Abstract
Background
Recurrent apnea is common in preterm infants, particularly at very early gestational ages. These episodes of loss of effective breathing can lead to hypoxemia and bradycardia, which may be severe enough to require resuscitation including use of positive pressure ventilation. Two forms of methylxanthine (caffeine and theophylline) have been used to stimulate breathing in order to prevent apnea and its consequences.
Objectives
To evaluate the effect of caffeine compared with theophylline treatment on the risk of apnea and use of mechanical ventilation in preterm infants with recurrent apnea.
Search methods
The standard search strategy of the Cochrane Neonatal Review Group was used. This included searches of electronic databases in August 2009: Oxford Database of Perinatal Trials; Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2009); MEDLINE (1966 to April 2009); and EMBASE Drugs and Pharmacology (1990 to April 2009), previous reviews including cross references.
Selection criteria
Randomized and quasi‐randomized trials comparing caffeine to theophylline for treating apnea in preterm infants and reporting effects on apnea event rates.
Data collection and analysis
Each author assessed eligibility and trial quality, extracted data separately and compared and resolved differences. Study authors were contacted for additional information.
Main results
Five trials involving a total of 108 infants were included. The quality of most of these small trials was fair to good. No difference in treatment failure rate (less than 50% reduction in apnea/bradycardia) was found between caffeine and theophylline after one to three days treatment (based on two studies) or five to seven days treatment (based on one study). There was no difference in mean apnea rate between caffeine and theophylline groups after one to three days treatment (based on five trials) and five to seven days treatment (based on four trials).
Adverse effects, indicated by tachycardia or feed intolerance leading to change in dosing, were lower in the caffeine group (summary relative risk 0.17, 95% CI 0.04 to 0.72). This was reported and consistent in three studies.
No trial reported the use of ventilation and no data were available to assess effects on growth and development.
Authors' conclusions
Caffeine appears to have similar short‐term effects on apnea/bradycardia as does theophylline although caffeine has certain therapeutic advantages over theophylline. Theophylline is associated with higher rates of toxicity. The possibility that higher doses of caffeine might be more effective in extremely preterm infants needs further evaluation in randomized clinical trials.
Keywords: Humans; Infant, Newborn; Apnea; Apnea/drug therapy; Bronchodilator Agents; Bronchodilator Agents/therapeutic use; Caffeine; Caffeine/therapeutic use; Central Nervous System Stimulants; Central Nervous System Stimulants/therapeutic use; Infant, Premature; Infant, Premature, Diseases; Infant, Premature, Diseases/drug therapy; Randomized Controlled Trials as Topic; Theophylline; Theophylline/therapeutic use
Plain language summary
Caffeine versus theophylline for apnea in preterm infants
There is some evidence that caffeine is as effective as theophylline in the short‐term for reducing apnea in premature babies, is better tolerated and is easier to give.
Apnea is a pause in breathing of greater than 20 seconds. It may occur repeatedly in preterm babies (born before 34 weeks gestation). Persistent apnea may be harmful to the developing brain or organs. Methylxanthines (such as theophylline and caffeine) are drugs that are believed to stimulate breathing efforts and have been used to reduce apnea. This review of trials found that caffeine has similar effects to theophylline but has a larger gap between levels that are therapeutic and those with toxic effects. Caffeine is more easily absorbed and has a longer half‐life that allows for once daily dosing.
Background
Description of the condition
Infant apnea has been defined as a pause in breathing of greater than 20 seconds or one of less than 20 seconds and associated with bradycardia and/or cyanosis (AAP 2003). Recurrent episodes of apnea are common in preterm infants and the incidence and severity increases at lower gestational ages (Henderson‐Smart 1995). Although apnea can occur spontaneously and be attributed to prematurity alone, it can also be provoked or made more severe if there is some additional insult such as infection, hypoxemia or intracranial pathology.
Description of the intervention
Methylxanthines are thought to stimulate breathing efforts and have been used in clinical practice to reduce apnea since the 1970's. Theophylline and caffeine are two forms that have been used. The efficacy of methylxanthines compared with control was evaluated in another review in the Cochrane Library (Henderson‐Smart 2004), which concluded that their use reduced apnea. The mechanism of their action is not certain. Possibilities include increased chemoreceptor responsiveness (based on increased breathing responses to CO2), enhanced respiratory muscle performance and generalized central nervous system excitation.
How the intervention might work
Caffeine has a potential therapeutic advantage over theophylline due to its higher therapeutic ratio, more reliable enteral absorption and longer half life, which allows once daily administration (Blanchard 1992).
Why it is important to do this review
If prolonged, apnea can lead to hypoxemia and reflex bradycardia, which may require active resuscitative efforts to reverse. There are clinical concerns that these episodes might be harmful to the developing brain or cause dysfunction of the gut or other organs, although there are no data to support this. Frequent episodes may be accompanied by respiratory failure of sufficient severity as to lead to intubation and the use of intermittent positive pressure ventilation (IPPV).
Objectives
To evaluate the effect of caffeine compared with theophylline treatment on the risk of apnea and use of mechanical ventilation in preterm infants with recurrent apnea.
Subgroup analyses:
1. Outcomes of different doses of caffeine or theophylline.
2. Outcomes of infants born at different gestational ages or birth weights.
Methods
Criteria for considering studies for this review
Types of studies
Trials utilising random or quasi‐random treatment assignment
Types of participants
Preterm neonates (born before 34 weeks gestation) requiring treatment for recurrent apnea of prematurity.
Types of interventions
Caffeine compared with theophylline for the treatment of apnea of prematurity. Trials in which caffeine and theophylline were compared as prophylactic therapy in preterm infants at risk of developing apnea or those in which they were used to assist extubation following IPPV were not eligible.
Types of outcome measures
Primary outcomes
1. Apnea (failed treatment defined as no clinically important reduction in apnea (>50% reduction), use of IPPV or death during study). 2. Mean rates of apnea
Secondary outcomes
3. Use of IPPV 4. Side effects such as tachycardia or feed intolerance leading to alteration in treatment 5. Longer term growth and development
Search methods for identification of studies
Electronic searches
This included searches of the Oxford Database of Perinatal Trials, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 2, 2009), MEDLINE (1966 to April 2009), and EMBASE Drugs and Pharmacology (1990 to April 2009) using key words 'caffeine', 'theophylline' and 'apnea', MeSH terms 'infant, preterm', and 'randomized controlled trial' or 'controlled clinical trial'.
Searching other resources
Searches were also made of previous reviews including cross references, abstracts, conferences and symposia proceedings, expert informants and journal handsearching mainly in the English language.
Clinical trials registries were also searched for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp)
Data collection and analysis
The standard strategy of the Neonatal Review Group was used.
Selection of studies
All randomized and quasi‐randomized controlled trials fulfilling the selection criteria described in the previous section were included. The investigators reviewed the results of the search and separately select the studies for inclusion. The review authors resolved any disagreement by discussion.
Data extraction and management
Data were extracted independently by the two review authors. Any standard error of the mean was replaced by the corresponding standard deviation. Differences were resolved by discussion and consensus of the reviewers. Where necessary, trial authors were contacted for additional information or data. Information was provided for four of these (Bairam 1987; Fuglsang 1989; Kumar 1992; Scanlon 1992).
Assessment of risk of bias in included studies
The methodological quality of the studies were assessed using the following key criteria: allocation concealment (blinding of randomization), blinding of intervention, completeness of follow‐up, and blinding of outcome measurement/assessment. For each criterion, assessment was yes, no, can't tell. Two review authors separately assessed each study. Any disagreement was resolved by discussion. This information was added to the table "Characteristics of Included Studies".
In addition, the following issues were evaluated and entered into the Risk of Bias Table:
1. Sequence generation: Was the allocation sequence adequately generated?
2. Allocation concealment: Was allocation adequately concealed?
3. Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
4. Incomplete outcome data: Were incomplete outcome data adequately addressed?
5. Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
6. Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
Measures of treatment effect
Statistical analyses were performed using Review Manager software. For individual trials, mean differences (and 95% confidence intervals) are reported for continuous variables. For categorical outcomes, the relative risk and risk difference (and 95% confidence intervals) are reported.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic.
Data synthesis
For the meta‐analysis, weighted mean differences (WMD) and 95% confidence intervals (CI) were reported for continuous variables. For meta‐analysis of categorical outcomes the summary relative risks (RR) and risk differences (RD) and 95% CI were calculated using a fixed effects model. To calculate the number needed to treat (NNT) the risk difference (RD) was used.
Subgroup analysis and investigation of heterogeneity
1. Different doses of caffeine or theophylline
2. Infants born at different gestational ages or birth weights.
Results
Description of studies
Results of the search
Nine trials were considered to be potentially eligible for this review.
Included studies
Five studies with a total of 108 preterm infants (54 in each group) were included. Details of the five included studies (Brouard 1985; Bairam 1987; Fuglsang 1989; Scanlon 1992; Kumar 1992) are available in the included table "Characteristics of Included Studies". Brouard 1985; Bairam 1987 and Scanlon 1992 were good quality trials documented in the publications. For the Fuglsang 1989 trial, the author provided study details. Kumar 1992 was only published as an abstract but the author provided missing information about study design and drug dosage.
Excluded studies
Four trials were excluded (Dani 2000; Zanardo 1995; Fang 1998; Laubscher 1998) as no clinical apnea outcome data were available. In addition to the published results, the third and fourth trials had no unpublished data on apnea outcomes (information provided by author Anne Greenough).
Risk of bias in included studies
The overall quality of the included studies was fair/good.
Allocation
Concealment of treatment allocation by blinded randomization was undertaken in three trials (Bairam 1987; Scanlon 1992; Kumar 1992). The method of randomization was not clear in the Brouard 1985 and Fuglsang 1989 trials.
Blinding
Blinding of the intervention outcome assessments was only undertaken in two trials (Bairam 1987; Fuglsang 1989).
Incomplete outcome data
In two trials some of the randomized infants were not analysed because of complications (Scanlon 1992, 8/44 in continuing apnea; Fuglsang 1989, 9/27 in continuing apnea and mean apnea). Despite the high rate of exclusion (30%) in Fuglsang 1989 the baseline information was similar in the two groups of included infants.
Other potential sources of bias
None known.
Effects of interventions
Caffeine vs. theophylline for apnea in preterm infants (all infants)(Comparison 1):
There is no difference in the failure rate (< 50% reduction in apnea/bradycardia) of treatment with caffeine or theophylline at one to three days [two studies, Bairam 1987; Scanlon 1992); summary relative risk (RR) 1.35, 95% confidence interval (CI) 0.41, 4.52] (Outcome 1.1) or at five to seven days (one study, Bairam 1987; RR 1.50, 95% CI 0.32, 7.14) (Outcome 1.2). There was a small borderline but insignificant increase in the mean rate/100 min of apnea in the caffeine group at one to three days [all five included studies, weighted mean difference (WMD) 0.11, 95% CI 0.00, 0.22] (Outcome 1.3). In individual studies at one to three days there was a significantly higher mean apnea rate in the caffeine group in Scanlon 1992 (0.46 95%CI 0.14, 0.78) and a non‐significant trend of increase in Bairam 1987. At five to seven days there was no difference in mean apnea rates in individual studies or in WMD between groups [four studies, Bairam 1987; Brouard 1985; Fuglsang 1989; Kumar 1992; (WMD 0.0; 95% CI ‐0.05, 0.05] (Outcome 1.4), or in individual studies.
Side effects, as indicated by tachycardia or feed intolerance leading to change in dosing, were lower in the caffeine group [RR 0.17; 95% CI 0.04, 0.72; risk difference (RD) ‐0.29; 95% CI ‐0.47, ‐0.10, number needed to treat (NNT) 3.5; 95% CI 2.1, 9.6] (Outcome 1.5). This was consistent across the three studies reporting side effects data (Bairam 1987; Brouard 1985; Scanlon 1992).
No trial reported the use of IPPV and no data are available to assess the effects on growth and neurological development.
There was insufficient data to undergo subgroup analyses of outcomes of different doses of caffeine or theophylline or outcomes of infants born at different gestational ages or birth weights.
Discussion
Summary of main results
There was no difference in the failure rate (number of infants with < 50% reduction in apnea) between caffeine and theophylline at one to three and five to seven days. There was also no significant differences in the weighted mean differences of apnea on day one to day three and on day five to day seven.
Three studies found standard caffeine treatment to have less short‐term side effects than theophylline, consistent with known caffeine and theophylline pharmacology. The NNT of 3.5 indicates that for every three to four patients treated with caffeine, one patient having a significant adverse event can be avoided.
There are no data from these studies on long‐term effectiveness.
Overall completeness and applicability of evidence
There is a need for clinical trials with larger numbers of infants born at a lower gestational age to demonstrate the effectiveness and safety of caffeine compared to theophylline treatment with respect to clinically important outcomes including safety and long‐term effects on neurodevelopmental outcome. The appropriate dose of methylxanthine therapy requires further investigation.
The daily maintenance dose of caffeine was 2.5 mg (equal to 5 mg caffeine citrate) in four trials and 3 mg (10 mg caffeine citrate) in one (Scanlon 1992). In most clinical practices, 5 mg (10 mg caffeine citrate) is used. The possibility that higher doses of caffeine might be more effective in extremely preterm infants needs further evaluation in randomized clinical trials.
Quality of the evidence
The results of this review should be interpreted with caution. The number of infants in each study was small. There was some variability in the characteristics of participants in terms of gestational age as well as clinical status. For example Scanlon 1992 examined infants who were of lower gestational age and who were oxygen dependent. This trial contributed the most weight to the difference in mean rates of apnea/bradycardia at one to three days. It also examined a third treatment arm of higher dose caffeine but this has not been included here.
Authors' conclusions
Implications for practice.
In treatment of preterm infants with apnea, caffeine appears to have similar short‐term reductions of apnea/bradycardia when compared to theophylline. In view of the other therapeutic advantages of caffeine (a higher therapeutic ratio, more reliable enteral absorption and a longer half life as well as less side effects than theophylline) caffeine is the preferred treatment for apnea in preterm infants. There are no data from these studies on the long‐term effectiveness and safety of the drugs.
Implications for research.
There is a need for clinical trials with larger numbers of infants born at lower gestation to demonstrate the effectiveness and safety of caffeine compared to theophylline treatment with respect to clinically important outcomes including safety and longterm effects on neurodevelopmental outcome. The appropriate dose of methylxanthine therapy requires further investigation.
The possibility that higher doses of caffeine might be more effective in extremely preterm infants needs further evaluation in randomized clinical trials.
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2013 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 16 April 2012 | Amended | Change of contact person only. |
| 7 December 2010 | Amended | Contact details updated. |
| 5 November 2009 | New citation required but conclusions have not changed | Two new trials included; no change to conclusions. |
| 17 August 2009 | New search has been performed | This review updates the review "Caffeine versus theophylline for apnea in preterm infants", published in the Cochrane Database of Systematic Reviews, Issue 1, 2003 (Steer 2003). Updated search August 2009 identified two new trials for inclusion. No change to conclusions. |
| 14 October 2002 | New search has been performed | This review is an update of the existing review: Steer P, Henderson‐Smart D. "Caffeine versus theophylline for apnea in preterm infants" published in The Cochrane Library, Issue 2, 1998. In an updated and extended search to October 2002, four potentially eligible studies were identified: two were excluded (Dani, Zanardo) and two are awaiting assessment (Fang, Laubscher). This update does not include any new studies. |
| 13 February 1998 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Trial authors Bairam, Scanlon, Fulsgang and Kumar communicated to provide data and methodology. Samantha Lain (previous Cochrane Neonatal Review assistant) provided assistance with author contacts.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Caffeine vs. theophylline for apnea in preterm infants (all infants).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Continuing apnea at 1‐3 days | 2 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.41, 4.52] |
| 2 Continuing apnea at 5‐7 days | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.32, 7.14] |
| 3 Mean apnea rate /100 mins at 1‐3 days | 5 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.00, 0.22] |
| 4 Mean apnea rate /100 mins at 5‐7 days | 4 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.05, 0.05] |
| 5 Side effects | 3 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.72] |
1.1. Analysis.
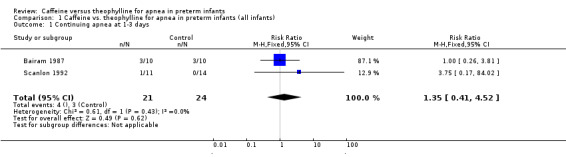
Comparison 1 Caffeine vs. theophylline for apnea in preterm infants (all infants), Outcome 1 Continuing apnea at 1‐3 days.
1.2. Analysis.
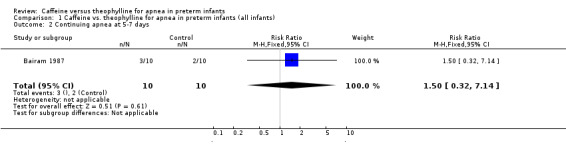
Comparison 1 Caffeine vs. theophylline for apnea in preterm infants (all infants), Outcome 2 Continuing apnea at 5‐7 days.
1.3. Analysis.
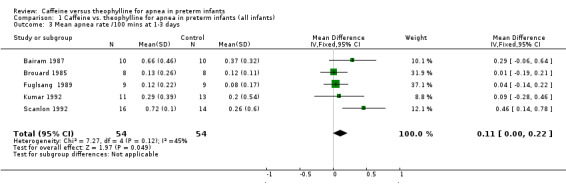
Comparison 1 Caffeine vs. theophylline for apnea in preterm infants (all infants), Outcome 3 Mean apnea rate /100 mins at 1‐3 days.
1.4. Analysis.
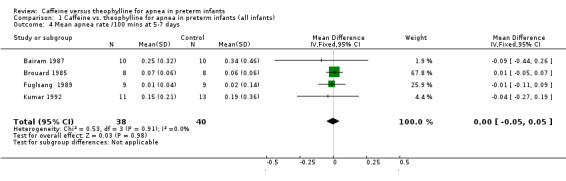
Comparison 1 Caffeine vs. theophylline for apnea in preterm infants (all infants), Outcome 4 Mean apnea rate /100 mins at 5‐7 days.
1.5. Analysis.
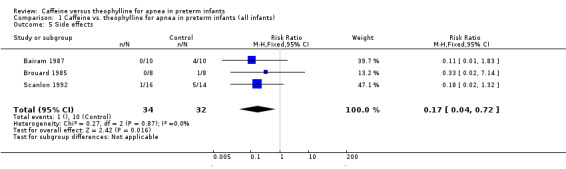
Comparison 1 Caffeine vs. theophylline for apnea in preterm infants (all infants), Outcome 5 Side effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bairam 1987.
| Methods | Single centre Blinding of randomization ‐ yes in sealed envelopes Blinding of intervention ‐ yes Complete followup ‐ yes * Blinding of outcome measure ‐ yes *extra information provided by the author (personal correspondence) | |
| Participants | 20 preterm infants (mean gestational age 30 weeks) included after 24 hour recording documented ≥ 3 apneas | |
| Interventions | Exp: standard caffeine = loading dose 10 mg/kg, maintenance dose 1.25 mg/kg/12hrs Control: theophylline = loading dose 6 mg/kg, maintenance dose 2 mg/kg/12hrs | |
| Outcomes | Frequency of apnea, bradycardia, apnea with bradycardia, systolic arterial pressure, tachycardia, weight gain, gastrointestinal intolerance, behavioural assessment (scaled‐score of motor activity, reactivity and sucking). | |
| Notes | Apnea defined as cessation of breathing of 15 seconds or more, or apnea plus bradycardia (<100). Author communication indicated that these were collected separately and in the review they were combined for primary outcome measure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both interventions and outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All studied |
| Selective reporting (reporting bias) | Low risk | None selected |
| Other bias | High risk | Assisted by author correspondence |
Brouard 1985.
| Methods | Single centre Blinding of randomization ‐ can't tell Blinding of intervention ‐ no Complete follow‐up ‐ yes Blinding of outcome measure ‐ can't tell | |
| Participants | 16 preterm infants (mean gestational age 30 weeks) enrolled infants where ≥ 3 severe apneas noted per 24 hours | |
| Interventions | Exp: standard caffeine = loading dose 10 mg/kg intramuscularly, daily maintenance dose 2.5 mg/kg orally to target serum level of 8 ‐ 16 mg/l) Control: Aminophylline used as theophylline = loading dose 5.5 mg/kg intravenously, maintenance dose adjusted to maintain plasma levels at 5 ‐ 10 mg/kg | |
| Outcomes | Apnea frequency on day 0, 1, and 5 Tachycardia Weight | |
| Notes | Severe apnea defined as cessation of breathing for 10 secs with heart rate < 80 for > 30 seconds or <60 for > 15 seconds | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of interventions, blinding of outcome assessments unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All studied |
| Selective reporting (reporting bias) | Low risk | No selective cases reported |
| Other bias | Unclear risk | None stated |
Fuglsang 1989.
| Methods | Single centre Blinding of randomization ‐ uncertain Blinding of intervention ‐ yes Complete followup ‐ no; 9 of the 27 randomized infants were excluded by day 5 because they had causes (6 with septicemia and need for assisted ventilation, 2 with cerebral hemorrhage) and 1 withdrawn by parents. Blinding of outcome measure ‐ yes, continuous polygraph research recording while treatment blinded. | |
| Participants | Data were reported for 18 preterm infants with idiopathic apnea requiring treatment. Mean (SD) comparisons between groups; weeks of gestational age at birth 31 (3) in the caffeine group and 30 (2) in the theophylline group; birth weight 1499 (467) grams and 1351 (489) respectively, age in days at randomization 8 (11) and 7 (13). | |
| Interventions | Caffeine citrate 20 mg/kg loading dose and maintenance dose of 5 mg/kg once daily Aminophylline used as theophylline 7.5 mg/kg loading dose and maintenance dose of 3.75 mg/kg 12 hourly. Ethylenediamine salt of theophylline (=aminophylline) was used. Both drugs were given orally. |
|
| Outcomes | Apnea ‐ cessation of breathing for more than 20 secs. In the paper the results were in a graph. The author provided us with a table of daily mean and SD of apnea per 24 hours for each group. Rates on day 2 (middle of 1‐3) and day 6 (middle of 5‐7) were used and converted to rates of apnea/100mins as in other studies. Bradycardia ‐ heart rate below 100 beats/min ‐ data not reported in the paper Side effects ‐ raised mean heart rate, excessive central nervous system stimulation or dyspepsia. These were not reported as individual data but verbal comments in the text of the paper indicated there was no side effects in either group. |
|
| Notes | In the first publication of this review and in updates this trial remained excluded because of the large proportion of infants excluded (9). The author provided valuable information and the baseline comparison of the remaining two groups (9 infants in each) was similar. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of interventions and outcome assessments ‐ stated in paper and from author |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 of the 27 randomized infants were excluded by day 5 because they had causes (6 with septicemia and need for assisted ventilation, 2 with cerebral hemorrhage) and 1 withdrawn by parents |
| Selective reporting (reporting bias) | High risk | As stated above in incomplete outcome data addressed |
| Other bias | Unclear risk | Not stated |
Kumar 1992.
| Methods | Single centre Randomization ‐ yes, undertaken in the pharmacy with sealed envelopes Blinding of intervention ‐ no Completenes of follow up ‐ unknown Blindness of outcome assessment ‐ unknown |
|
| Participants | 24 preterm infants with recurrent apnea, 11 allocated to caffeine and 13 to theophylline | |
| Interventions | Caffeine citrate 20 mgs/kg loading dose, 5 mg/kg/24 hrs maintenance Theophylline 5.5 mg/kg loading dose, 1.1 mg/kg 6 hrly maintenance |
|
| Outcomes | Days 1, 3 and 7 Apnea ≥ 15 sec / 12 hr Bradycardia < 80 / 12 hrs SaO2 < 85 / 12 hrs |
|
| Notes | Only published as an abstract but additional information regarding randomisation methods and dosages of caffeine and theophylline provided by author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomization ‐ yes, undertaken in pharmacy with sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of interventions or outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | High risk | Comment from author |
| Other bias | Low risk | Only published as abstract but additional information given by author |
Scanlon 1992.
| Methods | Single centre Blinding of randomization ‐ yes Blinding of intervention ‐ no Complete followup ‐ no success rates (>50% reduction in apnea) 4 infants on caffeine and 2 infants on theophylline were excluded from analysis because of a defined cause of apnea ‐ septicaemia, neurological abnormality or oesophageal reflux. All were examined for mean rates of apnea Blinding of outcome measure ‐ no | |
| Participants | 30 preterm infants <31 week gestation with apnea (≥ 10 in 8 hours or 4 in 1 hour) | |
| Interventions | Exp: standard caffeine = loading dose 12.5 mg/kg and maintenance 3 mg/kg/24 hours
Control : theophylline = loading dose 7.5 mg/kg/8hrs (aiming for plasma levels of 13 ‐ 20 mg/l) There was also a group that received higher dose caffeine, 25mg/kg load and 6 mg/kg/24 hrs, maintenance which is not included in this review. |
|
| Outcomes | Apnea frequency over 24 hours reported after one day and after 2 days Number of infants with > 50 % reduction in apnea frequency (12 in caffeine group and 14 in theophylline group) | |
| Notes | Apnea defined as a decrease in heart rate of 40 beats per minute with cessation of breathing and requiring stimulation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Interventions and outcomes not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All examined |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | None in publication |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dani 2000 | No clinical outcome data available |
| Fang 1998 | No outcome data on apnea is available |
| Laubscher 1998 | No outcome data on apnea is available |
| Sims 1989 | For weaning premature infants from the ventilator, not treatment for apnea |
| Zanardo 1995 | No clinical outcome data available |
Contributions of authors
The two review authors contributed equally to the review process and independently accessed trials for eligibility, trial quality, and performed data extraction. This updated was performed by Henderson‐Smart and approved by Steer.
Sources of support
Internal sources
Pediatrics, McMaster Children's Hospital, Hamilton, Ontario, Canada.
NSW Centre for Perinatal Health Services Research, University of Sydney, Australia.
School of Medicine, Faculty of Health Sciences, University of Queensland, Brisbane, Australia.
External sources
Department of Health and Ageing, Commonwealth Government, Canberra ACT, Australia.
Declarations of interest
Review authors Steer and Henderson‐Smart, were investigators in a trial of caffeine in preterm infants: "High dose caffeine for extubation of preterm Infants: a randomised controlled trial."
Edited (no change to conclusions)
References
References to studies included in this review
Bairam 1987 {published and unpublished data}
- Bairam A, Boutroy M, Badonnel Y, Vert P. The choice between theophylline and caffeine in the treatment of apnea in premature infants [Le choix entre theophylline et cafeine dans le traitement des apnees du premature]. Archives Francaises de Pediatrie 1990;47:461‐5. [PubMed] [Google Scholar]
- Bairam A, Boutroy MJ, Badonnel Y, Vert P. Theophylline versus caffeine: comparative effects in treatment of idiopathic apnea in the preterm infant. Journal of Pediatrics 1987;110:636‐9. [DOI] [PubMed] [Google Scholar]
Brouard 1985 {published data only}
- Brouard C, Moriette G, Murat I, Flouvat B, Pajot N, Walti H, Gamarra E, Relier JP. Comparative efficacy of theophylline and caffeine in the treatment of idiopathic apnea in premature infants. American Journal of Diseases of Children 1985;139:698‐700. [DOI] [PubMed] [Google Scholar]
Fuglsang 1989 {published data only}
- Fuglsang G, Nielsen K, Kjaer Nielsen L, Sennels F, Jakobsen P, Thelle T. The effect of caffeine compared with theophylline in the treatment of idiopathic apnea in premature infants. Acta Paediatrica Scandinavica 1989;78:786‐8. [DOI] [PubMed] [Google Scholar]
Kumar 1992 {published and unpublished data}
- Kumar SP, Mehta PN, Bradley BS, Ezhuthachan SG. Documented monitoring (DM) shows theophylline (T) to be more effective than caffeine (C) in prematurity apnea (PA). Pediatric Research 1992;31:208A. [Google Scholar]
Scanlon 1992 {published and unpublished data}
- Scanlon JE, Chin KC, Morgan ME, Durbin GM, Hale KA, Brown SS. Caffeine or theophylline for neonatal apnoea?. Archives of Disease in Childhood 1992;67:425‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Dani 2000 {published data only}
- Dani C, Bertini G, Reali M, Tronchin M, Wiechmann L, Martelli E, Rubaltelli F. Brain hemodynamic changes in preterm infants after maintenance dose caffeine and aminophylline treatment. Biology of the Neonate 2000;78:27‐32. [DOI] [PubMed] [Google Scholar]
Fang 1998 {published data only}
- Fang S, Sherwood R, Gamsu H, Marsden J, Peters T, Greenough A. Comparison of the effects of theophylline and caffeine on serum erythropoietin concentration in premature infants. European Journal of Pediatrics 1998;157:406‐9. [DOI] [PubMed] [Google Scholar]
Laubscher 1998 {published data only}
- Laubscher B, Greenough A, Dimitriou G. Camparative effects of theophylline and caffeine on respiratory function of prematurely born infants. Early Human Development 1998;50:185‐92. [DOI] [PubMed] [Google Scholar]
Sims 1989 {published data only}
- Sims ME, Rangasamy R, Lee S, Chung H, Cohen J, Walther FJ. Comparative evaluation of caffeine and theophylline for weaning premature infants from the ventilator. American Journal of Perinatology 1989;6:72‐5. [DOI] [PubMed] [Google Scholar]
Zanardo 1995 {published data only}
- Zanardo V, Dani C, Trevisanuto D, Meneghetti S, Guglielmi A, Zacchello G, Cantarutti F. Methylxanthines increase renal calcium excretion in preterm infants. Biology of the Neonate 1995;68:169‐74. [DOI] [PubMed] [Google Scholar]
Additional references
AAP 2003
- American Academy of Pediatrics. Policy statement. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 2003;111:914‐7. [PubMed] [Google Scholar]
Blanchard 1992
- Blanchard PW, Aranda JV. Pharmacotherapy of respiratory control disorders. In: Beckerman RC, Brouillette RT, Hunt CE editor(s). Respiratory Control Disorders in Infants and Children. Baltimore: Williams & Wilkins, 1992:352‐70. [Google Scholar]
Henderson‐Smart 1995
- Henderson‐Smart. Recurrent apnea. In: Yu VYH editor(s). Bailliere's Clinical Paediatrics. Pulmonary Problems in the Perinatal Period. Vol. 3, No. 1, London: Bailliere Tindall, 1995:203‐22. [Google Scholar]
Henderson‐Smart 2004
- Henderson‐Smart DJ, Steer P. Methylxanthine treatment for apnea in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000140] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Steer 1998
- Steer P, Henderson‐Smart D. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Steer 2003
- Steer P, Henderson‐Smart D. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]


