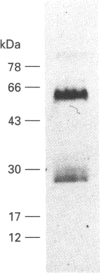
Abstract
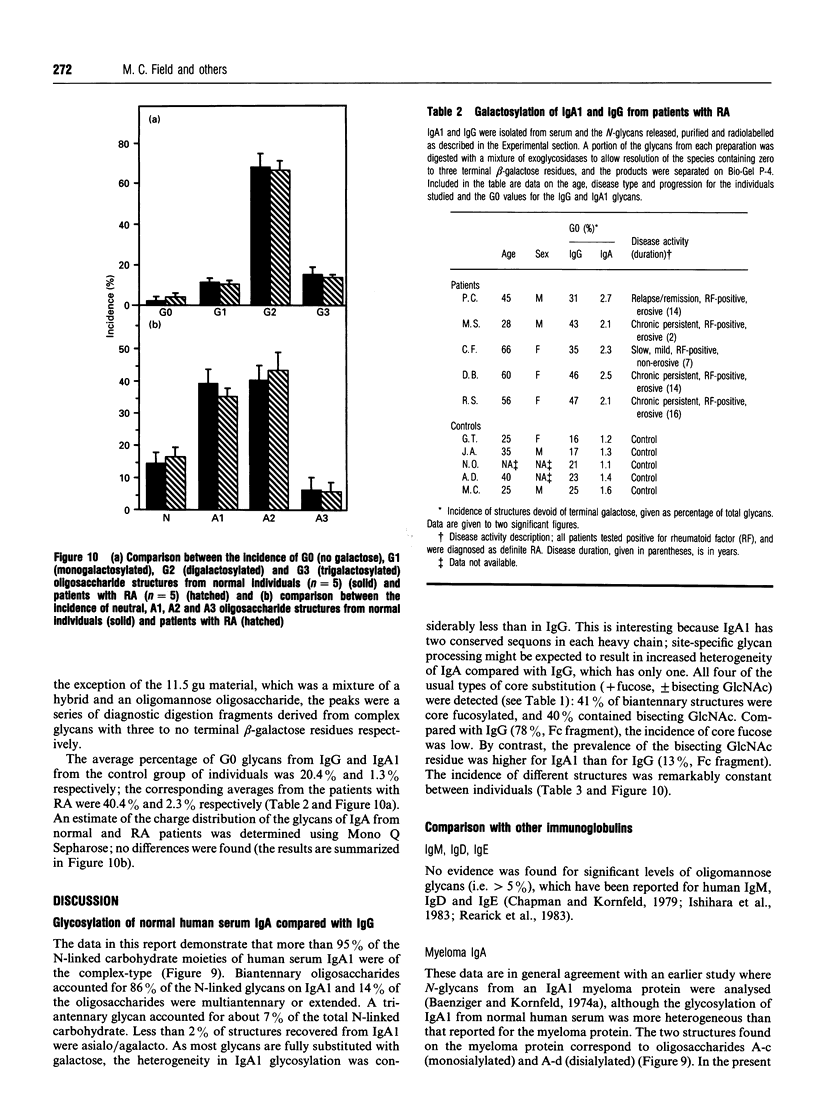
The primary structures of the N-linked oligosaccharides from normal human serum IgA1 were determined by a combination of sequential exoglycosidase digestion, Bio-Gel P-4 chromatography, anion-exchange chromatography and one-dimensional n.m.r. spectroscopy. Three major N-linked disialylated biantennary-complex-type structures were found (55%). The remaining N-linked oligosaccharides consisted of at least nine further structures, some of which (7%) were of the triantennary type and included disialylated triantennary oligosaccharides with outer-arm fucose substitution [Fuc alpha 1-3(4)]. Compared with IgG, the N-glycan structures on IgA are more completely processed: the outer arms have a higher proportion of galactose and sialic acid, and only trace levels of incompletely galactosylated oligosaccharides, commonly found on IgG, were detected. Analysis of the sialylated O-glycans revealed that 64% were [NeuAc2 alpha 3(6)]2Gal beta 3GalNAc and 9% were [NeuAc2 alpha 3(6)]-Gal beta 4GlcNAc beta 6[NeuAc2 alpha 3(6)Gal beta 3]GalNAc, and 27% were monosialylated. The N-linked glycosylation of both serum IgA1 and IgG isolated from a group of six normal individuals was compared with that from ten patients with rheumatoid arthritis (RA). In contrast with the hypogalactosylation found in IgG from diseased sera, there was no evidence of an equivalent decrease in the galactosylation of the IgA1 oligosaccharides. In addition, the N-glycosylation of IgA1 was remarkably consistent within the group of normal individuals. These data suggest that incomplete galactosylation of N-linked glycans and its augmentation in RA does not extend to IgA1 and that the RA-associated galactosyltransferase deficiency may be restricted to cells producing gamma-chain.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford D., Dwek R. A., Welply J. K., Amatayakul S., Homans S. W., Lis H., Taylor G. N., Sharon N., Rademacher T. W. The beta 1----2-D-xylose and alpha 1----3-L-fucose substituted N-linked oligosaccharides from Erythrina cristagalli lectin. Isolation, characterisation and comparison with other legume lectins. Eur J Biochem. 1987 Jul 15;166(2):311–320. doi: 10.1111/j.1432-1033.1987.tb13516.x. [DOI] [PubMed] [Google Scholar]
- Axford J. S., Sumar N., Alavi A., Isenberg D. A., Young A., Bodman K. B., Roitt I. M. Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J Clin Invest. 1992 Mar;89(3):1021–1031. doi: 10.1172/JCI115643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7260–7269. [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7270–7281. [PubMed] [Google Scholar]
- Borén T., Falk P., Roth K. A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993 Dec 17;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- Butters T. D., Scudder P., Rotsaert J., Petursson S., Fleet G. W., Willenbrock F. W., Jacob G. S. Purification to homogeneity of Charonia lampas alpha-fucosidase by using sequential ligand-affinity chromatography. Biochem J. 1991 Oct 1;279(Pt 1):189–195. doi: 10.1042/bj2790189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A., Kornfeld R. Structure of the high mannose oligosaccharides of a human IgM myeloma protein. I. The major oligosaccharides of the two high mannose glycopeptides. J Biol Chem. 1979 Feb 10;254(3):816–823. [PubMed] [Google Scholar]
- Chevailler A., Monteiro R. C., Kubagawa H., Cooper M. D. Immunofluorescence analysis of IgA binding by human mononuclear cells in blood and lymphoid tissue. J Immunol. 1989 Apr 1;142(7):2244–2249. [PubMed] [Google Scholar]
- Fanger M. W., Lydyard P. M. Receptors for IgA on human lymphocytes--I. detection and specificity. Mol Immunol. 1981 Mar;18(3):189–195. doi: 10.1016/0161-5890(81)90085-7. [DOI] [PubMed] [Google Scholar]
- Field M. C., Dwek R. A., Edge C. J., Rademacher T. W. O-linked oligosaccharides from human serum immunoglobulin A1. Biochem Soc Trans. 1989 Dec;17(6):1034–1035. doi: 10.1042/bst0171034. [DOI] [PubMed] [Google Scholar]
- Fortune F., Kingston J., Barnes C. S., Lehner T. Identification and characterization of IgA and Vicia villosa-binding T cell subsets in rheumatoid arthritis. Clin Exp Immunol. 1990 Feb;79(2):202–208. doi: 10.1111/j.1365-2249.1990.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter A., Hiemstra P. S., van der Voort E. A., van Es L. A., Daha M. R. Binding of human IgA1 to rat peritoneal macrophages. Immunology. 1988 Jun;64(2):207–212. [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Goroff D. K. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol. 1983 Jun;130(6):2882–2885. [PubMed] [Google Scholar]
- Hiemstra P. S., Gorter A., Stuurman M. E., Van Es L. A., Daha M. R. Activation of the alternative pathway of complement by human serum IgA. Eur J Immunol. 1987 Mar;17(3):321–326. doi: 10.1002/eji.1830170304. [DOI] [PubMed] [Google Scholar]
- Homans S. W., Dwek R. A., Fernandes D. L., Rademacher T. W. The analysis of coupling networks in a complex oligosaccharide mixture derived from the Fc region of rabbit immunoglobulin G using 1H-1H correlated NMR spectroscopy combined with double quantum NMR spectroscopy. Biochim Biophys Acta. 1984 Mar 22;798(1):78–83. doi: 10.1016/0304-4165(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Tejima S., Takahashi N., Takayasu T., Shinoda T. Structure and location of asparagine-linked oligosaccharides in the Fc region of A human immunoglobulin D. Biochem Biophys Res Commun. 1983 Jan 14;110(1):181–186. doi: 10.1016/0006-291x(83)91277-9. [DOI] [PubMed] [Google Scholar]
- Kerr M. A. The structure and function of human IgA. Biochem J. 1990 Oct 15;271(2):285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Weigle W. O., Spiegelberg H. L. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975 Feb;55(2):368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. O., Connolly J. M., Ramirez-Soto D., Poretz R. D. The polypeptide of immunoglobulin G influences its galactosylation in vivo. J Biol Chem. 1990 Apr 5;265(10):5833–5839. [PubMed] [Google Scholar]
- Loomes L. M., Stewart W. W., Mazengera R. L., Senior B. W., Kerr M. A. Purification and characterization of human immunoglobulin IgA1 and IgA2 isotypes from serum. J Immunol Methods. 1991 Aug 9;141(2):209–218. doi: 10.1016/0022-1759(91)90147-8. [DOI] [PubMed] [Google Scholar]
- Maliszewski C. R., March C. J., Schoenborn M. A., Gimpel S., Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990 Dec 1;172(6):1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazengera R. L., Kerr M. A. The specificity of the IgA receptor purified from human neutrophils. Biochem J. 1990 Nov 15;272(1):159–165. doi: 10.1042/bj2720159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Structures of the oligosaccharides present at the three asparagine-linked glycosylation sites of human IgD. J Biol Chem. 1983 Oct 10;258(19):11546–11556. [PubMed] [Google Scholar]
- Millet I., Panaye G., Revillard J. P. Expression of receptors for IgA on mitogen-stimulated human T lymphocytes. Eur J Immunol. 1988 Apr;18(4):621–626. doi: 10.1002/eji.1830180420. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Mizuochi T., Kobata A. Structures of the carbohydrate moieties of secretory component purified from human milk. J Biol Chem. 1982 Aug 25;257(16):9612–9621. [PubMed] [Google Scholar]
- Mizuochi T., Taniguchi T., Shimizu A., Kobata A. Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J Immunol. 1982 Nov;129(5):2016–2020. [PubMed] [Google Scholar]
- Monteiro R. C., Kubagawa H., Cooper M. D. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J Exp Med. 1990 Mar 1;171(3):597–613. doi: 10.1084/jem.171.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafson R. W., Thomas J. R., Ferguson M. A., Dwek R. A., Chaudhuri M., Chang K. P., Rademacher T. W. Structures of the N-linked oligosaccharides of Gp63, the major surface glycoprotein, from Leishmania mexicana amazonensis. J Biol Chem. 1990 Jul 25;265(21):12240–12247. [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Thomas J. R., Opdenakker G., Rademacher T. W., Wittwer A. J., Howard S. C., Nelson R., Siegel N. R., Jennings M. G. Cell-type-specific and site-specific N-glycosylation of type I and type II human tissue plasminogen activator. Biochemistry. 1989 Sep 19;28(19):7644–7662. doi: 10.1021/bi00445a021. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Roitt I. M., Isenberg D. A., Dwek R. A., Ansell B. M., Rademacher T. W. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988 Apr 30;1(8592):966–969. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- Parekh R., Isenberg D., Rook G., Roitt I., Dwek R., Rademacher T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun. 1989 Apr;2(2):101–114. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Parekh R., Roitt I., Isenberg D., Dwek R., Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988 May 1;167(5):1731–1736. doi: 10.1084/jem.167.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. J., Smith K. F., Amatayakul S., Ashford D., Rademacher T. W., Dwek R. A., Lachmann P. J., Harrison R. A. Two-domain structure of the native and reactive centre cleaved forms of C1 inhibitor of human complement by neutron scattering. J Mol Biol. 1990 Aug 5;214(3):751–763. doi: 10.1016/0022-2836(90)90290-3. [DOI] [PubMed] [Google Scholar]
- Pierce-Cretel A., Debray H., Montreuil J., Spik G., Van Halbeek H., Mutsaers J. H., Vliegenthart J. F. Primary structure of N-glycosidically linked asialoglycans of secretory immunoglobulins A from human milk. Eur J Biochem. 1984 Mar 1;139(2):337–349. doi: 10.1111/j.1432-1033.1984.tb08012.x. [DOI] [PubMed] [Google Scholar]
- Pierce-Cretel A., Pamblanco M., Strecker G., Montreuil J., Spik G., Dorland L., Van Halbeek H., Vliegenthart J. F. Primary structure of the N-glycosidically linked sialoglycans of secretory immunoglobulins A from human milk. Eur J Biochem. 1982 Jul;125(2):383–388. doi: 10.1111/j.1432-1033.1982.tb06694.x. [DOI] [PubMed] [Google Scholar]
- Pierce-Cretel A., Pamblanco M., Strecker G., Montreuil J., Spik G. Heterogeneity of the glycans O-glycosidically linked to the hinge region of secretory immunoglobulins from human milk. Eur J Biochem. 1981;114(1):169–178. doi: 10.1111/j.1432-1033.1981.tb06188.x. [DOI] [PubMed] [Google Scholar]
- Putnam F. W., Liu Y. S., Low T. L. Primary structure of a human IgA1 immunoglobulin. IV. Streptococcal IgA1 protease, digestion, Fab and Fc fragments, and the complete amino acid sequence of the alpha 1 heavy chain. J Biol Chem. 1979 Apr 25;254(8):2865–2874. [PubMed] [Google Scholar]
- Rademacher T. W., Homans S. W., Parekh R. B., Dwek R. A. Immunoglobulin G as a glycoprotein. Biochem Soc Symp. 1986;51:131–148. [PubMed] [Google Scholar]
- Rademacher T. W. Network theory of glycosylation--etiologic and pathogenic implications of changes in IgG glycoform levels in autoimmunity. Semin Cell Biol. 1991 Oct;2(5):327–337. [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A., Isenberg D., Rook G., Axford J. S., Roitt I. The role of IgG glycoforms in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1988;10(2-3):231–249. doi: 10.1007/BF01857227. [DOI] [PubMed] [Google Scholar]
- Rearick J. I., Kulczycki A., Jr, Kornfeld S. Structural studies of oligosaccharides of rat IgE and reexamination of the high-mannose oligosaccharide of human IgE. Arch Biochem Biophys. 1983 Jan;220(1):95–105. doi: 10.1016/0003-9861(83)90391-0. [DOI] [PubMed] [Google Scholar]
- Roque-Barreira M. C., Campos-Neto A. Jacalin: an IgA-binding lectin. J Immunol. 1985 Mar;134(3):1740–1743. [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986 Mar;64(3):163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Scudder P., Neville D. C., Butters T. D., Fleet G. W., Dwek R. A., Rademacher T. W., Jacob G. S. The isolation by ligand affinity chromatography of a novel form of alpha-L-fucosidase from almond. J Biol Chem. 1990 Sep 25;265(27):16472–16477. [PubMed] [Google Scholar]
- Shao M. C., Chin C. C., Caprioli R. M., Wold F. The regulation of glycan processing in glycoproteins. The effect of avidin on individual steps in the processing of biotinylated glycan derivatives. J Biol Chem. 1987 Mar 5;262(7):2973–2979. [PubMed] [Google Scholar]
- Stewart W. W., Kerr M. A. The specificity of the human neutrophil IgA receptor (Fc alpha R) determined by measurement of chemiluminescence induced by serum or secretory IgA1 or IgA2. Immunology. 1990 Nov;71(3):328–334. [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Ishii I., Ishihara H., Mori M., Tejima S., Jefferis R., Endo S., Arata Y. Comparative structural study of the N-linked oligosaccharides of human normal and pathological immunoglobulin G. Biochemistry. 1987 Feb 24;26(4):1137–1144. doi: 10.1021/bi00378a023. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Mizuochi T., Kobata A. Hydrazinolysis of asparagine-linked sugar chains to produce free oligosaccharides. Methods Enzymol. 1982;83:263–268. doi: 10.1016/0076-6879(82)83019-x. [DOI] [PubMed] [Google Scholar]
- Tomana M., Niedermeier W., Mestecky J., Skvaril F. The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry. 1976 Apr;13(4):325–328. doi: 10.1016/0019-2791(76)90342-6. [DOI] [PubMed] [Google Scholar]
- Toraño A., Putnam F. W. Complete amino acid sequence of the alpha 2 heavy chain of a human IgA2 immunoglobulin of the A2m (2) allotype. Proc Natl Acad Sci U S A. 1978 Feb;75(2):966–969. doi: 10.1073/pnas.75.2.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzukida Y., Wang C. C., Putnam F. W. Structure of the A2m(1) allotype of human IgA--a recombinant molecule. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1104–1108. doi: 10.1073/pnas.76.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Williams R. C., Jr Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med. 1976 Nov 2;144(5):1227–1242. doi: 10.1084/jem.144.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. B., Platt F. M., Isenberg D. A., Rademacher T. W. Aberrant control of galactosyltransferase in peripheral B lymphocytes and Epstein-Barr virus transformed B lymphoblasts from patients with rheumatoid arthritis. J Rheumatol. 1993 Aug;20(8):1282–1287. [PubMed] [Google Scholar]
- Wilton J. M. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978 Dec;34(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- Yeaman G. R., Kerr M. A. Opsonization of yeast by human serum IgA anti-mannan antibodies and phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1987 Apr;68(1):200–208. [PMC free article] [PubMed] [Google Scholar]
- Young N. M., Jackson G. E., Brisson J. R. The glycopeptides of the mouse immunoglobulin A T15. Mol Immunol. 1990 Nov;27(11):1083–1090. doi: 10.1016/0161-5890(90)90096-i. [DOI] [PubMed] [Google Scholar]