Abstract
Atrial fibrillation (AF) is associated with an increased risk of myocardial infarction (MI) and vice versa. This bidirectional association relies on shared risk factors as well as several direct and indirect mechanisms, through which one condition can predispose to the other — including inflammation, atrial ischaemia, left ventricular remodelling, myocardial oxygen supply–demand mismatch and coronary artery embolism. Patients with both AF and MI are at greater risk of stroke, heart failure (HF) and death than patients with only one of the conditions. In this Review, we describe the bidirectional association between AF and MI. We discuss the pathogenic basis of this bidirectional relationship, describe the risk of adverse outcomes when the two conditions co-exist, and review current data and guidelines on the prevention and management of both conditions. We also identify important gaps in the literature and propose directions for future research on the bidirectional association between AF and MI. The Review also features a summary of methodological approaches for studying bidirectional associations in population-based studies.
Introduction
Atrial fibrillation (AF) and myocardial infarction (MI) are major contributors to cardiovascular disease burden worldwide1. The numbers of prevalent cases of both AF and MI have almost doubled over the past 30 years, and both conditions are associated with a substantial risk of adverse outcomes1. Regardless of temporality, co-occurrence of AF and MI is common2.
The risk of MI in patients with AF is especially high in the first 30 days after AF diagnosis3, pointing to mechanisms beyond accumulation of underlying shared risk factors such as inflammation, coronary artery embolism and increased myocardial oxygen demand. In addition, the risk of new-onset AF is especially high in the first couple of days after acute MI (AMI)4, suggesting that AF is the result of atrial ischaemia, inflammation, remodelling and heart failure (HF). Patients with new-onset AF at the time of AMI have a higher risk of HF, stroke and death than those with AMI who do not develop AF, even several years after the cardiac event5–7. Studies published in the past 2 years have shown that the co-existence of AF and MI is associated with higher mortality than with one of these conditions alone8,9, highlighting the importance of future studies on prevention.
In this Review, we begin by summarizing historical data on the association between AF and MI and then describe studies, published in the past ~15 years, on the risk of MI in patients with AF and vice versa. We discuss the pathogenic basis of their bidirectional relationship, including shared risk factors and direct pathophysiological mechanisms. Furthermore, we describe the risk of adverse outcomes in patients with a coexistence of MI and AF and discuss potential preventive and management strategies. The Review also includes a summary of methodological approaches to the analysis of bidirectional associations in population-based studies (Box 1). Finally, we identify important gaps in the literature and propose directions for future research on the bidirectional association between AF and MI.
Box 1 |. Assessing bidirectional relationships.
Various statistical methodologies exist to assess bidirectional relationships between two conditions in population-based studies. The collection of longitudinal data allows the determination of temporality between exposure and outcome. In a classical analysis of survival data, by comparing Kaplan–Meier curves and using the Cox proportional hazards model, one would assess bidirectionality by testing two separate hypotheses to determine whether event ‘A’ is associated with event ‘B’ and vice versa154.
Multistate models are particularly well suited for analysing the temporality between multiple events155. These models use a life-course framework, in which individuals are allowed to transition between different disease ‘states’. Multistate models can be used to assess multiple disease pathways simultaneously, as was done in a study of the temporal association between myocardial infarction, chronic heart failure, atrial fibrillation (AF) and stroke2. These models are very flexible and allow for repeated events and multiple event types, and account for temporality. Special cases of multistate models include the competing risks model and the illness–death model. As an example, an illness–death model was used as a study of AF and fracture risk, which allowed the analysis to account for the time-varying nature of the exposure (AF status) and for the competing risk of death155. It should be noted that overly complex multistate models should be avoided as they can be difficult to interpret and can be limited by an insufficient sample size or number of events that prevents estimation of all transitions.
Mendelian randomization is one method that allows the assessment of causality in the setting of observational data156,157. Briefly, Mendelian randomization uses a genetic variant as an instrumental variable, or proxy, for the exposure variable. As the genetic variant is present at birth and is free from confounding by post-birth factors, the analysis resembles a randomized controlled trial, in which causal relationships can be identified. Bidirectional Mendelian randomization extends this technique by using two genetic instrumental variables, one for each exposure, A and B158–160. If a bidirectional relationship exists, the genetic instrumental variables for A will be associated with B, and the genetic instrumental variables for B will be associated with A. The challenges with assessing bidirectional relations include finding genetic instrumental variables that have a similar magnitude of association with each trait of interest, and ensuring that the two genetic variants are independent160. Three bidirectional Mendelian randomization studies showed that genetic predisposition to coronary artery disease was a causal risk factor for AF, but no causal association was found between genetic predisposition to AF and the risk of coronary artery disease66,161,162.
Historical data
Studies published in the 1930s describe AF complicating the clinical course of AMI10,11. Before the mid-1980s, when thrombolysis for the treatment of AMI was introduced12, the incidence of AF in the setting of AMI ranged from 3% to 12%13,14, and mortality was reported to be up to 38% in patients with concomitant AF and AMI15. Eldar and colleagues reported that the incidence of AF was similar in the periods immediately before (1981–1983) and after (1992) the introduction of thrombolysis (8.9% and 9.9%, respectively), but then decreased over time to 7.6% in 199616. Crude mortality did not change after the introduction of thrombolysis; however, after adjustment for comorbidities and conventional risk factors, mortality was >30% lower after thrombolysis was introduced16. In the ARIC study17, the prevalence of AF accompanying AMI increased slightly from 11% in 1987 to 15% in 2009, whereas survival did not change over time17. In patients with AMI, the prevalence of comorbidities increased over time, and substantial changes occurred in revascularization procedures and medication use over the study period. Temporal trends in AF prevalence differed by type of AMI; among patients with non-ST-segment elevation MI (NSTEMI), the prevalence of AF increased, whereas the prevalence of AF decreased in patients with ST-segment elevation MI (STEMI)17. This finding could possibly be explained by the introduction during the study period of primary percutaneous coronary intervention (PCI) as a treatment for STEMI, which decreases infarct size and reduces morbidity and mortality in these patients18,19.
Risk of MI in patients with AF
Although the risk of AF in the setting of AMI has been known and examined for a century, this association has been systematically studied and described only in the past decade (Fig. 1), and AF was not established as an independent risk factor for AMI in large observational studies until 201420–22. The first report from a cohort study that AF predisposes to MI was the population-based REGARDS study20. The risks of AMI and coronary heart disease were approximately twofold higher in patients with AF than in those without AF20. This observation was validated in several subsequent studies3,22,23. Among individuals with newly diagnosed AF or atrial flutter who were identified through Danish national health registries, the cumulative risk of MI at 10 years was 3.5%3. In meta-analyses published in 2016–2017, the rate of MI was approximately 50% higher for patients with AF than for patients without AF24,25. Observational studies on the risk of MI in patients with AF are summarized in Table 1.
Fig. 1 |. Timeline of selected literature on the bidirectional relationship between AF and MI.
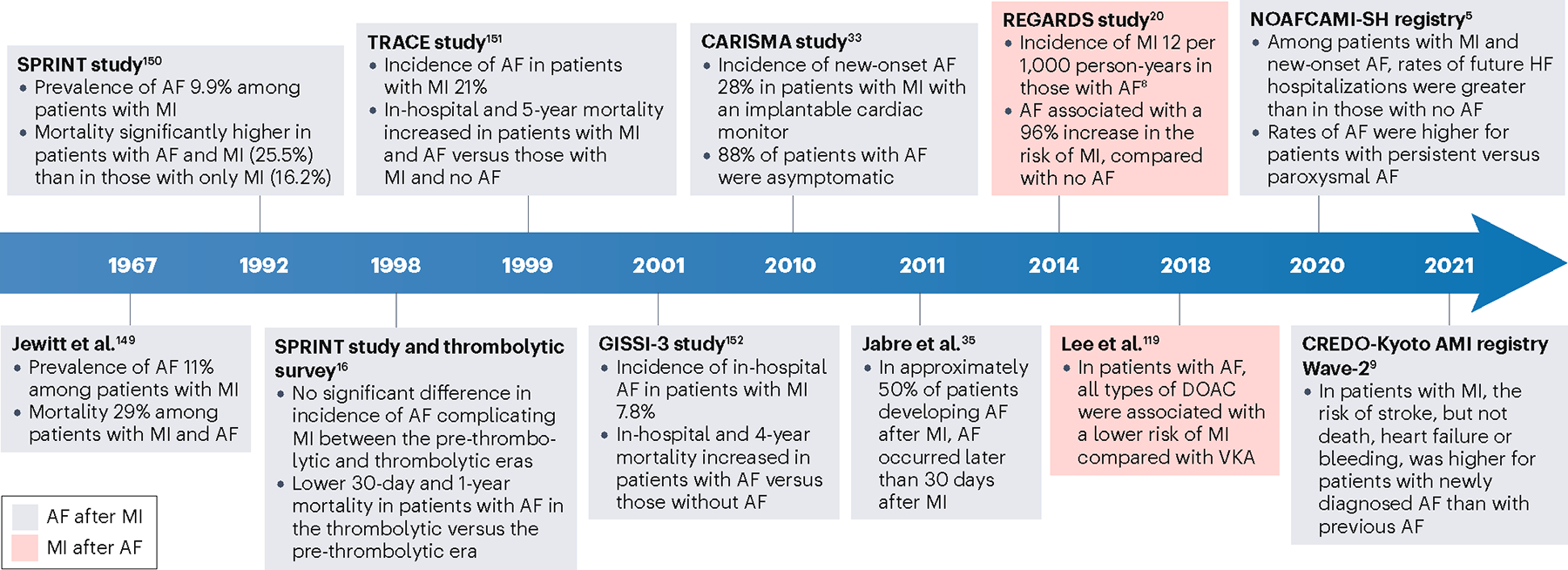
Grey boxes refer to studies examining patients with atrial fibrillation (AF) after myocardial infarction (MI), and red boxes refer to studies on patients with MI after AF. REFS.149–152. DOAC, direct oral anticoagulant; VKA, vitamin K antagonist.
Table 1 |.
Observational studies on the association between AF and MI
| Study (year) | Number of patients | Patient and study characteristics | Exposures | Outcomes | Main findings | Refs |
|---|---|---|---|---|---|---|
| Soliman et al. (2014) | 23,298 (57% women; 43% African American) | Mean age: 66.5 ± 9.7 years Median follow-up: 4.5 years |
AF | MI | Age-adjusted incidence of MI in those with AF: 12.0 per 1,000 person-years (95% CI 9.6–14.9), and in those without AF: 6.0 per 1,000 person-years (95% CI 5.6–6.6). AF associated with a higher risk of MI (HR 1.70, 95% CI 1.26–2.30) than no AF. Age-stratified analyses: no significant difference in risk between age groups (<75 years versus >75 years). | 20 |
| O’Neal et al. (2014) | 4,608 (60% women; 85% white) | Age 65–70 years: 44% Age 71–75 years: 24% Age 75–80 years: 22% Age >80 years: 10% Median follow-up: 12.2 years |
AF | MI | Incidence of MI in those with AF: 25.5 per 1,000 person‐years (95% CI 20.5–31.6), and in those without AF: 13.9 per 1,000 person‐years (95% CI 12.9–14.9). Risk of MI higher in those with AF than in those without AF (HR 1.7, 95% CI 1.4–2.2). | 21 |
| Chao et al. (2014) | 24,228 (40% women) | Mean age: 47 ± 11.5 years Mean follow-up: 5.7 years |
AF | MI | Annual incidence of MI in those with AF: 0.29%, and in those without AF: 0.10%. Risk of MI higher in those with AF than in those without AF (HR 2.93, 95% CI 2.21–3.87). Risk of MI higher in men with AF than in women with AF (HR 2.24, 95% CI 1.61–3.11). | 22 |
| Soliman et al. (2015) | 14,462 (56% women; 26% African American) | Mean age: 54 ± 5.7 years Median follow-up: 21.6 years |
AF | MI | Incidence of MI in those with AF: 11.6 per 1,000 person-years (95% CI 10.49–12.8), and in those without AF: 3.96 per 1,000 person-years (95% CI 3.71–4.22). Risk of MI higher in those with AF than in those without AF (HR 1.63, 95% CI 1.32–2.02). Association between AF and MI stronger for women than for men. No significant differences in risk by age or race. Stratified by MI type: AF significantly associated with NSTEMI but not with STEMI. | 23 |
| Magnani et al. (2016) | 15,080 (55% women; 75% white) | Mean age: 54.2 ± 5.8 years Mean follow-up: 20.6 years |
AF | CHD (defined by definite or probable MI) | Incidence of AF in white individuals: 8.1 per 1,000 person-years (95% CI 7.7–8.5), and in African American individuals: 5.8 per 1,000 person-years (95% CI 5.2–6.3). Risk of CHD higher in those with AF than in those without AF (rate ratio 6.8, 95% CI 6.2–7.5). | 28 |
| Sundbøll et al. (2017) | 623,924 (47% women) | Median age: 72.6 years (Q1–Q3 63.0–81.7 years) Median follow-up: 2.8 years |
AF | MI | 10-year cumulative risk of MI in AF: 3.5%. Adjusted incidence rate ratio for MI in first 30 days after AF: 8.0 (95% CI 6.8–9.5). No significant difference in adjusted incidence rate ratio for MI in those with AF versus those without AF after 5 years. | 3 |
| Rathore et al. (2000) | 106,780 (50.1% women; 92% white) | Median age: 79.2 years (Q1–Q3 73–85 years) Follow up: 1 year after hospitalization for MI |
MI | AF, mortality | Prevalence of AF in MI at hospital admission: 10.8%, and during hospitalization: 11.3%. AF versus no AF associated with higher in-hospital mortality (OR 1.21, 95% CI 1.17–1.26), 30-day mortality (OR 1.20, 95% CI 1.16–1.24) and 1-year mortality (OR 1.34, 95% CI 1.30–1.39). Mortality higher for AF onset during hospitalization than AF at hospital admission. | 7 |
| Jabre et al. (2011) | 3,220 (42% women) | Mean age: 68 ± 15 years Mean follow-up: 6.6 years |
First MI and AF | Mortality | AF before MI: 10%. AF onset after MI: 23%. Cumulative 5-year incidence of AF: 19%. AF events within 2 days after MI: 30%; 3–30 days after MI: 16%; >30 days after MI: 54%. AF associated with increased mortality (HR 3.77, 95% CI 3.37–4.21). Highest risk of death with AF onset >30 days after MI. | 35 |
| Krijthe et al. (2013) | 6,175 (54% women) | Mean age: 68.8 ± 9 years Mean follow-up: 11.7 years |
Clinically recognized and unrecognized MI | AF | Risk of AF higher for men with unrecognized MI than with no MI (HR 2.21, 95% CI 1.51–3.23). Risk of AF higher for men with recognized MI than with no MI (HR 1.66, 95% CI 1.21–2.29). No significant association between recognized or unrecognized MI and AF in women. | 34 |
| Almendro-Delia et al. (2014) | 39,237 (26% women | Mean age: 71.2 ± 9.8 years Maximum follow-up: 15 days |
AMI | In-hospital mortality | In-hospital mortality higher for AMI and previous AF than no AF (HR 1.89, 95% CI 1.6–2.4). In-hospital mortality higher for AMI and new-onset AF than no AF (HR 2.19, 95% CI 1.9–2.53). In-hospital mortality higher for AMI and new-onset AF than previous AF (HR 1.70, 95% CI 1.12–3.40). | 111 |
| Lee et al. (2020) | 2,523 (24% women) | Mean age: 61.6 ± 13.2 years Median follow-up: 7.2 years |
MI and AF | Mortality, stroke | Incidence of AF in MI: 10.7%. Higher risk of death for persistent (but not paroxysmal) AF than no AF (HR 1.75, 95% CI 1.03–2.96). Risk of stroke higher for persistent AF (HR 5.16, 95% CI 2.24–11.87) and paroxysmal AF (HR 1.97, 95% CI 1.16–3.35) than no AF. | 6 |
| Luo et al. (2021) | 2,399 (23% women) | Mean age: 64.7 ± 12.2 years Median follow-up: 2.7 years |
MI and AF (low or high burden defined by % time in AF) | HF hospitalization, all-cause death, ischaemic stroke | In-hospital AF: 11.6% (low-burden: 55.8%; high-burden: 44.2%). Risk of HF hospitalization higher in low-burden AF (HR 2.05, 95% CI 1.37–3.07) and high-burden AF (HR 4.50, 95% CI 3.05–6.66) than no AF. Risk of all-cause death and ischaemic stroke higher in high-burden AF (but not low-burden AF) than no AF. | 31 |
| Obayashi et al. (2021) | 6,228 (25% women) | Mean age: 68.1 ± 12.3 years Median follow-up: 5.5 years |
MI and AF | All-cause death, HF hospitalization, major bleeding, MI, stroke | In patients with MI, 9.5% had previous AF and 7.9% had new-onset AF. Higher risk of all-cause death with previous AF (HR 1.31, 95% CI 1.12–1.54) and new-onset AF (HR 1.32, 95% CI 1.14–1.52) than no AF. Risk of HF hospitalization higher with AF than without AF (no significant difference between previous and new-onset AF). Risk of major bleeding higher with AF than without AF. Risk of recurrent MI did not significantly differ with or without AF. Risk of stroke higher with AF than without AF. | 9 |
| Fauchier et al. (2021) | 797,212, (34% women) | Mean age: 67.5 ± 14.8 years Mean follow-up: 1.8 years |
MI and AF | All-cause death, cardiovascular death, HF hospitalization, ischaemic stroke | In patients with MI, 9.5% had previous AF and 4.4% had new-onset AF. Risk of all-cause death higher with previous AF (HR 1.17, 95% CI 1.16–1.19) and new-onset AF (HR 2.11, 95% CI 2.07–2.15) than no AF. Risks of cardiovascular death, HF hospitalization and ischaemic stroke higher with AF than without AF; higher risks with new-onset AF than with previous AF. | 8 |
AF, atrial fibrillation; CHD, coronary heart disease; HF, heart failure; Q1–Q3, 25th to 75th percentiles; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
Age and sex
In one study, the 5-year incidence of MI in patients with AF increased with advancing age, with a cumulative incidence of 3.3% in patients aged 67–69 years and 4.4% in patients aged 85–89 years26. However, in several other studies, no significant difference in the rate of AF-associated MI was found between age groups20,21,23.
In a large study conducted in Taiwan, men with AF had a higher absolute risk of AMI than women with AF (annual incidence of AF: 0.37% and 0.18%, respectively; HR for AMI: 2.24, 95% CI 1.61–3.11, P < 0.001)22. By contrast, the association between AF and subsequent AMI was stronger for women (HR for AF versus no AF: 2.47, 95% CI 1.87–3.25) than for men (HR for AF versus no AF: 1.08 95% CI 0.78–1.50) in the ARIC study23. The discrepancy in these findings could be due to diagnostic, preventive and treatment inequities, as well as hormonal variability and the differing effects of other risk factors27.
Race and ethnicity
In the ARIC study28, the rates of adverse outcomes after AF, including ischaemic stroke, HF, coronary artery disease and death, were greater in African American patients than in white patients. A greater risk of MI after AF in African American individuals than in white people has been reported in several other studies20,21,23,29. This finding is probably the result of racial inequities in the diagnosis, prevention and treatment of AF30. Comparative studies of AF incidence in other races and ethnicities are sparse; however, one study reported that the risk of MI did not significantly differ between white, Asian and Hispanic individuals with AF who were resident in the USA29.
Risk of AF in patients with AMI
The risk of AF in the setting of AMI has been extensively examined (Table 1). Patients with MI who developed AF are, on average, older and more likely to be women than those with MI who do not develop AF6,31. In addition, patients with new-onset AF after MI demonstrate signs of HF more frequently and have higher burdens of cardiovascular and non-cardiovascular comorbidities than patients with AMI and no AF6,31. Moreover, patients with newly diagnosed AF after MI are more likely to have reduced ejection fraction and a higher prevalence of STEMI and cardiogenic shock than those without AF9.
The prevalence of AF in patients with MI varies substantially across studies, depending on the population studied, the definition of AF and the duration of follow-up. AF was found in up to 11% of hospitalized patients with AMI assessed using 12-lead electrocardiograms or continuous electronic monitoring4,31. AF onset was often reported within days of AMI, suggesting a substantial risk of AF in the very short-term setting4,31.
Intense and long-term monitoring of patients with an implantable cardiac monitor revealed a greater incidence of AF after AMI than detected with in-hospital continuous electronic monitoring or consecutive 12-lead electrocardiograms32,33. In the CARISMA study33, the incidence of AF (no previous MI) was about 28%, whereas the incidence of AF was 58% in patients with STEMI in the ARREST study32, in which the majority of confirmed AF (93%) was asymptomatic32. In a report from the prospective, community-based Rotterdam study34, clinically recognized and unrecognized MI were both associated with a higher risk of AF compared with no MI. Observational studies on the risk of AF in patients with MI are summarized in Table 1.
Temporality of AF and MI
In the ULSAM study2 from Sweden, in which men enrolled at 50 years of age have been followed up for 40 years, participants with concomitant AF and MI more frequently had MI first and then developed AF than the other way around. In an observational study of 3,220 individuals hospitalized with MI, most cases of incident AF occurred after, rather than before, the MI35. In 30% of the individuals who developed AF after MI, AF occurred on the same day or within 2 days of MI. In 16%, AF onset was from 3–30 days after MI and, in 54%, AF occurred >30 days after MI. The risk of AF gradually decreased after the first year following AMI35. In both the ARREST32 and CARISMA33 studies, in which patients had implantable cardiac monitors, the majority of AF onset occurred in the first year after AMI.
Among patients who had AF before MI, the rate of MI has been reported to be particularly high during the first months after AF diagnosis3,36. In a study from Danish health registries3, during the first 30 days after AF diagnosis, the incidence of MI was similar to the risk of ischaemic stroke (adjusted incidence rate ratio 8.0, 95% CI 6.8–9.5 and 9.9, 95% CI 8.5–11.5, respectively) compared with individuals who did not have AF. The rate of MI decreased gradually after AF diagnosis, and after 6–10 years was similar to that of individuals without AF (adjusted incidence rate ratio 0.91, 95% CI 0.77–1.08)3.
Shared risk factors for AF and MI
Many risk factors are common to both AF and MI. These include non-modifiable demographic factors, modifiable risk factors, social determinants and comorbidities (Box 2). Some of these factors are discussed in more detail below.
Box 2 |. Shared risk factors for AF and MI.
Shared risk factors for atrial fibrillation (AF) and myocardial infarction (MI) overlap and interact.
Demographic
Age
Sex
Race/ethnicity
Lifestyle
Alcohol intake
Smoking
Physical inactivity
Comorbidities
Heart failure
Hypertension
Obesity
Type 2 diabetes mellitus
Chronic kidney disease
Social determinants
Education
Employment
Income and wealth
Social network
Rurality and neighbourhood
Structural racism
Environmental
Air pollution
Demographic factors
AF and MI share several non-modifiable risk factors. Advancing age is a prominent risk factor for both AF and MI. In a report from the Framingham Heart Study37, the prevalence and incidence of AF increased for each decade beyond 60 years of age. Similarly, hospitalization rates for AMI increased with advancing age and peaked in individuals aged >85 years38.
Biological sex is also strongly associated with both AF and MI. In the Framingham Heart Study39, lifetime risk of AF was higher in men than in women. The incidence of AF worldwide was also higher in men in the 2017 Global Burden of Disease Study40. However, using the clinical risk factors, researchers reported that the risk of AF conferred by male sex was no longer observed after accounting for height, weight and other risk factors41. In the ARIC study42, the lifetime risk of AF was similar for African American men and women (21% and 22%, respectively). For NSTEMI, a study from Finland showed a 2.4-fold higher risk in men than in women43, while the same researchers found a threefold higher risk of STEMI in men than in women in another study44.
Race and ethnicity are also associated with variation in incidence and outcome in both AF and MI. The incidence per 1,000 person-years of diagnosed AF has been estimated to be higher among white (11.2, 95% CI 9.8–12.8) than Hispanic (6.1, 95% CI 4.7–7.8), African American (5.8, 95% CI 4.8–7.0) or Chinese (3.9, 95% CI 2.5–6.1) individuals45. Part of the variation in clinically diagnosed AF reflects ascertainment biases46. In the ARIC study47, white participants had a higher rate of clinically recognized MI than African American participants (5.04 versus 3.24 per 1,000 person years, P = 0.002). This finding could be partly explained by social determinants, including residential environment (urban versus rural), availability of health care, access to treatment and socioeconomic position48.
Modifiable risk factors and comorbidities
AF and MI also share several modifiable risk factors. In the ARIC study49, the prevalence of cardiovascular risk factors in patients with AF was higher than in matched AF-free controls, even >15 years before AF diagnosis. Trajectories of multiple risk factors over time were associated with future risk of AF. In addition, the prevalence of stroke, MI and HF increased gradually in the period close to AF diagnosis, suggesting that shared modifiable risk factors have an important role in the co-occurrence of the two conditions49.
Smoking.
Tobacco smoking is a risk factor for both AF and MI50. In an Australian study, current smoking was associated with a 2.5-fold higher risk of acute MI and a 1.3-fold higher risk of AF than non-smoking51. In a large, multinational study, the risk of recurrent MI was reduced in patients who stopped smoking after AMI compared with those who persisted with smoking (OR 0.57, 95% CI 0.36–0.89)52. Smoking cessation has also been reported to be associated with a reduced risk of incident AF53.
Alcohol intake.
Observational studies have demonstrated a protective association between light-to-moderate alcohol intake and MI54,55. However, in a Mendelian randomization study, a causal relationship was found between alcohol intake and risk of MI at all levels of intake56. A linear dose–response relationship between alcohol and AF was reported in an observational study57. This finding is supported by the results of a Mendelian randomization study, in which each additional drink of alcohol per day was associated with an increased risk of AF (OR 1.26, 95% CI 1.07–1.48)58. In observational studies, the association between alcohol intake and the risk of disease is subject to residual confounding, which is likely to be the reason for conflicting results between observational studies and Mendelian randomization studies. In a study on patients with AF who had a regular alcohol intake (≥10 standard drinks per week) and were randomly assigned to abstinence from alcohol or no intervention, the reduction in AF recurrence and AF burden was greatest among individuals in the abstinence group (HR 0.55, 95% CI 0.36–0.84)59.
Body weight and physical activity.
Obesity is a causal risk factor for both AF and MI50. In addition, weight gain and fluctuating weight are associated with a higher risk of incident AF than steady weight60,61, and weight loss is associated with a reduced AF burden62. In addition, weight changes have been associated with an increased risk of first AMI compared with stable weight63. In pooled analyses and meta-analyses, inverse dose–response associations have been found between guideline-recommended levels of physical activity and both AF64 and fatal MI65.
Hypertension.
Elevated blood pressure (BP) is a major risk factor for both AF and MI50,66. In the SPRINT trial67,68, participants were randomly assigned to intensive (systolic BP <120 mmHg) or standard (<140 mmHg) BP reduction. Intensive BP lowering led to a significant reduction in the risk of new-onset AF (HR 0.74, 95% CI 0.56–0.98) and a composite outcome including MI, acute coronary syndrome, stroke, HF or death from cardiovascular disease (HR 0.75, 95% CI 0.64–0.89)67,68. A meta-analysis of 14 trials demonstrated an overall relative risk of MI of 0.86 (95% CI 0.78–0.96) in individuals treated with intensive BP-lowering therapy compared with less-intensive therapy69.
Diabetes mellitus.
Type 2 diabetes mellitus (T2DM) is a causal risk factor for MI50. In a meta-analysis of randomized controlled trials, glycaemic control in patients with T2DM reduced the risk of MI (relative risk 0.90, 95% CI 0.82–0.98)70. Mendelian randomization studies do not support a causal relationship between T2DM and AF50,71. However, an earlier observational study showed that, in patients with T2DM, longer disease duration and poor glycaemic control were associated with an increased risk of AF72. The association between T2DM and AF might be confounded by shared risk factors, such as obesity and hypertension, or mediated by coronary artery disease.
Social determinants
Both AF and MI have been found to be more frequent among individuals with lower socioeconomic status48,73. Low levels of education and wealth have been shown to be independent predictors of MI73, and the risk of incident and prevalent AF has been reported to be greater in individuals with lower levels of education, income and employment48,74. The incidence of adverse events related to AF, such as death and stroke, is also increased among patients with low income48. Results from a Finnish study published in 2001 also showed substantial differences in survival after MI according to income75. Approximately 49% of men with low income died within 12 months of MI, compared with 27% in the high-income group. The difference in mortality after MI among women with low or high income was less marked (30% and 20%, respectively)75.
Rurality could also have an influence on AF and MI incidence. Individuals living in rural areas are at higher risk of AF than those in urban areas, possibly due to an increased burden of risk factors such as older mean age, smoking and obesity, and social factors, such as lower levels of income and education48. In addition, access to health care can be limited in rural areas48. In a large study of 70,424 patients with STEMI from the USA, patients living in rural areas were less likely to receive primary PCI and had longer times to reperfusion compared with patients living in urban areas76. However, the investigators found no significant difference in adjusted in-hospital mortality between patients from rural or urban areas76.
Environmental risk factors
Air pollution is associated with both AF and MI. In a meta-analysis of five studies, long-term exposure to air pollution was associated with increased risk of AF incidence77. In addition, a large Swedish study showed that higher 24-h mean levels of air pollutants recorded in Stockholm were associated with an increased incidence of AF in study participants aged >75 years compared with lower levels of pollutants78. A strong association was reported between short-term exposure to air pollution as well as weather changes and increased risk of STEMI in populations from two large urban areas of Italy79. Moreover, a meta-analysis of 24 studies, including >70 million individuals, showed a significant association between long-term exposure to air pollution and the risk of MI80.
Pathophysiology
We have discussed how shared risk factors can lead to the co-occurrence of AF and MI; however, several direct mechanisms explain how one condition might predispose to the other. AF and MI are both associated with inflammation81–83, which contributes to the bidirectional association. The onset of AF in the setting of MI is partially a result of structural changes caused by ischaemia and inflammation84–86, whereas MI in patients with a history of AF can be the result of coronary artery embolism87 or a mismatch between oxygen supply and demand88. The various pathophysiological mechanisms involved in the bidirectional interaction between AF and MI are shown in Figs 2,3 and discussed in more detail below.
Fig. 2 |. Inflammatory cells and mediators of inflammation modulate cardiac electrophysiology and structural properties leading to atrial fibrillation.
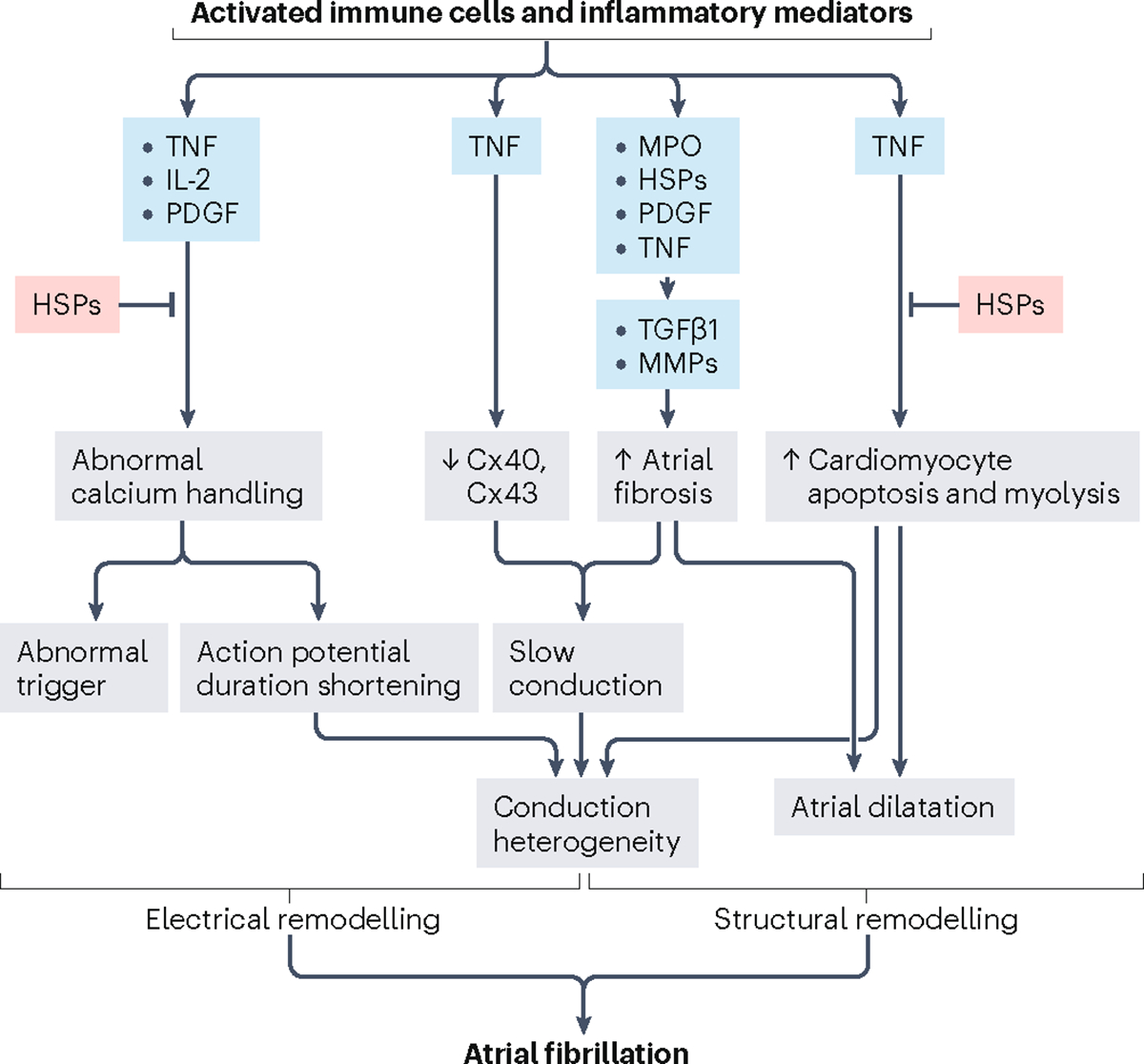
Calcium homeostasis in cardiomyocytes is regulated by tumour necrosis factor (TNF), IL-2 and platelet-derived growth factor (PDGF), which are associated with increased triggering and shortening of the action potential duration. The atrial expression of connexin 40 (Cx40) and Cx43 is downregulated by inflammation via TNF. Myeloperoxidase (MPO), heat shock proteins (HSPs), PDGF and TNF activate fibroblasts, which express transforming growth factor-β1 (TGFβ1) and matrix metallopeptidases (MMPs), leading to increased collagen synthesis and atrial fibrosis. TNF also increases cardiomyocyte apoptosis and myolysis. These changes contribute to heterogeneous atrial conduction and increased vulnerability to atrial fibrillation. HSPs protect cardiomyocytes against abnormal calcium handling, apoptosis, and myolysis. Adapted with permission from REF.153, Springer Nature.
Fig. 3 |. Pathophysiology of MI leading to AF and AF leading to MI.
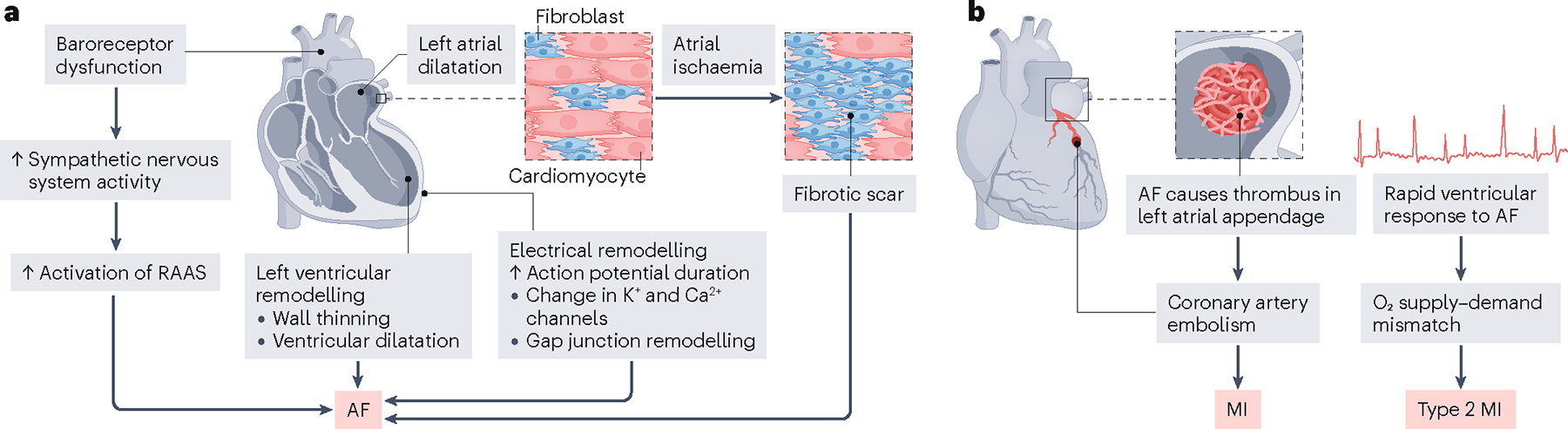
a, Acute myocardial infarction (MI) can initiate several mechanisms that ultimately result in atrial fibrillation (AF). Left ventricular remodelling with wall thinning and left ventricular dysfunction potentiate left atrial dilatation (due to pressure and/or volume overload), electrical remodelling (increases in action potential duration, alterations to K+ and Ca2+ ion channels and gap junction remodelling) and neurohumoral modulation (stimulation of the renin–angiotensin–aldosterone system (RAAS), vasopressin and atrial natriuretic peptide). In addition, atrial ischaemia caused by MI can lead to cardiomyocyte death and fibrotic replacement, producing an arrhythmogenic substrate that alters electrical conduction and can result in AF. b, In the setting of AF, blood can pool in the left atrial appendage, leading to thrombus formation, which can be dispersed as a coronary artery embolism. During episodes of AF with a rapid ventricular response rate (tachycardia), myocardial oxygen demand increases and can cause a mismatch with oxygen supply, which can lead to type 2 MI.
Inflammation
Inflammation has a major role in coronary artery disease89. Immune cells are involved in the development of atherosclerotic plaques and the transformation from stable to vulnerable plaque morphology89. In MI, inflammatory cells and mediators are involved in erosion and rupture of the atherosclerotic plaque89. In addition, inflammation in the setting of systemic disease, including obesity and hypertension, and ischaemia can cause electrical and structural remodelling of the atria, leading to AF90 (Fig. 2). In turn, AF might also promote inflammation, but the underlying mechanisms are poorly understood90.
Increased systemic levels of inflammatory markers, such as IL-6 and C-reactive protein, are associated with incident AF81 and MI82,83. In a Mendelian randomization study, a causal link was found between levels of circulating interleukins and both AF and coronary artery disease91. In patients with AF, biomarkers of inflammation and platelet activation are independent predictors of cardiovascular events, including MI92,93. Furthermore, markers of oxidative stress and inflammation are associated with AF in the setting of AMI94,95.
In the CANTOS trial96, anti-inflammatory therapy in patients with previous MI was associated with a reduced risk of recurrent MI. Whether treatment with anti-inflammatory drugs reduces incident or recurrent AF is unclear. The use of colchicine, prednisolone and canakinumab in the treatment and prevention of AF has been reviewed previously97. In small studies, these drugs tended to have beneficial effects, but larger trials are warranted to validate these findings97.
Mechanisms involved in AF after MI
Atrial ischaemia.
Several factors contribute to the development of AF after AMI, including atrial ischaemia (Fig. 3a). Ischaemia causes damage to cardiomyocytes and leads to their replacement by fibrotic tissue, which can disrupt electrical conduction in the atria, causing the initiation of AF98. In animal models and small clinical studies, occlusion of atrial coronary branches leading to atrial ischaemia independently predicted incident AF after AMI84–86. In a dog model, chronic ventricular MI without atrial involvement caused alterations in atrial electrical restitution and sympathetic hyperinnervation99. This finding suggests mechanisms beyond atrial ischaemia in the risk of AF after AMI.
Left ventricular dysfunction.
Ventricular ischaemia caused by MI can lead to left ventricular dysfunction and HF100,101, which can induce AF, possibly due to secondary processes such as atrial dilatation (due to pressure or volume overload), neurohumoral modulation and atrial ion channel remodelling102 (Fig. 3a). Left ventricular dysfunction has been associated with new-onset AF in patients with AMI in several studies9,103. Therefore, left ventricular dysfunction is a possible pathophysiological explanation for AF occurring in the setting of MI. However, in a study of 786 patients with STEMI, no correlation was found between infarct size, assessed by cardiac MRI, and the development of AF103.
Mechanisms involved in MI after AF
Type II MI.
AF with a rapid ventricular response (tachycardia) can cause oxygen supply–demand mismatch due to an increased demand for oxygen and less time for subendocardial perfusion and thus lead to type II MI104 (Fig. 3b). In an assessment of coronary blood flow and myocardial perfusion in patients with AF, both were found to be impaired, despite the absence of coronary obstructive disease105, and coronary blood flow worsened with AF burden105. The reduction in coronary blood flow in AF is partly reversible after cardioversion88.
Coronary artery embolism.
During episodes of AF, blood can pool, coagulate and form thrombi in the left atrial appendage106. Thrombi can then embolize directly to the coronary arteries and lead to AMI106 (Fig. 3b). In a study of 1,232 patients with STEMI, the prevalence of coronary artery embolism was low (4.3%)87. However, among those patients with coronary artery embolism, AF was the most common underlying cause (28%)87.
Clinical outcomes
Mortality
In a meta-analysis of 43 studies, mortality was 46% higher among patients with concomitant AF and MI than in patients with MI who were free from AF107. Across these studies, mortality was assessed over varying follow-up periods, from the time of hospital admission until discharge, to up to 8 years after the index MI107. In-hospital, short-term and long-term mortality were all higher in patients with MI and either previous or new-onset AF than in patients without AF107,108. Whether the temporality of AF and MI has an influence on mortality is unclear. In several studies, increased mortality has been reported among patients with new-onset AF compared with patients with a history of AF at the time of AMI8,109–111. A community-based study conducted in Olmsted County, MN, USA, demonstrated that mortality was highest for patients with new-onset AF developing >30 days after MI35. However, other studies have shown no significant difference in mortality between patients with previous or new-onset AF after MI9,107.
Heart failure
HF is a common complication after AMI38, and the risk of subsequent HF hospitalization is increased in patients with AMI accompanied by AF5,9. In patients with AF after AMI, the rate of HF hospitalization was 5.8-fold higher in persistent and 2.6-fold in transient new-onset AF (P = 0.008 for interaction) compared with patients with AMI and no AF5. However, whether the risk of HF is dependent on the temporality of AF and MI is unclear8,9,109.
Stroke
AF and MI are both associated with an increased risk of ischaemic stroke2. In patients with AMI, concomitant AF is associated with a greater risk of ischaemic stroke compared with patients with AMI and no AF8,9,108,109. Studies published in the past 2 years have demonstrated that the risk of stroke is higher in patients with new-onset AF than in patients with previous AF, and the risk was especially high during the first 30 days after AMI8,9. In an observational study, the incidence of stroke among patients with new-onset AF after AMI was higher in those with permanent AF than in those with paroxysmal AF (22.0% versus 8.3%; HR 5.16, 95% CI 2.24–11.87 for permanent AF and HR 1.97, 95% CI 1.16–3.35 for paroxysmal AF)6.
Prevention
Prevention of MI in patients with AF
In the European and US guidelines on AF, recommendations largely focus on anticoagulation therapy for the prevention of stroke, but they do not specifically address the prevention of MI in patients with AF112,113. In a study of data from US registries, less than half of patients with AF received all the indicated, evidence-based preventive therapies for comorbid cardiovascular risk factors and conditions114. Anticoagulation is pivotal to reducing the risk of stroke in patients with AF and might also reduce the risk of MI115. Importantly, however, randomized trials of oral anticoagulation (OAC) therapy are designed with stroke, rather than MI, as the primary outcome. In the RE-LY trial116, patients with AF were randomly assigned to receive dabigatran or a vitamin K antagonist (VKA) for stroke prevention. The rate of MI was higher among patients receiving dabigatran than among those receiving a VKA (0.74% versus 0.53% per year)116. However, trials of apixaban or rivaroxaban versus VKA have shown lower absolute rates of MI in patients assigned to direct OACs than in those in the VKA groups117,118. In an observational study, patients receiving a VKA had a higher risk of MI than those receiving OACs, whereas no significant differences in the rate of MI were found between the various direct OACs (apixaban, dabigatran, edoxaban and rivaroxaban)119. However, the study design is subject to confounding by indication.
The management of comorbidities and risk factors in patients with AF is essential for the prevention of subsequent MI. In the ORBIT-AF registry120, every 5% increase in systolic BP from baseline was associated with a 5% increase in the risk of MI (adjusted HR 1.05, 95% CI 1.00–1.11) in patients with AF. In a study of 2,372 South Korean men, smoking cessation after AF onset was associated with a reduction in the rate of adverse outcomes, including incident CVD121. However, smoking cessation was not significantly associated with a reduction in the risk of MI alone, probably due to the low number of events and low statistical power121.
Prevention of AF in patients with MI
Currently, no specific guidelines exist for the prevention of AF in patients with MI. Angiotensin-converting enzyme (ACE) inhibition after MI attenuates left ventricular remodelling and thus prevent HF122. As left ventricular dysfunction is associated with a risk of AF102, studies have been conducted to assess whether treatment with ACE inhibitors is associated with a reduced risk of AF after MI. In the randomized TRACE study123, patients with HF secondary to AMI who received an ACE inhibitor had a significantly reduced incidence of AF than those in the placebo group (HR 0.45, 95% CI 0.26–0.76)123. However, in two large, population-based studies, no association was found between renin–angiotensin–aldosterone inhibition and AF incidence in patients with AMI or coronary artery disease124,125. The divergent findings could be related to differences in study population characteristics, such as age, sex and comorbidities. In the CAPRICORN study126, patients with AMI and HF were randomly assigned to receive a β-blocker or placebo. The investigators found a significant reduction in the incidence of AF in the treatment group (HR 0.41, 95% CI 0.25–0.68). Data from observational and clinical preventive studies in patients with AF, MI or both are presented in Table 2.
Table 2 |.
Studies on the prevention of AF in patients with MI and vice versa
| Study (year) | Number of patients | Patient and study characteristics | Exposures or interventions | Outcomes | Main findings | Refs |
|---|---|---|---|---|---|---|
| Prevention of MI in patients with AF | ||||||
| Connolly et al. (2006) | 6,706 (34% women) | Mean age: 70.2 ± 9.4 years RCT Follow up: 1.28 years |
OAC or clopidogrel plus aspirin | Composite (MI, stroke, embolus, vascular death), MI | Higher risk of composite end point with clopidogrel plus aspirin than with OAC (HR 1.44, 95% CI 1.18–1.76); no significant difference in the risk of MI alone between groups (HR 1.58, 95% CI 0.94–2.67) | 148 |
| Lee et al. (2017) | 71,959 (47% women) | Median age: 75 years Retrospective cohort study Follow up: 4.1 years |
Aspirin monotherapy, VKA monotherapy or dual therapy | First-time MI | Higher risk of MI with aspirin monotherapy than with VKA monotherapy (IRR 1.54, 95% CI 1.40–1.68); higher risk of MI with dual therapy than with VKA monotherapy (IRR 1.22, 95% CI 1.06–1.40) | 115 |
| Lee et al. (2018) | 31,739 (47% women) | Median age: 74 years Retrospective cohort study Follow up: 3 years |
Apixaban, dabigatran, rivaroxaban or VKA | MI | Standardized absolute 1-year risk of MI with apixaban 1.16% (95% CI 0.94–1.39%), dabigatran 1.20% (95% CI 0.95–1.47%), rivaroxaban 1.07% (95% CI 0.83–1.32%) and VKA 1.56% (95% CI 1.33–1.80%); no significant difference in the risk of MI between DOACs; higher risk of MI with VKA than with any of the three DOACs | 119 |
| Vemulapalli et al. (2019) | 10,098 (42% women) | Mean age 73.5±11 years Prospective cohort study Follow up: 2 years |
Changes in systolic blood pressure | MI | Risk of MI increased by 5% (HR 1.05, 95% CI 1.00–1.11) for every 5-mmHg increase in systolic blood pressure from baseline | 120 |
| Prevention of AF in patients with MI | ||||||
| Pedersen et al. (1999) | 1,577 (28% women) | Mean age: 68 years Reduced LVEF RCT Follow up: 4 years |
ACEi versus placebo | New-onset AF | AF in ACEi group: 2.8%; AF in placebo group: 5.3%; lower risk of AF with ACEi than with placebo (HR 0.45, 95% CI 0.26–0.76) | 123 |
| Batra et al. (2017) | 112,648 (35.5% women) | Median age: 72 years (Q1–Q3 62–81) Retrospective cohort study Follow up: 3 years |
ACEi or ARB | New-onset AF | No reduction in the risk of new-onset AF with ACEi or ARB (HR 1.07, 95% CI 1.00–1.15) | 124 |
| Singh et al. (2012) | 28,620 (72.9% women) | Mean age: 78.3 ± 7.1 years Retrospective cohort study Mean follow up: 3.8 years |
ACEi or ARB | New-onset AF | No reduction in risk of new-onset AF with ACEi or ARB (HR 0.99, 95% CI 0.94–1.04) | 125 |
| McMurray et al. (2005) | 1,959 (26% women) | Mean age: 63 years (range: 25–90 years) RCT Follow up: 1.3 years |
β-Blocker versus placebo | AF | AF in β-blocker group: 2.3%; AF in placebo group: 5.4%; lower risk of AF with β-blocker than with placebo (HR 0.41, 95% CI 0.25–0.68) | 126 |
AF, atrial fibrillation; ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin-receptor blocker; DOAC, direct oral anticoagulant; Q1–Q3, 25th to 75th percentiles; IRR, incidence rate ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OAC, oral anticoagulant; RCT, randomized controlled trial; VKA, vitamin K antagonist.
Management
ST-segment elevation MI
In the European and US guidelines, the recommendation is for all patients with AMI (either STEMI or NSTEMI), including those with AF, to be treated with a loading dose of 150–300 mg of aspirin (class IA)127–130. Patients with STEMI and AF who are treated with OACs should also be given parenteral anticoagulation (unfractionated heparin and low-molecular-weight heparin) before primary PCI127. Fibrinolysis is often contraindicated in patients treated with OACs, so primary PCI should be the first choice when possible127,130. If PCI is not possible, fibrinolysis can be used despite OAC therapy.
Non-ST-segment elevation MI
In addition to the loading dose of aspirin, the European and US guidelines recommend parenteral anticoagulation at the time of diagnosis of NSTEMI and during revascularization procedures128,129. However, in patients with AF who are already receiving OACs, the European guidelines recommend that oral treatment be continued128. This recommendation is based on a subgroup analysis of the randomized WOEST trial131, in which there was no significant difference in the risk of bleeding and major adverse events between patients receiving uninterrupted OAC therapy and those with bridging therapy during PCI. Therefore, the safety of bridging direct OAC with parenteral anticoagulation in patients undergoing PCI is unclear128. Whether direct OAC can be discontinued without parenteral anticoagulation is also unclear. Therefore, the European guidelines recommend that low-dose parenteral anticoagulation is added to direct OAC128. For patients with AF who are receiving VKA and have an international normalized ratio >2.5, no additional parenteral anticoagulation is recommended128. In an observational study, no association was found between cardiovascular events and adding parenteral anticoagulation to VKA treatment, whereas dual treatment was associated with procedural complications132.
AF and chronic ischaemic heart disease
For patients with AF and chronic ischaemic heart disease, OAC therapy is recommended in both the European and the US guidelines133,134. For patients who undergo PCI, VKA should be continued, whereas direct OACs should be discontinued 12–48 h before an elective procedure133.
Post-procedural management
In patients with AF and acute or chronic ischaemic heart disease, triple antithrombotic therapy with direct OAC and dual antiplatelet therapy is associated with a high risk of major bleeding compared with dual antithrombotic therapy with OACs and a P2Y12 inhibitor135–138. Uncertainty exists as to whether dual antithrombotic therapy increases the risk of stent thrombosis and recurrent MI compared with triple therapy, as four large trials comparing the two strategies were underpowered to assess ischaemic outcomes139–142. However, meta-analyses have demonstrated a significantly higher risk of stent thrombosis and MI with dual antithrombotic therapy than with triple therapy, but no significant difference in all-cause mortality135,137,138. Clopidogrel is preferred over prasugrel or ticagrelor in patients receiving concomitant OAC therapy, due to the lower risk of major bleeding with clopidogrel143.
The European guidelines recommend early cessation of triple antithrombotic therapy (≤1 week after PCI) and continuation of dual therapy for up to 12 months if the risk of stent thrombosis is low112. Monotherapy with an OAC is recommended after 12 months, or after 6 months in patients with a high risk of bleeding or medically treated MI112,128,133. However, if the risk of stent thrombosis outweighs the bleeding risk, triple therapy for >1 week, but ≤1 month, should be considered112. The US guidelines recommend dual therapy with a P2Y12 inhibitor and an OAC for patients with AF and a CHA2DS2-VASc risk score of ≥2 undergoing PCI113. If triple therapy is prescribed, a transition to dual therapy should be considered at 4–6 weeks after intervention113.
Rate and rhythm control
In patients with AMI and haemodynamically compromising AF, rate control in the acute setting can be pursued with an intravenous β-blocker if there are no signs of acute HF and the treatment does not compromise BP112,113,127. In patients with AMI and acute HF, digoxin can be considered for acute rate control113. If rate control is not adequate, acute rhythm control with intravenous amiodarone or electrical cardioversion is recommended113,127.
When pursuing long-term rhythm control in patients with AF and ischaemic heart disease, amiodarone, β-blockers, digoxin and calcium-channel blockers (verapamil or diltiazem) can be used, although calcium-channel blockers are contraindicated in patients with HF with reduced ejection fraction due to negative inotropic effects112. In addition, vernakalant can be used, but is contraindicated in the first month after AMI112. This recommendation is made because patients with recent AMI (<30 days) were excluded from placebo-controlled randomized trials of vernakalant144,145. Flecainide is not recommended in patients with ischaemic heart disease112,134 due to the findings of the CAST study146, in which 1,498 patients were randomly assigned to either class Ib antiarrhythmics (flecainide or encainide) or placebo after AMI to reduce ventricular ectopy146. The investigators observed excess deaths due to arrhythmia and recurrent MI in the treatment group146. In another study, treatment with propafenone in patients with structural heart disease resulted in a greater incidence of serious adverse events than placebo147. Therefore, propafenone is not recommended in patients with known coronary artery disease112,134. Finally, AF ablation can be considered for long-term rhythm control112,113.
Knowledge gaps and future directions
There are several gaps in our knowledge about the bidirectional association between AF and MI. First, there is a lack of studies in individuals of African ancestry on the inequities in the prevention, diagnosis and treatment of both conditions that should be addressed to mitigate the increased risk of MI in individuals with AF and vice versa. It is also unclear whether biological sex is a modifier of the risk of AF in patients with MI and on adverse outcomes, such as HF, stroke and death. Therefore, we encourage the future study of the risk and temporality of AF in patients with MI, and MI in patients with AF, according to race, ethnicity and sex. Moreover, whether the bidirectional risk of AF and MI differs according to socioeconomic status, residential environments, availability of health care and health literacy has not been adequately explored.
In the prevention of MI in patients with AF, there is a lack of knowledge on the protective role of OAC therapy and modifiable risk factors on MI risk. Ideally, types of OAC drug should be compared in randomized controlled trials powered for MI as a primary outcome. In addition, we suggest future studies comparing rate and rhythm control, as well as risk factor modification, in patients with AF for the prevention of MI. Another potential study direction is the comparison of catheter ablation and pharmacological rhythm control and risk of MI in patients with AF. For the prevention of AF in patients with MI, the potential effects of new drugs, such as sodium–glucose cotransporter 2 inhibitors should be investigated. In addition, antithrombotic regimens for NSTEMI, STEMI and chronic ischaemic heart disease in patients with AF treated with OACs are still challenging and should be investigated further.
Conclusions
The association between AF and MI is bidirectional, and the pathogenesis of their co-occurrence is multifactorial. AF and MI not only share common risk factors leading to their co-existence, but each condition also increases the risk of subsequently experiencing the other through direct and indirect mechanisms. The coexistence of AF and MI is associated with worse prognosis than each condition alone, with increased mortality and risk of HF and ischaemic stroke, emphasizing the importance of prevention and management of both conditions. Understanding of the increased risk of adverse events in patients with both conditions should lead future studies to focus on preventive measures for MI in patients with AF and vice versa. Finally, balancing the risk between bleeding and thrombotic events is challenging in the management of patients with concomitant AF and MI and needs to be further explored.
Key points.
Atrial fibrillation (AF) is a risk factor for myocardial infarction (MI); the rate of MI is approximately 50% higher in patients with AF than in those without AF.
MI is associated with subsequent AF, and the rate of AF is particularly high in the first days after MI.
The bidirectional association between AF and MI might be partly explained by indirect mechanisms related to shared risk factors such as age, sex, modifiable risk factors, comorbidities and social determinants of health.
There are several mechanisms through which one condition can lead directly to the other, such as coronary embolism, oxygen supply–demand mismatch, atrial ischaemia, cardiac remodelling and inflammation.
Patients with coexisting AF and MI have an increased risk of stroke, heart failure and death compared with those with either condition alone, emphasizing the importance of prevention and management.
Medical treatment in patients with both AF and MI is challenging, owing to the need to balance the risks of thromboembolic complications, bleeding and stent thrombosis.
Acknowledgements
T.C.F. is supported by Aarhus University, Region Midtjyllands Sundhedsvidenskabelig Forskningsfond (A3116), Helsefonden (20-B-0206) and the Danish Agency for Higher Education and Science (EliteForsk 2025-00072B). S.R.P. is supported by NIH grant 5R01HL128914-04. H.L. is supported by the European Commission Grant (Agreement No 847770) and NIH U01AG068221. L.T. is supported by the American Heart Association (18SFRN34150007). E.J.B. is supported by the NIH (2R01 HL092577, 1R01 HL141434 01A1, 2U54HL120163, 1R01AG066010 and 1R01AG066914) and the American Heart Association (18SFRN34110082). J.K. received funding from the Marie Sklodowska-Curie Actions under the European Union’s Horizon 2020 research and innovation programme (agreement No. 838259).
Footnotes
Competing interests
The authors declare no competing interests.
Review criteria
Several PubMed searches were conducted, using different combinations of MESH terms and non-MESH terms. These searches yielded 14,645 studies that were screened by title, after which, 723 studies were eligible for inclusion and assessed by abstract; 516 studies were excluded 207 studies were assessed by full text. Finally, 172 studies were included in the Review.
References
- 1.Roth GA et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol 76, 2982–3021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lind L et al. Life-Time Covariation of Major Cardiovascular Diseases. Circ Genom Precis Med 14, e002963 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundbøll J et al. Risk of arterial and venous thromboembolism in patients with atrial fibrillation or flutter: A nationwide population-based cohort study. Int J Cardiol 241, 182–187 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Lopes RD et al. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX-AMI trial. Eur Heart J 30, 2019–2028 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J et al. Long-term impact of new-onset atrial fibrillation complicating acute myocardial infarction on heart failure. ESC Heart Fail 7, 2762–2772 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH et al. New-onset paroxysmal atrial fibrillation in acute myocardial infarction: increased risk of stroke. BMJ Open 10, e039600 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathore SS et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation 101, 969–974 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Fauchier L et al. Outcomes in patients with acute myocardial infarction and new atrial fibrillation: a nationwide analysis. Clin Res Cardiol 110, 1431–1438 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Obayashi Y et al. Newly Diagnosed Atrial Fibrillation in Acute Myocardial Infarction. J Am Heart Assoc 10, e021417 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington AW & Wright JH Cardiac Infarction: A Study of 148 Cases. Glasgow Med J 119, 1–12 (1933). [PMC free article] [PubMed] [Google Scholar]
- 11.Master AM, Dack S & Jaffe HL Disturbances of Rate and Rhythm in Acute Coronary Artery Thrombosis. Ann Intern Med 11, 735 (1937). [Google Scholar]
- 12.Simoons ML et al. Improved Survival After Early Thrombolysis in Acute Myocardial Infarction: A Randomised Trial by the Interuniversity Cardiology Institute in The Netherlands. Lancet 326, 578–581 (1985). [DOI] [PubMed] [Google Scholar]
- 13.Mintz SS & Katz LN Recent Myocardial Infarction: An Analysis of Five Hundred and Seventy-Two Cases. Arch Intern Med 80, 205–236 (1947). [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum FF & Levine SA Prognostic Value of Various Clinical and Electrocardiographic Features of Acute Myocardial Infarction: I. Immediate Prognosis. Arch Intern Med 68, 913–944 (1941). [Google Scholar]
- 15.Hurwitz M & Eliot RS Arrhythmias in Acute Myocardial Infarction. Dis Chest 45, 616–626 (1964). [DOI] [PubMed] [Google Scholar]
- 16.Eldar M et al. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and Thrombolytic Survey Groups. Circulation 97, 965–970 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Bengtson LG et al. Temporal trends in the occurrence and outcomes of atrial fibrillation in patients with acute myocardial infarction (from the Atherosclerosis Risk in Communities Surveillance Study). Am J Cardiol 114, 692–697 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grines CL et al. A Comparison of Immediate Angioplasty with Thrombolytic Therapy for Acute Myocardial Infarction. N Engl J Med 328, 673–679 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Schömig A et al. Coronary Stenting plus Platelet Glycoprotein IIb/IIIa Blockade Compared with Tissue Plasminogen Activator in Acute Myocardial Infarction. N Engl J Med 343, 385–391 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Soliman EZ et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 174, 107–114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neal WT, Sangal K, Zhang ZM & Soliman EZ Atrial fibrillation and incident myocardial infarction in the elderly. Clin Cardiol 37, 750–755 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao TF et al. Acute myocardial infarction in patients with atrial fibrillation with a CHA2DS2-VASc score of 0 or 1: a nationwide cohort study. Heart Rhythm 11, 1941–1947 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Soliman EZ et al. Atrial Fibrillation and Risk of ST-Segment-Elevation Versus Non-ST-Segment-Elevation Myocardial Infarction: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 131, 1843–1850 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruddox V et al. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol 24, 1555–1566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo XY et al. Atrial fibrillation is associated with an increased risk of myocardial infarction: Insights from a meta-analysis. Atherosclerosis 254, 1–7 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Piccini JP et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J 35, 250–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yahagi K, Davis HR, Arbustini E & Virmani R Sex differences in coronary artery disease: Pathological observations. Atherosclerosis 239, 260–267 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Magnani JW et al. Racial Differences in Atrial Fibrillation-Related Cardiovascular Disease and Mortality: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol 1, 433–441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neal WT et al. Sex and racial differences in cardiovascular disease risk in patients with atrial fibrillation. PLoS One 14, e0222147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lett E, Asabor E, Beltrán S, Cannon AM & Arah OA Conceptualizing, Contextualizing, and Operationalizing Race in Quantitative Health Sciences Research. Ann Fam Med 20, 157–163 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J et al. Long-term impact of the burden of new-onset atrial fibrillation in patients with acute myocardial infarction: results from the NOAFCAMI-SH registry. Europace 23, 196–204 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Romanov A et al. Incidence of atrial fibrillation detected by continuous rhythm monitoring after acute myocardial infarction in patients with preserved left ventricular ejection fraction: results of the ARREST study. Europace 20, 263–270 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Bloch Thomsen PE et al. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation 122, 1258–1264 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Krijthe BP et al. Unrecognized myocardial infarction and risk of atrial fibrillation: The Rotterdam Study. Int J Cardiol 168, 1453–1457 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Jabre P et al. Atrial fibrillation and death after myocardial infarction: a community study. Circulation 123, 2094–2100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massicotte-Azarniouch D et al. Incident Atrial Fibrillation and the Risk of Congestive Heart Failure, Myocardial Infarction, End-Stage Kidney Disease, and Mortality Among Patients With a Decreased Estimated GFR. Am J Kidney Dis 71, 191–199 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Schnabel RBD et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 386, 154–162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang J, Alderman MH, Keenan NL & Ayala C Acute myocardial infarction hospitalization in the United States, 1979 to 2005. Am J Med 123, 259–266 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Staerk L et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ 361, k1453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai H et al. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes 7, 574–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonso A et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2, e000102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mou L et al. Lifetime Risk of Atrial Fibrillation by Race and Socioeconomic Status: ARIC Study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol 11, e006350 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kytö V, Sipilä J & Rautava P Association of age and gender with risk for non-ST-elevation myocardial infarction. Eur J Prev Cardiol 22, 1003–1008 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Kytö V, Sipilä J & Rautava P Gender, age and risk of ST segment elevation myocardial infarction. Eur J Clin Invest 44, 902–909 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Virani SS et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 143, e254–e743 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Heckbert SR et al. Differences by Race/Ethnicity in the Prevalence of Clinically Detected and Monitor-Detected Atrial Fibrillation: MESA. Circ Arrhythm Electrophysiol 13, e007698 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang ZM et al. Race and Sex Differences in the Incidence and Prognostic Significance of Silent Myocardial Infarction in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 133, 2141–2148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Essien UR et al. Social determinants of atrial fibrillation. Nat Rev Cardiol 18, 763–773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norby FL et al. Trajectories of Cardiovascular Risk Factors and Incidence of Atrial Fibrillation Over a 25-Year Follow-Up: The ARIC Study (Atherosclerosis Risk in Communities). Circulation 134, 599–610 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lind L, Ingelsson M, Sundstrom J & Ärnlöv J Impact of risk factors for major cardiovascular diseases: a comparison of life-time observational and Mendelian randomisation findings. Open Heart 8, e001735 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banks E et al. Tobacco smoking and risk of 36 cardiovascular disease subtypes: fatal and non-fatal outcomes in a large prospective Australian study. BMC Med 17, 128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow CK et al. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 121, 750–758 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Aune D, Schlesinger S, Norat T & Riboli E Tobacco smoking and the risk of atrial fibrillation: A systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol 25, 1437–1451 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Gémes K et al. Alcohol consumption is associated with a lower incidence of acute myocardial infarction: results from a large prospective population-based study in Norway. J Intern Med 279, 365–375 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Leong DP et al. Patterns of Alcohol Consumption and Myocardial Infarction Risk. Circulation 130, 390–398 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Biddinger KJ et al. Association of Habitual Alcohol Intake With Risk of Cardiovascular Disease. JAMA Network Open 5, e223849 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larsson SC, Drca N & Wolk A Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol 64, 281–289 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Lankester J, Zanetti D, Ingelsson E & Assimes TL Alcohol use and cardiometabolic risk in the UK Biobank: A Mendelian randomization study. PLoS One 16, e0255801 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voskoboinik A et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N Engl J Med 382, 20–28 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Jones NR, Taylor KS, Taylor C,J & Aveyard P Weight change and the risk of incident atrial fibrillation: a systematic review and meta-analysis. Heart 105, 1799–1805 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huxley RR et al. Physical Activity, Obesity, Weight Change, and Risk of Atrial Fibrillation. Circ Arrhythm Electrophysiol 7, 620–625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abed HS et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 310, 2050–2060 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Janszky I et al. Weight and weight change and risk of acute myocardial infarction and heart failure - the HUNT Study. J Intern Med 280, 312–322 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Mishima RS et al. Self-reported physical activity and atrial fibrillation risk: A systematic review and meta-analysis. Heart Rhythm 18, 520–528 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Hansen KW et al. Association of fatal myocardial infarction with past level of physical activity: a pooled analysis of cohort studies. Eur J Prev Cardiol 28, 1590–1598 (2021). [DOI] [PubMed] [Google Scholar]
- 66.Wang Q et al. A phenome-wide bidirectional Mendelian randomization analysis of atrial fibrillation. Int J Epidemiol 51, 1153–1166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soliman EZ et al. Effect of Intensive Blood Pressure Lowering on the Risk of Atrial Fibrillation. Hypertension 75, 1491–1496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright JT et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 373, 2103–2116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie XMD et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 387, 435–443 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Fang H-J et al. Effects of intensive glucose lowering in treatment of type 2 diabetes mellitus on cardiovascular outcomes: a meta-analysis of data from 58,160 patients in 13 randomized controlled trials. Int J Cardiol 218, 50–58 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Harati H et al. No evidence of a causal association of type 2 diabetes and glucose metabolism with atrial fibrillation. Diabetologia 62, 800–804 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dublin S et al. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med 25, 853–858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kilpi F, Silventoinen K, Konttinen H & Martikainen P Disentangling the relative importance of different socioeconomic resources for myocardial infarction incidence and survival: a longitudinal study of over 300 000 Finnish adults. Eur J Public Health 26, 260–266 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Soliman EZ, Zhang ZM, Judd S, Howard VJ & Howard G Comparison of Risk of Atrial Fibrillation Among Employed Versus Unemployed (from the REasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol 120, 1298–1301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salomaa V et al. Relation of socioeconomic position to the case fatality, prognosis and treatment of myocardial infarction events; the FINMONICA MI Register Study. J Epidemiol Community Health 55, 475–482 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hillerson D et al. Characteristics, Process Metrics, and Outcomes Among Patients With ST-Elevation Myocardial Infarction in Rural vs Urban Areas in the US: A Report From the US National Cardiovascular Data Registry. JAMA Cardiol 7, 1016–1024 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen M, Zhao J, Zhuo C & Zheng L The Association Between Ambient Air Pollution and Atrial Fibrillation - A Systematic Review and Meta-Analysis. Int Heart J 62, 290–297 (2021). [DOI] [PubMed] [Google Scholar]
- 78.Dahlquist M et al. Short-term associations between ambient air pollution and acute atrial fibrillation episodes. Environ Int 141, 105765 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Biondi-Zoccai G et al. Impact of environmental pollution and weather changes on the incidence of ST-elevation myocardial infarction. Eur J Prev Cardiol 28, 1501–1507 (2021). [DOI] [PubMed] [Google Scholar]
- 80.Khosravipour M, Safari-Faramani R, Rajati F & Omidi F The long-term effect of exposure to respirable particulate matter on the incidence of myocardial infarction: a systematic review and meta-analysis study. Environ Sci Pollut Res Int 29, 42347–42371 (2022). [DOI] [PubMed] [Google Scholar]
- 81.Schnabel RB et al. Large-Scale Candidate Gene Analysis in Whites and African Americans Identifies IL6R Polymorphism in Relation to Atrial Fibrillation. Circ Cardiovasc Genet 4, 557–564 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ridker PM, Cushman M, Stampfer MJ, Tracy RP & Hennekens CH Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336, 973–979 (1997). [DOI] [PubMed] [Google Scholar]
- 83.Ridker PM, Hennekens CH, Buring JE & Rifai N C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N Engl J Med 342, 836–843 (2000). [DOI] [PubMed] [Google Scholar]
- 84.Alasady M et al. Coronary artery disease affecting the atrial branches is an independent determinant of atrial fibrillation after myocardial infarction. Heart Rhythm 8, 955–960 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Nishida K et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation 123, 137–146 (2011). [DOI] [PubMed] [Google Scholar]
- 86.Alasady M et al. Myocardial infarction and atrial fibrillation: importance of atrial ischemia. Circ Arrhythm Electrophysiol 6, 738–745 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Popovic B et al. Coronary Embolism Among ST-Segment-Elevation Myocardial Infarction Patients: Mechanisms and Management. Circ Cardiovasc Interv 11, e005587 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Range FT et al. Impaired myocardial perfusion and perfusion reserve associated with increased coronary resistance in persistent idiopathic atrial fibrillation. Eur Heart J 28, 2223–2230 (2007). [DOI] [PubMed] [Google Scholar]
- 89.Hansson GK Mechanisms of disease: Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med 352, 1685 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Hu YF, Chen YJ, Lin YJ & Chen SA Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 12, 230–243 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Yuan S, Lin A, He QQ, Burgess S & Larsson SC Circulating interleukins in relation to coronary artery disease, atrial fibrillation and ischemic stroke and its subtypes: A two-sample Mendelian randomization study. Int J Cardiol 313, 99–104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aulin J et al. Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. Am Heart J 170, 1151–1160 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Ferro D et al. Soluble CD40 ligand predicts ischemic stroke and myocardial infarction in patients with nonvalvular atrial fibrillation. Arterioscler Thromb Vasc Biol 27, 2763–2768 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Bas HA et al. The association of plasma oxidative status and inflammation with the development of atrial fibrillation in patients presenting with ST elevation myocardial infarction. Scand J Clin Lab Invest 77, 77–82 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Aronson D et al. Relation of C-reactive protein and new-onset atrial fibrillation in patients with acute myocardial infarction. Am J Cardiol 100, 753–757 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Ridker PM et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377, 1119–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Varghese B et al. Inflammation, atrial fibrillation, and the potential role for colchicine therapy. Heart Rhythm 2, 298–303 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nattel S Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin Electrophysiol 3, 425–435 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Miyauchi Y et al. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation 108, 360–366 (2003). [DOI] [PubMed] [Google Scholar]
- 100.Sciagrà R et al. Detection of infarct size safety threshold for left ventricular ejection fraction impairment in acute myocardial infarction successfully treated with primary percutaneous coronary intervention. Eur J Nucl Med Mol Imaging 40, 542–547 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Stone GW et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol 67, 1674–1683 (2016). [DOI] [PubMed] [Google Scholar]
- 102.Cha Y-M, Redfield MM, Shen W-K & Gersh BJ Atrial Fibrillation and Ventricular Dysfunction. Circulation 109, 2839–2843 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Reinstadler SJ et al. Impact of Atrial Fibrillation During ST-Segment–Elevation Myocardial Infarction on Infarct Characteristics and Prognosis. Circ Cardiovasc Imaging 11, e006955 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Sandoval Y & Jaffe AS Type 2 Myocardial Infarction: JACC Review Topic of the Week. J Am Coll Cardiol 73, 1846–1860 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Luo C et al. Documentation of impaired coronary blood flow by TIMI frame count method in patients with atrial fibrillation. Int J Cardiol 167, 1176–1180 (2013). [DOI] [PubMed] [Google Scholar]
- 106.Raphael CE et al. Coronary Embolus: An Underappreciated Cause of Acute Coronary Syndromes. JACC Cardiovasc Interv 11, 172–180 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Jabre P et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation 123, 1587–1593 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petersen JK et al. Incidence of ischaemic stroke and mortality in patients with acute coronary syndrome and first-time detected atrial fibrillation: a nationwide study. Eur Heart J 42, 4553–4561 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Wang CL, Chen PC, Juang HT & Chang CJ Adverse Outcomes Associated with Pre-Existing and New-Onset Atrial Fibrillation in Patients with Acute Coronary Syndrome: A Retrospective Cohort Study. Cardiol Ther 8, 117–127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gourronc Y et al. De novo atrial fibrillation as an independent prognostic marker after ST-segment elevation myocardial infarction: Results from the RIMA registry. J Cardiol 74, 123–129 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Almendro-Delia M et al. Prognostic impact of atrial fibrillation in acute coronary syndromes: results from the ARIAM registry. Eur Heart J Acute Cardiovasc Care 3, 141–148 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Hindricks G et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42, 373–498 (2021). [DOI] [PubMed] [Google Scholar]
- 113.January CT et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 140, e125–e151 (2019). [DOI] [PubMed] [Google Scholar]
- 114.Hess PL et al. Use of evidence-based cardiac prevention therapy among outpatients with atrial fibrillation. Am J Med 126, 625–632.e621 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee CJ et al. Antithrombotic Therapy and First Myocardial Infarction in Patients With Atrial Fibrillation. J Am Coll Cardiol 69, 2901–2909 (2017).28619189 [Google Scholar]
- 116.Connolly SJ et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 361, 1139–1151 (2009). [DOI] [PubMed] [Google Scholar]
- 117.Patel MR et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N Engl J Med 365, 883–891 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Granger CB et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 365, 981–992 (2011). [DOI] [PubMed] [Google Scholar]
- 119.Lee CJ et al. Risk of Myocardial Infarction in Anticoagulated Patients With Atrial Fibrillation. J Am Coll Cardiol 72, 17–26 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Vemulapalli S et al. Blood Pressure Control and Cardiovascular Outcomes in Patients With Atrial Fibrillation (From the ORBIT-AF Registry). Am J Cardiol 123, 1628–1636 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Choi S et al. Association of smoking cessation after atrial fibrillation diagnosis on the risk of cardiovascular disease: a cohort study of South Korean men. BMC Public Health 20, 168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanif K, Bid HK & Konwar R Reinventing the ACE inhibitors: some old and new implications of ACE inhibition. Hypertens Res 33, 11–21 (2010). [DOI] [PubMed] [Google Scholar]
- 123.Pedersen OD, Bagger H, Kober L & Torp-Pedersen C Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation 100, 376–380 (1999). [DOI] [PubMed] [Google Scholar]
- 124.Batra G et al. Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers Are Associated With Improved Outcome but Do Not Prevent New-Onset Atrial Fibrillation After Acute Myocardial Infarction. J Am Heart Assoc 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh JP et al. Renin-angiotensin-system modulators and the incidence of atrial fibrillation following hospitalization for coronary artery disease. Europace 14, 1287–1293 (2012). [DOI] [PubMed] [Google Scholar]
- 126.McMurray J et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol 45, 525–530 (2005). [DOI] [PubMed] [Google Scholar]
- 127.Ibanez B et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39, 119–177 (2017). [DOI] [PubMed] [Google Scholar]
- 128.Collet J-P et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 42, 1289–1367 (2020). [DOI] [PubMed] [Google Scholar]
- 129.Amsterdam EA et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 64, e139–e228 (2014). [DOI] [PubMed] [Google Scholar]
- 130.O’Gara PT et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61, e78–e140 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Dewilde WJ et al. Uninterrupted oral anticoagulation versus bridging in patients with long-term oral anticoagulation during percutaneous coronary intervention: subgroup analysis from the WOEST trial. EuroIntervention 11, 381–390 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Kiviniemi T et al. Comparison of additional versus no additional heparin during therapeutic oral anticoagulation in patients undergoing percutaneous coronary intervention. Am J Cardiol 110, 30–35 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Knuuti J et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 41, 407–477 (2019). [DOI] [PubMed] [Google Scholar]
- 134.January CT et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation 130, e199–e267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luo CF, Mo P, Li GQ & Liu SM Aspirin-omitted dual antithrombotic therapy in non-valvular atrial fibrillation patients presenting with acute coronary syndrome or undergoing percutaneous coronary intervention: results of a meta-analysis. Eur Heart J Cardiovasc Pharmacother 7, 218–224 (2021). [DOI] [PubMed] [Google Scholar]
- 136.Lopes RD et al. Optimal Antithrombotic Regimens for Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: An Updated Network Meta-analysis. JAMA Cardiol 5, 582–589 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Galli M, Andreotti F, Porto I & Crea F Intracranial haemorrhages vs. stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta-analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients. Europace 22, 538–546 (2020). [DOI] [PubMed] [Google Scholar]
- 138.Potpara TS et al. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. Europace 22, 33–46 (2020). [DOI] [PubMed] [Google Scholar]
- 139.Cannon CP et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med 377, 1513–1524 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Gibson CM et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med 375, 2423–2434 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Lopes RD et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med 380, 1509–1524 (2019). [DOI] [PubMed] [Google Scholar]
- 142.Vranckx P et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet 394, 1335–1343 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Lupercio F et al. P2Y(12) inhibitors with oral anticoagulation for percutaneous coronary intervention with atrial fibrillation: a systematic review and meta-analysis. Heart 106, 575–583 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Camm AJ et al. A Randomized Active-Controlled Study Comparing the Efficacy and Safety of Vernakalant to Amiodarone in Recent-Onset Atrial Fibrillation. J Am Coll Cardiol 57, 313–321 (2011). [DOI] [PubMed] [Google Scholar]
- 145.Roy D et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation 117, 1518–1525 (2008). [DOI] [PubMed] [Google Scholar]
- 146.Echt DS et al. Mortality and Morbidity in Patients Receiving Encainide, Flecainide, or Placebo. N Engl J Med 324, 781–788 (1991). [DOI] [PubMed] [Google Scholar]
- 147.Podrid PJ & Anderson JL Safety and Tolerability of Long-term Propafenone Therapy for Supraventricular Tachyarrhythmias. Am J Cardiol 78, 430–434 (1996). [DOI] [PubMed] [Google Scholar]
- 148.Connolly S et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 367, 1903–1912 (2006). [DOI] [PubMed] [Google Scholar]
- 149.Jewitt DE, Balcon R, Raftery EB & Oram S Incidence and management of supraventricular arrhythmias after acute myocardial infarction. Lancet 2, 734–738 (1967). [DOI] [PubMed] [Google Scholar]
- 150.Behar S, Zahavi Z, Goldbourt U & Reicher-Reiss H Long-term prognosis of patients with paroxysmal atrial fibrillation complicating acute myocardial infarction. SPRINT Study Group. Eur Heart J 13, 45–50 (1992). [DOI] [PubMed] [Google Scholar]
- 151.Pedersen OD, Bagger H, Køber L & Torp-Pedersen C The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evalution. Eur Heart J 20, 748–754 (1999). [DOI] [PubMed] [Google Scholar]
- 152.Pizzetti F, et al. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart 86, 527–532 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu Y‑F et al. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 12, 230–243 (2015). [DOI] [PubMed] [Google Scholar]
- 154.Watanabe H et al. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J 158, 629–636 (2009). [DOI] [PubMed] [Google Scholar]
- 155.Le-Rademacher JG, Therneau TM & Ou F-S The Utility of Multistate Models: A Flexible Framework for Time-to-Event Data. Curr Epidemiol Rep 9, 182–189 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Evans DM & Davey Smith G Mendelian Randomization: New Applications in the Coming Age of Hypothesis-Free Causality. Annu Rev Genomics Hum Genet 16, 327–350 (2015). [DOI] [PubMed] [Google Scholar]
- 157.Lawlor DA, Harbord RM, Sterne JA, Timpson N & Davey Smith G Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27, 1133–1163 (2008). [DOI] [PubMed] [Google Scholar]
- 158.Davey Smith G & Hemani G Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23, R89–R98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zheng J et al. Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep 4, 330–345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Richmond RC & Davey Smith G Commentary: Orienting causal relationships between two phenotypes using bidirectional Mendelian randomization. Int J Epidemiol 48, 907–911 (2019). [DOI] [PubMed] [Google Scholar]
- 161.Yan T et al. Coronary Artery Disease and Atrial Fibrillation: A Bidirectional Mendelian Randomization Study. J Cardiovasc Dev Dis 9, 69 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kwok MK & Schooling CM Mendelian randomization study on atrial fibrillation and cardiovascular disease subtypes. Sci Rep 11, 18682 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


