Abstract
Background
Hypertriglyceridaemia is associated with many diseases including atherosclerosis, diabetes, hypertension and chylomicronaemia. Chinese herbal medicines have been used for a long time as lipid‐lowering agents.
Objectives
To assess the effects and safety of Chinese herbal medicines for hypertriglyceridaemia.
Search methods
We searched a number of databases including The Cochrane Library, MEDLINE, EMBASE and several Chinese databases (all until May 2012).
Selection criteria
Randomised controlled trials in participants with hypertriglyceridaemia comparing Chinese herbal medicines with placebo, no treatment, and pharmacological or non‐pharmacological interventions.
Data collection and analysis
Two review authors independently extracted data and assessed the risk of bias. Any disagreement was resolved by discussion and a decision was achieved based on consensus. We assessed trials for risk of bias against key criteria: random sequence generation, allocation concealment, blinding of participants, incomplete outcome data, selective outcome reporting and other sources of bias.
Main results
We included three randomised trials with 170 participants. Ninety participants were randomised to the Chinese herbal medicines groups and 80 to the comparator groups with numbers ranging from 50 to 60 participants per trial. The duration of treatment varied from four to six weeks. All the included trials were conducted in China and published in Chinese. Overall, the risk of bias of included trials was unclear. There were no outcome data in any of the trials on death from any cause, cardiovascular or cerebrovascular events, health‐related quality of life, or costs.
Three different herbal medicines, including Zhusuan Huoxue decoction, Huoxue Huayu Tongluo decoction, and Chushi Huayu decoction were evaluated. All three trials investigating Chinese herbal medicines treatment alone (two studies) or in combination with gemfibrozil (one study) reported results on serum triglyceride (TG) in favour of the herbal treatment. We did not perform a meta‐analysis due to significant clinical heterogeneity between the studies.
No relevant differences in adverse effects occurred and no serious adverse events were noted.
Authors' conclusions
The present systematic review suggests that Chinese herbal medicines may have positive effects on hypertriglyceridaemia. The trials did not report serious adverse effects following Chinese herbal medicines treatment. However, based on an unclear risk of bias in included studies and lack of patient‐important long‐term outcomes, no definite conclusion could be reached.
Keywords: Humans; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Gemfibrozil; Gemfibrozil/therapeutic use; Hypertriglyceridemia; Hypertriglyceridemia/drug therapy; Hypolipidemic Agents; Hypolipidemic Agents/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Chinese herbal medicines for hypertriglyceridaemia
Hypertriglyceridaemia is a condition characterised by increased blood levels of triglycerides, which constitute one of the blood lipid components. Hypertriglycerideamia can be divided into primary and secondary types. Hypertriglyceridaemia is associated with many diseases including atherosclerosis, diabetes and hypertension.
To evaluate the effects of various herbal formulations (including single herbs, Chinese proprietary medicines, and mixtures of different herbs) for treating hypertriglyceridaemia, we examined all available randomised controlled trials of Chinese herbal medicines. We identified three studies lasting from four to six weeks and recruiting 170 participants with hypertriglyceridaemia. There were no data on death from any cause, cardiovascular or cerebrovascular events (such as heart attacks or strokes), health‐related quality of life, or costs.
We found that Chinese herbal medicines used alone or in combination with lipid‐lowering drugs or 'life style' changes may have positive effects on reducing the blood levels of triglycerides. No relevant differences in adverse effects occurred and no serious adverse events were noted.
On the basis of the current evidence, no definite conclusion is possible especially because of the unclear risk of bias in the included studies and lack of reporting on patient‐important long‐term outcomes.
Summary of findings
Summary of findings for the main comparison. Chinese herbal medicines for hypertriglyceridaemia.
| Traditional Chinese herbal medicines compared with western medicine for hypertriglyceridaemia | |||
|
Patient or population: participants with hypertriglyceridaemia
Settings: not always specified
Intervention: traditional Chinese herbal medicines Comparison: benzbromarone, gemfibrozil, fenofibrate | |||
| Outcomes | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Death from any cause | See comment | See comment | Not investigated |
| Cardiovascular or cerebrovascular events | See comment | See comment | Not investigated |
|
Adverse events [follow‐up: 4 to 6 weeks] |
See comment | See comment | No serious adverse events were reported in the three included studies |
| Health‐related quality of life | See comment | See comment | Not investigated |
| Costs | See comment | See comment | Not investigated |
|
Serum triglyceride concentrations (mmol/L) [follow‐up: 4 to 6 weeks] a. Zhusuan Huoxue decoction + lifestyle intervention vs fenofibrate + lifestyle intervention b. Huoxue Huayu Tongluo decoction plus gemfibrozil vs gemfibrozil c. Chushi Huayu decoction vs benzbromarone |
a. 60 (1) b. 60 (1) c. 60 (1) |
⊕⊝⊝⊝ very lowa | a. Statistically significant higher triglyceride levels after Zhusuan Huoxue decoction b. No statistically significant differences c. Statistically significant lower triglyceride levels after Chushi Huayu decoction |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
aDue to very serious indirectness, low number of studies and patients, and inconsistency of results
Background
Description of the condition
Hypertriglyceridaemia (HTG) is a condition characterised by high blood levels of triglycerides, constituting one of the blood lipid components. Hypertriglyceridaemia can be divided into primary and secondary types. The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) defined elevated triglycerides as 1.69 mmol/L (150 mg/dL) and higher (NCEP 2001). The Third National Health and Nutrition Examination Survey (NHANES) found that approximately 1.7% of the sample had HTG, equating to roughly 3.4 million Americans in the US, adults aged 20 years and older (Christian 2011). The participants with severe HTG tended to be men (75.3%), non‐Hispanic whites (70.1%), and aged 40 to 59 years (58.5%) (Christian 2011). The prevalence of HTG was approximately 35% in men and 25% in women in US adults aged 20 years and older (NCEP 2001). The adjusted prevalence of HTG (above 1.70 mmol/L) among adults aged 18 years and older was reported to be 11.9% in China (Li 2005). The MONICA survey, carried out in three sub‐districts in Jakarta in 1993 reported that the prevalence of HTG was 32.1% in males and 20.6% in females (WHO 2000).
Hypertriglyceridaemia is often associated with other lipid abnormalities and co‐exists with other cardiac and vascular risk factors including the 'metabolic syndrome' (Li 2005; NCEP 2001). Elevated triglycerides often occur in parallel with high levels of cholesterol. While mild‐to‐moderate HTG is not usually associated with overt physical signs, lipaemia retinalis and eruptive xanthomas have been associated with severe HTG which has also been associated with life‐threatening acute pancreatitis (Kumar 2005; Martinez 2008). Hypertriglyceridaemia may be further associated with many diseases including atherosclerosis, diabetes, hypertension and chylomicronaemia syndrome (Anderson 2011; Chait 1992; Le 2007; Mota 2004; Zhang 2011). The relationship between HTG and atherosclerosis remains controversial. Meta‐analyses of thousands of patients followed up for more than 10 years in prospective studies showed that a triglyceride elevation of 1 mmol/L (88.8 mg/dL) increased the risk of cardiovascular disease by 32% in men and 76% in women, independent of HDL‐C levels (Hokanson 1996).
Description of the intervention
Clinical practice guidelines by the National Cholesterol Education Program suggest that pharmacotherapy should be considered for a triglyceride level over 200 mg/dL which is defined as severe HTG (NCEP 2001).
With regards to pharmacological treatment, current fibric acid derivatives such as gemfibrozil, bezafibrate and fenofibrate are considered the first choice as they are relatively safe and, in standard doses, it is possible to reduce plasma triglyceride levels by up to 50% (Barter 2006). Other pharmacological therapy used for the treatment of hypertriglyceridaemia includes statins, niacin, bile acid sequestrants, rimonabant, Omega‐3, and gene therapy. Although statins and other cholesterol‐lowering drugs are generally well tolerated, adverse reactions may occur in some patients (Beltowski 2009; Jacobson 2009).
For HTG, herbal treatments have been used in patients with cardiovascular problems, pancreatitis, and diabetic ketoacidosis. For example, a composite formula consisting of Panax notoginseng and borneol has been known to decrease serum lipid levels without adverse effects on liver function (Zhang 2007). Another more complex herbal formula, Daming capsule, has demonstrated benefits in hypercholesterolaemia and combined lipidaemia (Jing 2009). Chinese herbal medicines used to treat HTG include Yinchenwulingsan, Yiqihuoxue Formula, Buyang Huanwu decoction and Huayuchushisan (Sun 2009; Wang 2010; Wei 2006; Zhai 2007).
For the purpose of this review, Chinese herbal medicines are defined as drugs from medicinal plants used for the treatment of HTG. They can be in a form of raw herbs, herbal extracts or herbs formulated into capsules, tablets, decoctions, or injections. Substances such as garlic, evening primrose oil and those that could not be classified as Chinese materia medica are not included.
Adverse effects of the intervention
Herbal medicines used for lowering blood lipid levels have been associated with symptoms and signs including reduced appetite, nausea, abdominal discomfort, abdominal distension, diarrhoea, increased blood urea nitrogen (BUN) and hepatic dysfunction with isolated elevation of alanine‐aminotransferase levels (ALT). No serious adverse effects have been reported (Liu J 2006; Liu 2008), and the safety profile of herbal medicines in long‐term use has not been sufficiently assessed.
How the intervention might work
The mechanisms of action of herbal medicines in lowering blood triglycerides are largely unclear and may be complex; the small body of work in this area has been limited largely to animal experiments. Some potentially active ingredients from medicinal herbs have been identified. For example, purified Salvia miltiorrhiza extract as a farnesoid X receptor/liver X receptor alpha co‐agonist has been shown to improve the lipid profiles in hyperlipidaemic rats (Ji 2008), and the mechanism of Daming capsule on lowering blood lipids may be related to the change of Cx43 (Xing 2007). However, the mechanisms of most herbal medicines for lowering triglycerides are not well defined.
Why it is important to do this review
Although there is evidence from randomised clinical trials (RCTs) that herbal medicines improve lipid profiles, the evidence is by no means conclusive. One review on Chinese red yeast rice for primary hyperlipidaemia inferred short‐term beneficial effects of red yeast rice preparations on lipid modification (Liu J 2006). A systematic review objectively evaluating the existing evidence on the potential benefits and harms of the use of herbal medicines in HTG is needed to update the body of evidence and to help inform clinical decisions. Compared with a previous review on hyperlipidaemia (Liu J 2006), this review will focus on HTG.
Objectives
To assess the effects and safety of herbal medicines for hypertriglyceridaemia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (RCTs) with an adequate method of random sequence generation.
Adequate methods of sequence generation included computer‐generated random numbers, tables of random numbers or drawing of lots. We planned to contact authors of RCTs with unclear randomisation methods to decide eligibility of the inclusion when necessary.
Types of participants
Adults (18 years or older) with hypertriglyceridaemia (HTG).
Diagnostic criteria
We included studies of participants with HTG, defined as a mean triglyceride levels greater than 2.3 mmol/L (200 mg/dL). Other definitions could be accepted because the diagnostic criteria might have changed during the last decade. We excluded trials of secondary HTG. We did not include studies of participants with borderline triglycerides levels of 150 to 199 mg/dL and participants with hypercholesterolaemia as a co‐factor.
Types of interventions
Intervention
Chinese herbal medicines (including medicines from mixtures of herbs, single herbs, Chinese proprietary medicines, or a formula of herbs prescribed by a Chinese medicine practitioner; we planned to group each category separately when conducting comparative analyses; we excluded herbal food or nutritional supplements).
Control
No treatment.
Placebo.
Non‐traditional Chinese active agents.
Types of outcome measures
Outcome measures sought at the end of treatment and at maximal follow‐up after completion of the treatment included the following.
Primary outcomes
Cardiovascular and cerebrovascular events (cardiac death, non‐fatal myocardial infarction, acute coronary syndrome, coronary revascularisation (percutaneous coronary intervention and coronary artery bypass graft), transient ischaemic events (TIA), stroke (fatal and non‐fatal, haemorrhagic and non‐haemorrhagic)).
Death from any cause.
Serum triglyceride concentrations.
Secondary outcomes
Health‐related quality of life (evaluated by a validated instrument).
Serum cholesterol levels (including total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C)).
Weight, body mass index (BMI), waist‐to‐hip ratio (WHR).
Adverse events (two types of adverse events were analysed, serious adverse events and adverse events not considered serious. A serious adverse event was defined as any untoward medical occurrence that resulted in death, is life‐threatening, requires hospitalisation or prolongation of hospitalisations, results in persistent or significant disability/incapacity, is an event that may jeopardise the patient or requires intervention to prevent one of the former serious adverse events (ICH‐GCP 1997). All other adverse events were considered non‐serious).
Costs.
Timing of outcome measurement
The minimum treatment duration was four weeks. We wanted to explore short‐term (less than six months) and long‐term (more than six months) duration in a subgroup analysis.
'Summary of findings' table
We established a 'Summary of findings table' reporting the following outcomes listed according to priority (Table 1).
Death from any cause.
Cardiovascular events.
Cerebrovascular events.
Adverse events.
Health‐related quality of life.
Costs.
Serum triglyceride levels.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials from inception to specified date.
The Cochrane Library (2012, Issue 5)
MEDLINE (to May, 2012)
EMBASE (to May, 2012)
Chinese Biomedical Database (CBM) (to May, 2012)
China National Knowledge Infrastructure (to May, 2012)
Chinese VIP Information (to May, 2012)
Chinese Academic Conference Papers Database and Chinese Dissertation Database (to May, 2012)
We also searched databases of ongoing trials (http://www.controlled‐trials.com/ with links to several databases and https://www.clinicaltrialsregister.eu/). We planned to provide information, including trial identifier, about recognised studies in the table 'Characteristics of ongoing studies'.
For detailed search strategies, please see under Appendix 1.
If additional key words of relevance had been detected during any of the electronic or other searches, we would have modified the electronic search strategies to incorporate these terms. We planned to include studies published in any language.
Searching other resources
We tried to identify additional studies by searching the reference lists of retrieved included trials and planned to scrutinise (systematic) reviews, meta‐analyses, and health technology assessment reports.
Data collection and analysis
Selection of studies
To determine the studies to be assessed further, two review authors (ZLL, GQL) independently scanned the abstract, title or both sections of every record retrieved. We investigated all potentially relevant articles as full text. Where differences in opinion existed, they were resolved by a third party. If resolving disagreement had not been possible, we planned to add the article to those 'awaiting classification' and we planned to contact authors for clarification. We attached an adapted PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart of study selection (Figure 1) (Liberati 2009).
1.
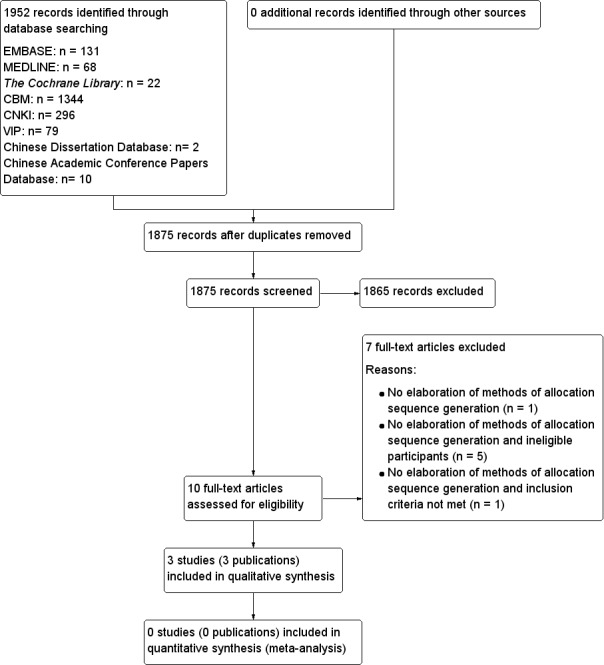
Study flow diagram.
Data extraction and management
For studies that fulfilled the inclusion criteria, two review authors (ZLL, GQL) independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details see Characteristics of included studies; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7) with any disagreements resolved by discussion, or if required, by a third party.
We planned to send an email request to contact persons of published studies to enquire whether authors were willing to answer questions regarding their trials if required. The results of this survey are included in Appendix 8. Thereafter, we planned to seek relevant missing information on the trial from the original author(s) of the article, if required.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we wanted to maximise yield of information by simultaneous evaluation of all available data.
Assessment of risk of bias in included studies
Two review authors (ZLL, GQL) assessed each trial independently. We resolved any disagreements by consensus, or by consultation with a third party.
We assessed risk of bias using The Cochrane Collaboration’s tool (Higgins 2011). We used the following criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We judged risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and used individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We present a 'Risk of bias' graph (Figure 2) and a 'Risk of bias' summary (Figure 3).
2.
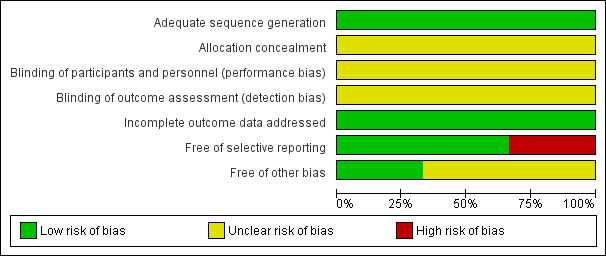
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
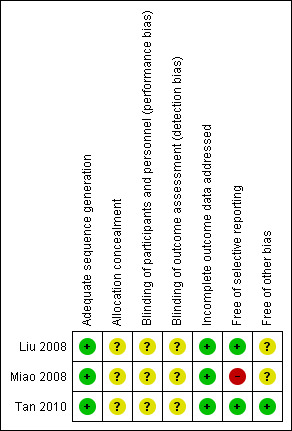
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
We assessed the impact of individual bias domains on study results at endpoint and study levels.
Measures of treatment effect
We expressed dichotomous data as risk ratio (RR) with 95% confidence intervals (CI) and continuous data as differences in means (MD) with 95% CI.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome, if required.
Dealing with missing data
We performed evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat (ITT), as‐treated and per‐protocol (PP) populations. We investigated attrition rates, for example drop‐outs, losses to follow‐up and withdrawals and critically appraised issues of missing data and imputation methods (for example last‐observation‐carried‐forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical or methodological or statistical heterogeneity, we planned not to report study results as meta‐analytically pooled effect estimates.
We wanted to identify heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We would have specifically examined heterogeneity employing the I2 statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% and more indicated a considerable level of inconsistency (Higgins 2011).
When heterogeneity was found, we planned to determine potential reasons for it by examining individual study and subgroup characteristics.
We expected the following characteristics to introduce clinical heterogeneity.
Age.
Gender.
Compliance.
Comorbidity.
Disease duration.
Dose.
Treatment course.
Dosing frequency.
Assessment of reporting biases
Funnel plots would have been used for 10 or more studies to assess small study bias.
Data synthesis
We planned primarily to summarise low‐risk of bias data by means of a random‐effects model. We would have performed statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses of the primary outcome parameter(s) (see above) and investigate interaction. If a sufficient number of randomised trials were identified, we would have performed subgroup analyses according to formulation of herbs (extract, single herb, or mixture of herbs), and treatment duration (short‐term and long‐term).
The following subgroup analyses were planned.
Formulation of herbs (extract, single herb, or mixture of herbs).
Treatment duration (no more than six months and more than six months).
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size.
Restricting the analysis to published studies.
Restricting the analysis taking account risk of bias, as specified above.
Restricting the analysis to very long or large studies to establish how much they dominate the results.
Restricting the analysis to studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country, trials with significant skewed distributions of participants in groups (Pocock 1990).
We also wanted to test the robustness of the results by repeating the analysis using different measures of effect size (RR, odds ratio (OR), etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
Results of the search
The initial search using the electronic search strategies listed in the appendices yielded 1952 studies. Twelve unpublished studies were identified. After duplicates of different databases were removed, 1875 potentially relevant articles were kept for further assessment. After scanning the studies identified, we recognised 10 studies that could not be excluded by scrutiny of the title and abstract only. Further investigation of the full articles revealed three included studies (three included publications). Seven studies were excluded and the reasons for exclusion are listed under 'Characteristics of excluded studies'. We prepared a PRISMA flow diagram to describe the articles found from our searches (Figure 1). No difference on the assessment on the eligibility of the studies was found between the two authors.
Missing data
There were no missing data In the three included studies.
Dealing with duplicate or companion publications
We did not find duplicate or companion documents of primary publications.
Included studies
Three randomised clinical trials (RCTs) were included in this review. Further details are given in the 'Characteristics of included studies' table. All trials employed a parallel two‐arm design. None of the studies compared Chinese herbal medicines versus placebo. Each of the three comparisons: Chinese herbal medicines versus benzbromarone, Chinese herbal medicines plus gemfibrozil versus gemfibrozil and Chinese herbal medicines plus lifestyle intervention versus fenofibrate plus lifestyle intervention was tested in one trial, respectively. All the included studies were published studies.
Participants
One trial did not specify the diagnostic criteria (Liu 2008); the other two trials specified the diagnostic criteria of the participants in the publications (Miao 2008; Tan 2010). All three trials specified exclusion criteria . None of the included trials specified the number of screened populations. A total of 170 participants with hypertriglyceridaemia were included in the three trials; all recruited from Chinese populations. All of the randomised participants completed the study. One trial reported that the age of the participants ranged from 25 to 71 years (Miao 2008). The other two trials did not report the age range of the participants (Liu 2008; Tan 2010). One trial did not report the number of male and female participants (Tan 2010). Among the 120 participants in two trials, the proportion of males to females was 65 to 55. One trial included both inpatients and outpatients (Liu 2008). Two trials did not specify the setting (Miao 2008; Tan 2010). The pertinent patient populations of the included studies are shown in Table 2.
1. Overview of study populations.
|
Characteristic Study ID |
Intervention(s) and comparator(s) | [N] screened / eligible | [N] randomised | [N] safety | [N] ITT | [N] finishing study | [%] of randomised participants finishing study |
| Liu 2008 | Zhusuan Huoxue decoction plus lifestyle intervention | ‐ | 30 | 30 | 30 | 30 | 100 |
| Fenofibratea | 30 | 30 | 30 | 30 | 100 | ||
| total: | 60 | 60 | 60 | 60 | 100 | ||
| Miao 2008 | Huoxue Huayu Tongluo decoction plus gemfibrozil | ‐ | 30 | 30 | 30 | 30 | 100 |
| Gemfibrozilb | 30 | 30 | 30 | 30 | 100 | ||
| total: | 60 | 60 | 60 | 60 | 100 | ||
| Tan 2010 | Chushi Huayu decoction | ‐ | 30 | 30 | 30 | 30 | 100 |
| Benzbromaronec | 20 | 20 | 20 | 20 | 100 | ||
| total: | 50 | 50 | 50 | 50 | 100 | ||
| Total | All interventions | 90 | 90 | ||||
| All comparators | 80 | 80 | |||||
| All interventions and comparators | 170 | 170 | |||||
"‐" denotes not reported aFenofibrate: a cholesterol‐lowering drug belonging to the fibric acid derivatives group bGemfibrozil: a triglycerides‐lowering drug belonging to the fibric acid derivatives group cBenzbromarone: a potent uricosuric agent for treatment of gout introduced in the 1970 but also used as a triglycerides lowering drug
ITT: intention‐to‐treat
Interventions
In total, three different herbal medicines were tested (Table 3; Appendix 2). The formulations of all the tested herbal medicines were decoctions. Three different herbal medicines, including Zhusuan Huoxue decoction, Huoxue Huayu Tongluo decoction,and Chushi Huayu decoction, which contain Astragalus membranaceous, were evaluated in the included trials. The three formulae each contained more than nine herbal ingredients, belonging to three classes in traditional Chinese medicine: herbs for tonifying qi, such as Astragalus membranaceous, which appeared in all the three formulas, herbs for removing dampness; such as Poria cocos,Rheum palmatum, Plantago asiatica; and herbs for promoting blood circulation, such as Saliva miltiorrhiza, Carthamus tinctorius, and Panax notoginseng.
2. Chinese herbal medicines and their ingredients.
| Study ID | Chinese formula | Botanical name of ingredient |
| Liu 2008 | Zhusuan Huoxue decoction |
Astragalus membranaceous,Poria cocos,Alisma plantago‐aquatica, Plantago asiatica, Rheum palmatum, Eucommia ulmoides, Codonopsis pilosula,Panax notoginseng, Carthamus tinctorius, Saliva miltiorrhiza, Imperata cylindrica. |
| Miao 2008 | Huoxue Huayu Tongluo decoction |
Saliva miltiorrhiza, Carthamus tinctorius, Angelica sinensis, Ligusticum chuanxiong, Paeonia lactiflora, Rehmannia glutinosa, Astragalus membranaceous. Additional herbs for hypertension: Gastrodia elata, Uncaria macrophylla. Additional herbs for coronary heart disease: Curcuma aromatica, Citrus aurantium. Additional herbs for peripheral neuropathy: Lonicera japonica, Trachelospermum jasminoides. Additional herbs for retinopathy: Lycium barbarum, Chrysanthemum morifolium. Additional herbs for nephropathy: Rehmannia glutinosa, Cornus officinalis. |
| Tan 2010 | Chushi Huayu decoction | Smilax glabra, Dioscorea hypoglauca, Coix lacryma‐jobi var. ma‐yuen, Leonurus heterophylus, Lysimachia christinae, Plantago asiatica, Saliva miltiorrhiza, Astragalus membranaceous,Rheum palmatum, Glycyrrhiza uralensis. |
No trial reported quality standards of the herbal preparations. The control interventions included lifestyle intervention, fenofibrate capsules, gemfibrozil tablets and benzbromarone tablets. The duration of treatment varied from four to six weeks.
Outcomes
None of the trials reported outcomes on cardiovascular and cerebrovascular events or death from any cause. Serum triglyceride levels (TG) and adverse effects were reported in all studies. Uric acid levels were reported in two studies (Liu 2008; Tan 2010). Symptoms were reported in one study (Tan 2010). Detailed information is shown in Appendix 5.
Study details
None of the three studies mentioned whether they had a run‐in period and all the studies terminated as scheduled.
Publication details
Two of the three studies were published in 2008 (Liu 2008; Miao 2008) and one in 2010 (Tan 2010). All three studies were conducted in China and all were published in peer‐reviewed journals in Chinese. None of the trials were claimed as commercially funded.
Excluded studies
Seven studies were excluded. They were excluded due to ineligible participants, or non‐eligible interventions, or missing elaboration of methods of random sequence generation. Detailed information is shown in Characteristics of excluded studies.
Risk of bias in included studies
The trial reports provided very limited information about design and methodology. No multi‐centre RCTs were identified in our searches. None of the trials reported a sample size calculation. None of the trials stated that they performed an intention‐to‐treat analysis, but missing data were not reported in the trials. Assessment of risk of bias is described for each included study in the Characteristics of included studies table. Review authors' judgements about each 'Risk of bias' item is presented as percentages across all included studies in Figure 2, and review authors' judgements about each 'Risk of bias' item for each included study is shown in Figure 3. No difference emerged on the assessment of risk of bias of the included studies between review authors.
Allocation
All the studies reported an adequate random sequence generation. Detailed information is shown in Characteristics of included studies and Figure 3.
Blinding
None of the studies reported blinding. Detailed information is shown in the Characteristics of included studies table and Figure 3.
Incomplete outcome data
Incomplete outcome data were adequately addressed in all studies. Detailed information is shown in the Characteristics of included studies table and Figure 3.
Selective reporting
None of the trials had a published protocol. According to the nominated outcomes in the methods section and the reported outcomes in the results section, we made a judgement on this domain. Two trials were free of selective reporting (Liu 2008; Tan 2010). Detailed information is shown in the Characteristics of included studies table and Figure 3.
Other potential sources of bias
We did not detect any specific type of other potential risk of bias.
Effects of interventions
See: Table 1
Primary Outcomes
Cardiovascular and cerebrovascular events
None of the trials reported these outcomes.
Death from any cause
None of the trials reported this outcome.
Serum triglyceride concentrations
Serum triglyceride concentrations (TG) were reported in all three studies. Compared to benzbromarone tablets, Chushihuayu decoction showed beneficial effects on lowering TG with a mean difference (MD) of ‐1.14 mmol/L (95% confidence interval (CI) ‐1.40 to ‐0.88; 50 participants, 1 trial; Analysis 1.1). Compared to gemfibrozil, Huoxue Huayu Tongluo decoction plus gemfibrozil did not show beneficial effects on lowering TG with a MD of ‐0.32 mmol/L (95% CI ‐0.69 to ‐0.05; 60 participants; 1 trial; Analysis 2.1). Compared to gemfibrozil, Huoxue Huayu Tongluo decoction plus gemfibrozil showed beneficial effects on reducing the number of patients with TG 2.2 mmol/L or more with a RR of 0.20 (95% CI 0.05 to 0.84; 60 participants, 1 trial; Analysis 2.2). Compared to fenofibrate plus lifestyle intervention, significantly higher TG levels were reported in the Zhusuan Huoxue decoction plus lifestyle intervention group with a MD of 0.51 mmol/L (95% CI 0.31 to 0.71; 60 participants; 1 trial; Analysis 3.1).
1.1. Analysis.
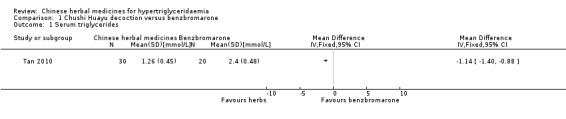
Comparison 1 Chushi Huayu decoction versus benzbromarone, Outcome 1 Serum triglycerides.
2.1. Analysis.
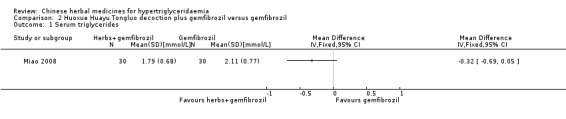
Comparison 2 Huoxue Huayu Tongluo decoction plus gemfibrozil versus gemfibrozil, Outcome 1 Serum triglycerides.
2.2. Analysis.
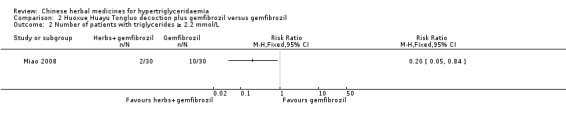
Comparison 2 Huoxue Huayu Tongluo decoction plus gemfibrozil versus gemfibrozil, Outcome 2 Number of patients with triglycerides ≥ 2.2 mmol/L.
3.1. Analysis.
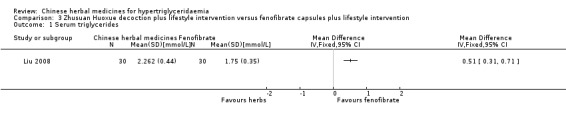
Comparison 3 Zhusuan Huoxue decoction plus lifestyle intervention versus fenofibrate capsules plus lifestyle intervention, Outcome 1 Serum triglycerides.
Secondary Outcomes
Health‐related quality of life
None of the trials reported this outcome.
Serum cholesterol levels
None of the trials reported this outcome.
Weight, body mass index (BMI), waist‐to‐hip ratio (WHR)
None of the trials reported these outcomes.
Adverse events
Adverse events were observed in all three studies. Adverse reactions including gastrointestinal adverse reactions, renal colic, acute arthritis (Tan 2010), abdominal discomfort (Miao 2008) and elevated alanine‐aminotransferase levels (ALT) (Liu 2008) were reported. When comparing fenofibrate plus lifestyle intervention versus Zhusan Huoxue decoction plus lifestyle intervention (Liu 2008), there was a higher number of individuals with elevated ALT (two versus zero cases, respectively ‐ Analysis 3.2). Other comparisons also did not reveal statistically significant differences between intervention and comparator groups (Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 2.3).
3.2. Analysis.
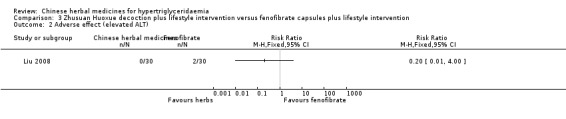
Comparison 3 Zhusuan Huoxue decoction plus lifestyle intervention versus fenofibrate capsules plus lifestyle intervention, Outcome 2 Adverse effect (elevated ALT).
1.2. Analysis.
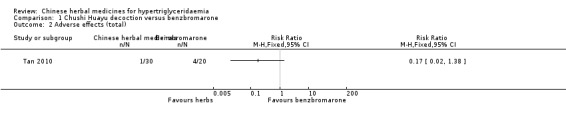
Comparison 1 Chushi Huayu decoction versus benzbromarone, Outcome 2 Adverse effects (total).
1.3. Analysis.
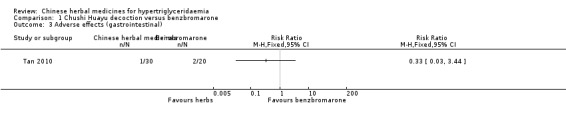
Comparison 1 Chushi Huayu decoction versus benzbromarone, Outcome 3 Adverse effects (gastrointestinal).
1.4. Analysis.
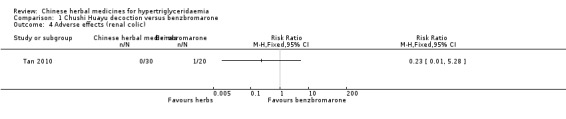
Comparison 1 Chushi Huayu decoction versus benzbromarone, Outcome 4 Adverse effects (renal colic).
1.5. Analysis.
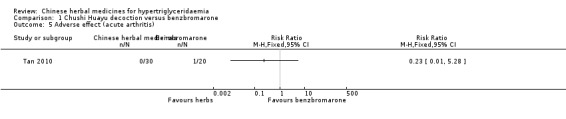
Comparison 1 Chushi Huayu decoction versus benzbromarone, Outcome 5 Adverse effect (acute arthritis).
2.3. Analysis.
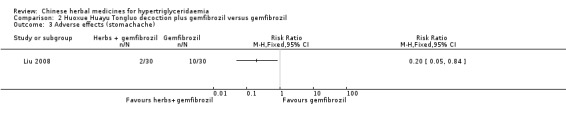
Comparison 2 Huoxue Huayu Tongluo decoction plus gemfibrozil versus gemfibrozil, Outcome 3 Adverse effects (stomachache).
Costs
None of the trials reported this outcome.
Reporting biases
We did not construct funnel plots to examine for publication and small study bias because we could not perform a meta‐analysis in this systematic review. When there are fewer than 10 studies in a comparison, it is difficult to assess asymmetry by visual examination, and the power of the funnel plot test is too low to distinguish chance from real asymmetry (Higgins 2011). None of the included trials was a non‐published study. None of the included trials had multiple publications.
Subgroup analyses
No subgroup analyses were done due to the limited number of trials.
Sensitivity analyses
No sensitivity analyses were done due to the limited number of trials.
Discussion
Summary of main results
Three randomised controlled trials (RCTs) with 170 participants are included in this review. The present systematic review suggests that Chinese herbal medicines used alone or in combination with other lipid‐lowering drugs or lifestyle changes may have positive effects on reducing the blood levels of triglycerides (TG). However, the Chinese herbal medicine interventions did not show consistent effects on serum TG concentrations. Chushihuayu decoction showed beneficial effects on lowering TG, Huoxue Huayu Tongluo decoction plus gemfibrozil did not. Huoxue Huayu Tongluo decoction plus gemfibrozil showed beneficial effects on reducing the number of patients with a TG of 2.2 mmol/L and more, while higher TG levels were reported in the Zhusuan Huoxue decoction plus lifestyle intervention group. No serious adverse events were reported. However, definite conclusions on the efficacy of Chinese herbal medicines could not be reached because of the mostly unclear risk of bias in studies and lack of reporting on patient‐important and long‐term outcomes.
Overall completeness and applicability of evidence
The age and gender of participants in the included trials were representative of hypertriglyceridaemic persons. All participants were recruited from Chinese populations and this could impact the applicability of the interventions to other populations. No long‐term data on outcomes were reported in the included trials. Many types of Chinese herbal medicines with possible lipid‐lowering effects have not been investigated in RCTs.
Quality of the evidence
All of the RCTs included in this review showed a mostly unclear risk of bias in more than one 'Risk of bias' domains. Study publications provided only limited descriptions of study design, allocation concealment and baseline data. No multi‐centre, large scale RCTs were found. Methodologically poorly designed trials show larger differences between experimental and control groups than those conducted rigorously (Kjaergard 2001; Moher 1998; Schulz 1995). However, the insufficient number of adequate trials prohibited us from performing meaningful sensitivity analyses to show how robust the results of the review are after restricting analyses to trials with adequate methodology. Moreover, the included trials were heterogeneous in the interventions (three different herbal medicines tested only once).
Potential biases in the review process
Although we conducted comprehensive searches, we only identified and included three trials; all of them were conducted and published in Chinese. All of the trials had small sample sizes. We tried to avoid language bias and location bias, but we could not exclude potential dissemination bias. We have undertaken extensive searches for unpublished material, few of the trials identified qualified for inclusion, but at the same time we cannot disregard the fact that trials with negative findings might remain unpublished.
Agreements and disagreements with other studies or reviews
To our knowledge, no systematic review has been undertaken with a focus on Chinese herbal medicines for hypertriglyceridaemia. A systematic review on Chinese red yeast rice for dyslipidaemia was published in 2006 (Liu J 2006). This review included 93 trials with red yeast rice as the intervention and placebo as the control. Patients with primary hyperlipidaemia were included. Meta‐analyses were conducted by grouping different controls and the conclusion of the review was that current evidence from randomised trials shows short‐term beneficial effects of red yeast rice preparations on lipid modification (Liu J 2006). None of the trials from Liu J 2006 were included in our review. We excluded studies mostly because the participants were not patients with hypertriglyceridaemia, and therefore, did not meet our inclusion criteria. Though the two reviews did not focus on the same events, the conclusions of the review (Liu J 2006) and this review are similar as hyperlipidaemia and hypertriglyceridaemia are closely related.
Authors' conclusions
Implications for practice.
Based on this systematic review, we cannot be certain of the efficacy and safety of Chinese herbal medicines in the treatment of hypertriglyceridaemia. The evidence is inconclusive due to small number of included studies, unclear risk of bias in major domains and the inconsistency of results in the included studies.
Implications for research.
For herbal mixtures, rigorous description of the pharmacology of the interventions and clinical outcomes should be emphasised. Future studies should be carried out on a formula based on our three included trials and in multiple trial centres. Standardised monitoring and reporting should be used for assessment of adverse events. Evaluation of patient‐important and long‐term outcomes is necessary.
Acknowledgements
We thank Ruan Yao and Wu Qiong for their contributions on this protocol. Jian Ping Liu was in part supported by grant number R24 AT001293 from the National Center for Complementary and Alternative Medicine of the US National Institutes of Health.
We thank Karla Bergerhoff of the Cochrane Metabolic and Endocrine Disorders Group for her help in the development of the search strategy.
ZLL acknowledges the support from the Australian Endeavour Award Program (Award holder number 2758_2012).
Appendices
Appendix 1. Search strategies
| Search terms |
| Unless otherwise stated, search terms are free text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| #1 MeSH descriptor Hypertriglyceridemia explode all trees #2 (hypertriglyceridaemia* in All Text or hypertriglyceridaemia* in All Text or hypertriglyceridaemia* in All Text or hypertriglyceridaemia* in All Text) #3 (#1 or #2) #4 MeSH descriptor Drugs, Chinese herbal explode all trees #5 MeSH descriptor Phytotherapy explode all trees #6 MeSH descriptor Herbal medicine explode all trees #7 MeSH descriptor Plants, medicinal explode all trees #8 MeSH descriptor Plant preparations explode all trees #9 MeSH descriptor Plant extracts explode all trees #10 MeSH descriptor Medicine, Chinese traditional explode all trees #11 MeSH descriptor Medicine, Kampo explode all trees #12 ((herbal in All Text near/6 remed* in All Text) or (herbal in All Text near/6 extract* in All Text) or (herbal in All Text near/6 preparation* in All Text) or (herbal in All Text near/6 mixture* in All Text) or (herbal in All Text near/6 medic* in All Text) ) #13 ((phyto in All Text near/6 drug* in All Text) or (phyto in All Text near/6 pharmaceutical* in All Text) or (phyto in All Text near/6 therap* in All Text) or (phyto in All Text near/6 treatment* in All Text) or (phyto in All Text near/6 medici* in All Text) ) #14 ((Chinese in All Text near/6 herb* in All Text) or (Chinese in All Text near/6 plant* in All Text) or (Chinese in All Text near/6 medic* in All Text) or (Chinese in All Text near/6 drug* in All Text) or (Chinese in All Text near/6 formul* in All Text) or (Chinese in All Text near/6 prescri* in All Text) ) #15 ((plant* in All Text near/6 preparation* in All Text) or (plant* in All Text near/6 extract* in All Text) or (plant* in All Text near/6 medic* in All Text) ) #16 ((Chinese in All Text near/6 traditional in All Text) and medic* in All Text) #17 (botanical in All Text and extract* in All Text) #18 (#4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or a#14 in All Text or #15 or #16 or #17) #19 (#3 and #18) |
| MEDLINE |
| 1 exp Hypertriglyceridemia/ 2 (hypertrigly?erid?emia* or Hypertriacylgly?erid?emia*).tw,ot. 3 1 or 2 4 exp Drugs, Chinese Herbal/ 5 exp Phytotherapy/ 6 exp Herbal Medicine/ 7 exp Plants, Medicinal/ 8 exp Plant Preparations/ 9 exp Plant Extracts/ 10 exp Medicine, Kampo/ 11 exp Medicine, Chinese traditional/ 12 (herbal adj6 (remed* or extract* or preparation* or mixture* or medic*)).tw,ot. 13 (phyto adj6 (drug* or pharmaceutical* or therap* or treatment* or medici*)).tw,ot. 14 (Chinese adj6 (herb* or plant* or medic* or drug* or formul* or prescri*)).tw,ot. 15 (plant* adj6 (preparation* or extract* or medic*)).tw,ot. 16 (Chinese adj6 traditional medic*).tw,ot. 17 botanical extract*.tw,ot. 18 or/4‐17 19 3 and 18 20 (animals not (humans and animals)).sh. 21 19 not 20 |
| EMBASE |
| 1 exp hypertriglyceridaemia/ 2 (hypertrigly?erid?emia* or hypertriacylgly?erid?emia*).tw,ot. 3 1 or 2 4 exp medicinal plant/ or exp plant extract/ or exp Chinese drug/ or exp Chinese herb/ or exp herbaceous agent/ or exp Chinese medicine/ or exp herbal medicine/ 5 exp phytotherapy/ 6 (herbal adj6 (remed* or extract* or preparation* or mixture* or medic*)).tw,ot. 7 (phyto adj6 (drug* or pharmaceutical* or therap* or treatment* or medici*)).tw,ot. 8 (Chinese adj6 (herb* or plant* or medic* or drug* or formul* or prescri*)).tw,ot. 9 (plant* adj6 (preparation* or extract* or medic*)).tw,ot. 10 (Chinese adj6 traditional medic*).tw,ot. 11 botanical extract*.tw,ot. 12 or/4‐11 13 3 and 12 14 limit 13 to human |
| Chinese BioMedical Database |
| 1 MeSH =="hypertriglyceridaemia /all subtitles/all trees" 2 MeSH ==" hyperlipoproteinemias/all subtitles/all trees" 3 hypertriglyceridaemia 4 MeSH==" triglyceride /BL/all trees" 5 triglyceride ascend 6 hyperlipoproteinemias 7 MeSH=="medicine, eastern tradition/all subtitles/all trees" 8 MeSH=="complementary medicine/" 9 MeSH=="plant extracts/all subtitles/all trees" 10 MeSH=="Plant, Therapeutic Use /All subtitles" 11 MeSH=="drugs, over the counter" 12 MeSH=="Phytotherapy/All subtitles/all trees" 13 Herbs or herbal or herb 14 Alternative medicine* 15 Complementary medicine* 16 Traditional medicine* 17 plant or plants 18 (China or The East) with medicine* 19 Plant medicinal product * or medicinal materials or herbal medicine 20 Chinese herbal drugs * or Chinese medicine* 21 Traditional Chinese Medicine * 22 Traditional Chinese Medicine and Western Medicine* 23 MeSH=="animals/all trees" 24 not #23 25 MeSH="Chinese herbal drugs/all subtitles" 26 #6 or #5 or #4 or #3 or #2 or #1 27 #25 or #22 or #21 or #20 or #19 or #18 or #17 or #16 or #15 or #14 or #13 or #12 or #11 or #10 or #9 or #8 or #7 28 #27 and #26 and #24 29 Title: rats or rabbits 30 (not #29) and #28 31 #30 and (summary in MH or summary in PT) 32 (not #31) and #30 |
| China National Knowledge Infrastructure |
| 1 triglyceride ascend or high triglyceride or hypertriglyceridaemia or hyperlipidaemia 2 Chinese herbal medicine or Chinese medicine or Chinese and western or plants or herbs 3 1 and 2 |
| Chinese VIP Information |
| 1 triglyceride ascend or high triglyceride or hypertriglyceridaemia or hyperlipidaemia 2 Chinese herbal medicine or Chinese medicine or Chinese and western or plants or herbs 3 1 and 2 |
| Chinese Academic Conference Papers Database and Chinese Dissertation Database |
| hypertriglyceridaemia and (Chinese medicine or Chinese herbal medicine or Chinese and Western or plants or herbs) and randomizations |
Appendix 2. Description of interventions
|
Characteristic Study ID |
Intervention(s) [route, frequency, total dose/day] | Comparator(s) [route, frequency, total dose/day] |
| Liu 2008 | Zhusuan Huoxue decoction plus lifestyle intervention (oral, twice daily) | Fenofibrate capsules plus lifestyle intervention (oral, once daily, 0.2 g/day) |
| Miao 2008 | Huoxue Huayu Tongluo decoction (oral, twice daily, 300 mL/day) plus gemfibrozil (oral, twice daily, 0.4 g/day) | Gemfibrozil tablets (oral, twice daily, 0.4 g/day) |
| Tan 2010 | Chushi Huayu decoction, oral, twice/day, 400 mL/day, one dose/day | Benzbromarone tablets, oral, once/day, 50 mg/day |
Appendix 3. Baseline characteristics (I)
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Duration of intervention | Duration of follow‐up | Participating population | Country | Setting | Ethnic groups [%] | Duration of disorder [mean years (SD)/range] |
| Liu 2008 | Zhusuan Huoxue decoction plus lifestyle intervention | 4 weeks | No follow‐up | Participants with hypertriglyceridaemia and hyperuricaemia | China | in‐ and outpatients | ‐ | ‐ |
| Fenofibrate capsules plus lifestyle intervention | ‐ | ‐ | ||||||
| Miao 2008 | Huoxue Huayu Tongluo decoction plus gemfibrozil | 6 weeks | No follow‐up | Participants with hypertriglyceridaemia and type 2 diabetes | China | ‐ | ‐ | ‐ |
| Gemfibrozil tablets | ‐ | ‐ | ||||||
| Tan 2010 | Chushi Huayu decoction | 30 days | No follow‐up | Participants with hypertriglyceridaemia and hyperuricaemia | China | ‐ | ‐ | ‐ |
| Benzbromarone tablets | ‐ | ‐ | ||||||
|
Footnotes "‐" denotes not reported SD: standard deviation | ||||||||
Appendix 4. Baseline characteristics (II)
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Sex [female %] | Age [mean years (SD)] | BMI [mean kg/m2 (SD)] | Co‐medications / Co‐interventions | Co‐morbidities |
| Liu 2008 | Zhusuan Huoxue decoction plus lifestyle intervention | 20 | 60 (13) | ‐ | Lifestyle intervention | Hyperuricaemia |
| Fenofibrate capsules plus lifestyle intervention | 13 | 60 (11) | ‐ | Lifestyle intervention | Hyperuricaemia | |
| Miao 2008 | Huoxue Huayu Tongluo decoction plus gemfibrozil | 40 | 54 (8) | ‐ | Gemfibrozil tablets | Type 2 diabetes, hypertension (12), coronary heart disease (9) |
| Gemfibrozil tablets | 43 | 54 (8) | ‐ | Gemfibrozil tablets | Type 2 diabetes, hypertension (12), coronary heart disease (7) | |
| Tan 2010 | Chushi Huayu decoction | ‐ | ‐ | ‐ | None | Hyperuricaemia, hypertension (9), diabetes (3), coronary heart disease (8), kidney stones (4) |
| Benzbromarone tablets | ‐ | ‐ | ‐ | None | Hyperuricaemia, hypertension (6), diabetes (1), coronary heart disease (6), kidney stones (3) | |
|
Footnotes "‐" denotes not reported; BMI: body mass index; SD: standard deviation | ||||||
Appendix 5. Matrix of study endpoints (publications)
|
Characteristic Study ID |
Primary endpoint(s)a [time of measurement] | Secondary endpoint(s)a [time of measurement] | Other endpoint(s)a [time of measurement] |
| Liu 2008 | ‐ | ‐ | Uric acid (0, 4 wk), triglycerides (0, 4 wk), adverse effects (0, 4 wk) |
| Miao 2008 | ‐ | ‐ | Triglycerides (‐1, 7 wk), adverse effects (‐1, 7 wk) |
| Tan 2010 | ‐ | ‐ | Symptoms (0, 30 d), uric acid (0, 30 d), triglycerides (0, 4 wk), adverse effects (0, 30 d) |
|
Footnotes aPrimary or secondary endpoint(s) refer to verbatim statements in the publication, other endpoints relate to outcomes which were not specified as 'primary' or 'secondary' outcomes in the publication "‐" denotes not reported; d: days; wk: weeks | |||
Appendix 6. Adverse events (I)
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Deaths [n/N] | All adverse events [n/N (%)] | Severe/serious adverse events [n/N (%)] | Drop‐outs due to adverse events [n/N (%)] |
| Liu 2008 | Zhusuan Huoxue decoction plus lifestyle intervention | 0/30 | 0/30 | 0/30 | 0/30 |
| Fenofibrate capsules plus lifestyle intervention | 0/30 | 2/30 (6.7) | 0/30 | 0/30 | |
| all: | 0/60 | 2/60 (3.3) | 0/60 | 0/60 | |
| Miao 2008 | Huoxue Huayu Tongluo decoction plus gemfibrozil | 0/30 | 0/30 | 0/30 | 0/30 |
| Gemfibrozil tablets | 0/30 | 3/30 (10.0) | 0/30 | 0/30 | |
| all: | 0/60 | 3/60 (5.0) | 0/60 | 0/60 | |
| Tan 2010 | Chushi Huayu decoction | 0/30 | 1/30 (3.3) | 0/30 | 0/30 |
| Benzbromarone tablets | 0/20 | 4/20 (20.0) | 0/20 | 0/20 | |
| all: | 0/50 | 5/50 (10.0) | 0/50 | 0/50 |
Appendix 7. Adverse events (II)
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Hospitalisation [n/N (%)] | Out‐patient treatment [n/N (%)] | Symptoms [n/N (%)] |
| Liu 2008 | Zhusuan Huoxue decoction plus lifestyle intervention | 0/30 | 0/30 | 0/30 |
| Fenofibrate capsules plus lifestyle intervention | 0/30 | 0/30 | 0/30 | |
| all: | 0/60 | 0/60 | 0/60 | |
| Miao 2008 | Huoxue Huayu Tongluo decoction plus gemfibrozil | 0/30 | 0/30 | 0/30 |
| Gemfibrozil tablets | 0/30 | ‐ | 3/30 | |
| all: | 0/60 | ‐ | 3/60 | |
| Tan 2010 | Chushi Huayu decoction | 0/30 | 0/30 | 1/30 (3.3) |
| Benzbromarone tablets | 0/20 | 0/20 | 4/20 (20.0) | |
| all: | 0/50 | 0/50 | 5/50 (10.0) | |
|
Footnotes "‐" denotes not reported | ||||
Appendix 8. Survey of authors providing information on included trials
|
Characteristic Study ID |
Study author contacted | Study author replied | Study author provided data |
| Liu 2008 | Y | Y (author's email: docterabcde@163.com) | The detailed compositions of Zhusuan Huoxue decoction were: Astragalus membranaceous,Poria cocos,Alisma plantago‐aquatica, Plantago asiatica, Rheum palmatum, Eucommia ulmoides, Codonopsis pilosula,Panax notoginseng, Carthamus tinctorius, Saliva miltiorrhiza, Imperata cylindrica. |
| Miao 2008 | N | N/A | N/A |
| Tan 2010 | N | N/A | N/A |
|
Footnotes N: no; Y: yes; N/A: not applicable | |||
Data and analyses
Comparison 1. Chushi Huayu decoction versus benzbromarone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse effects (total) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects (gastrointestinal) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse effects (renal colic) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse effect (acute arthritis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Huoxue Huayu Tongluo decoction plus gemfibrozil versus gemfibrozil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients with triglycerides ≥ 2.2 mmol/L | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects (stomachache) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Zhusuan Huoxue decoction plus lifestyle intervention versus fenofibrate capsules plus lifestyle intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse effect (elevated ALT) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Liu 2008.
| Methods |
Parallel randomised controlled clinical trial Comparative study design (2‐sided confidence interval) Randomisation ratio: 1:1 |
|
| Participants |
Inclusion criteria: primary hyperuricaemia, blood uric acid concentration > 420 mmol/L (male), > 360 mmol/L (female); TG > 117 mmol/L; age: 18 ‐ 75 years old; participants without using uric acid‐lowering drugs Exclusion criteria: acute gout episode patients; secondary hyperuricaemia patients; patients with severe primary co‐morbidities on heart, liver, renal and blood systems and mental disorders Diagnostic criteria: not mentioned in the original article |
|
| Interventions |
Number of study centres: 1 Treatment before study: not mentioned in the original article Titration period: 4 weeks |
|
| Outcomes | Outcomes reported in abstract of publication: blood uric acid, TG | |
| Study details |
Run‐in period: not mentioned in the original article Study terminated before regular end: no |
|
| Publication details |
Language of publication: Chinese Unclear funding Publication status: peer review journal |
|
| Stated aim of study | Quote: "To investigate the clinical effectiveness and safety of Zhusuanhuoxue decoction on the patients suffered from primary hyperuricaemia combined with hypertriglyceridaemia." | |
| Notes | TG: triglycerides | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Quote: "Random number table was used to distribute the 60 participants to two groups" |
| Allocation concealment | Unclear risk | Comment: no detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data addressed All outcomes | Low risk | Comment: no missing data |
| Free of selective reporting | Low risk | Comment: all the prespecified outcomes in the methods section of the article were reported in the results section |
| Free of other bias | Unclear risk | Comment: no information on the basic characteristics of the two groups of participants and no information on funding |
Miao 2008.
| Methods |
Parallel randomised controlled clinical trial Comparative study design (2‐sided confidence interval) Randomisation ratio: 1:1 |
|
| Participants |
Inclusion criteria: not specified Exclusion criteria: participants with severe liver or kidney diseases or hypothyroidism or drug‐induced liver injury, or familial dyslipidaemia and those refused to comply with the trial Diagnostic criteria: TG ≥ 2.26 mmol/L, FBG < 7mmol/L, PBG < 10mmol/L; TCM differentiation: vein blood stasis syndrome |
|
| Interventions |
Number of study centres: 1 Treatment before study: not mentioned in the original article Titration period: 6 weeks |
|
| Outcomes | Outcomes reported in abstract of publication: TG | |
| Study details |
Run‐in period: not mentioned in the original article Study terminated before regular end: no |
|
| Publication details |
Language of publication: Chinese Unclear funding Publication status: peer review journal |
|
| Stated aim of study | Quote: "To investigate the clinical effectiveness and safety of Chinese integrative medicine on patients suffering from hypertriglyceridaemia with type 2 diabetes" | |
| Notes | FBG: fasting blood glucose; PBG: plasma blood glucose; TCM: traditional Chinese medicine; TG: triglycerides | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Quote: "Random number table was used to distribute the 60 participants to two groups" |
| Allocation concealment | Unclear risk | Comment: no detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data addressed All outcomes | Low risk | Comment: no missing data |
| Free of selective reporting | High risk | Comment: adverse effects were reported in the results section but were not prespecified in the methods section of the article |
| Free of other bias | Unclear risk | Comment: no information on the basic characteristics of the two group participants and no information on funding |
Tan 2010.
| Methods |
Parallel randomised controlled clinical trial Comparative study design (2‐sided confidence interval) Randomisation ratio: 3:2 |
|
| Participants |
Inclusion criteria: not mentioned in the original article Exclusion criteria: secondary hyperuricaemia; active stage of gouty arthritis; patients with severe primary co‐morbidities on heart, liver, renal and blood systems and mental disorders; age less than 18 years old; pregnant or breast feeding period; allergic condition or allergic to the tested medicine; do not meet the inclusion criteria; do not comply the trial or data missing; illegible TCM differentiation. Diagnostic criteria: TG > 1.7 mmol/L; SUA > 440 mmol/L with acute gouty arthritis attack at least twice a year or SUA > 535 mmol/L without symptoms; TCM differentiation: damp and hot clip stasis. |
|
| Interventions |
Number of study centres: 1 Treatment before study: not mentioned in the original article Titration period: 30 days |
|
| Outcomes | Outcomes reported in abstract of publication: blood triglycerides, SUA and symptoms | |
| Study details |
Run‐in period: not mentioned in the original article Study terminated before regular end: no |
|
| Publication details |
Language of publication: Chinese No commercial funding Publication status: peer review journal |
|
| Stated aim of study | Quote: "To observe the clinical effects of Chushihuayu decoction on the level of serum uric acid and triglycerides in patients with hyperuricaemia and hypertriglyceridaemia" | |
| Notes | SUA: serum uric Acid; TCM: traditional Chinese medicine; TG: triglycerides | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Quote: "Random number table was used to distribute the 60 participants to two groups" |
| Allocation concealment | Unclear risk | Comment: no detailed information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data addressed All outcomes | Low risk | Comment: no missing data |
| Free of selective reporting | Low risk | Comment: all the prespecified outcome in the methods section of the article were reported in the results section |
| Free of other bias | Low risk | Comment: the basic characteristics of the two group participants were comparable and no commercial funding; other risk of bias features not detected |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cui 2005 | Claimed randomised clinical study without elaboration of the methods of random sequence generation. The intervention and the control group both used Chinese herbal medicines and the intervention group did not meet the inclusion criteria of this review. |
| Feng 2004 | Claimed randomised clinical study without elaboration of the methods of random sequence generation. The participants included hypercholesterolaemic and hypertriglyceridaemic patients and did not meet the inclusion criteria. |
| Li 2010 | Claimed randomised clinical study without elaboration of the methods of random sequence generation. The participants included hypercholesterolaemic and hypertriglyceridaemic patients and did not meet the inclusion criteria. |
| Lin 1999 | Claimed randomised clinical study without elaboration of the methods of random sequence generation. The participants included hypercholesterolaemic and hypertriglyceridaemic patients and did not meet the inclusion criteria. |
| Liu 2006 | Claimed randomised clinical study without elaboration of the methods of random sequence generation. The participants included hypercholesterolaemic and hypertriglyceridaemic patients and did not meet the inclusion criteria. |
| Lv 2005 | Claimed randomised clinical study without elaboration of the methods of random sequence generation. The participants included hypercholesterolaemic and hypertriglyceridaemic patients and did not meet the inclusion criteria. |
| Yang 2009 | Claimed randomised clinical study without elaboration of the methods of random sequence generation. |
Differences between protocol and review
Review authors have changed since the published protocol. One review author (Qiong Wu) was removed and one review author (Kelvin Chan) was added.
Contributions of authors
Zhao Lan Liu (ZLL): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and update draft.
George Qian Li (GQL): protocol draft, search strategy development, trial selection, data extraction, data analysis, data interpretation and update draft.
Alan Bensoussan (LB): protocol draft, data interpretation and update draft.
Hosen Kiat (HK): protocol draft, review draft and update draft.
Kelvin Chan (KC): data interpretation and update draft.
Jian Ping Liu (JPL): protocol draft, search strategy development, trial selection, data extraction, data analysis, data interpretation, review draft and update draft.
Sources of support
Internal sources
Beijing University of Chinese Medicine (2011‐CXTD‐09), Beijing, China.
External sources
Grant number 2011ZX09302‐006 from the Ministry of Science and Technology, China.
Grant number JYBZZ‐JS006 from Beijing University of Chinese Medicine, China.
Grant number CSO‐51 from Global fund for HIV, China.
The Project for Standard Operation Procedure of Clinical Appraisal in the Program for Significant New Drugs Development (2011ZX09302‐006‐01‐03(5), China.
Declarations of interest
None known.
New
References
References to studies included in this review
Liu 2008 {published data only}
- Liu YQ, Shi SQ, Zhao Y, Lu JS, Li YF, Yan WH, et al. Therapeutic effect of uric‐acid‐sequester herbal extract on the primary hyperuricemia and hypertriglyceridemia. Clinical Practice Medicine 2008;3(34):60‐6. [Google Scholar]
Miao 2008 {published data only}
- Miao GZ, Wang YJ, Cao PL, Zhu XM, Wang LQ, Miu Juan, et al. The clinical observation of integrative medicine for type 2 diabetes and hypertriglyceridaemia patients. Beijing Journal of Traditional Chinese Medicine 2008;27(6):458‐9. [Google Scholar]
Tan 2010 {published data only}
- Tan N, Huang SG, Zhou RY, Li DY, Zhu HJ. Clinical observation of Chushihuayu decoction on the treatment of hyperuricemia and hypertriglyceridemia. Chinese Journal of Information on Traditional Chinese Medicine 2010;17(3):9‐11. [Google Scholar]
References to studies excluded from this review
Cui 2005 {published data only}
- Cui AQ, Liu GR. The observation of clinical effectiveness of Lipi Tiaozhi capsule on hypertriglyceridaemia. Shandong Journal of Traditional Chinese Medicine 2005;24(7):398‐9. [Google Scholar]
Feng 2004 {published data only}
- Feng YY, Li NF. The observation on effectiveness and safety of Xuezhikang on hyperlipidaemia patients. Journal of Chinese Medicine Research 2004;4(10):883‐5. [Google Scholar]
Li 2010 {published data only}
- Li H. The observation of effectiveness of Tiedanxin soft capsule for hypertriglyceridaemia. Chinese Medicine Modern Distance 2010;8(16):79‐80. [Google Scholar]
Lin 1999 {published data only}
- Lin SM, Chen SW, Chen C. A clinical study of Guben Jiangzhi pill for old patients with hyperlipoproteinemia ‐ with the data analysis of 160 cases. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai 1999;13(1):16‐9. [Google Scholar]
Liu 2006 {published data only}
- Liu YT, Wang ZY. The effectiveness of Cattail pollen capsule on 58 dyslipidaemia patients. Chinese Journals of Practical Medicine 2006;1(6):63‐4. [Google Scholar]
Lv 2005 {published data only}
- Lv SF, Cao KQ, Wang PY. The clinical observation of Modified Zexie decoction on hyperlipidaemia. Clinical Journal of Traditional Chinese Medicine 2005;17(5):454‐5. [Google Scholar]
Yang 2009 {published data only}
- Yang AN, Xu YX, Yang BP, Li D. 40 cases of familial hypertriglyceridaemia treated with Qiwu Taozhi granule. Gansu Journal of Traditional Chinese Medicine 2009;22(3):27‐8. [Google Scholar]
Additional references
Anderson 2011
- Anderson F, Mbatha SZ, Thomson SR. The early management of pancreatitis associated with hypertriglyceridaemia. South African Journal of Surgery 2011;49(2):82‐4. [PUBMED: 21614978] [PubMed] [Google Scholar]
Barter 2006
Beltowski 2009
Chait 1992
- Chait A, Brunzell JD. Chylomicronemia syndrome. Advances in Internal Medicine 1992;37:249‐73. [PUBMED: 1557997] [PubMed] [Google Scholar]
Christian 2011
- Christian JB, Bourgeois N, Snipes R, Lowe KA. Prevalence of severe (500 to 2,000 mg/dl) hypertriglyceridemia in United States adults. American Journal of Cardiology 2011;107(6):891‐7. [PUBMED: 21247544] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hokanson 1996
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high‐density lipoprotein cholesterol level: a meta‐analysis of population‐based prospective studies. Journal of Cardiovascular Risk 1996;3(2):213‐9. [PUBMED: 8836866] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. Code of Federal Regulations & International Conference on Harmonisation Guidelines. Pennsylvania: Parexel/Barnett, 1997. [Google Scholar]
Jacobson 2009
Ji 2008
- Ji W, Gong BQ. Hypolipidemic activity and mechanism of purified herbal extract of Salvia miltiorrhiza in hyperlipidemic rats. Journal of Ethnopharmacology 2008;119(2):291‐8. [PUBMED: 18691646] [DOI] [PubMed] [Google Scholar]
Jing 2009
- Jing A, Li‐Mei Z, Yan‐Jie L, Ben‐Zhi C, Yong Z, Bao‐Feng Y. A randomized, multicentre, open‐label, parallel‐group trial to compare the efficacy and safety profile of daming capsule in patients with hypercholesterolemia. Phytotherapy Research 2009;23(7):1039‐42. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kumar 2005
- Kumar J, Wierzbicki AS. Images in clinical medicine. Lipemia retinalis. The New England Journal of Medicine 2005;353(8):823. [PUBMED: 16120862] [DOI] [PubMed] [Google Scholar]
Le 2007
- Le NA, Walter MF. The role of hypertriglyceridemia in atherosclerosis. Current Atherosclerosis Reports 2007;9(2):110‐5. [PUBMED: 17877919] [DOI] [PubMed] [Google Scholar]
Li 2005
- Li LM, Rao KQ, Kong LZ, Yao CH, Xiang HD, Zhai FY, et al. [A description on the Chinese national nutrition and health survey in 2002]. Zhonghua Liu Xing Bing Xue Za Zhi 2005;26(7):478‐84. [PUBMED: 16334996] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu J 2006
- Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fonnebo V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta‐analysis of randomized controlled trials. Chinese Medical Journal 2006;23:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2008
- Martinez DP, Diaz JO, Bobes CM. Eruptive xanthomas and acute pancreatitis in a patient with hypertriglyceridemia. International Archives of Medicine 2008;1(1):6. [PUBMED: 18474088] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Mota 2004
- Mota M, Panus C, Mota E, Lichiardopol C, Vladu D, Toma E. The metabolic syndrome ‐ a multifaced disease. Romanian Journal of Internal Medicine 2004;42(2):247‐55. [PUBMED: 15529615] [PubMed] [Google Scholar]
NCEP 2001
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in AdultsNational Cholesterol Education Program (NCEP). Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285(19):2486‐97. [DOI] [PubMed] [Google Scholar]
Pocock 1990
- Pocock, S.J. Clinical Trials: A Practical Approach. John Wiley & Sons, UK, 1990. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Sun 2009
- Sun YC. Treatment of 60 cases of hypertriglyceridemia with Huayuchushisan. Shanxi Journal of Traditional Chinese Medicine 2009;30(9):1163. [Google Scholar]
Wang 2010
- Wang YZ, Han XL. Clinical observation on hypertriglyceridemia treated by Yiqisanju formula in 24 patients. Hebei Journal of Traditional Chinese Medicine 2010;32(7):999. [Google Scholar]
Wei 2006
- Wei ASH, Ye JH, Chen P, Jian KK, Liu T, Lang JM. Effects of Yinchenwulingsan on insulin resistance of hypertriglyceridemia patients. New Journal of Traditional Chinese Medicine 2006;38(11):44. [Google Scholar]
WHO 2000
- WHO. Noncommunicable diseases in South‐East Asia region: a profile. http://209.61.208.233/LinkFiles/NCD_InforBase_ncd‐profile.pdf (accessed April 10, 2013).
Xing 2007
- Xing Y, Yue P, Sun LH, Zhao WX, Wang Y, Zhang Y, et al. [Effect of Daming capsule on expression of connexin43 isoforms in hyperlipemic rat's cardiac muscle]. Zhongguo Zhong Yao Za Zhi 2007;32(14):1440‐5. [PUBMED: 17966361] [PubMed] [Google Scholar]
Zhai 2007
- Zhai ZS, Han XL. Treatment of 24 cases of hypertriglyceridemia with Buyang Huanwu decoction. Hebei Journal of Traditional Chinese Medicine 2007;29(9):807. [Google Scholar]
Zhang 2007
- Zhang SJ, Cheng ZX, Lin YW, Qin J, Cheng YH, Liu SL. [Effection of composite salviae dropping pill on hyperlipemia patients with phlegm and blood stasis syndrome]. Zhongguo Zhong Yao Za Zhi 2007;32(5):440‐3. [PubMed] [Google Scholar]
Zhang 2011
- Zhang J, Dong Z, Li G, Jiao SF, Xie J, Zhou Y, et al. A cross‐sectional study on risk factors of associated type 2 diabetes mellitus among adults in Beijing. Zhonghua Liu Xing Bing Xue Za Zhi 2011;32(4):357‐60. [PUBMED: 21569666] [PubMed] [Google Scholar]


