD and may therefore not be representative of our non-CAD patients. The study nevertheless demonstrated an association between DM duration and DM-related microvascular disease possibly contributing to the excess mortality. Diabetic nephropathy is one of the most serious DM-related microvascular diseases and the leading cause of end-stage renal disease. Patients with advanced diabetic nephropathy are at high risk of cardiovascular death as well as renal failure.26,29 In our study, DM patients without CAD were much likely to have nephropathy than patients with neither DM nor CAD (7.0% versus 0.1%). Thus, diabetic nephropathy may have contributed to the observed excess circulatory death, as well as being an overall important driver of excess mortality in DM patients without CAD. A possible relationship between diabetic nephropathy and cardiovascular death could be the presence of microalbuminuria since microalbuminuria has been found to be a strong predictor for the development of both microvascular and macrovascular complications in DM patients.30,31 A Swedish cohort study that examined mortality and CAD extent in patients with type 1 DM referred for CAG found that patients with normal CAG findings (ie no CAD) died predominantly of renal failure (HR 2.29 [95% CI 1.77–2.96]).32 Renal failure was also found to be a strong predictor of mortality in patients with type 2 DM when compared to the general population of Italy.33
There is a known relationship between different microvascular diseases in diabetes, likely due to shared risk factors. Thus, the occurrence of one microvascular disease is a risk factor for developing another microvascular disease.34 In particular, diabetic nephropathy and diabetic retinopathy are strongly correlated with each other,35 followed by the association between diabetic retinopathy and diabetic peripheral neuropathy, as opposed to the weakest association between diabetic nephropathy and diabetic peripheral neuropathy.36 In the context of this study, this indicates that DM patients with any pre-existing microvascular disease at index CAG may have had a higher risk of developing diabetic nephropathy, which in turn may have led to renal failure and death. This explains perhaps why DM patients without CAD but with existing microvascular disease were observed to have a greater all-cause mortality.
We observed that DM patients had a higher risk of circulatory death despite the absence of CAD. While it was previously assumed that a DM diagnosis carried the same high risk of adverse cardiovascular events as having coronary heart disease,37 new evidence suggests that DM is indeed not a risk equivalent. According to a meta-analysis of 13 studies involving 45,108 patients, DM patients had a lower risk of adverse cardiovascular events compared to patients with known coronary heart disease.38 Consistently, we have previously shown that DM patients without CAD had a similar risk of MI as patients with neither DM nor CAD, suggesting that prophylactic treatment may have modified the risk of adverse cardiovascular events in DM patients without CAD.8 Nationwide Danish data has shown substantial reduction in cardiovascular morbidity and mortality in recent decades due to increased primary prophylactic treatment, including statin treatment.39 Continued statin treatment after CAG is associated with improved CVD risk among DM patients despite absence of CAD.40 In this cohort, we found that 69% of DM patients without CAD redeemed prescription on statin within 180 days after CAG, compared to 39% of patients with neither DM nor CAD. Although we did not find the risk of circulatory death to be equivalent between DM patients without CAD and patients with neither DM nor CAD in this study, the absolute 10-year risk difference was only 2.2%. While type 2 DM has a 2-fold increase in CVD risk,41 patients with type 1 DM have been reported have an up to 9-fold increased risk of CVD.42 However, circulatory mortalities were similarly increased in patients with type 1 DM and type 2 DM compared to patients with no DM in our study.
The relative risks of cancer-related death among DM patients were similarly increased in patients with and without CAD. While prophylactic treatment targeting cardiovascular risk factors in DM patients has been proven successful, resulting in lower cardiovascular mortality, it has also paved the way for DM-related cancers to become more prevalent causes of death in DM patients.43 While this might not be true in low-income countries with poor DM care, where infections and CAD are the primary causes of death, it is very much the case in high-income countries including Denmark.43–45 Cancer mortality has not decreased at the same rate as cardiovascular death in developed countries.46 A meta-analysis of 144 studies with over 32 million patients found that patients with type 2 DM had a 15–25% higher risk of incident all-site cancer and mortality compared to patients without type 2 DM.47 Several other studies have recognized DM as a potentially independent aetiological factor of mortality in a variety of cancers.48–51 This may explain why excess mortality due to cancer was observed among DM patients without CAD in this study.
Given these results, appropriate preventive measures should be implemented to reduce excess mortality associated with diabetic microvascular disease in DM patients despite the absence of CAD. Optimized prophylactic management targeting modifiable risk factors of DM-related microvascular disease could help reduce DM-related mortality. In other words, risk factors associated with disease onset and progression should be identified and managed as early as possible, especially early signs and symptoms of progressive chronic kidney disease. This requires systematic screening and increased awareness of DM-related microvascular disease.52
In the Steno-2 study, DM type 2 patients with microalbuminuria received intensified multifactorial intervention, targeting risk factors of both macrovascular- and microvascular diseases. The risk factors included microalbuminuria, hyperglycemia, dyslipidemia, and hypertension. After 21 years of follow-up, these patients had a 51% relative risk reduction for incident CVD, when compared to patients who received conventional multifactorial treatment according to the national guidelines. Furthermore, in the intensive-therapy group, the progression of retinopathy, autonomic neuropathy, and diabetic nephropathy was reduced by 33%, 41%, and 48%, respectively. A trend toward a lower risk of end-stage renal disease was also observed.53–55 In the future, newer reno-protective medications including sodium glucose transport protein 2 inhibitors,56,57 glucagon like peptide 1 analogues, and non-steroidal mineralocorticoid receptor antagonists58 may prevent the development of chronic kidney disease (CKD) and delay the worsening of CKD and thus prevent deaths due to CKD in patients with DM type 2.59 We note that only few DM patients in our study were in treatment with sodium glucose transport protein 2 inhibitors or glucagon like peptide 1 analogues.
Limitations
Firstly, we did not have access to HbA1c measurements. As a result, DM status was determined using multiple validated, high-quality, national healthcare registries. Secondly, CAG does not describe lesion morphology and vessel wall vulnerability. This means that some patients may have had vulnerable plaques that could not be identified by CAG. Thirdly, we obtained our outcomes from the national databases instead of reviewing medical records on an individual basis. This may have influenced our results, as the accuracy of the register on causes of death depends solely on the coding done by individual physicians.19 Also, diagnostic codes are often provided by junior doctors, who may not always be familiar enough with patients’ medical history to provide accurate codes for the underlying cause of death. There may be a bias in classifying circulatory death in patients with pre-existing circulatory disease, as they may be assigned circulatory death more often than others. The same might be true for DM patients and death due to DM-related diseases. Furthermore, changes in coding practice and procedures, as well as low autopsy rates may also have influenced the validity of the reported cause-specific death.19 Fourthly, we do not have access to cholesterol or triglyceride measurements. Finally, since WHO has classified Denmark as a cardiovascular low-risk country, our findings may not be representative for intermediate and high-risk countries.
Conclusion
In conclusion, excess mortality was partly due to increased risks of circulatory- and cancer-related deaths. However, endocrinologic death emerged as a leading cause of death, with DM-related microvascular disease being an important driver of death in DM patients without CAD when compared to patients with neither DM nor CAD.
Acknowledgment
The abstract of this paper was presented at the European Society of Cardiology Congress 2022 as a moderated poster presentation with interim findings. The poster’s abstract was published in the European Heart Journal, Volume 43, Issue Supplement 2.
Funding Statement
This study was funded by the Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Abbreviations
CAG, Coronary angiography; CAD, Coronary artery disease; DM, Diabetes mellitus; MI, Myocardial infarction; CVD, Cardiovascular disease; PAD, Peripheral artery disease; BMI, Body Mass Index; CABG, Coronary artery bypass grafting; PCI, Percutaneous coronary intervention; WHO, World Health Organization; ICD-10, International Classification of Diseases 10th revision; ATC, Anatomical Therapeutic Chemical; CIP, Cumulative incidence proportion; HR, Hazard ratio.
Data Sharing Statement
According to Danish data protection regulations, data cannot be made publicly available.
Ethics Approval and Informed Consent
This study was approved by the Danish Data Protection Agency (record no. 1-16-02-193-18), and the requirement for informed consent was waived by our local institutional ethical board (record no. 14-45-70-24-22).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Consent for Publication
Per Danish regulation, observational, noninterventional registry-based studies require only a waiver from the local ethics board to fulfill informed consent requirements.
Disclosure
MM is supported by a grant from the Novo Nordisk Foundation (grant number NNF22OC0074083); has received institutional research grants from Bayer and Novo Nordisk; and has received lecture and/or advisory board fees from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Novo Nordisk. He also has an institutional research contract with Philips and Janssen and reports equity interests from Eli Lilly and Verve Therapeutics. KWO has received a research grant from the Danish Cardiovascular Academy. All other authors report no conflicts of interest in this work.
References
- 1.Folsom AR, Szklo M, Stevens J, Liao F, Smith R, Eckfeldt JH. A prospective study of coronary heart disease in relation to fasting insulin, glucose, and diabetes. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 1997;20(6):935–942. doi: 10.2337/diacare.20.6.935 [DOI] [PubMed] [Google Scholar]
- 2.Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–1258. doi: 10.4239/wjd.v6.i13.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain MS, Sharma G. The Burden of Cardiovascular Diseases Due to COVID-19 Pandemic. Thorac Cardiovasc Surg. 2024;72(1):40–50. doi: 10.1055/s-0042-1755205 [DOI] [PubMed] [Google Scholar]
- 4.Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2014;7:169–183. doi: 10.2147/DMSO.S61438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain MS, Gupta G, Goyal A, et al. From nature to therapy: luteolin’s potential as an immune system modulator in inflammatory disorders. J Biochem Mol Toxicol. 2023;37(11):e23482. doi: 10.1002/jbt.23482 [DOI] [PubMed] [Google Scholar]
- 6.Hussain MS, Altamimi ASA, Afzal M, et al. Kaempferol: paving the path for advanced treatments in aging-related diseases. Exp Gerontol. 2024;188:112389. doi: 10.1016/j.exger.2024.112389 [DOI] [PubMed] [Google Scholar]
- 7.Saely CH, Aczel S, Marte T, Langer P, Drexel H. Cardiovascular complications in Type 2 diabetes mellitus depend on the coronary angiographic state rather than on the diabetic state. Diabetologia. 2004;47(1):145–146. doi: 10.1007/s00125-003-1274-6 [DOI] [PubMed] [Google Scholar]
- 8.Olesen KKW, Madsen M, Egholm G, et al. Patients With Diabetes Without Significant Angiographic Coronary Artery Disease Have the Same Risk of Myocardial Infarction as Patients Without Diabetes in a Real-World Population Receiving Appropriate Prophylactic Treatment. Diabetes Care. 2017;40(8):1103–1110. doi: 10.2337/dc16-2388 [DOI] [PubMed] [Google Scholar]
- 9.Olesen KKW, Madsen M, Gyldenkerne C, et al. Ten-year cardiovascular risk in diabetes patients without obstructive coronary artery disease: a retrospective Western Denmark cohort study. Cardiovasc Diabetol. 2021;20(1):23. doi: 10.1186/s12933-021-01212-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyldenkerne C, Olesen KK, Thrane PG, et al. Diabetes is not a risk factor for myocardial infarction in patients without coronary artery disease: a study from the Western Denmark Heart Registry. Diab Vasc Dis Res. 2020;17(4):1479164120941809. doi: 10.1177/1479164120941809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olesen KKW, Madsen M, Gyldenkerne C, et al. Diabetes Mellitus Is Associated With Increased Risk of Ischemic Stroke in Patients With and Without Coronary Artery Disease. Stroke. 2019;50(12):3347–3354. doi: 10.1161/STROKEAHA.119.026099 [DOI] [PubMed] [Google Scholar]
- 12.Seyed Ahmadi S, Svensson AM, Pivodic A, Rosengren A, Lind M. Risk of atrial fibrillation in persons with type 2 diabetes and the excess risk in relation to glycaemic control and renal function: a Swedish cohort study. Cardiovasc Diabetol. 2020;19(1):9. doi: 10.1186/s12933-019-0983-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pop-Busui R, Januzzi JL, Bruemmer D, et al. Heart Failure: an Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care. 2022;45(7):1670–1690. doi: 10.2337/dci22-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt M, Maeng M, Madsen M, Sorensen HT, Jensen LO, Jakobsen CJ. The Western Denmark Heart Registry: its Influence on Cardiovascular Patient Care. J Am Coll Cardiol. 2018;71(11):1259–1272. doi: 10.1016/j.jacc.2017.10.110 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 16.Mainz J, Hess MH, Johnsen SP. The Danish unique personal identifier and the Danish Civil Registration System as a tool for research and quality improvement. Int J Qual Health Care. 2019;31(9):717–720. doi: 10.1093/intqhc/mzz008 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pottegard A, Schmidt SAJ, Wallach-Kildemoes H, Sorensen HT, Hallas J, Schmidt M. Data Resource Profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46(3):798–798f. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39(7 Suppl):26–29. doi: 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 20.Isaksen AA, Sandbaek A, Bjerg L. Validation of Register-Based Diabetes Classifiers in Danish Data. Clin Epidemiol. 2023;15:569–581. doi: 10.2147/CLEP.S407019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gribsholt SB, Farkas DK, Thomsen RW, Richelsen B, Sorensen HT. Mortality Among Danish Patients with a Hospital Diagnosis of Overweight or Obesity Over a 40-Year Period. Clin Epidemiol. 2022;14:309–325. doi: 10.2147/CLEP.S350459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boisen AB, Dalager-Pedersen M, Sogaard M, Mortensen R, Thomsen RW. Relationship between death and infections among patients hospitalized in internal medicine departments: a prevalence and validation study. Am J Infect Control. 2014;42(5):506–510. doi: 10.1016/j.ajic.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 24.Olesen K, Gyldenkerne C, Thrane PG, Maeng M. Causes of excess mortality in diabetes patients without coronary artery disease. Eur Heart J. 2022;43(Supplement_2). doi: 10.1093/eurheartj/ehac544.2402 [DOI] [Google Scholar]
- 25.American Diabetes A. Microvascular Complications and Foot Care: standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S124–S138. doi: 10.2337/dc19-S011 [DOI] [PubMed] [Google Scholar]
- 26.Girach A, Manner D, Porta M. Diabetic microvascular complications: can patients at risk be identified? A review. Int J Clin Pract. 2006;60(11):1471–1483. doi: 10.1111/j.1742-1241.2006.01175.x [DOI] [PubMed] [Google Scholar]
- 27.Silbernagel G, Rosinger S, Grammer TB, et al. Duration of type 2 diabetes strongly predicts all-cause and cardiovascular mortality in people referred for coronary angiography. Atherosclerosis. 2012;221(2):551–557. doi: 10.1016/j.atherosclerosis.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 28.Zoungas S, Woodward M, Li Q, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–2474. doi: 10.1007/s00125-014-3369-7 [DOI] [PubMed] [Google Scholar]
- 29.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 30.Gall MA, Borch-Johnsen K, Hougaard P, Nielsen FS, Parving HH. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes. 1995;44(11):1303–1309. doi: 10.2337/diab.44.11.1303 [DOI] [PubMed] [Google Scholar]
- 31.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310(6):356–360. doi: 10.1056/NEJM198402093100605 [DOI] [PubMed] [Google Scholar]
- 32.Ritsinger V, Hero C, Svensson AM, et al. Mortality and extent of coronary artery disease in 2776 patients with type 1 diabetes undergoing coronary angiography: a nationwide study. Eur J Prev Cardiol. 2017;24(8):848–857. doi: 10.1177/2047487316687860 [DOI] [PubMed] [Google Scholar]
- 33.Bo S, Ciccone G, Rosato R, et al. Renal damage in patients with Type 2 diabetes: a strong predictor of mortality. Diabet Med. 2005;22(3):258–265. doi: 10.1111/j.1464-5491.2004.01394.x [DOI] [PubMed] [Google Scholar]
- 34.Teliti M, Cogni G, Sacchi L, et al. Risk factors for the development of micro-vascular complications of type 2 diabetes in a single-centre cohort of patients. Diab Vasc Dis Res. 2018;15(5):424–432. doi: 10.1177/1479164118780808 [DOI] [PubMed] [Google Scholar]
- 35.El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, Moharram OA, Kangave D. Retinopathy as a predictor of other diabetic complications. Int Ophthalmol. 2001;24(1):1–11. doi: 10.1023/a:1014409829614 [DOI] [PubMed] [Google Scholar]
- 36.Girach A, Vignati L. Diabetic microvascular complications--can the presence of one predict the development of another? J Diabetes Complications. 2006;20(4):228–237. doi: 10.1016/j.jdiacomp.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 37.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404 [DOI] [PubMed] [Google Scholar]
- 38.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26(2):142–148. doi: 10.1111/j.1464-5491.2008.02640.x [DOI] [PubMed] [Google Scholar]
- 39.Gyldenkerne C, Knudsen JS, Olesen KKW, et al. Nationwide Trends in Cardiac Risk and Mortality in Patients With Incident Type 2 Diabetes: a Danish Cohort Study. Diabetes Care. 2021;44(10):2353–2360. doi: 10.2337/dc21-0383 [DOI] [PubMed] [Google Scholar]
- 40.Olesen KKW, Heide-Jørgensen U, Thim T, et al. Statin but not aspirin treatment is associated with reduced cardiovascular risk in patients with diabetes without obstructive coronary artery disease: a cohort study from the Western Denmark Heart Registry. Eur Heart J Cardiovasc Pharm. 2021;8(5):434–441. doi: 10.1093/ehjcvp/pvab040 [DOI] [PubMed] [Google Scholar]
- 41.Risk Factors C E, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai X, Li J, Cai W, et al. Meta-analysis of type 1 diabetes mellitus and risk of cardiovascular disease. J Diabetes Complications Apr. 2021;35(4):107833. doi: 10.1016/j.jdiacomp.2020.107833 [DOI] [PubMed] [Google Scholar]
- 43.Pearson-Stuttard J, Bennett J, Cheng YJ, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9(3):165–173. doi: 10.1016/S2213-8587(20)30431-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zargar AH, Wani AI, Masoodi SR, et al. Causes of mortality in diabetes mellitus: data from a tertiary teaching hospital in India. Postgrad Med J. 2009;85(1003):227–232. doi: 10.1136/pgmj.2008.067975 [DOI] [PubMed] [Google Scholar]
- 45.Zargar AH, Wani AI, Masoodi SR, Laway BA, Bashir MI. Mortality in diabetes mellitus--data from a developing region of the world. Diabet Res Clin Pract. 1999;43(1):67–74. doi: 10.1016/s0168-8227(98)00112-0 [DOI] [PubMed] [Google Scholar]
- 46.Song M, Vogelstein B, Giovannucci EL, Willett WC, Tomasetti C. Cancer prevention: molecular and epidemiologic consensus. Science. 2018;361(6409):1317–1318. doi: 10.1126/science.aau3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ling S, Brown K, Miksza JK, et al. Association of Type 2 Diabetes With Cancer: a Meta-analysis With Bias Analysis for Unmeasured Confounding in 151 Cohorts Comprising 32 Million People. Diabetes Care. 2020;43(9):2313–2322. doi: 10.2337/dc20-0204 [DOI] [PubMed] [Google Scholar]
- 48.Ling S, Brown K, Miksza JK, et al. Risk of cancer incidence and mortality associated with diabetes: a systematic review with trend analysis of 203 cohorts. Nutr, Metab Cardiovasc Dis. 2021;31(1):14–22. doi: 10.1016/j.numecd.2020.09.023 [DOI] [PubMed] [Google Scholar]
- 49.Forssas E, Sund R, Manderbacka K, Arffman M, Ilanne-Parikka P, Keskimaki I. Increased cancer mortality in diabetic people treated with insulin: a register-based follow-up study. BMC Health Serv Res. 2013;13:267. doi: 10.1186/1472-6963-13-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavallari I, Bhatt DL, Steg PG, et al. Causes and Risk Factors for Death in Diabetes: a Competing-Risk Analysis From the SAVOR-TIMI 53 Trial. J Am Coll Cardiol. 2021;77(14):1837–1840. doi: 10.1016/j.jacc.2021.02.030 [DOI] [PubMed] [Google Scholar]
- 51.Harborg S, Kjaergaard KA, Thomsen RW, Borgquist S, Cronin-Fenton D, Hjorth CF. New Horizons: epidemiology of Obesity, Diabetes Mellitus, and Cancer Prognosis. J Clin Endocrinol Metab. 2023. doi: 10.1210/clinem/dgad450 [DOI] [PubMed] [Google Scholar]
- 52.Crasto W, Patel V, Davies MJ, Khunti K. Prevention of Microvascular Complications of Diabetes. Endocr Metab Clinics. 2021;50(3):431–455. [DOI] [PubMed] [Google Scholar]
- 53.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778 [DOI] [PubMed] [Google Scholar]
- 54.Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:9153):617–22. doi: 10.1016/S0140-6736(98)07368-1 [DOI] [PubMed] [Google Scholar]
- 55.Gaede P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016;59(11):2298–2307. doi: 10.1007/s00125-016-4065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cha AS, Chen Y, Fazioli K, Rivara MB, Devine EB. Microvascular Benefits of New Antidiabetic Agents: a Systematic Review and Network Meta-Analysis of Kidney Outcomes. J Clin Endocrinol Metab. 2021;106(4):1225–1234. doi: 10.1210/clinem/dgaa894 [DOI] [PubMed] [Google Scholar]
- 57.Rangaswami J, Bhalla V, de Boer IH, et al. Cardiorenal Protection With the Newer Antidiabetic Agents in Patients With Diabetes and Chronic Kidney Disease: a Scientific Statement From the American Heart Association. Circulation. 2020;142(17):e265–e286. doi: 10.1161/CIR.0000000000000920 [DOI] [PubMed] [Google Scholar]
- 58.Forst T, Mathieu C, Giorgino F, et al. New strategies to improve clinical outcomes for diabetic kidney disease. BMC Med. 2022;20(1):337. doi: 10.1186/s12916-022-02539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perkovic V, Tuttle KR, Rossing P, et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N Engl J Med. doi: 10.1056/NEJMoa2403347 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
According to Danish data protection regulations, data cannot be made publicly available.